Background: Cdk5 activated by p39 has not been characterized, likely because of its instability.
Results: Hydrogen bond interaction was reduced between p39 and Cdk5 and experimentally confirmed with amino acid substitution mutants of p35 and p39.
Conclusion: Instability of p39-Cdk5 is caused by decreased hydrogen bond interaction.
Significance: Structural basis of the instability of p39-Cdk5 was unveiled.
Keywords: CDK (Cyclin-dependent Kinase), Computer Modeling, Cyclins, Neurons, Serine-Threonine Protein Kinase, Cdk5, Hydrogen Bond, p35, p39, Subunit Interaction
Abstract
Cyclin-dependent kinase 5 (Cdk5) is a brain-specific membrane-bound protein kinase that is activated by binding to the p35 or p39 activator. Previous studies have focused on p35-Cdk5, and little is known regarding p39-Cdk5. The lack of functional understanding of p39-Cdk5 is due, in part, to the labile property of p39-Cdk5, which dissociates and loses kinase activity in nonionic detergent conditions. Here we investigated the structural basis for the instability of p39-Cdk5. p39 and p35 contain N-terminal p10 regions and C-terminal Cdk5 activation domains (AD). Although p35 and p39 show higher homology in the C-terminal AD than the N-terminal region, the difference in stability is derived from the C-terminal AD. Based on the crystal structures of the p25 (p35 C-terminal region including AD)-Cdk5 complex, we simulated the three-dimensional structure of the p39 AD-Cdk5 complex and found differences in the hydrogen bond network between Cdk5 and its activators. Three amino acids of p35, Asp-259, Asn-266, and Ser-270, which are involved in hydrogen bond formation with Cdk5, are changed to Gln, Gln, and Pro in p39. Because these three amino acids in p39 do not participate in hydrogen bond formation, we predicted that the number of hydrogen bonds between p39 and Cdk5 was reduced compared with p35 and Cdk5. Using substitution mutants, we experimentally validated that the difference in the hydrogen bond network contributes to the different properties between Cdk5 and its activators.
Introduction
Cyclin-dependent kinases (Cdks)2 are Ser/Thr kinases that are activated by binding to a cyclin regulatory subunit (1, 2). The Cdk-cyclin complexes play a critical role in cell cycle progression at several cell cycle points, including the G1/S and G2/M checkpoints. In yeast, a single Cdk, Cdk1, is utilized throughout the entire cell cycle by activation with different cyclins at different cell cycle phases. Thus, Cdk1 mediates different cell cycle events in combination with different cyclins. In contrast, in mammalian cells, several Cdk family members are activated by their respective partner cyclins at specific cell cycle phases. Nevertheless, many mammalian cell cycle Cdks are still activated by multiple cyclins. Only a few cyclin-specific functions are known for particular Cdks, and the others remain to be investigated.
Cdk5, a unique member of the Cdks, is activated in postmitotic neurons by the p35 and p39 activators, which share no amino acid sequence homology with cyclins. Cdk5 is a multifunctional protein kinase, playing an important role in neuronal development, survival, and synaptic activity (3–5). However, how p35 and p39 contribute to these various Cdk5 functions remains unknown. Mice lacking Cdk5 die perinatally with abnormal positioning of neurons in many brain regions, including the cerebral and cerebellar cortices (6, 7). p35 and p39 double-null mice show identical phenotypes to Cdk5−/− mice (8). Mice deficient in p35 are viable but exhibit inverted layering of neurons in the cerebral cortex (9). In contrast, p39−/− mice show no obvious defects (8). These observations suggest that Cdk5-p39 may have a distinct physiological role, even if most p39 functions could be compensated by p35-Cdk5.
In addition to the physiological functions described above, Cdk5 is involved in pathological neurodegeneration. Abnormal activation of Cdk5, which is induced by the cleavage of p35 to the p25 C-terminal activation domain (AD) by calpain (10–12), is associated with Alzheimer and Parkinson diseases (5, 10, 13, 14). Calpain cleavage alters the cellular localization of active Cdk5 (10–12, 15, 16) and lengthens the half-life (10, 17). On the other hand, Cdk5 activity is shown to be protective against Huntingtin aggregation toxicity (18–20). Thus, regulation of Cdk5 kinase activity is critical for protection against these diseases.
p39 is an isoform of p35 and was isolated from a cDNA library of the mouse brain hippocampus by PCR using the nucleotide sequence of p35 (21, 22). p35 and p39 have 57% amino acid identity, with a particularly high homology of 72% in the C-terminal Cdk5 AD, which is also called the globular domain after similar folding to cyclin A (23). However, in contrast to p35-Cdk5, which has been studied intensively, little is known about p39-Cdk5. This is due to the difficulty of handling the p39-Cdk5 complex. p39-Cdk5 is inactivated by dissociation in the presence of nonionic detergent (24), in contrast to Cdk5-p35, which is activated in these conditions. Nonetheless, nonionic detergent must be used for isolation of the active Cdk5 complex, because p35 and p39 associate with membranes through N-terminal myristoylation (10, 15).
Here, we studied the structural basis of the different stabilities of Cdk5 complexed with p35 or p39. We found that three amino acids in α-helices 5 and 6 in the AD of p35 and p39 are the source for the different observed stabilities.
EXPERIMENTAL PROCEDURES
Materials
The anti-Cdk5 antibodies C8 and DC17 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FLAG monoclonal antibody (M2) was obtained from Sigma. Peroxidase-conjugated goat anti-mouse IgG and alkaline phosphatase-conjugated swine anti-rabbit IgG were purchased from Dako (Glostrup, Denmark). Histone H1 was obtained from Roche Diagnostics. Leupeptin was purchased from the Peptide Institute (Osaka, Japan), and 4-[2-aminoethyl]benzenesulfonyl fluoride hydrochloride (Pefabloc SC) was from Merck (Darmstadt, Germany). Protein A/G Plus-Agarose was purchased from Santa Cruz Biotechnology. [γ-32P]ATP was obtained from PerkinElmer Life Sciences.
