Background: Plasmodium apicoplast protein synthesis is essential, but few apicoplast tRNA synthetases have been characterized.
Results: Apicoplast glutamyl-tRNA synthetase aminoacylates tRNAGlu and tRNAGln, is sensitive to a bacterial inhibitor, and is essential in blood stages.
Conclusion: Formation of apicoplast Gln-tRNAGln is via indirect aminoacylation.
Significance: We demonstrate that the apicoplast glutamyl-tRNA synthetase is a potential drug target.
Keywords: Aminoacyl tRNA Synthetase, Malaria, Nucleic Acid Enzymology, Plasmodium, Transfer RNA (tRNA), Apicoplast
Abstract
The malaria parasite Plasmodium falciparum and related organisms possess a relict plastid known as the apicoplast. Apicoplast protein synthesis is a validated drug target in malaria because antibiotics that inhibit translation in prokaryotes also inhibit apicoplast protein synthesis and are sometimes used for malaria prophylaxis or treatment. We identified components of an indirect aminoacylation pathway for Gln-tRNAGln biosynthesis in Plasmodium that we hypothesized would be essential for apicoplast protein synthesis. Here, we report our characterization of the first enzyme in this pathway, the apicoplast glutamyl-tRNA synthetase (GluRS). We expressed the recombinant P. falciparum enzyme in Escherichia coli, showed that it is nondiscriminating because it glutamylates both apicoplast tRNAGlu and tRNAGln, determined its kinetic parameters, and demonstrated its inhibition by a known bacterial GluRS inhibitor. We also localized the Plasmodium berghei ortholog to the apicoplast in blood stage parasites but could not delete the PbGluRS gene. These data show that Gln-tRNAGln biosynthesis in the Plasmodium apicoplast proceeds via an essential indirect aminoacylation pathway that is reminiscent of bacteria and plastids.
Introduction
Malaria is a mosquito-borne disease caused by the apicomplexan parasite Plasmodium. The World Health Organization estimated that there are ∼216 million malaria cases and 655,000 deaths annually, the latter mostly of young children in sub-Saharan Africa (1). Others recently reported that worldwide deaths are 2-fold higher (2). Resistance to most approved anti-malarials has been documented or is emerging (3), and despite encouraging progress against the disease with insecticide-impregnated bed nets and other tools, malaria control is difficult to maintain. This concern highlights the continual requirement for new anti-malarial drugs that are directed against novel targets.
The apicoplast (4) is a relict plastid and the remnant of an ancient secondary endosymbiotic event in which the eukaryotic progenitor of the malaria parasite engulfed a photosynthetic eukaryote. The circular 35-kb Plasmodium apicoplast genome (5) encodes components of the organelle's transcriptional and translational machinery (5–7) as well as the SufB protein involved in FeS cluster formation (8) and the ClpC protease. Most apicoplast proteins, however, are encoded by the nuclear genome and imported into the organelle post-translationally (9). Over 500 apicoplast-targeted proteins have been identified (10, 11), revealing apicoplast biosynthetic pathways for fatty acids (9, 12), isoprenoid precursors (13), and heme (14), as well as enzymes for tRNA modification (11) and lipoylation (15). Several of these pathways exhibit prokaryotic-like features and contain potential drug targets (11, 13, 16). Recent studies have shown that apicoplast isoprenoid precursor biosynthesis is essential in P. falciparum asexual stages (17), indicating that the pathway cannot be bypassed by salvage of lipids from the host and may be a good drug target in asexual stages. The type II fatty acid and heme biosynthetic pathways, however, are not essential in the asexual stages (16), and although not good targets for asexual stage chemotherapy, they may prove to be valuable prophylactic targets in liver stages (18).
Antibiotics that inhibit protein synthesis in prokaryotes can inhibit growth of blood stage parasites in vitro (19, 20) and possess anti-malarial properties in vivo (21, 22). Although slow acting against blood stage parasites and not used as first line drugs, antibiotics such as doxycycline have been used to treat patent blood stage infections that are resistant to fast acting drugs or when these drugs are unavailable (23). They are also used for prophylaxis against malaria transmitted by anopheline mosquitoes infected with Plasmodium sporozoites (24). Most, if not all, of these antibiotics inhibit apicoplast protein synthesis (25), suggesting that other processes that support this pathway might be useful drug targets.
Aminoacylated tRNAs synthesized by aminoacyl-tRNA synthetases (aaRSs)4 are essential substrates for protein synthesis. Consequently, aaRSs have emerged as targets for new antibiotics (26). Mupirocin, for example, is a topical antibiotic already in clinical use, and other aaRS inhibitors such as the boronated antifungal compound AN2690 (27) are being developed. Aminoacylation in malaria parasites had been little studied despite its critical role in parasite biology and potential as a drug target, but studies describing the apicoplast (28) and cytoplasmic aspartyl-tRNA synthetases (29), lysyl-tRNA synthetases (30, 31), and tryptophanyl-tRNA synthetases (32, 33) were recently published.
The classical route for aminoacylated tRNA formation, direct aminoacylation, is catalyzed by aaRSs specific for each cognate amino acid:tRNA pair, but some aminoacylated tRNAs are made via alternative pathways. Most bacteria lack glutaminyl-tRNA synthetase (GlnRS) and produce Gln-tRNAGln via a two-step indirect aminoacylation pathway (34) shown as Reactions 1 and 2. First, tRNAGln is glutamylated by a nondiscriminating glutamyl-tRNA synthetase (GluRS). The misacylated Glu-tRNAGln is subsequently converted into Gln-tRNAGln by glutamyl-tRNA amidotransferase (Glu-AdT).
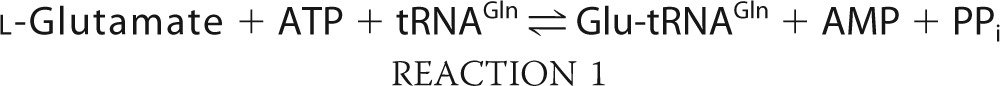 |
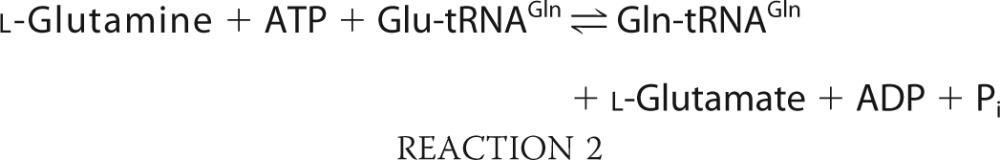 |
The second step (Reaction 2) catalyzed by Glu-AdT is essential because the misacylated Glu-tRNAGln is toxic if it is not converted to Gln-tRNAGln by Glu-AdT (35).
By analyzing conserved apicoplast-targeted proteins in the genomes of several Plasmodia (10, 36, 37) and the related parasite Theileria parva (38), we noticed that both organisms encoded apicoplast-targeted GluRS and Glu-AdT enzymes but lacked an apicoplast-targeted GlnRS. We hypothesized that the apicoplast utilizes the indirect pathway for Gln-tRNAGln biosynthesis and that inhibition of this pathway might provide a new way to inhibit apicoplast protein synthesis. We began to explore these hypotheses by characterizing the first enzyme in the pathway, the apicoplast-targeted GluRS.
EXPERIMENTAL PROCEDURES
Bioinformatics
A list of putative apicoplast-targeted proteins conserved in Plasmodium falciparum, T. parva, and Toxoplasma gondii was obtained from the supplementary information in Gardner et al. (38). Nucleotide or amino acid sequences of Plasmodium genes or proteins (10, 39) and those from other species were obtained from PlasmoDB (40), the Wellcome Trust Sanger Institute GeneDB website, or UniProt (41). Multiple sequence alignments were generated using tCoffee-Expresso (42) and formatted for display using ESPript (43) with SimilarityGlobalScore = 0.7, SimilarityDiffScore 0.5, SimilarityType = R (Risler), and Consensus = C. The phylogenetic tree was constructed using the tools provided on line (44). The “one-click” mode was used, employing MUSCLE (45) for sequence alignment and Gblocks (46), PhyML (47), and TreeDyn (48) for curation of the multiple sequence alignment and tree construction and rendering, respectively. The final tree was constructed using 200 bootstraps.
Isolation of the PfGluRS cDNA from Blood Stage Parasites
P. falciparum 3D7 asexual stage parasites (MRA-102, MR4 ATCC (BEI Resources), Manassas, VA) were cultured as described (49). Infected erythrocytes were lysed with 0.5% saponin (Sigma) and total RNA was isolated using TRIzol (Invitrogen). A cDNA of the PfGluRS gene (Pf3D7_1357200) was amplified by RT-PCR using the GR31_F primer (5′-GAGAGGAAATAGGTGTAATGT-3′), which maps 25 nucleotides 5′ to the predicted start codon and the reverse primer G33_R (5′-AAACTTAAATAGATTTTTCAAATGTAA-3′) that is complementary to the 3′ end of the predicted coding sequence. The resulting PCR product was cloned into the pCR-XL-TOPO vector (Invitrogen) and sequenced.
Expression and Purification of Mature PfGluRS in Wheat Germ Extracts
Two versions of the PfGluRS coding sequence were cloned for expression in wheat germ extracts. The native PfGluRS coding sequence (amino acids 78–574), minus the predicted apicoplast leader sequence (amino acids 1–77), was amplified from first strand cDNA using forward (5′-CCACATGGAGGGTAAAGTACGGTTAAG-3′) and reverse (5′-CTATTCAGTGGTGGTGGTGGTG-3′) primers, and a synthetic DNA segment encoding the same amino acid sequence but codon-optimized for Triticum aestivum (GeneArt AG) was amplified by PCR using the forward 5′-CACTATGGAGGGCAAGGTGCGC-3′ and reverse 5′-GTACTCAGTGGTGATGGTGGTGGTG-3′ primers. The PCR products encoding the native and codon-optimized PfGluRS enzymes were cloned into a cell-free expression vector that carries SP6 RNA polymerase promoter and Ω sequence for the wheat germ ribosome-binding site. In vitro transcription and translation were carried out as described (50). PfGluRS was purified from the extracts using Ni-NTA affinity chromatography. Pure fractions observed via SDS-PAGE were pooled, dialyzed against aminoacylation buffer (100 mm Hepes-KOH, pH 7.2, 30 mm KOH, 12 mm MgCl2), and concentrated using Amicon ultracentrifugal filters (Millipore). The yield was 0.75 mg of protein in a total volume of 1 ml.
Expression and Purification of the Mature PfGluRS in Escherichia coli
PfGluRS was also expressed in E. coli KRX cells (Promega) containing the pRARE2 plasmid (EMD4BioSciences) and the pET-29a vector encoding the predicted mature PfGluRS coding sequence (Pf3D7_1357200, amino acids 78–574) codon-optimized for E. coli (GeneArt, Inc.) with N-terminal His6 tags. After Ni-NTA chromatography and gel filtration (51), pure fractions were concentrated and dialyzed overnight against aminoacylation buffer containing 0.5 mm DTT and stored at −20 °C in 30% glycerol. The absence of contaminating pyrophosphatase activity was confirmed as described (52).
tRNA Substrates
Synthetic genes encoding P. falciparum apicoplast tRNAGlu and tRNAGln (both from GenBankTM accession number X95276) were prepared by annealing overlapping oligonucleotides, ligated into the pGFIB expression vector (53), and transformed into E. coli BL21 cells. Cultures were grown, and crude total tRNA was extracted (54), deacylated in 200 mm Tris-HCl, pH 9, precipitated with isopropyl alcohol, washed with 80% ethanol, air-dried, and resuspended in 500 μl of RNase-free water. Samples were stored at −80 °C until use. Total E. coli tRNA (Sigma catalog no. 9014-25-9) was used for the experiment in Fig. 4. The crude P. falciparum tRNAGlu and tRNAGln preparations were each separately 32P-labeled on their 3′ termini using the E. coli CCA-adding enzyme and [α-32P]ATP as outlined in Ref. 55.
FIGURE 4.
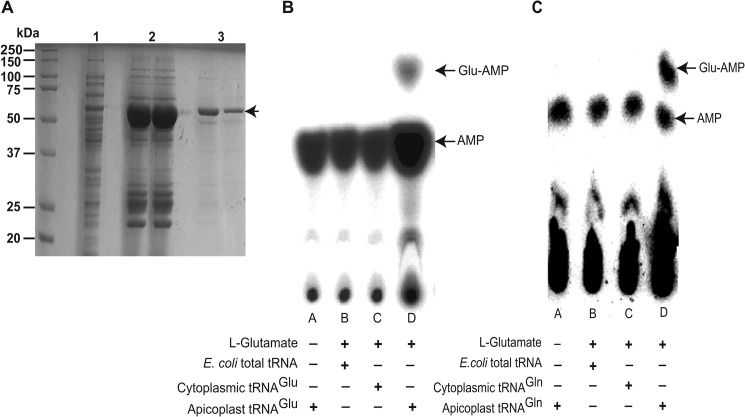
Mature apicoplast PfGluRS purification and aminoacylation assay. A, purification of the mature PfGluRS assessed by 7.5% SDS-PAGE. Lane 1, crude E. coli lysate; lane 2, eluate from an Ni-NTA column; lane 3, eluate from gel filtration purification. B and C, representative phosphorimages of the separation of Glu-[α-32P]AMP and [α-32P]AMP by PEI-cellulose TLC after PfGluRS-catalyzed aminoacylation. The aminoacylation assays were performed as described under “Experimental Procedures” with the following modifications. B, reactions in lanes A and D contained apicoplast tRNAGlu, but l-glutamate was omitted from lane A. Lane B contained E. coli tRNA, and lane C contained Pf cytoplasmic tRNAGlu. C, reactions in lanes A and D contained apicoplast tRNAGln but l-glutamate was omitted from lane A. Lane B contained E. coli tRNA and lane C contained P. falciparum cytoplasmic tRNAGln. Apicoplast PfGluRS is a nondiscriminating enzyme that is capable of specifically glutamylating both of the apicoplast tRNA substrates.
Aminoacylation Assay and Activation by Pyrophosphatase
The aminoacylation assay using recombinant PfGluRS expressed in E. coli was performed and quantified as described in Ref. 55. In experiments testing the effect of PPase, two reactions were initiated simultaneously without PPase and allowed to proceed for 20 min. PPase (10 units/ml) was then added to one reaction and both reactions were incubated for an additional 40 min.
Determination of Kinetic Parameters
The Km value for l-Glu was determined (55–57) in reactions consisting of varying concentrations of l-Glu (5–400 μm). The Km values for tRNAGlu and tRNAGln were determined in varying concentrations of 32P-labeled tRNA (from 0.02 to 5 μm) at an l-Glu concentration of 200 μm. The Km values for all substrates were determined simultaneously with enzyme aliquots from the same dilution. In all cases the concentration of PfGluRS used was chosen so as to obtain linear kinetics of glutamyl-tRNA formation in a 3-min reaction. Steady-state kinetic parameters were calculated using nonlinear regression based on the Michaelis-Menten equation (GraphPad Prism 5). The aminoacylation discrimination factor D (58) was calculated using the following formula: D = (kcat/Km)tRNAGlu/(kcat/Km)tRNAGlu.
GluRS Inhibition Assay
The GluRS inhibitor 5′-O-[N-(l-glutamyl)sulfamoyl]adenosine (Glu-SA) (59) was obtained from IDT (Coralville, IA). To determine the Ki value for Glu-SA inhibition of PfGluRS with respect to l-Glu, we measured the Km value for l-Glu in the absence of the inhibitor and the Kmapp values for l-Glu in the presence of various fixed concentrations of Glu-SA (10 nm to 100 μm). The Ki value was determined from a Kmapp versus [I] plot according to the equation Kmapp = Km (1 + [I]/Ki).
Myc Tagging and Attempted Deletion of the Endogenous Plasmodium berghei GluRS Gene
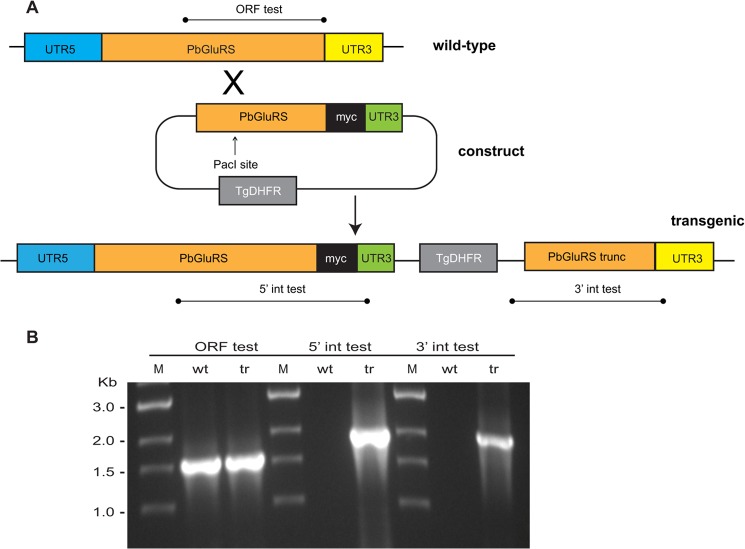
A 4× myc tag was appended to the 3′ end of the gene encoding the putative apicoplast-targeted P. berghei GluRS (PlasmoDB ID PBANKA_113350) as described (16). A 1.6-kb fragment of the 3′ end of the gene without the stop codon was amplified from P. berghei ANKA genomic DNA using primers PbGluRS_F1 (TACCGCGGAAGTGCATATATATGCAAATGCA) and PbGluRS_R (ATACTAGTTATGTTAAATATACTCTTTATGTATTG); the SacII and SpeI restriction sites are underlined. Polymerase chain reactions (PCR) (50 liters) contained 50 ng of genomic DNA, 0.1 μm of each primer, 5 μl of 10× buffer, and 1 μl of Advantage2 polymerase (Clontech). The PCR product was digested with SacII and SpeI and cloned into the b3D myc vector (16). P. berghei ANKA parasites were transfected, and parasites with myc insertions in the GluRS gene were selected and cloned as described (16). Plasmid integration at the 5′ and 3′ insertion sites was confirmed by PCR using primer pairs PbGluRS_For2 (TACCGCGGAAAAGCTTATTATTGCTTTTGCAC) and PbGluRS_int5_Rev2 (GAGACAGCTCAATTCTTTATGTCC) for the 5′ integration test, and PbGluRS_int3_For2 (CCTCTTCGCTATTACGCCAGCT) and PbGluRS_int3_Rev2 (GTGAAATACGAGTATTAATATACAGG) for the 3′ integration test.
The strategy described previously (60) was used to attempt deletion of the PbGluRS gene by double crossover recombination. The primers used to amplify the genomic regions were as follows: PbGluRS_apico_KO.Pr1For (GCCCGCGGAATTTATTGAAAATATGGTTATCAGC); PbGluRS_apico_KO.Pr2Rev (TCATACTGTGGGCCCATAGAAGGATTACACATAACAAGG); PbGluRS_apico_KO.Pr3For, TCCTTCTATGGGCCCACAGTATGAATTAAATTTGGTCAC); and PbGluRS_apico_KO.Pr4Rev (GCGCGGCCGCTTACTTTGTGATTCAGTTGCGC). Primers 1 and 2 were designed to amplify a 952-bp fragment containing the last 102 bp of the PbGluRS coding sequence and 850 bp of the 3′ UTR. Primers 3 and 4 were designed to amplify an 813-nucleotide fragment containing the first 62 bp of the PbGluRS coding sequence and 751 bp of the 5′ UTR. Primer 1 contained an added 5′-terminal GC dinucleotide and a SacII site. Primer 4 contained an added 5′-terminal GC dinucleotide and a NotI site. Primers 2 and 3 contained an ApaI site flanked by complementary sequences (underlined) for recombinatorial PCR. The two genomic fragments were first amplified in separate reactions using the cycling parameters described above, and the resulting products were combined in a second PCR to form a single product, which was digested with SacII and NotI and cloned into the B3D KO Red vector. This construct was linearized with ApaI, and P. berghei ANKA parasites were transfected as described (61). Pyrimethamine-resistant parasites were genotyped by PCR using the following primer pairs: ORF test, PbGluRS_For1 and PbGluRS_Rev; Test 1 For (ATATTCTGCGATTTTTCTTG) and Test 1 Rev (GCAAGGCGATTAAGTTGGGT); Test 2 For (GGCTACGTCCCGCACGGACGAATCCAGATGG) and Test 2 Rev (AGTATAACACTCTCTATCCTAAAA).
Subcellular Localization
Analysis of the P. berghei clones expressing Myc-tagged PbGluRS was performed as outlined in Ref. 62. Double staining was performed using a rabbit polyclonal anti-acyl carrier protein (ACP) primary antibody (diluted 1:500) (63) as an apicoplast marker and mouse monoclonal anti-Myc antibody (Santa Cruz Biotechnology, diluted 1:500) to detect PbGluRS-myc. Fluorescent staining was achieved with Alexa Fluor-conjugated secondary antibodies (Invitrogen) specific to rabbit (Alexa Fluor 594, red) and mouse (Alexa Fluor 488, green) IgG. DAPI was used to stain nucleic acids, and the mitochondrion was stained by incubating the parasites in culture media for 30 min with 20 nm MitoTracker Red (Invitrogen), and fixing the cells as outlined above. Images were acquired using an Olympus Delta Vision imaging system (Applied Precision) with a ×100 objective and deconvolved using the Softworx package (Applied Precision) with the default parameters.