Construction of Expression Plasmids for p35 and p39 Mutants
Generation of mouse p35 and p39 cDNA in pFLAG-CMV2 vector was described previously (24). Truncation mutants or amino acid substitution mutants of p35 or p39 were generated by PCR-based mutagenesis. The primers used are as follows: 5′-GGGAAGCTTGGGCCCAAACGGGTCATCGTCC-3′ and 5′-AAAGGATCCAAACTACTCATTCTTCAAGTCAGAG-3′ for p35 AD; 5′-CCCAAGCTTGGGGGCTCGCCGCGGCGG-3′ and 5′-CGGGATCCCGCTAGCCCTCGTTCTTGAGG-3′ for p39 AD; 5′-TGTAAGGAAGCCTTTTGGCAACGTTGCCTCTCAGTTATC-3′ and 5′-GATAACTGAGAGGCAACGTTGCCAAAAGGCTTCCTTACA-3′ for p35 AD-D259Q; 5′-GCCTCTCAGTTATCCAGCTCATGAGCTCCA-3′ and 5′-TGGAGCTCATGAGCTGGATAACTGAGAGGC-3′ for p35 AD-N266Q; and 5′-CTCATGAGCTCCAAGATGCTGCAG-3′ and 5′-CTGCAGCATCTTGGAGCTCATGAG-3′ for p35 AD-S270P.
The double amino acid mutants, p35 AD-D259Q/N266Q, p35 AD-D259Q/S270P, and p35 AD-N266Q/S270P, were constructed using the single amino acid mutants above as templates and the primers described above. p35 and p35 AD with triple amino acid mutations, p35-D259Q/N266Q/S270P (p35-TM) and p35 AD-D259Q/N266Q/S270P (p35 AD-TM), were constructed using p35-D259Q/N266Q as template and primers for S270P.
p39 and p39 AD with triple amino acid mutations, p39-Q295D/Q302N/P306S (p39-TM) and p39 AD-Q295D/Q302N/P306S (p39 AD-TM), we constructed by mutating three amino acids sequentially using p39 or p39 AD as template and the following primers: 5′-CGCTTCTGGGACCGCTGCCTG-3′ and 5′-CAGGCAGCGGTCCCAGAAGCG-3′ for the Q295D mutation; 5′-CGCCTCATCAACCGGCTCAGC-3′ and 5′-GCTGAGCCGGTTGATGAGGCG-3′ for the Q302N mutation; and 5′-CTCATGAGCTCCAAGATGCTGCAG-3′ and 5′-CTGCAGCATCTTGGAGCTCATGAG-3′ for the P306S mutation. All mutations were confirmed by DNA sequencing.
Cell Culture and Transfection
HEK293 cells were maintained in DMEM supplemented with 10% fetal bovine serum (Biowest, Nuaille, France) and transfected with expression vectors encoding Cdk5, p39, p35, or their mutants using PolyFect transfection reagent (Qiagen) according to the manufacturer's instructions. The cells were harvested 24 h after transfection and lysed in 20 mm MOPS, pH 7.5, 50 mm NaCl, 2 mm MgCl2,1 mm EGTA, 1 mm dithiothreitol, 0.2 mm Pefabloc SC, 10 μg/ml leupeptin. After centrifugation at 15,000 × g for 30 min at 4 °C, the supernatant extracts were removed for experimental analysis.
Immunoprecipitation
HEK293 cell extracts were incubated with anti-FLAG antibody M2 for 1 h at 4 °C, followed by incubation with Protein A/G Plus-Agarose for 1 h at 4 °C. After agarose beads were washed five times with cell lysis buffer in the presence or absence of 1% Nonidet P-40, the beads were collected by brief centrifugation and examined by immunoblotting. For the Cdk5 kinase assay, the agarose beads were further washed three times with kinase assay buffer (10 mm MOPS, pH 6.8, 1 m MgCl2, 0.1 mm EGTA, 0.1 mm EDTA).
Cdk5 Kinase Assay
The kinase activity of Cdk5 was measured in kinase assay buffer containing 0.1 mg/ml histone H1 and 0.1 mm [γ-32P]ATP at 35 °C for 30–60 min. The reaction was stopped by the addition of Laemmli's sample buffer and boiling for 3 min. After SDS-PAGE, the radioactivity incorporated into histone H1 was measured with a FLA7000 Bioimage analyzer (GE Healthcare).
Molecular Graphic Analysis of Hydrogen Bonds in the p25-Cdk5 Complex and Structural Modeling of p39AD-Cdk5
Hydrogen bonds between p25 and Cdk5 in the crystal structure of the p25-Cdk5 complexes (Protein Data Bank codes 1UNG, 1UNH, 1UNL, 3O0G, and 1H4L) (23, 25, 26) were explored using the viewing system of the Discovery Studio software (Accelrys, Inc., San Diego, CA) (supplemental Table S1). The structural model of the p39 AD-Cdk5 complex was simulated by the “Build Mutants” tool of the Discovery Studio software based on the crystal structures of p25-Cdk5.
RESULTS
The Different Stability between p35-Cdk5 and p39-Cdk5 Is Derived from the Cdk5 Activation Domain of p35 and p39
We previously reported that p39-Cdk5 prepared from rat brains or Sf9 cells is inactivated in the presence of nonionic detergent, in contrast to p35-Cdk5, which is activated in these conditions (24). The inactivation of p39-Cdk5 was due to dissociation of p39 from Cdk5. To investigate the molecular basis of the different binding affinity of p35-Cdk5 and p39-Cdk5, we overexpressed FLAG-tagged p35 and p39 in HEK293 cells, which do not express detectable levels of endogenous p35 or p39, allowing us to directly compare protein levels and analyze them by immunoprecipitation using the same antibody (Fig. 1A). First, we tested whether p39, as well as p35, activates Cdk5 in HEK293 cells, as determined by phosphorylation of co-transfected Tau. Tau was shifted upward to similar levels by Cdk5 when co-transfected with p35 or p39 (Fig. 1B), indicating that p39-Cdk5 has similar kinase activity as p35-Cdk5 in HEK293 cells. Next, we isolated the complexes by immunoprecipitation with anti-FLAG antibody in buffer containing low (0.1%) Nonidet P-40, under conditions in which we could isolate active p39-Cdk5 from brain extracts. In the kinase activity using histone H1 as a substrate, p39 immunoprecipitates showed little kinase activity, whereas p35 immunoprecipitates showed robust activity (Fig. 1C, left panel, H1). We noticed that Cdk5 levels were considerably lower in the p39 immunoprecipitates than in the p35 immunoprecipitates. Therefore, we increased the amount of p39 immunoprecipitates and found that H1 kinase activity was roughly similar to that of the p35 immunoprecipitates containing similar amounts of Cdk5 (Fig. 1C, right panel, Cdk5 and H1). In this case, p39 levels were considerably higher than p35, indicating that p39 has a lower binding affinity to Cdk5 even in low concentrations of Nonidet P-40.
FIGURE 1.