RESULTS
Components of the Apicoplast Indirect Aminoacylation Pathway
The first step in the indirect aminoacylation pathway for Gln-tRNAGln biosynthesis is glutamylation of tRNAGln by a nondiscriminating GluRS. The P. falciparum genome (10) encodes two putative GluRS enzymes, one of which (Pf3D7_1357200, 68.4 kDa, 574 amino acids) possesses a predicted N-terminal apicoplast-targeting sequence (64, 65). The other, Pf3D7_1349200, appears to be cytoplasmic (66), suggesting that the enzyme encoded by Pf3D7_1357200 is the apicoplast GluRS and therefore should possess nondiscriminating activity. Apicoplast-targeted PfGluRS orthologs are present in all sequenced plasmodia, Theileria and Toxoplasma, but not in apicomplexans that lack apicoplasts. Organisms, or organelles, that utilize the indirect aminoacylation pathway for Gln-tRNAGln biosynthesis often, but not always (67), lack GlnRS. Consistent with this, malaria parasites possess only one GlnRS (Pf3D7_1331700) that lacks an apicoplast targeting signal and is probably cytoplasmic (66, 68). Nuclear genes encoding apicoplast-targeted P. falciparum orthologs of the GatA and GatB subunits of bacterial Glu-AdT were also identified, but not GatC.5 GatC is a small, poorly conserved protein (12 kDa) that appears to perform a purely structural role at the interface between the GatA and GatB subunits in bacterial Glu-AdTs (69). A potential Plasmodium GatC ortholog may be difficult to detect by similarity searches. Furthermore, we did not find a Plasmodium ortholog of the GatF subunit of yeast mitochondrial Glu-AdT (70). Finally, putative tRNAGlu and tRNAGln substrates of GluRS and Glu-AdT are encoded by the apicoplast genome (5). Thus, except for an ortholog of GatC, we identified all components of the indirect aminoacylation pathway in P. falciparum (Table 1) and in other apicoplast-containing apicomplexan parasites.
TABLE 1.
Components of the apicoplast indirect aminoacylation pathway in P. falciparum and P. berghei
PlasmoDB identifiers for the nucleus-encoded proteins are indicated.
Isolation of the PfGluRS cDNA from Blood Stages
The predicted Pf3D7_1357200 gene encoding PfGluRS contained seven exons, but the gene structure had not yet been confirmed experimentally. We isolated a RT-PCR product from P. falciparum blood stage total RNA that confirmed the annotated gene structure. Microarray-based gene expression analyses also showed that the PfGluRS gene was transcribed in asexual parasites, with moderate induction in late trophozoites, and that its expression occurred in-phase with genes transcribed from the apicoplast genome (71). PfGluRS transcripts were identified in blood stage parasites using RNA-Seq (72), and the protein was also detected in gametocytes via proteomics (73, 74).
Sequence Comparison of the PfGluRS and Bacterial Orthologs
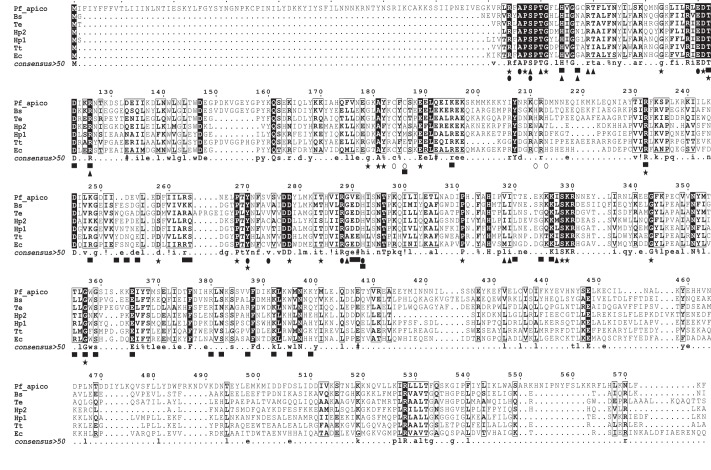
Aminoacyl tRNA synthetases are classified into two major groups. Class I enzymes contain an N-terminal catalytic domain and generally acylate the 2′-hydroxyl of the terminal tRNA adenosine, whereas the catalytic domains of class II enzymes possess a seven-stranded anti-parallel β-sheet fold flanked by α-helices and acylate the 3′-hydroxyl of the tRNA adenosine. Glutamyl-tRNA synthetases are class I enzymes (75). The PfGluRS amino acid sequence was aligned with those of several prokaryotic orthologs (Fig. 1) to identify conserved and divergent features. The PfGluRS possesses the “HIGH” and “KMSKF” ATP-binding motifs that are characteristic of the Rossman fold (75). Other features common to class I GluRSs that were well conserved in PfGluRS included residues that interact with the l-glutamate, ATP, and tRNAGlu substrates (76). A zinc-binding SWIM motif (CXCX24CX24H; open circles in Fig. 1) in the E. coli GluRS is essential for catalytic activity (77) but is not completely conserved in the PfGluRS (CXCX24CX24D). The His residue in the bacterial SWIM motif is required for Zn2+ binding but is replaced in PfGluRS by an Asp residue, which is a potential Zn2+ ligand. Other GluRSs also lack a canonical SWIM motif and do not bind or require Zn2+ for activity (78). We found that PfGluRS did not require Zn2+ supplementation for in vitro activity, and the ZincPred server (79) did not identify any zinc-binding sites in the protein. Finally, the Plasmodium enzyme has an N-terminal extension, the bipartite apicoplast-targeting signal, that prokaryotic enzymes lack (64, 65).
FIGURE 1.
Alignment of the P. falciparum apicoplast PfGluRS amino acid sequence with bacterial orthologs. The PfGluRS amino acid sequence was aligned with those of bacterial and cyanobacterial orthologs using tCoffee-Expresso (42). Sequences and UniProt accessions are as follows: Pf_apico, P. falciparum apicoplast GluRS (Q8IDD3); Bs, Bacillus subtilis (P22250); Te, Thermosynechococcus elongatus (Q8DLI5); Hp1, Helicobacter pylori GluRS1 (P96551); Hp2, H. pylori GluRS2 (O25360); Tt, T. thermophilus (P27000); Ec, E. coli (P04805). Residues are highlighted as follows (76): residues conserved in the GluRS/GlnRS family, stars; interactions with l-glutamate, solid circles; productive interactions with ATP, solid triangles; interactions with tRNA substrate, solid black squares. The open circles indicate the zinc-binding residues identified in the E. coli GluRS (107). Residues 94–94 and 326–330 correspond to the HIGH and “KMSKF” ATP-binding motifs of class I aaRSs (75). The N-terminal extension (gray highlighting) in the PfGluRS sequence is the bipartite apicoplast targeting sequence.
Nondiscriminating GluRSs differ from discriminating enzymes in that they can glutamylate the cognate substrate tRNAGlu as well as the noncognate substrate tRNAGln. Recognition and discrimination of tRNA substrates by GluRS (78, 80) requires that the enzyme is able to distinguish between the tRNAGlu UUC and tRNAGln UUG anticodons. The alignment (Fig. 1) revealed similarities between the PfGluRS and nondiscriminating bacterial and cyanobacterial enzymes at two residues critical for tRNA anticodon discrimination. The thermophilic eubacterium Thermus thermophilus possesses a single discriminating GluRS (81). Structural analysis of the T. thermophilus GluRS revealed that the bulky side chain of the Arg-358 residue in the anti-codon binding pocket (position 442 in Fig. 1) recognizes the pyrimidine base of C36 in the anticodon of the tRNAGlu substrate. However, in the cyanobacterium Thermosynechococcus elongatus, which has a single nondiscriminating GluRS, Arg-358 is replaced by an uncharged Gly residue. The smaller Gly side chain forms a larger pocket that allows recognition of the purine base of G36 in the noncognate tRNAGln as well as the tRNAGlu C36 nucleotide in the cognate tRNAGlu (78). Six other amino acid substitutions of this Arg residue (Gln, Ser, Glu, Asn, Tyr, and His) have been observed in other nondiscriminating GluRSs (80). A second residue involved in anticodon discrimination is Thr-444 in the T. thermophilus GluRS (position 537 in Fig. 1), which is substituted by Gly in T. elongatus and most other nondiscriminating GluRSs (80). The PfGluRS contains Glu and Gly at these positions, respectively, which is consistent with other nondiscriminating GluRSs and our analyses of PfGluRS aminoacylation activity (see below).
Phylogenetic Analysis
A maximum likelihood phylogenetic tree was constructed of the apicoplast PfGluRS, the human and P. falciparum cytoplasmic GluRSs, and GluRSs from several bacteria and an archaeon. As shown in Fig. 2, PfGluRS resolved with bacterial enzymes, consistent with an organellar origin, and not with eukaryotic cytoplasmic or archaeal enzymes.
FIGURE 2.
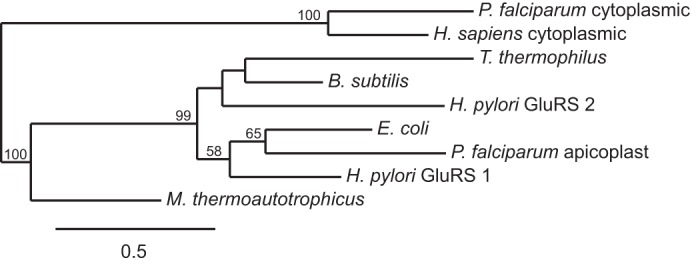
Phylogenetic analysis of the apicoplast PfGluRS and archaeal, bacterial, and eukaryotic orthologs. A maximum likelihood tree of representative GluRS sequences from the three domains of life. Scale bar, 0.5 changes/site.
Expression of Recombinant PfGluRS in Wheat Germ Extracts and E. coli
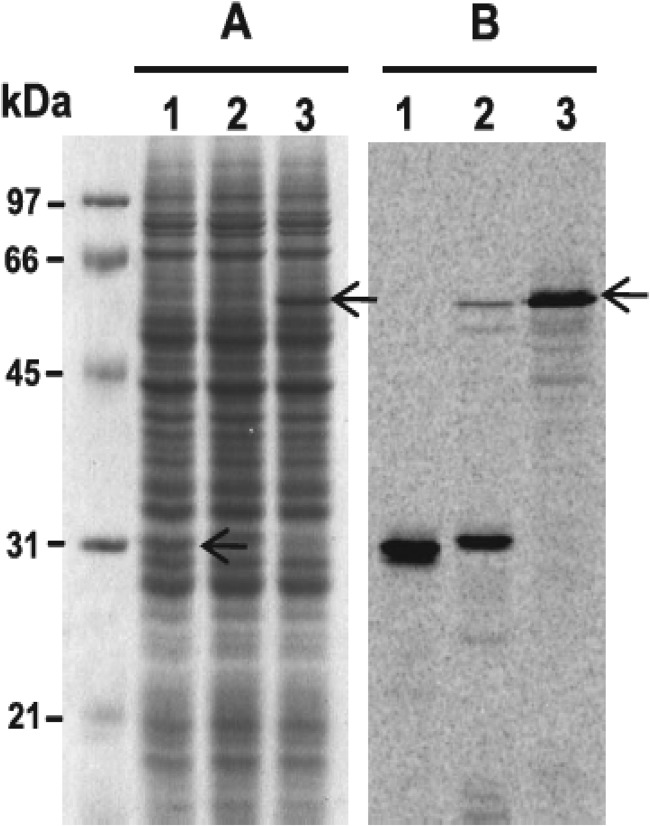
We used the multiple sequence alignment of PfGluRS and bacterial orthologs (Fig. 1) and predictions from PlasmoAP (65) and PATS (64) to estimate the N-terminal residue of the mature PfGluRS. Residue Glu-78 was selected because it is N-terminal to the conserved ATP-binding site and within the N-terminal extension of the PfGluRS. Initial attempts to express PfGluRS in E. coli were not successful, but a wheat germ system was used to first validate that the gene coded for the aminoacylation activity. Wheat germ extracts had been used previously to express catalytically active P. falciparum dihydrofolate reductase (DHFR-TS) (50). Recombinant PfGluRS was expressed in the extracts at a level similar to that observed with PfDHFR-TS. Furthermore, the PfGluRS produced in wheat germ could glutamylate apicoplast tRNAGlu, an activity that was not present in control extracts that expressed green fluorescent protein (GFP), suggesting that the Plasmodium enzyme was functional.5 In an attempt to increase the yield, we subsequently expressed a codon-optimized version (for wheat) of the PfGluRS coding sequence in extracts, and we included [14C]Leu to detect newly synthesized proteins. Codon optimization dramatically increased the amount of PfGluRS produced in comparison with the same protein encoded by the native sequence. This is clearly evident in an SDS-polyacrylamide gel stained with Coomassie Blue (Fig. 3A) and autoradiographed (Fig. 3B). Most of the 14C-labeled protein in extracts that expressed the wild-type PfGluRS migrated at 32 kDa, about half of the predicted size, whereas virtually all of the 14C-labeled protein in extracts expressing the codon-optimized version appeared to be full length. PfGluRS produced using the codon-optimized construct also possessed glutamylation activity.5 We therefore applied the same codon optimization strategy to express PfGluRS in E. coli, and we purified the enzyme via Ni-NTA and gel filtration chromatography. SDS-PAGE revealed a single band of ∼63 kDa (Fig. 4A) that reacted with anti-His antibody in a Western blot. However, a faster migrating band formed upon storage and the specific activity of the enzyme declined, indicating that PfGluRS was susceptible to oxidation. Consequently, DTT was included in both storage and reaction buffers, which prevented formation of the faster migrating band and preserved enzyme activity. We routinely obtained 20 mg of pure enzyme/liter of culture. PfGluRS produced in E. coli was used for the enzyme kinetics and inhibition assays.
FIGURE 3.
Analysis of PfGluRS and GFP expressed in wheat germ extracts. A, Coomassie-stained SDS-polyacrylamide gel of soluble proteins in wheat germ extracts expressing GFP or PfGluRS in the presence of [14C]leucine. Lane 1, GFP; lane 2, native (wild type) PfGluRS; lane 3, codon-optimized PfGluRS. B, autoradiograph of the gel in A.
PfGluRS Is a Nondiscriminating Enzyme
We hypothesized that the PfGluRS encoded by Pf3D7_1357200 was the first of two enzymes in an indirect aminoacylation pathway. As such, it should possess nondiscriminating activity and glutamylate apicoplast tRNAGln as well as tRNAGlu. To test this hypothesis, aminoacylation assays were performed with mature PfGluRS in the presence of either apicoplast tRNAGlu or tRNAGln substrates (52, 82). Briefly, crude preparations of each apicoplast tRNA expressed in E. coli were 32P-labeled at their 3′-terminal AMP with [α-32P]AMP using the nucleotide exchange activity of E. coli CCA-adding enzyme. The labeled tRNAs were then incubated with recombinant PfGluRS, and at various time points an aliquot of the reaction was withdrawn and added to an acidic solution of nuclease P1 to simultaneously quench the reaction, prevent deacylation, and digest the tRNA to mononucleotides. Next, the reaction products were subjected to TLC to separate [32P]AMP from glutamyl-[32P]AMP, and the extent of aminoacylation was calculated from the ratio of the radioactivity of the two spots as determined by phosphorimaging. As shown in Fig. 4, B and C, both of the apicoplast tRNA substrates were glutamylated, demonstrating that the PfGluRS is a nondiscriminating enzyme. PfGluRS did not utilize l-glutamine as a substrate5 and did not glutamylate cytoplasmic P. falciparum tRNAGlu and tRNAGln or E. coli tRNA (Fig. 4, B and C). Because nondiscriminating GluRSs are often found in cells or organelles that lack GlnRS and must use indirect aminoacylation for Gln-tRNAGln biosynthesis, this finding provides strong biochemical evidence that the indirect pathway is used in the Plasmodium apicoplast.
Effect of PPase on PfGluRS Aminoacylation Activity
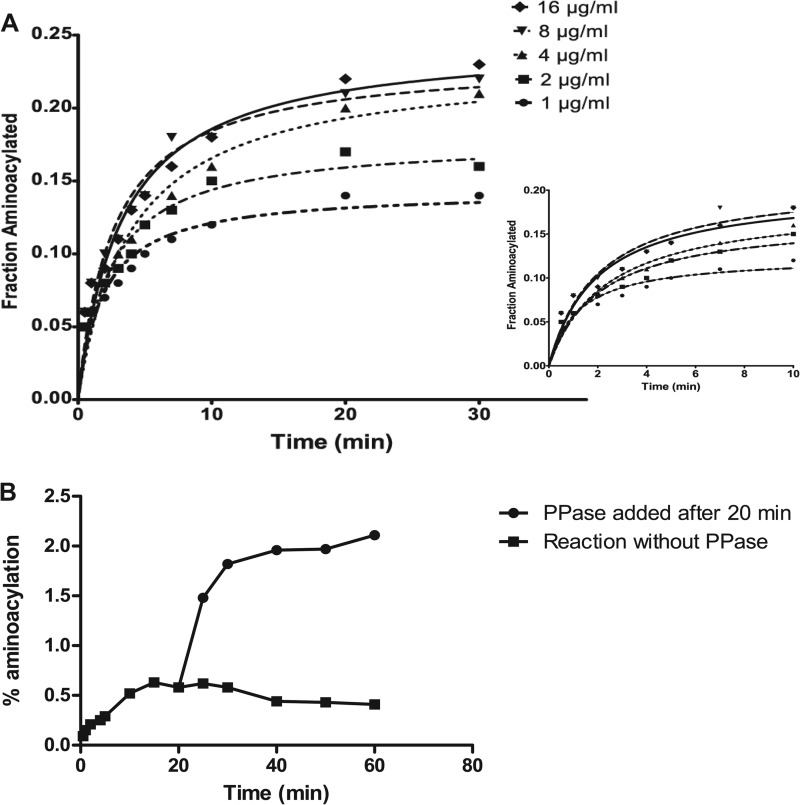
Aminoacylation reactions performed with varying amounts of PfGluRS clearly showed that an increase in enzyme concentration was accompanied by an increase in the reaction rate (Fig. 5A). However, the aminoacylation activity plateaued within a few minutes after enzyme addition, an effect suggestive of product inhibition. Adding more PfGluRS to the reaction after it had plateaued did not increase aminoacylation, implying that this effect was not an artifact caused by enzyme denaturation. Because inorganic pyrophosphate (PPi) can inhibit tRNA synthetase activity (52), we added PPase to an aminoacylation assay after the reaction had plateaued. This dramatically stimulated the PfGluRS reaction rate and increased the steady-state level of the Glu-tRNAGlu product ∼3-fold compared with reactions without PPase (Fig. 5B). Enhanced PfGluRS activity in the presence of PPase indicated that the strong inhibition observed in its absence was due to the generation of PPi during the reaction.
FIGURE 5.
PfGluRS aminoacylation time course assays and stimulation of activity by pyrophosphatase. A, normalized plot of tRNAGlu aminoacylation time course assays performed using the indicated concentrations of PfGluRS. Inset, enlarged view of the period between 0 and 10 min. B, two aminoacylation assays containing 4 μg/ml of PfGluRS were initiated simultaneously and allowed to proceed for 20 min in the absence of PPase. After 20 min, PPase (10 units/ml) was added to one reaction, and both reactions were incubated for an additional 40 min.
Kinetic Analyses
Having demonstrated PfGluRS aminoacylation activity and inhibition by PPi, we next conducted kinetic analyses to determine optimal reaction parameters and to compare the Plasmodium enzyme to bacterial orthologs. PfGluRS demonstrated higher affinities toward the cognate as compared with the noncognate tRNA substrate (Table 2). We then determined the same kinetic values for PfGluRS in reactions containing PPase. Here, the stimulatory effect of PPase was dramatic, increasing turnover by 8.4- and 5-fold and catalytic efficiency by 13- and 4-fold, for tRNAGlu and tRNAGln substrates, respectively, in comparison with reactions performed in its absence. The discrimination factor, D, a measure that compares enzyme turnover rates for cognate and noncognate substrates at the same concentration (83) was also calculated, revealing that PfGluRS glutamylated the cognate tRNAGlu substrate more efficiently than the noncognate tRNAGln substrate, an effect that was most pronounced in the presence of PPase.
TABLE 2.
Kinetic analyses of PfGluRS
The Km value for the tRNA substrates was determined in reactions containing 100 mm Hepes-KOH, pH 7.2, 30 mm KOH, 12 mm MgCl2, 2 mm DTT, 4 mm ATP, 200 μm l-glutamate, 4 μg/ml recombinant PfGluRS expressed in E. coli and varying concentrations of the indicated 32P-labeled tRNA from 0.02 to 5 μm. NA means not applicable.
| Substrate | PPase | Km | kcat | kcat/Km | D-factora |
|---|---|---|---|---|---|
| μm | s−1 | s−1 μm−1 | |||
| tRNAGlu | − | 0.0497 ± 0.0097 | 0.43 ± 0.027 | 8.65 | 1.59 |
| tRNAGln | − | 0.0767 ± 0.0031 | 0.42 ± 0.047 | 5.43 | |
| tRNAGlu | + | 0.0032 ± 0.0007 | 3.61 ± 1.15 | 112.67 | 4.86 |
| tRNAGln | + | 0.0911 ± 0.0054 | 2.11 ± 1.58 | 23.17 | |
| l-Glu | − | 1.401 ± 0.61 | 1.39 ± 0.098 | 0.99 | NA |
| ATP | − | 2.35 ± 0.67 | 1.18 ± 0.307 | 0.704 | NA |
a D-factor = (kcat/Km tRNAGlu)/(kcat/Km tRNAGln).
PfGluRS Inhibition by a Bacterial GluRS Inhibitor
Glu-SA, a GluRS inhibitor, is a stable analog of the glutamyl-AMP intermediate of the aminoacylation reaction (59). It belongs to a group of synthetic aminoacyl adenylates called aminoacylsulfamoyladenosines in which the labile mixed anhydride function of the aminoacyladenylate intermediate in the aminoacylation reaction is replaced by an isosteric, nonhydrolyzable sulfamoyl group (84). Glu-SA is a strong competitive inhibitor of the E. coli GluRS (Ki = 2.8 nm) but is a weaker competitive inhibitor of mammalian (mouse liver) GluRS (Ki = 70 nm) (59). Using the aminoacylation assay employed above to determine the kinetic values for PfGluRS, we found that Glu-SA is a competitive inhibitor of the mature PfGluRS (Fig. 6). However, Glu-SA is much less potent against the P. falciparum enzyme (Ki = 0.7 μm) than it is against either the E. coli or mammalian GluRSs.