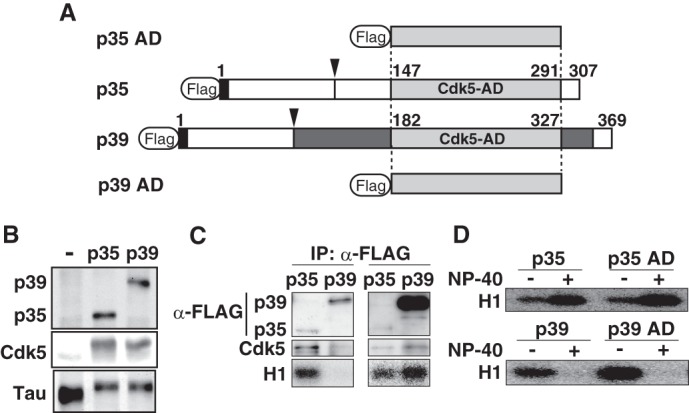
Different stability of p35-Cdk5 and p39-Cdk5 in nonionic detergent is caused by differences in the Cdk5-activation domain. A, schematic representation of full-length p35 and p39, and both C-terminal Cdk5-AD, p35 AD and p39 AD. p35 and p39 contain a Cdk5-AD (light gray) in the C-terminal region. The p35 AD and p39 AD consist of 145 and 146 amino acids, respectively, and share 72% identity. p39 has a Pro-rich sequence and additional residues flanking the Cdk5-AD (dark gray). The calpain cleavage site in p35 and p39 is indicated by the arrowhead. The extreme N-terminal nine amino acids (black), which include a myristoylation signal, are conserved between p35 and p39. B, kinase activity of p35-Cdk5 and p39-Cdk5 in HEK293 cells. The kinase activity of p35-Cdk5 and p39-Cdk5 was assessed by examining the phosphorylation-dependent upward shift of Tau, which was co-expressed and evaluated by immunoblotting (bottom panel). C, kinase activity of p35-Cdk5 and p39-Cdk5 expressed in HEK293 cells. p35 or p39 tagged with FLAG was expressed together with Cdk5 in HEK293 cells. p35-Cdk5 (left lanes) or p39-Cdk5 (right lanes) was immunoprecipitated (IP) with anti-FLAG antibody, and the immunoprecipitates were immunoblotted with anti-FLAG (top panels) or anti-Cdk5 (middle panels) antibody. The kinase activity of immunoprecipitates was measured using histone H1 and [γ-32P]ATP as substrates. D, kinase activity of p35-Cdk5, p35 AD-Cdk5, p39-Cdk5, and p39 AD-Cdk5. p35-Cdk5 and p35 AD-Cdk5 were immunoprecipitated in the absence of Nonidet P-40, followed by measurement of histone H1 kinase activity in the absence (−) or presence (+) of 1% Nonidet P-40 (upper panel). The kinase activity of p39-Cdk5 and p39 AD-Cdk5 was also measured (lower panel).
p39 shows high amino acid sequence identity (72%) to p35 in the α-helix-rich Cdk5 AD, with a longer N-terminal stretch immediately upstream of the AD and a C-terminal specific insertion (Fig. 1A, dark gray). We speculated that the N- or C-terminal regions flanking the AD could make p39 unstable and, if so, the AD alone might form a stable complex with Cdk5. We next constructed p39 AD and p35 AD (Fig. 1A) and compared their Cdk5 activating abilities in the presence of Nonidet P-40. However, they showed the same properties as the full-length molecules: p35 AD-Cdk5 was activated, and p39 AD-Cdk5 was inactivated in the presence of 1% Nonidet P-40 (Fig. 1D). At present, we do not know the mechanism by which p35 (p35 AD)-Cdk5 is activated by nonionic detergent. However, these results indicate that the different stability originates from the AD of p35 and p39.
The Difference in Hydrophobicity of the AD of p35 and p39
p35-Cdk5 and p39-Cdk5 show different sensitivity to nonionic detergents. We suspected their hydrophobicity might be different. Hydrophobicity of p35 AD and p39 AD was calculated by the method of Eisenberg et al. (27) (Fig. 2A). The globular AD consists of eight α-helices, including an N-terminal helix (Fig. 2A, αNT) and α1–α7 (Fig. 2A, α1–α7) (23). There are several apparent differences between p35 AD and p39 AD, but the biggest is in C-terminal α-helices α5 and α6 (Fig. 2A), which constitute the interface to Cdk5. We compared amino acid sequences in α5 and α6 and found that Ala-256, Ser-262, Leu-267, and Gln-274 in p35 are replaced with Arg at the corresponding amino acids 292, 299, 304, and 310 in p39 (Fig. 2A). When these amino acids in p35 were computationally replaced with Arg, the hydrophobicity of α5-α6 became almost identical to p39 (Fig. 2A, middle and bottom panels).
FIGURE 2.