FIGURE 6.
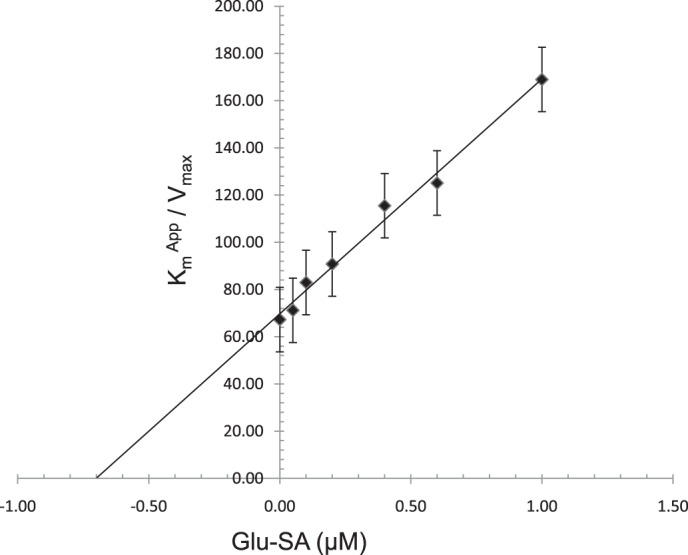
Competitive inhibition of PfGluRS by Glu-SA. The Kmapp for glutamate was determined with 400 μm l-glutamate in the presence of 0–1 μm Glu-SA at 37 °C. The data show that Glu-SA is a competitive inhibitor of PfGluRS with respect to l-glutamate with a Ki of 0.7 μm.
Subcellular Localization
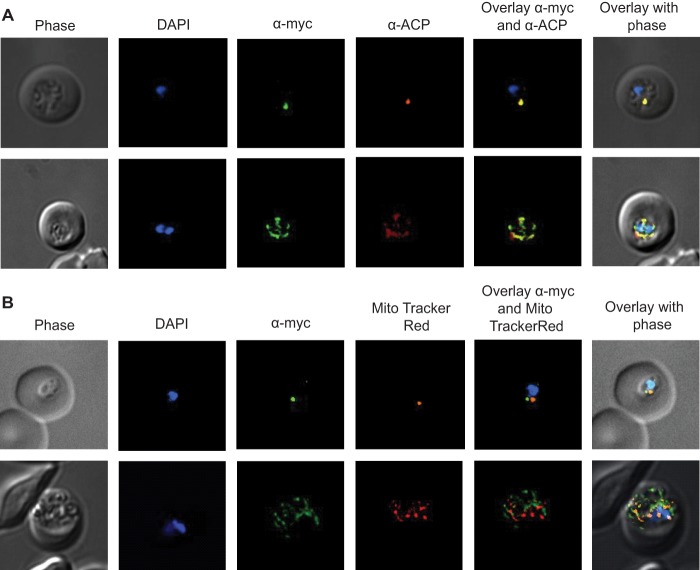
The P. falciparum GluRS encoded by Pf3D7_1357200 contains a predicted apicoplast targeting sequence (10, 65, 85), but the subcellular localization of the enzyme has never been established experimentally. To determine whether the enzyme is targeted to the apicoplast in vivo, we tagged the endogenous gene encoding the P. berghei ortholog of PfGluRS (Table 1) with a quadruple Myc tag (Fig. 7). We tagged the endogenous PbGluRS coding sequence so that the native promoter controlled the timing and level of expression, minimizing the possibility that the fusion protein would be mis-targeted. Fixed blood stage parasites were stained with anti-Myc antibody to detect PbGluRS and ACP antisera (9) to detect the apicoplast, and the samples were observed using Deltavision deconvolution fluorescence microscopy. Structures containing the Myc-tagged PbGluRS (PbGluRS-myc) exhibited a typical apicoplast appearance (Fig. 8A). PbGluRS-Myc marked a small spherical compartment in ring stage parasites (Fig. 8A), which then elongated and developed into a complex branched form at the trophozoite stage (Fig. 8A) prior to splitting into numerous individual structures in schizonts, one for each daughter merozoite. PbGluRS-Myc co-localized with ACP (Fig. 8A, α-Myc/α-ACP overlay), confirming that it is targeted to the apicoplast. This result demonstrated that the PbGluRS genomic locus is susceptible to integration of plasmid DNA.
FIGURE 7.
Integration of a second copy of GluRS fused to a quadruple Myc tag (PbGluRS-myc) into the P. berghei genome. A, 1.6-kb fragment of the 3′ end of the GluRS gene without the stop codon was amplified from P. berghei ANKA genomic DNA and ligated upstream of the quadruple Myc tag in the b3D myc vector (16). Following linearization of the construct with PacI, it was transfected into P. berghei blood stage schizonts that were subsequently injected into mice. The vector contains a mutated T. gondii DHFR/TS gene as a pyrimethamine selectable marker (TgDHFR), and transgenic parasites were selected by pyrimethamine treatment and cloned by limiting dilution. B, PCR analysis of the integration site in a cloned PbGluRS-myc transgenic (tr) parasite. Ethidium bromide-stained agarose gels showing the integration of the PbGluRS-myc vector into the P. berghei genome. Only PbGluRS-myc is positive in the 3′ and 5′ integration (int) tests, whereas both PbGluRS-myc and wild-type (wt) parasite genomic DNAs are positive for the PbGluRS open reading frame test (ORF test). M indicates the size ladder.
FIGURE 8.
PbGluRS localizes to the apicoplast. Transgenic PbGluRS-myc parasites were generated in which the protein was expressed under the control of the endogenous promoter with a C-terminal quadruple Myc tag. Differential interference contrast and fluorescent images were captured and processed using deconvolution microscopy; a merge of the images is presented on the far right column (overlay). A, PbGluRS-Myc apicoplast localization was monitored by immunofluorescence assay using an anti-Myc antibody (green), and the apicoplast was detected by staining with anti-ACP antibody (red). Nucleic acid was stained with DAPI (blue). PbGluRS-Myc marks a characteristically small and round compartment early in the infection cycle (top row), which then elongates and develops into a complex, multiply branched form at the trophozoite stage prior to splitting into individual spots, one for each merozoite, in the schizont stage (bottom row). PbGluRS-Myc co-localized with ACP (α-Myc/α-ACP overlay) confirming localization to the plastid. B, PbGluRS is not localized to the mitochondrion in erythrocytic stages. The mitochondrion was labeled using MitoTracker Red, and PbGluRS-Myc was detected by immunofluorescence assay using an anti-Myc antibody (green). Nucleic acid was stained with DAPI. In the early stages of parasite development (top row), PbGluRS-Myc and the mitochondria are discrete single organelles. In the late trophozoite stages, the mitochondria and PbGluRS-Myc are heavily branched but mostly distinct with a few points of overlapping signal (bottom row). In late schizonts, PbGluRS-Myc and mitochondria form single organelles. Scale bar, 1 μm.
To investigate potential localization to the mitochondrion, live parasites expressing PbGluRS-myc were incubated with MitoTracker Red and then fixed, stained with anti-Myc monoclonal antibody, and examined by fluorescence microscopy. In ring stages, the anti-Myc antibody and MitoTracker Red marked distinct organelles that were closely apposed to each other (Fig. 8B). Little co-localization between the mitochondrion and PbGluRS-Myc was observed in late trophozoites or early schizont stages with the mitochondrial staining largely distinct from that of PbGluRS-Myc, apart from a few apparent points of contact between the two organelles (Fig. 8B). Close apposition of the mitochondrion and apicoplast has been previously observed in Plasmodium spp. (86). At the late schizont stage, the mitochondria and the apicoplast again appeared as closely apposed but distinct organelles adjacent to the nuclei. Together with our demonstration above that PbGluRS co-localizes with the apicoplast protein ACP (Fig. 8A), these observations show that PbGluRS-myc is located within the apicoplast but not the mitochondrion in erythrocytic parasites. Finally, a bipartite apicoplast-targeting sequence from a Babesia bovis GluRS was recently shown (87) to direct GFP into the Babesia apicoplast. This is consistent with our localization of the PfGluRS by an analogous approach,5 and by epitope tagging of the endogenous PbGluRS enzyme (Figs. 8 and 9).
FIGURE 9.
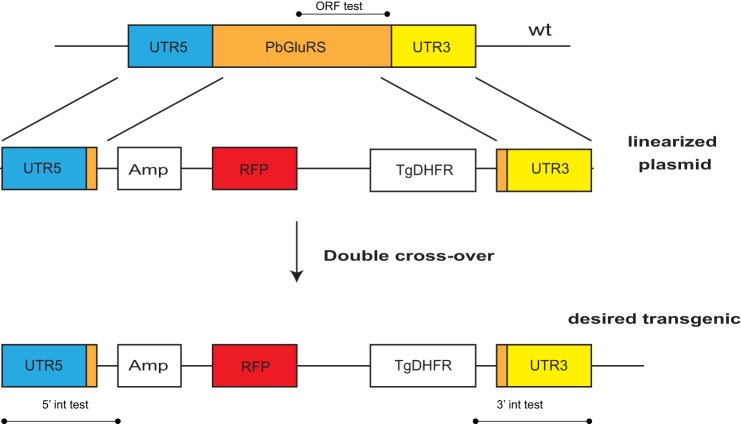
Attempted knock-out of the PbGluRS gene by double crossover recombination (replacement). Two fragments containing 5′- and 3′-UTRs of the PbGluRS gene, with ∼100 nucleotides of the start and end, respectively, of the PbGluRS coding sequence were cloned into the B3D KO Red vector (60). After linearization with ApaI, the construct was transfected into P. berghei ANKA parasites (108). In three independent experiments, we were unable to delete the endogenous PbGluRS gene by integration of the deletion construct via double crossover recombination.
Attempted Deletion of the PbGluRS Gene
The apicoplast GluRS should be essential because it produces two substrates for apicoplast protein synthesis, Glu-tRNAGlu directly, and Gln-tRNAGln indirectly, in concert with a Glu-AdT. Because bioinformatic analyses suggest that Plasmodium lacks an apicoplast-targeted GlnRS (66, 88), indirect aminoacylation is probably the sole route for Gln-tRNAGln formation in the apicoplast. To test whether PbGluRS was required for blood-stage growth, we transfected P. berghei parasites with a construct (Fig. 9) designed to delete the endogenous PbGluRS gene by double crossover recombination. As a control, we transfected parasites from the same batch with the same construct (Fig. 7) used to generate the PbGluRSmyc transgenic parasites. In three independent experiments, we were unable to integrate the deletion construct into the PbGluRS genomic locus via double crossover recombination. As shown earlier, however, transgenic PbGluRSmyc parasites were readily obtained (data not shown). These results indicate that the PbGluRS locus was accessible to recombination but that the gene could not be deleted, strongly suggesting that the apicoplast-targeted GluRS is essential in blood stage parasites.