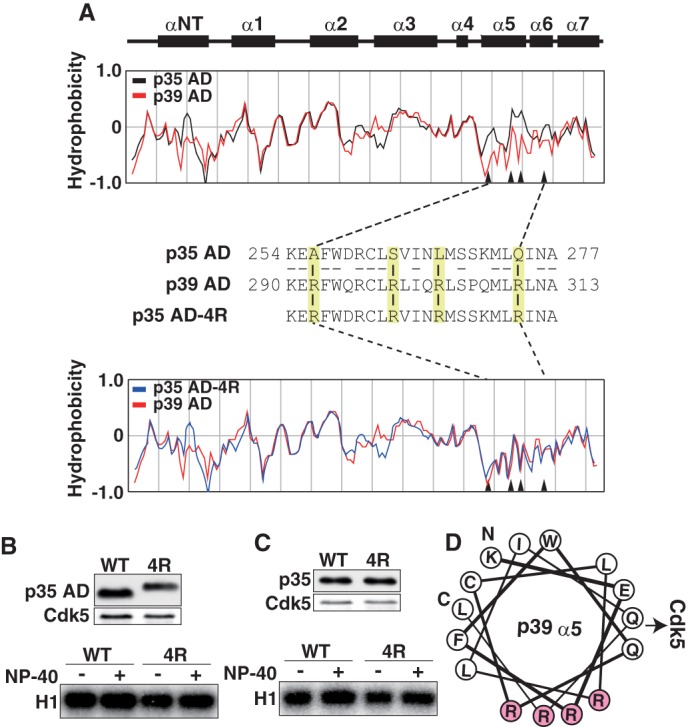
Different hydrophobicity of p35AD and p39 AD is not the cause of the different stability of Cdk5 complexes. A, the hydrophobicity of p35 AD and p39 AD. Upper two panels show the α-helices in p35 AD (23). The hydrophobicity of p35 AD (black) and p39 AD (red) is shown in the second panel. The third panel shows amino acid sequences of the α5-α6 region of p35 AD and p39 AD. Four amino acids in p35 AD, Ala-256, Ser-262, Leu-267, and Gln-274, are changed to arginine in p39 AD (yellow). The amino acid sequence of mutant p35 AD (p35 AD-4R) containing Arg at the above four sites is shown in the third line. The hydrophobicity of p35 AD-4R (blue) is compared with that of p39 AD (red) in the bottom panel. Arrowheads in hydrophobicity graphs indicate the four amino acids. B, kinase activity of p35 AD and p35 AD-4R. Expression of p35 AD (WT) or p35 AD-4R (4R) and Cdk5 in HEK293 cells was examined by immunoblotting with anti-FLAG (p35 AD) and anti-Cdk5 (Cdk5). p35 AD or p35 AD-4R was immunoprecipitated with anti-FLAG antibody. After washing in the absence (−) or presence (+) of 1% Nonidet P-40, the kinase activity of the immunoprecipitates was measured using histone H1 (H1) and [γ-32P]ATP as substrates (lower panel). C, kinase activity of p35 or p35–4R. Expression of p35 (WT) or p35–4R (4R) and Cdk5 in HEK293 cells is shown by immunoblotting with anti-FLAG (p35) or anti-Cdk5 (Cdk5). p35 or p35–4R was immunoprecipitated, and after washing in the absence (−) or presence (+) of 1% Nonidet P-40, the kinase activity was measured using histone H1 and [γ-32P]ATP as substrates (lower panel). D, diagram of the arrangement of amino acid residues in α5-helix, viewed down from the N-terminal Lys-290 (K) to the C-terminal Leu-311 (L) of the helix. All four Arg residues (R, red) are positioned in one side of the α-helix, perpendicular to the direction of Cdk5 binding.
We next constructed the 4R mutant of p35 AD (p35 AD-4R) and evaluated the kinase activity of Cdk5 associated with p35 AD or p35 AD-4R. p35 AD-4R showed a slower electrophoretic mobility than p35 AD on SDS-PAGE (Fig. 2B, upper panel), likely because of its higher isoelectric point. p35 AD-4R prepared in the presence of Nonidet P-40 displayed slightly higher histone H1 kinase activity than p35 AD-4R prepared in the absence of Nonidet P-40, as was observed with p35 AD (Fig. 2B, lower panel, WT). The effect of 4R mutation was also examined in the context of full-length p35. The 4R mutant did not change the sensitivity of the p35-Cdk5 complex to Nonidet P-40 (Fig. 2C).
Hydrogen Bond Interactions between p25 and Cdk5 and a Structural Model of the p39 AD-Cdk5 Complex
Next we considered hydrogen bonds as a possible source for the difference between p35 and p39 in binding to Cdk5. We first explored the hydrogen bond interactions between p25 (p35 AD) and Cdk5 in the five complexes of p25 (p35 AD)-Cdk5 registered in the Protein Data Bank (codes 1UNG, 1UNH, 1UNL, 3O0G, and 1H4L) (23, 25, 26). The hydrogen bond interactions are variable among the five crystal structures and also between the two asymmetric units consisting of A chain (p25)-D chain (Cdk5) and B chain (p25)-E chain (Cdk5) in respective crystal structures. The amino acid residues of p25 that interact with Cdk5 are listed in supplemental Table S1 and shown in the amino acid alignment of p35 AD and p39AD (Fig. 3A, blue). Most are conserved between p35 and p39, and only three amino acids at Asp-259, Asn-266, and Ser-270 in p35AD were changed to Gln-295, Gln-302, and Pro-306 in p39 AD (Fig. 3A, red). To evaluate the participation of the three amino acids of p39 in the interaction with Cdk5, the structural model of the p39-Cdk5 complex was made based on the crystal structure (Protein Data Bank code 1UNH) of the p25-Cdk5 complex (Fig. 3B, left panel). The overall conformation was similar, but the model revealed that the three amino acids of p39 do not participate in hydrogen bond formation with any amino acids of Cdk5 (Fig. 3B, right panel).
FIGURE 3.
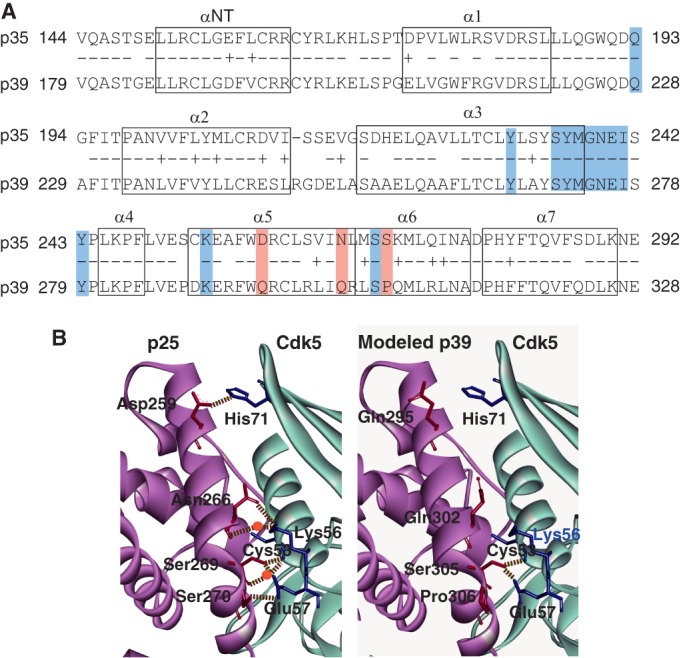
Hydrogen bond network between Cdk5 and p25 (p35 AD) or p39 AD. A, amino acid alignment and α-helix regions in p35 AD and p39 AD. Eight α-helices are indicated by boxes (23). Identical amino acids are indicated by −, and homologous amino acids are indicated by +. The amino acids that participate in the hydrogen bond network are shaded: the amino acids common to p35 and p39 are indicated in blue, and the three different amino acids are red. B, ribbon diagrams of the three-dimensional structure around the interface between Cdk5 and p25 (p35 AD) or p39 AD. The left panel is the crystal structure of p25 (A-chain)-Cdk5 (D-chain) registered in Protein Data Bank code 1UNH (25). p25 is represented by magenta, and Cdk5 is in light blue. The right panel is the three-dimensional structure of the corresponding region of p39 AD-Cdk5 predicted by in silico modeling; p39 AD is represented by magenta, and Cdk5 is in light blue. Hydrogen bonds are shown by dashed lines, and the water molecules that make a hydrogen bridge are indicated by red circles. Although Asp-259, Asn-266, and Ser-270 of p25 (p35 AD) form hydrogen bonds or hydrogen bridges with His-71, Lys-56, and Glu-57 of Cdk5, respectively, the corresponding amino acids, Gln-295, Gln-302, and Pro-306, of p39 AD do not form hydrogen bond networks with any amino acids of Cdk5.