DISCUSSION
Plasmodium aaRSs have not been widely studied despite their critical role in protein synthesis, a process absolutely required for parasite replication. One early study reported aaRS activity in P. berghei cell-free extracts (89), and genome sequencing later revealed the entire complement of aaRSs in Plasmodium (10, 68, 88). But to date, cytoplasmic and apicoplast P. falciparum aspartyl-tRNA synthetase (28, 29), lysyl-tRNA synthetase (30, 31), and tryptophanyl-tRNA synthetase (32, 33) are the only other malarial aaRSs besides PfGluRS to be functionally or structurally characterized.
To our knowledge, the apicoplast PfGluRS is only the second apicoplast aaRS to be biochemically characterized. Recently, aminoacylation by recombinant apicoplast LysRS (PF3D7_1416800) was shown to be inhibited by a series of novel compounds that mimicked the lysyl-adenylate reaction intermediate, and two compounds exhibited IC50 values in the 40–85 nm range against asexual parasites in vitro (28). Istvan et al. (90) also showed that apicoplast isoleucyl-tRNA synthetase (PF3D7_1225100) is the target of mupirocin, an inhibitor of bacterial isoleucyl-tRNA synthetases used to treat skin infections. Mupirocin also inhibited parasite growth in vitro at very low concentrations (EC50 ∼58 nm). Together, these results suggest that inhibitors of other apicoplast aaRSs might also exhibit good anti-parasitic activity (91).
We used recombinant PfGluRS produced in E. coli (Fig. 3) to conduct biochemical analyses to determine whether the enzyme had nondiscriminating aminoacylation activity required for indirect aminoacylation and to establish optimal reaction conditions. We chose to use an aminoacylation assay (52) that was sensitive and could be adapted to measure Glu-AdT activity (92, 93). PfGluRS glutamylated apicoplast tRNAGlu and tRNAGln, but not cytoplasmic tRNAGlu, tRNAGln, or E. coli tRNA (Fig. 4, B and C). The aminoacylation activity was therefore both nondiscriminating and specific for the apicoplast tRNAs. The kinetic analyses showed that, like other nondiscriminating GluRSs (94), PfGluRS-catalyzed glutamylation of the cognate and noncognate substrates at similar rates (Table 2). We also found that PfGluRS was sensitive to oxidation because DTT was required to maintain enzyme activity. The sensitivity of PfGluRS to oxidation might provide a means to regulate apicoplast protein synthesis in response to changes in the organelle's redox status (95).
As with other aaRSs (52), PfGluRS-catalyzed glutamylation was subject to product inhibition by PPi, which was reversed by adding PPase to the reaction (Fig. 5B). Aminoacylation of tRNAs proceeds in two steps. First, the tRNA synthetase binds ATP and amino acid to form an enzyme-bound aminoacyl-adenylate and PPi. Second, upon binding of the tRNA substrate to the enzyme-aminoacyl-adenylate complex, the amino acid is transferred from the aminoacyl-adenylate to the 3′ end of the tRNA with the concomitant release of PPi. Subsequent PPi hydrolysis provides a thermodynamic push for the reaction, so PPi must be removed to prevent aaRS inhibition. Inorganic pyrophosphatase performs this function in vivo. PPase is essential in E. coli (96) and yeast (97), and chloroplasts possess a plastid-specific PPase. Transient repression of plastid PPase expression in tobacco leaves increased PPi levels, reduced total soluble leaf protein by 60%, and inhibited expression of nucleus- and plastid-encoded subunits of the carbon-fixing enzyme ribulose-1,5-bisphosphate carboxylase oxygenase, suggesting that repressing plastid PPase levels reduced cytoplasmic and plastid protein synthesis (98). The P. falciparum genome (99) encodes a soluble pyrophosphatase (PF3D7_0316300) that may be dual-targeted to the apicoplast and the cytoplasm via two alternatively spliced isoforms. The enzyme is expressed in asexual stages and gametocytes (71, 100); an apicoplast isoform might regulate PPi levels within the organelle to prevent PPi-mediated inhibition of protein synthesis or PPi-sensitive enzymes in pathways such as isoprenoid biosynthesis (13).
Next, we tested whether a potent bacterial GluRS inhibitor, Glu-SA, was able to inhibit the Plasmodium enzyme. Glu-SA inhibited PfGluRS activity (Ki = 0.7 μm, Fig. 6) but with a 250- and 10-fold lower efficiency than for E. coli or murine liver GluRSs, respectively (59). The wide range of susceptibility to Glu-SA inhibition exhibited by enzymes from different lineages probably reflects structural differences between their active sites. These differences between Plasmodium and host enzymes might be exploited to produce inhibitors with selective activity against the Plasmodium apicoplast GluRS.
The PfGluRS and its apicomplexan orthologs possess a bipartite apicoplast targeting sequence, but the enzyme's subcellular localization had never been determined experimentally. We found via immunofluorescence microscopy of Myc-tagged PbGluRS that the enzyme was localized in the apicoplast in erythrocytic stage parasites (Fig. 8A). Minor overlaps between the anti-Myc and MitoTracker Red signals were observed where the apicoplast and mitochondrion appeared to contact one another (Fig. 8B), a phenomenon observed with other apicoplast-targeted proteins (86, 101). Localization of PbGluRS solely in the apicoplast differs from the situation in Arabidopsis, where at least 15 nucleus-encoded aaRSs, including GluRS, are targeted to the plastid and the mitochondrion (102). Plasmodial genomes encode a second GluRS in addition to the one studied here, but that enzyme, encoded in P. falciparum by the gene Pf3D7_1349200, is predicted to be cytoplasmic (66, 68, 88). Dual targeting of aaRSs to the plastid and mitochondrion is common in higher plants (102), but this is unlikely in Plasmodium because the mitochondrion in the related parasite Toxoplasma imports aminoacylated tRNAs from the cytoplasm (66).
In this work, we identified components of an indirect aminoacylation pathway to produce Gln-tRNAGln for apicoplast protein synthesis. We demonstrated that the first enzyme in the pathway, GluRS, is targeted to the apicoplast in blood stage parasites and can glutamylate both tRNAGlu and tRNAGln in vitro. The latter property is associated with GluRSs that play a role in indirect aminoacylation, providing convincing evidence that the apicoplast indeed uses this pathway. The second enzyme in the pathway, Glu-AdT, has not been studied in Plasmodium, but we have expressed recombinant P. falciparum GatA and GatB subunits5 and are testing whether they can amidate Glu-tRNAGln produced by the PfGluRS to form Gln-tRNAGln. We are also investigating a potential novel feature of the Plasmodium Glu-AdT, the apparent absence of a GatC subunit that is required to stabilize the bacterial Glu-AdT (GatCAB) (69, 103), and whether the GluRS, Glu-AdT, and tRNAGln form a ribonucleoprotein complex (the transamidosome) as they do in bacteria (104–106). Finally, apicoplast indirect aminoacylation is probably essential in malaria parasites because the parasite genome does not encode an apicoplast-targeted GlnRS (66, 68, 88). This is consistent with our inability to delete the gene encoding the P. berghei apicoplast GluRS in three independent experiments, despite the fact that it was accessible to myc tagging. This pathway may be a potential drug target because apicoplast protein synthesis is essential in both blood and liver stages. Further investigations to examine the requirements for apicoplast indirect aminoacylation across the Plasmodium life cycle and to identify specific inhibitors of plasmodial GluRS or Glu-AdT enzymes may lead to novel ways in which to target this pathway for chemotherapy.
Acknowledgments
We thank Geoffrey McFadden for providing anti-ACP antibody, J. Lapointe, and A. Weiner for plasmids expressing E. coli glutamyl-tRNA synthetase and CCA-adding enzyme, Y. M. Hou for the pGFIB vector, and Kelly Sheppard, A. Vaughan, and S. Mikolajczak for advice. We thank MR4 for providing P. falciparum 3D7 parasites contributed by Dan Carucci and Alister Craig. Unpublished P. berghei genome sequence data were produced by the Pathogen Sequencing Group at the Wellcome Trust Sanger Institute and can be obtained on line.
This work was supported, in whole or in part, by National Institutes of Health Grant R21AI81097 from the NIAID (to M. J. G.) and Grants AI 093380 and AI 099280 (to P. K. R.). This work was also supported by the Murdock Trust, Seattle Biomedical Research Institute, and Puget Sound Partners for Global Health (to M. J. G.).
M. J. Gardner and B. M. Mailu, unpublished observations.
- aaRS
- aminoacyl-tRNA synthetase
- ACP
- acyl-carrier protein
- GluRS
- glutamyl-tRNA synthetase
- Glu-SA
- 5′-O-[N-(l-glutamyl)sulfamoyl]adenosine
- GlnRS
- glutaminyl-tRNA synthetase
- Pb
- P. berghei
- Pf
- P. falciparum
- PPase
- inorganic pyrophosphatase
- AspRS
- Glu-AdT, glutamyl-tRNA amidotransferase
- Ni-NTA
- nickel-nitrilotriacetic acid
- For
- forward
- Rev
- reverse
- DHFR
- dihydrofolate reductase
- -TS
- -thymidylate synthase.
REFERENCES
- 1. World Health Organization (2012) World Malaria Report 2012, World Health Organization, Geneva [Google Scholar]
- 2. Murray C. J., Rosenfeld L. C., Lim S. S., Andrews K. G., Foreman K. J., Haring D., Fullman N., Naghavi M., Lozano R., Lopez A. D. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379, 413–431 [DOI] [PubMed] [Google Scholar]
- 3. Dondorp A. M., Nosten F., Yi P., Das D., Phyo A. P., Tarning J., Lwin K. M., Ariey F., Hanpithakpong W., Lee S. J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S. S., Yeung S., Singhasivanon P., Day N. P., Lindegardh N., Socheat D., White N. J. (2009) Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361, 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McFadden G. I., Reith M. E., Munholland J., Lang-Unnasch N. (1996) Plastid in human parasites. Nature 381, 482–483 [DOI] [PubMed] [Google Scholar]
- 5. Wilson R. J., Denny P. W., Preiser P. R., Rangachari K., Roberts K., Roy A., Whyte A., Strath M., Moore D. J., Moore P. W., Williamson D. H. (1996) Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 261, 155–172 [DOI] [PubMed] [Google Scholar]
- 6. Gardner M. J., Feagin J. E., Moore D. J., Spencer D. F., Gray M. W., Williamson D. H., Wilson R. J. (1991) Organization and expression of small subunit ribosomal RNA genes encoded by a 35-kb circular DNA in Plasmodium falciparum. Mol. Biochem. Parasitol. 48, 77–88 [DOI] [PubMed] [Google Scholar]
- 7. Gardner M. J., Williamson D. H., Wilson R. J. (1991) A circular DNA in malaria parasites encodes an RNA polymerase like that of prokaryotes and chloroplasts. Mol. Biochem. Parasitol. 44, 115–123 [DOI] [PubMed] [Google Scholar]
- 8. Seeber F. (2002) Biogenesis of iron-sulphur clusters in amitochondriate and apicomplexan protists. Int. J. Parasitol. 32, 1207–1217 [DOI] [PubMed] [Google Scholar]
- 9. Waller R. F., Keeling P. J., Donald R. G., Striepen B., Handman E., Lang-Unnasch N., Cowman A. F., Besra G. S., Roos D. S., McFadden G. I. (1998) Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 95, 12352–12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., Paulsen I. T., James K., Eisen J. A., Rutherford K., Salzberg S. L., Craig A., Kyes S., Chan M. S., Nene V., Shallom S. J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M. W., Vaidya A. B., Martin D. M., Fairlamb A. H., Fraunholz M. J., Roos D. S., Ralph S. A., McFadden G. I., Cummings L. M., Subramanian G. M., Mungall C., Venter J. C., Carucci D. J., Hoffman S. L., Newbold C., Davis R. W., Fraser C. M., Barrell B. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ralph S. A., van Dooren G. G., Waller R. F., Crawford M. J., Fraunholz M. J., Foth B. J., Tonkin C. J., Roos D. S., McFadden G. I. (2004) Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2, 203–216 [DOI] [PubMed] [Google Scholar]
- 12. Gardner M. J., Tettelin H., Carucci D. J., Cummings L. M., Aravind L., Koonin E. V., Shallom S., Mason T., Yu K., Fujii C., Pederson J., Shen K., Jing J., Aston C., Lai Z., Schwartz D. C., Pertea M., Salzberg S., Zhou L., Sutton G. G., Clayton R., White O., Smith H. O., Fraser C. M., Adams M. D., Venter J. C., Hoffman S. L. (1998) Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science 282, 1126–1132 [DOI] [PubMed] [Google Scholar]
- 13. Jomaa H., Wiesner J., Sanderbrand S., Altincicek B., Weidemeyer C., Hintz M., Türbachova I., Eberl M., Zeidler J., Lichtenthaler H. K., Soldati D., Beck E. (1999) Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285, 1573–1576 [DOI] [PubMed] [Google Scholar]
- 14. Nagaraj V. A., Arumugam R., Chandra N. R., Prasad D., Rangarajan P. N., Padmanaban G. (2009) Localisation of Plasmodium falciparum uroporphyrinogen III decarboxylase of the heme-biosynthetic pathway in the apicoplast and characterisation of its catalytic properties. Int. J. Parasitol. 39, 559–568 [DOI] [PubMed] [Google Scholar]
- 15. Günther S., Matuschewski K., Müller S. (2009) Knockout studies reveal an important role of Plasmodium lipoic acid protein ligase A1 for asexual blood stage parasite survival. PLoS One 4, e5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaughan A. M., O'Neill M. T., Tarun A. S., Camargo N., Phuong T. M., Aly A. S., Cowman A. F., Kappe S. H. (2009) Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11, 506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeh E., DeRisi J. L. (2011) Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9, e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodman C. D., McFadden G. I. (2013) Targeting apicoplasts in malaria parasites. Expert Opin. Ther. Targets 17, 167–177 [DOI] [PubMed] [Google Scholar]
- 19. Geary T. G., Jensen J. B. (1983) Effects of antibiotics on Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 32, 221–225 [DOI] [PubMed] [Google Scholar]
- 20. Ginsburg H., Divo A. A., Geary T. G., Boland M. T., Jensen J. B. (1986) Effects of mitochondrial inhibitors on intraerythrocytic Plasmodium falciparum in in vitro cultures. J. Protozool. 33, 121–125 [DOI] [PubMed] [Google Scholar]
- 21. Puri S. K., Singh N. (2000) Azithromycin: antimalarial profile against blood- and sporozoite-induced infections in mice and monkeys. Exp. Parasitol. 94, 8–14 [DOI] [PubMed] [Google Scholar]
- 22. Sullivan M., Li J., Kumar S., Rogers M. J., McCutchan T. F. (2000) Effects of interruption of apicoplast function on malaria infection, development, and transmission. Mol. Biochem. Parasitol. 109, 17–23 [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization (2010) Guidelines for the Treatment of Malaria, 2nd Ed., pp. 53–62, World Health Organization, Geneva [Google Scholar]
- 24. Tan K. R., Magill A. J., Parise M. E., Arguin P. M. (2011) Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 84, 517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dahl E. L., Rosenthal P. J. (2008) Apicoplast translation, transcription and genome replication: targets for antimalarial antibiotics. Trends Parasitol. 24, 279–284 [DOI] [PubMed] [Google Scholar]
- 26. Lv P. C., Zhu H. L. (2012) Aminoacyl-tRNA synthetase inhibitors as potent antibacterials. Curr. Med. Chem. 19, 3550–3563 [DOI] [PubMed] [Google Scholar]
- 27. Baker S. J., Zhang Y. K., Akama T., Lau A., Zhou H., Hernandez V., Mao W., Alley M. R., Sanders V., Plattner J. J. (2006) Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), for the potential treatment of onychomycosis. J. Med. Chem. 49, 4447–4450 [DOI] [PubMed] [Google Scholar]
- 28. Hoen R., Novoa E. M., López A., Camacho N., Cubells L., Vieira P., Santos M., Marin-Garcia P., Bautista J. M., Cortés A., Ribas de Pouplana L., Royo M. (2013) Selective inhibition of an apicoplastic aminoacyl-tRNA synthetase from Plasmodium falciparum. Chembiochem 14, 499–509 [DOI] [PubMed] [Google Scholar]
- 29. Bour T., Akaddar A., Lorber B., Blais S., Balg C., Candolfi E., Frugier M. (2009) Plasmodial aspartyl-tRNA synthetases and peculiarities in Plasmodium falciparum. J. Biol. Chem. 284, 18893–18903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoepfner D., McNamara C. W., Lim C. S., Studer C., Riedl R., Aust T., McCormack S. L., Plouffe D. M., Meister S., Schuierer S., Plikat U., Hartmann N., Staedtler F., Cotesta S., Schmitt E. K., Petersen F., Supek F., Glynne R. J., Tallarico J. A., Porter J. A., Fishman M. C., Bodenreider C., Diagana T. T., Movva N. R., Winzeler E. A. (2012) Selective and specific inhibition of the Plasmodium falciparum lysyl-tRNA synthetase by the fungal secondary metabolite cladosporin. Cell Host Microbe 11, 654–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan S., Garg A., Camacho N., Van Rooyen J., Kumar Pole A., Belrhali H., Ribas de Pouplana L., Sharma V., Sharma A. (2013) Structural analysis of malaria-parasite lysyl-tRNA synthetase provides a platform for drug development. Acta Crystallogr. D Biol. Crystallogr. 69, 785–795 [DOI] [PubMed] [Google Scholar]
- 32. Koh C. Y., Kim J. E., Napoli A. J., Verlinde C. L., Fan E., Buckner F. S., Van Voorhis W. C., Hol W. G. (2013) Crystal structures of Plasmodium falciparum cytosolic tryptophanyl-tRNA synthetase and its potential as a target for structure-guided drug design. Mol. Biochem. Parasitol. 189, 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khan S., Garg A., Sharma A., Camacho N., Picchioni D., Saint-Léger A., Ribas de Pouplana L., Yogavel M., Sharma A. (2013) An appended domain results in an unusual architecture for malaria parasite tryptophanyl-tRNA synthetase. PLoS One 8, e66224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheppard K., Yuan J., Hohn M. J., Jester B., Devine K. M., Söll D. (2008) From one amino acid to another: tRNA-dependent amino acid biosynthesis. Nucleic Acids Res. 36, 1813–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baick J. W., Yoon J. H., Namgoong S., Söll D., Kim S. I., Eom S. H., Hong K. W. (2004) Growth inhibition of Escherichia coli during heterologous expression of Bacillus subtilis glutamyl-tRNA synthetase that catalyzes the formation of mischarged glutamyl-tRNA1 Gln. J. Microbiol. 42, 111–116 [PubMed] [Google Scholar]
- 36. Carlton J. M., Angiuoli S. V., Suh B. B., Kooij T. W., Pertea M., Silva J. C., Ermolaeva M. D., Allen J. E., Selengut J. D., Koo H. L., Peterson J. D., Pop M., Kosack D. S., Shumway M. F., Bidwell S. L., Shallom S. J., van Aken S. E., Riedmuller S. B., Feldblyum T. V., Cho J. K., Quackenbush J., Sedegah M., Shoaibi A., Cummings L. M., Florens L., Yates J. R., Raine J. D., Sinden R. E., Harris M. A., Cunningham D. A., Preiser P. R., Bergman L. W., Vaidya A. B., van Lin L. H., Janse C. J., Waters A. P., Smith H. O., White O. R., Salzberg S. L., Venter J. C., Fraser C. M., Hoffman S. L., Gardner M. J., Carucci D. J. (2002) Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature 419, 512–519 [DOI] [PubMed] [Google Scholar]
- 37. Carlton J. M., Adams J. H., Silva J. C., Bidwell S. L., Lorenzi H., Caler E., Crabtree J., Angiuoli S. V., Merino E. F., Amedeo P., Cheng Q., Coulson R. M., Crabb B. S., Del Portillo H. A., Essien K., Feldblyum T. V., Fernandez-Becerra C., Gilson P. R., Gueye A. H., Guo X., Kang'a S., Kooij T. W., Korsinczky M., Meyer E. V., Nene V., Paulsen I., White O., Ralph S. A., Ren Q., Sargeant T. J., Salzberg S. L., Stoeckert C. J., Sullivan S. A., Yamamoto M. M., Hoffman S. L., Wortman J. R., Gardner M. J., Galinski M. R., Barnwell J. W., Fraser-Liggett C. M. (2008) Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 455, 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gardner M. J., Bishop R., Shah T., de Villiers E. P., Carlton J. M., Hall N., Ren Q., Paulsen I. T., Pain A., Berriman M., Wilson R. J., Sato S., Ralph S. A., Mann D. J., Xiong Z., Shallom S. J., Weidman J., Jiang L., Lynn J., Weaver B., Shoaibi A., Domingo A. R., Wasawo D., Crabtree J., Wortman J. R., Haas B., Angiuoli S. V., Creasy T. H., Lu C., Suh B., Silva J. C., Utterback T. R., Feldblyum T. V., Pertea M., Allen J., Nierman W. C., Taracha E. L., Salzberg S. L., White O. R., Fitzhugh H. A., Morzaria S., Venter J. C., Fraser C. M., Nene V. (2005) Genome sequence of Theileria parva, a bovine pathogen that transforms lymphocytes. Science 309, 134–137 [DOI] [PubMed] [Google Scholar]