The Triple Amino Acid Substitution Decreases the Stability of the p35-Cdk5 Complex
To validate the above simulation model, we first constructed single amino acid substitution mutants of p35 AD, from Asp-259 to Gln (D259Q), Asn-266 to Gln (N266Q), and Ser-270 to Pro (S270P) (Fig. 4A). Each mutant was transfected together with Cdk5 in HEK293 cells, and Cdk5 complex formation and kinase activity was examined in the absence or presence of 1% Nonidet P-40 (Fig. 4B). All mutants bound to Cdk5 and showed higher kinase activity when they were prepared in the presence of Nonidet P-40 compared with the absence of Nonidet P-40 (Fig. 4B, lower panel). We next generated double substitution mutants of p35 AD (D259Q/N266Q, D259Q/S270P, and N266Q/S270P; Fig. 4A) and examined Cdk5 binding and associated kinase activity. All of the double mutants bound Cdk5 and showed stronger kinase activity when prepared in the presence of Nonidet P-40 (Fig. 4C).
FIGURE 4.
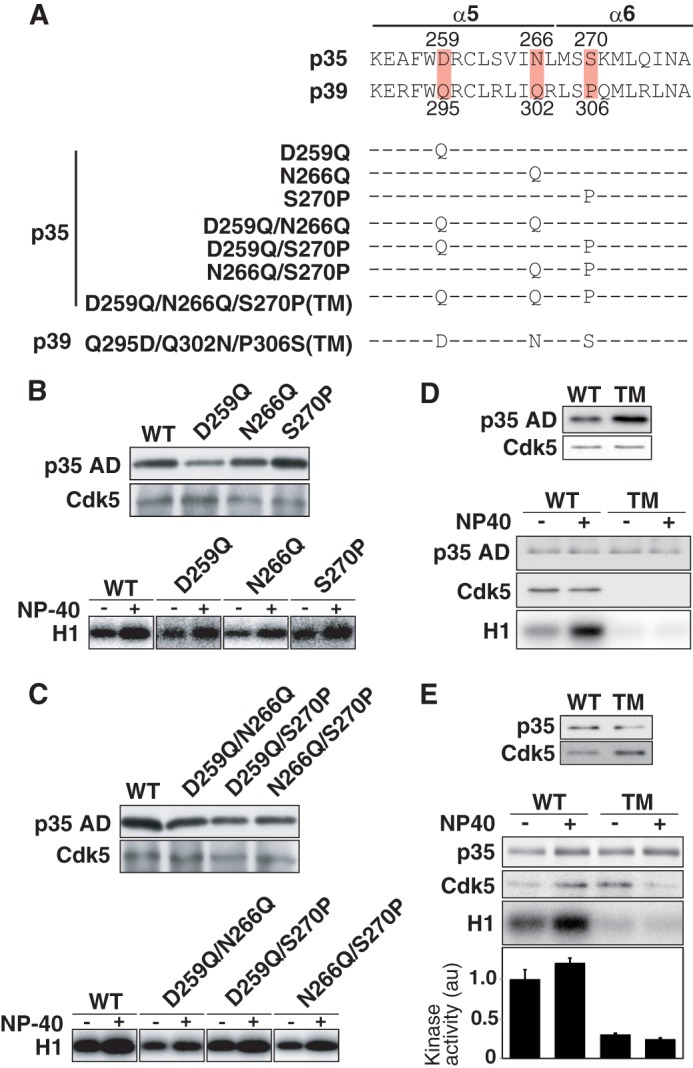
Amino acid substitution mutants of p35 AD and p39 AD. A, amino acid sequences of α5-α6 of p35 and p39. The three amino acids that are involved in hydrogen bond formation are highlighted. Substitution mutants used are shown below. B, p35 AD (WT) or one of the single amino acid mutants (D259Q, N266Q, and S270P) were co-expressed with Cdk5 in HEK293 cells. Their complexes with Cdk5 were immunoprecipitated with anti-FLAG and detected by immunoblotting with anti-FLAG (p35 AD or mutants) and anti-Cdk5 (upper panel). The kinase activity was measured using histone H1 (H1) and [γ-32P]ATP as substrates in the absence (−) or presence (+) of 1% Nonidet P-40 (lower panel). C, p35 AD (WT) or the double amino acid mutants (D259Q/N266Q, D259Q/S270P, and N266Q/S270P) were co-expressed with Cdk5 in HEK293 cells. Their complexes with Cdk5 were immunoprecipitated and detected by immunoblotting with anti-FLAG (p35 AD or mutants) and anti-Cdk5 (upper panel). The kinase activity was measured using histone H1 (H1) and [γ-32P]ATP as substrates in the absence (−) or presence (+) of 1% Nonidet P-40 (lower panel). D, p35 AD or its triple amino acid mutant (p35 AD-TM) was co-expressed with Cdk5 in HEK293 cells. Immunoblotting shows the expression of p35 AD (WT) or p35 AD-TM (TM) and Cdk5. After washing the immunoprecipitates in the absence (−) or presence (+) of 1% Nonidet P-40, p35 AD and Cdk5 (Cdk5) were examined by immunoblotting, and the kinase activity (H1) was measured using histone H1 and [γ-32P]ATP as substrates. E, expression of p35 (WT) or p35-TM (TM) and Cdk5 in HEK293 cells (upper panel). After washing the immunoprecipitates in the absence (−) or presence (+) of 1% Nonidet P-40, p35 or Cdk5 was examined by immunoblotting, and the kinase activity (H1) was measured using histone H1 and [γ-32P]ATP as substrates. Quantification is shown as the relative ratio to the kinase activity of Cdk5-p35 in the absence of Nonidet P-40 (means ± S.E., n = 3).
We next replaced three amino acids at Asp-259, Asn-266, and Ser-270 in p35 AD with corresponding amino acids of p39 AD, Gln, Gln, and Pro (p35 AD triple mutation (TM); Fig. 4A). p35 AD bound similar amounts of Cdk5 in the presence or absence of Nonidet P-40 (Fig. 4D, upper panel, WT) and showed higher H1 kinase activity when prepared in the presence of Nonidet P-40 (Fig. 4D, lower panel, left two lanes). In contrast, Cdk5 was not found in the immunoprecipitates of p35 AD-TM, and no H1 kinase activity was detected in the absence or presence of Nonidet P-40 (Fig. 4D, lower panel, right two lanes). We applied the triple mutation to full-length p35 (p35-TM) and examined its binding to Cdk5 and the associated kinase activity. The triple mutation destabilized the complex, which dissociated in the presence of Nonidet P-40 (Fig. 4E, right lane), and also decreased the kinase activity even in the absence of Nonidet P-40 (Fig. 4E, lower panel, H1, and quantification in graph below).
The Triple Amino Acid Substitution Increases the Stability of the p39-Cdk5 Complex
Finally, we examined whether the TM of p39 AD or p39 at Gln-295, Gln-302, and Pro-306 to Asp, Asn, and Ser, respectively, stabilizes complex formation with Cdk5. In p39 AD-TM immunoprecipitates from HEK293 cells co-expressing Cdk5, a strong Cdk5 signal was detected both in the presence and absence of Nonidet P-40, which was different from p39 AD (Fig. 5A, lower panel). The p39 AD-TM immunoprecipitates exhibited strong H1 kinase activity only in the absence of Nonidet P-40 (Fig. 5A, lower panel, H1), indicating that the H1 kinase activity was still abolished by the detergent treatment. We applied the TM to full-length p39 (p39-TM) and examined binding to Cdk5 and kinase activity. p39-TM bound Cdk5 and displayed H1 kinase activity both in the presence and absence of Nonidet P-40 (Fig. 5B). However, the kinase activity was reduced in the presence of Nonidet P-40, despite the association with Cdk5 (Fig. 5B, lower panel, H1). These results indicate that the hydrogen bond network between Cdk5 and its activator contributes to binding strength but not to the detergent-dependent activation of kinase activity.
FIGURE 5.
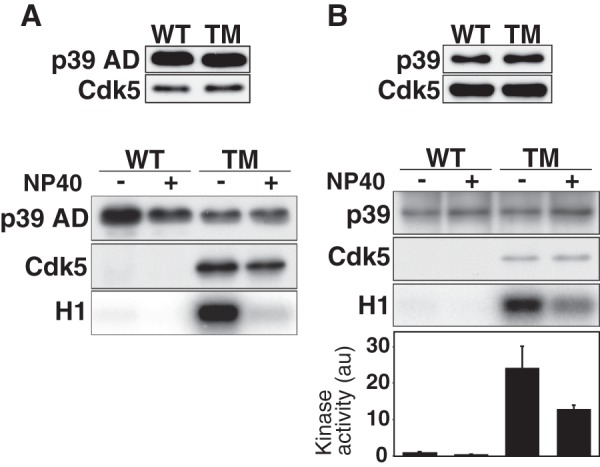
Triple mutation of p39AD or p39 increases the binding affinity to Cdk5. A, wild-type p39 AD or the triple amino acid mutant of p39 AD (p39 TM-AD, Q295D/Q302N/P306S) was co-expressed with Cdk5 in HEK293 cells. Immunoblotting shows expression of p39 AD (WT) or p39 AD-TM (TM) and Cdk5 (upper panel). p39 AD or p39 AD-TM complexed with Cdk5 was immunoprecipitated with anti-FLAG. After washing the immunoprecipitates in the absence (−) or presence (+) of 1% Nonidet P-40, p39 AD or p39 AD-TM and Cdk5 were examined by immunoblotting, and the kinase activity was measured using histone H1 (H1) and [γ-32P]ATP as substrates. B, wild-type p39 AD or the triple amino acid mutant, p39-TM, was co-expressed with Cdk5 in HEK293 cells. Immunoblots show expression of p39 (WT) or p39-TM (TM) and Cdk5 (upper panel). After washing the immunoprecipitates in the absence (−) or presence (+) of 1% Nonidet P-40, p39 WT or p39-TM and Cdk5 were examined by immunoblotting, and the kinase activity was measured using histone H1 (H1) and [γ-32P]ATP as substrates. Quantification is shown as the relative ratio to the kinase activity of Cdk5-p39 in the absence of Nonidet P-40 (means ± S.E., n = 3).
DISCUSSION
p39 is a dispensable Cdk5 activator (8). However, it is likely that p39 plays an unidentified role in activating Cdk5, although currently only limited knowledge is available for p39. One major reason for the lack of data on p39 may be the difficulty in handling the p39-Cdk5 complex. We previously showed that p39-Cdk5 is a labile complex that dissociates and is inactivated in the presence of nonionic detergents, which is in contrast to p35-Cdk5 that is stable and activated under the same conditions (24, 28). In this study, we used computer simulation to identify differences in p35-Cdk5 and p39-Cdk5 at the molecular level that could explain the apparent and different stability. Three amino acids in α-helices 5 and 6 in the globular AD of p35 that form the hydrogen bond network with Cdk5 were altered in p39, resulting in reduced hydrogen bond interactions. We experimentally confirmed this simulation using substitution mutants of these three amino acids in p35 and p39. The triple mutations destabilized p35-Cdk5 and increased the stability of p39-Cdk5.
p39-Cdk5 and p35-Cdk5 are both membrane-associated protein kinases (10, 15–17). Therefore, nonionic detergent is required for solubilization and isolation. Because p39-Cdk5 is dissociated in the presence of nonionic detergent, however, careful attention must be paid to the concentrations of nonionic detergent upon its preparation. For an example, p39-Cdk5 can be prepared from rat brain lysates by immunoprecipitation in reduced concentrations of nonionic detergent in isolation buffer (24, 29) and isolated p39-Cdk5 exhibited comparable kinase activity to p35-Cdk5 when the protein amounts of Cdk5 were adjusted (24). However, the p39 immunoprecipitates displayed much less activity compared with the p35 immunoprecipitates when they were expressed in HEK293 cells (Fig. 1B). Because we used FLAG-tagged versions of p35 and p39 in this study, we could directly compare the abundance of p35 or p39 associated with Cdk5, in contrast to the previous report (24), in which we used respective antibodies to p35 and p39 and could not directly compare their protein ratio. We found that a considerably large amount of p39 was required to obtain levels of Cdk5 comparable to that in p35 immunoprecipitates, indicating that p39 has weaker affinity to Cdk5 even in low concentrations of nonionic detergent. Nevertheless, comparable kinase activity was detected when the amount of Cdk5 was adjusted (Fig. 1B), suggesting that Cdk5 exhibits similar histone H1 kinase activity when bound to either p35 or p39.
p39 has unique amino acid sequences in the regions flanking the globular Cdk5-AD (Fig. 1A). The N-terminal region is an extension of the proline-rich sequence, and the C-terminal sequence is an insertion, where muskelin is reported to bind (30). First we speculated that these flanking sequences might compromise the stability of p39-Cdk5. However, this was not the case. The activation domain of p39 itself was found to contain the sequences that contribute to instability, despite the high homology of AD between p35 and p39. Major differences are found in α-helices 5 and 6, which constitute the interface to Cdk5 (Fig. 3A). The hydrophobicity was one of the differences found in the regions. The lower hydrophobicity of p39 was attributed to Arg at amino acids 292, 299, 304, and 310, whereas the corresponding amino acids in p35 are Ala-256, Ser-262, Leu-267, and Gln-274. These Arg residues also contribute to the higher isoelectric point of 11.9 of p39, compared with 8.29 of p35. However, the 4R mutation did not change the stability of Cdk5-p35 AD and Cdk5-p35 in nonionic detergent (Fig. 2B). Arg residues are positioned in intervals of three or four amino acids, indicating that these Arg residues reside at one side of the α-helix (Fig. 2A) that is perpendicular to the side that binds to Cdk5 (Fig. 2D). Therefore, these Arg residues are not involved in the direct interaction with Cdk5, although it is still possible that the basic nature of p39 may modulate the stability of the p39-Cdk5 complex in cells by providing the site for interaction with other proteins.
The three-dimensional structure modeling of p39 AD-Cdk5 based on five p25 (p35 AD)-Cdk5 complexes (Protein Data Bank codes 1UNG, 1UNH, 1UNL, 3O0G, and 1H4L) (23, 25, 26) pointed to the difference in the hydrogen bond network between p35-Cdk5 and p39-Cdk5, although their overall conformation is similar. The hydrogen bond network is formed between Asp-259, Asn-266, and Ser-270 of p35 and His-71, Lys-56, and Glu-57 of Cdk5 (Fig. 3B). In contrast, in p39, these amino acids are changed to Gln-295, Gln-302, and Pro-306, and they cannot form hydrogen bonds with Cdk5. The importance of these three amino acids in the interaction with Cdk5 was experimentally validated using substitution mutants; triple mutations in p35 dramatically reduced the stability of the complex with Cdk5 (Fig. 4), and triple mutations in p39 increased the stability of the complex with Cdk5 (Fig. 5). These results indicate the importance of the hydrogen bond network for the stability of Cdk5-activator complexes.
The physiological significance of the different affinity of p35 and p39 to Cdk5 remains elusive. We used mouse cDNA of p35 or p39 to activate Cdk5 in HEK293 cells. The amino acid sequences of α5-α6 of these activators are completely conserved in the mammalian species sequenced thus far, such as human, mouse, rat, bovine, and porcine, suggesting that these two activators play a similar role with similar properties in mammals. In other vertebrates, Xenopus and zebrafish are also reported to have two Cdk5 activators, which were assigned to mammalian p35 and p39 based on amino acid sequence homology. The AD of Xenopus p35 and p39 shares 84.2 and 84.1% identities with mammalian p35 and p39, respectively, and those of zebrafish show 91.7 and 77.1% identities, respectively (Fig. 6A). In specific analysis of the α5-α6 region, however, the two activators of both Xenopus and zebrafish show greater similarity to mammalian p35 rather than p39 (Fig. 6B). Two of the three amino acids, Asp-259, Asn-266, and Ser-270, of mammalian p35 are conserved in p35 of Xenopus and zebrafish, suggesting that Xenopus or zebrafish p35 forms a stable complex with Cdk5, as does mammalian p35. In contrast, whereas Xenopus p39 has Gln at the position of Gln-295 of mammalian p39, all three amino acids of zebrafish p39 were different from mammalian p39 (Fig. 6B). Looking at the entire sequence of α5–6 (Fig. 6B), it is known that p39 of both Xenopus and zebrafish have a closer resemblance to p35 than p39 (Fig. 6B), suggesting diversity of the stability and kinase activity of the complex with Cdk5. It may be interesting to examine the stability and activity of not only Xenopus and zebrafish Cdk5-activator complexes, but also various combinations of Cdk and cyclin complexes.
FIGURE 6.
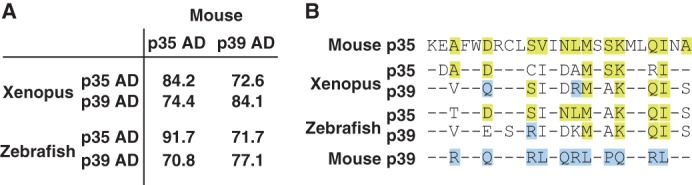
Amino acid comparisons of the AD of p35 and p39 among mouse, Xenopus and zebrafish. A, homology of p35 AD and p39 AD between mouse and Xenopus or zebrafish. Identity of amino acids of p35 AD and p39 AD between mouse and Xenopus or zebrafish is indicated as a percentage. B, amino acid alignment of α-helices 5 and 6 of p35 and p39 between mouse and Xenopus or zebrafish. Amino acids common to mouse p35 and p39 are represented by −. Amino acids identical to p35 but not p39 are indicated in yellow, whereas those identical to that of p39 are indicated in blue.
This work was supported by Grants-in-Aid for Scientific Research on Priority Area from MEXT of Japan (to S. H.).

This article contains supplemental Table S1.
- Cdk
- cyclin-dependent kinase
- AD
- activation domain
- TM
- triple amino acid mutation.
REFERENCES
- 1. Morgan D. O. (1997) Cyclin-dependent kinases. Engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13, 261–291 [DOI] [PubMed] [Google Scholar]
- 2. Murray A. W. (2004) Recycling the cell cycle. Cyclins revisited. Cell 116, 221–234 [DOI] [PubMed] [Google Scholar]
- 3. Hisanaga S., Endo R. (2010) Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J. Neurochem. 115, 1309–1321 [DOI] [PubMed] [Google Scholar]
- 4. Su S. C., Tsai L.-H. (2011) Cyclin-dependent kinases in brain development and disease. Annu. Rev. Cell Dev. Biol. 27, 465–491 [DOI] [PubMed] [Google Scholar]
- 5. Cheung Z. H., Ip N. Y. (2012) Cdk5. A multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 22, 169–175 [DOI] [PubMed] [Google Scholar]
- 6. Ohshima T., Ward J. M., Huh C. G., Longenecker G., Veeranna, Pant H. C., Brady R. O., Martin L. J., Kulkarni A. B. (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. U.S.A. 93, 11173–11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilmore E. C., Ohshima T., Goffinet A. M., Kulkarni A. B., Herrup K. (1998) Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci. 18, 6370–6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ko J., Humbert S., Bronson R. T., Takahashi S., Kulkarni A. B., Li E., Tsai L.-H. (2001) p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J. Neurosci. 21, 6758–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chae T., Kwon Y. T., Bronson R., Dikkes P., Li E., Tsai L.-H. (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18, 29–42 [DOI] [PubMed] [Google Scholar]
- 10. Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L.-H. (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 [DOI] [PubMed] [Google Scholar]
- 11. Kusakawa G., Saito T., Onuki R., Ishiguro K., Kishimoto T., Hisanaga S. (2000) Calpain-dependent proteolytic cleavage of the p35 CDK5 activtor to p25. J. Biol. Chem. 275, 17166–17172 [DOI] [PubMed] [Google Scholar]
- 12. Lee M. S., Kwon Y. T., Li M., Peng J., Friedlander R. M., Tsai L.-H. (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360–364 [DOI] [PubMed] [Google Scholar]
- 13. Cruz J. C., Tsai L. H. (2004) Cdk5 deregulation in the pathogenesis of Alzheimer's disease. Trends Mol. Med. 10, 452–458 [DOI] [PubMed] [Google Scholar]
- 14. Shukla V., Skuntz S., Pant H. C. (2012) Deregulated Cdk5 activity is involved in inducing Alzheimer's disease. Arch. Med. Res. 43, 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asada A., Yamamoto N., Gohda M., Saito T., Hayashi N., Hisanaga S. (2008) Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cycline-dependent kinase 5 (Cdk5) complexes. J. Neurochem. 106, 1325–1336 [DOI] [PubMed] [Google Scholar]
- 16. Asada A., Saito T., Hisanaga S. (2012) Subcellular localization of active Cdk5 is determined by its own kinase activity. J. Cell Sci. 125, 3421–3429 [DOI] [PubMed] [Google Scholar]
- 17. Minegishi S., Asada A., Miyauchi S., Fuchigami T., Saito T., Hisanaga S. (2010) Membrane association facilitates degradation and cleavage of the cyclin-dependent kinase 5 activators p35 and p39. Biochemistry 49, 5482–5493 [DOI] [PubMed] [Google Scholar]
- 18. Luo S., Vacher C., Davies J. E., Rubinsztein D. C. (2005) Cdk5 phosphorylation of huntingtin reduces its cleavage by caspases. Implications for mutant huntingtin toxicity. J. Cell Biol. 169, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schilling B., Gafni J., Torcassi C., Cong X., Row R. H., LaFevre-Bernt M. A., Cusack M. P., Ratovitski T., Hirschhorn R., Ross C. A., Gibson B. W., Ellerby L. M. (2006) Huntingtin phosphorylation sites mapped by mass spectrometry. Modulation of cleavage and toxicity. J. Biol. Chem. 281, 23686–23697 [DOI] [PubMed] [Google Scholar]
- 20. Kaminosono S., Saito T., Oyama F., Ohshima T., Asada A., Nagai Y., Nukina N., Hisanaga S. (2008) Suppression of mutant huntingtin aggregate formation by Cdk5/p35 through the effect on microtubule stability. J. Neurosci. 28, 8747–8755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang D., Yeung J., Lee K. Y., Matsushita M., Matsui H., Tomizawa K., Hatase O., Wang J. H. (1995) An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J. Biol. Chem. 270, 26897–26903 [DOI] [PubMed] [Google Scholar]
- 22. Zheng M., Leung C. L., Liem R. K. (1998) Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J. Neurobiol. 35, 141–159 [DOI] [PubMed] [Google Scholar]
- 23. Tarricone C., Dhavan R., Peng J., Areces L. B., Tsai L. H., Musacchio A. (2001) Structure and regulation of the CDK5-p25(nck5a) complex. Mol. Cell 8, 657–669 [DOI] [PubMed] [Google Scholar]
- 24. Yamada M., Saito T., Sato Y., Kawai Y., Sekigawa A., Hamazumi Y., Asada A., Wada M., Doi H., Hisanaga S. (2007) Cdk5-p39 is a labile complex with the similar substrate specificity to Cdk5-p35. J. Neurochem. 102, 1477–1487 [DOI] [PubMed] [Google Scholar]
- 25. Mapelli M., Massimiliano L., Crovace C., Seeliger M. A., Tsai L. H., Meijer L., Musacchio A. (2005) Mechanism of CDK5/p25 binding by CDK inhibitors. J. Med. Chem. 48, 671–679 [DOI] [PubMed] [Google Scholar]
- 26. Ahn J. S., Radhakrishnan M. L., Mapelli M., Choi S., Tidor B., Cuny G. D., Musacchio A., Yeh L. A., Kosik K. S. (2005) Defining Cdk5 ligand chemical space with small molecule inhibitors of tau phosphorylation. Chem. Biol. 12, 811–823 [DOI] [PubMed] [Google Scholar]
- 27. Eisenberg D., Weiss R. M., Terwilliger T. C. (1982) The helical hydrophobic moment. A measure of the amphiphilicity of a helix. Nature 299, 371–374 [DOI] [PubMed] [Google Scholar]
- 28. Zhu Y.-S., Saito T., Asada A., Maekawa S., Hisanaga S. (2005) Activation of latent cyclin-dependent kinase 5 (Cdk5)–p35 complexes by membrane dissociation. J. Neurochem. 94, 1535–1545 [DOI] [PubMed] [Google Scholar]
- 29. Takahashi S., Saito T., Hisanaga S., Pant H. C., Kulkarni A. B. (2003) Tau phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J. Biol. Chem. 278, 10506–10515 [DOI] [PubMed] [Google Scholar]
- 30. Ledee D. R., Gao C. Y., Seth R., Fariss R. N., Tripathi B. K., Zelenka P. S. (2005) A specific interaction between muskelin and the cyclin-dependent kinase 5 activator p39 promotes peripheral localization of muskelin. J. Biol. Chem. 280, 21376–21383 [DOI] [PubMed] [Google Scholar]