- 39. Hall N., Karras M., Raine J. D., Carlton J. M., Kooij T. W., Berriman M., Florens L., Janssen C. S., Pain A., Christophides G. K., James K., Rutherford K., Harris B., Harris D., Churcher C., Quail M. A., Ormond D., Doggett J., Trueman H. E., Mendoza J., Bidwell S. L., Rajandream M. A., Carucci D. J., Yates J. R., 3rd, Kafatos F. C., Janse C. J., Barrell B., Turner C. M., Waters A. P., Sinden R. E. (2005) A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307, 82–86 [DOI] [PubMed] [Google Scholar]
- 40. Aurrecoechea C., Brestelli J., Brunk B. P., Dommer J., Fischer S., Gajria B., Gao X., Gingle A., Grant G., Harb O. S., Heiges M., Innamorato F., Iodice J., Kissinger J. C., Kraemer E., Li W., Miller J. A., Nayak V., Pennington C., Pinney D. F., Roos D. S., Ross C., Stoeckert C. J., Jr., Treatman C., Wang H. (2009) PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 37, D539–D543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. UniProt Consortium (2010) The Universal Protein Resource (UniProt) in 2010. Nucleic Acids Res. 38, D142–D148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Armougom F., Moretti S., Poirot O., Audic S., Dumas P., Schaeli B., Keduas V., Notredame C. (2006) Expresso: automatic incorporation of structural information in multiple sequence alignments using 3D-Coffee. Nucleic Acids Res. 34, W604–W608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gouet P., Courcelle E., Stuart D. I., Métoz F. (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 [DOI] [PubMed] [Google Scholar]
- 44. Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J. F., Guindon S., Lefort V., Lescot M., Claverie J. M., Gascuel O. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edgar R. C. (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Castresana J. (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552 [DOI] [PubMed] [Google Scholar]
- 47. Guindon S., Gascuel O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 [DOI] [PubMed] [Google Scholar]
- 48. Chevenet F., Brun C., Bañuls A. L., Jacq B., Christen R. (2006) TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trager W., Jensen J. B. (1976) Human malaria parasites in continuous culture. Science 193, 673–675 [DOI] [PubMed] [Google Scholar]
- 50. Mudeppa D. G., Pang C. K., Tsuboi T., Endo Y., Buckner F. S., Varani G., Rathod P. K. (2007) Cell-free production of functional Plasmodium falciparum dihydrofolate reductase-thymidylate synthase. Mol. Biochem. Parasitol. 151, 216–219 [DOI] [PubMed] [Google Scholar]
- 51. Jortzik E., Mailu B. M., Preuss J., Fischer M., Bode L., Rahlfs S., Becker K. (2011) Glucose-6-phosphate dehydrogenase 6-phosphogluconolactonase: a unique bifunctional enzyme from Plasmodium falciparum. Biochem. J. 436, 641–650 [DOI] [PubMed] [Google Scholar]
- 52. Wolfson A. D., Uhlenbeck O. C. (2002) Modulation of tRNAAla identity by inorganic pyrophosphatase. Proc. Natl. Acad. Sci. U.S.A. 99, 5965–5970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Masson J. M., Miller J. H. (1986) Expression of synthetic suppressor tRNA genes under the control of a synthetic promoter. Gene 47, 179–183 [DOI] [PubMed] [Google Scholar]
- 54. Jahn C. E., Charkowski A. O., Willis D. K. (2008) Evaluation of isolation methods and RNA integrity for bacterial RNA quantitation. J. Microbiol. Methods 75, 318–324 [DOI] [PubMed] [Google Scholar]
- 55. Oshikane H., Sheppard K., Fukai S., Nakamura Y., Ishitani R., Numata T., Sherrer R. L., Feng L., Schmitt E., Panvert M., Blanquet S., Mechulam Y., Söll D., Nureki O. (2006) Structural basis of RNA-dependent recruitment of glutamine to the genetic code. Science 312, 1950–1954 [DOI] [PubMed] [Google Scholar]
- 56. Dubois D. Y., Blais S. P., Huot J. L., Lapointe J. (2009) A C-truncated glutamyl-tRNA synthetase specific for tRNA(Glu) is stimulated by its free complementary distal domain: mechanistic and evolutionary implications. Biochemistry 48, 6012–6021 [DOI] [PubMed] [Google Scholar]
- 57. Feng L., Sheppard K., Tumbula-Hansen D., Söll D. (2005) Gln-tRNAGln formation from Glu-tRNAGln requires cooperation of an asparaginase and a Glu-tRNAGln kinase. J. Biol. Chem. 280, 8150–8155 [DOI] [PubMed] [Google Scholar]
- 58. Freist W., Sternbach H., Cramer F. (1989) Arginyl-tRNA synthetase from yeast. Discrimination between 20 amino acids in aminoacylation of tRNA(Arg)-C-C-A and tRNA(Arg)-C-C-A(3′NH2). Eur. J. Biochem. 186, 535–541 [DOI] [PubMed] [Google Scholar]
- 59. Bernier S., Dubois D. Y., Habegger-Polomat C., Gagnon L.-P., Lapointe J., Chênevert R. (2005) Glutamylsulfamoyladenosine and pyroglutamylsulfamoyladenosine are competitive inhibitors of E. coli glutamyl-tRNA synthetase. J. Enzyme Inhib. Med. Chem. 20, 61–67 [DOI] [PubMed] [Google Scholar]
- 60. Mikolajczak S. A., Aly A. S., Dumpit R. F., Vaughan A. M., Kappe S. H. (2008) An efficient strategy for gene targeting and phenotypic assessment in the Plasmodium yoelii rodent malaria model. Mol. Biochem. Parasitol. 158, 213–216 [DOI] [PubMed] [Google Scholar]
- 61. Labaied M., Harupa A., Dumpit R. F., Coppens I., Mikolajczak S. A., Kappe S. H. (2007) Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 75, 3758–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tonkin C. J., van Dooren G. G., Spurck T. P., Struck N. S., Good R. T., Handman E., Cowman A. F., McFadden G. I. (2004) Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol. Biochem. Parasitol. 137, 13–21 [DOI] [PubMed] [Google Scholar]
- 63. Waller R. F., Reed M. B., Cowman A. F., McFadden G. I. (2000) Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19, 1794–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zuegge J., Ralph S., Schmuker M., McFadden G. I., Schneider G. (2001) Deciphering apicoplast targeting signals–feature extraction from nuclear-encoded precursors of Plasmodium falciparum apicoplast proteins. Gene 280, 19–26 [DOI] [PubMed] [Google Scholar]
- 65. Foth B. J., Ralph S. A., Tonkin C. J., Struck N. S., Fraunholz M., Roos D. S., Cowman A. F., McFadden G. I. (2003) Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum. Science 299, 705–708 [DOI] [PubMed] [Google Scholar]
- 66. Pino P., Aeby E., Foth B. J., Sheiner L., Soldati T., Schneider A., Soldati-Favre D. (2010) Mitochondrial translation in absence of local tRNA aminoacylation and methionyl tRNA Met formylation in Apicomplexa. Mol. Microbiol. 76, 706–718 [DOI] [PubMed] [Google Scholar]
- 67. Saad N. Y., Schiel B., Brayé M., Heap J. T., Minton N. P., Dürre P., Becker H. D. (2012) Riboswitch (T-box)-mediated control of tRNA-dependent amidation in Clostridium acetobutylicum rationalizes gene and pathway redundancy for asparagine and asparaginyl-trnaasn synthesis. J. Biol. Chem. 287, 20382–20394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jackson K. E., Habib S., Frugier M., Hoen R., Khan S., Pham J. S., Ribas de Pouplana L., Royo M., Santos M. A., Sharma A., Ralph S. A. (2011) Protein translation in Plasmodium parasites. Trends Parasitol. 27, 467–476 [DOI] [PubMed] [Google Scholar]
- 69. Nakamura A., Yao M., Chimnaronk S., Sakai N., Tanaka I. (2006) Ammonia channel couples glutaminase with transamidase reactions in GatCAB. Science 312, 1954–1958 [DOI] [PubMed] [Google Scholar]
- 70. Frechin M., Senger B., Brayé M., Kern D., Martin R. P., Becker H. D. (2009) Yeast mitochondrial Gln-tRNA(Gln) is generated by a GatFAB-mediated transamidation pathway involving Arc1p-controlled subcellular sorting of cytosolic GluRS. Genes Dev. 23, 1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bozdech Z., Llinas M., Pulliam B. L., Wong E. D., Zhu J., DeRisi J. L. (2003) The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. October;1(1):E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vignali M., Armour C. D., Chen J., Morrison R., Castle J. C., Biery M. C., Bouzek H., Moon W., Babak T., Fried M., Raymond C. K., Duffy P. E. (2011) NSR-seq transcriptional profiling enables identification of a gene signature of Plasmodium falciparum parasites infecting children. J. Clin. Invest. 121, 1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lasonder E., Ishihama Y., Andersen J. S., Vermunt A. M., Pain A., Sauerwein R. W., Eling W. M., Hall N., Waters A. P., Stunnenberg H. G., Mann M. (2002) Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419, 537–542 [DOI] [PubMed] [Google Scholar]
- 74. Florens L., Washburn M. P., Raine J. D., Anthony R. M., Grainger M., Haynes J. D., Moch J. K., Muster N., Sacci J. B., Tabb D. L., Witney A. A., Wolters D., Wu Y., Gardner M. J., Holder A. A., Sinden R. E., Yates J. R., Carucci D. J. (2002) A proteomic view of the Plasmodium falciparum life cycle. Nature 419, 520–526 [DOI] [PubMed] [Google Scholar]
- 75. Eriani G., Delarue M., Poch O., Gangloff J., Moras D. (1990) Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 347, 203–206 [DOI] [PubMed] [Google Scholar]
- 76. Sekine S., Nureki O., Dubois D. Y., Bernier S., Chênevert R., Lapointe J., Vassylyev D. G., Yokoyama S. (2003) ATP binding by glutamyl-tRNA synthetase is switched to the productive mode by tRNA binding. EMBO J. 22, 676–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Banerjee R., Dubois D. Y., Gauthier J., Lin S. X., Roy S., Lapointe J. (2004) The zinc-binding site of a class I aminoacyl-tRNA synthetase is a SWIM domain that modulates amino acid binding via the tRNA acceptor arm. Eur. J. Biochem. 271, 724–733 [DOI] [PubMed] [Google Scholar]
- 78. Schulze J. O., Masoumi A., Nickel D., Jahn M., Jahn D., Schubert W. D., Heinz D. W. (2006) Crystal structure of a non-discriminating glutamyl-tRNA synthetase. J. Mol. Biol. 361, 888–897 [DOI] [PubMed] [Google Scholar]
- 79. Shu N., Zhou T., Hovmöller S. (2008) Prediction of zinc-binding sites in proteins from sequence. Bioinformatics 24, 775–782 [DOI] [PubMed] [Google Scholar]
- 80. Lee J., Hendrickson T. L. (2004) Divergent anticodon recognition in contrasting glutamyl-tRNA synthetases. J. Mol. Biol. 344, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sekine S., Nureki O., Shimada A., Vassylyev D. G., Yokoyama S. (2001) Structural basis for anticodon recognition by discriminating glutamyl-tRNA synthetase. Nat. Struct. Biol. 8, 203–206 [DOI] [PubMed] [Google Scholar]
- 82. Bullock T. L., Uter N., Nissan T. A., Perona J. J. (2003) Amino acid discrimination by a class I aminoacyl-tRNA synthetase specified by negative determinants. J. Mol. Biol. 328, 395–408 [DOI] [PubMed] [Google Scholar]
- 83. Freist W. (1989) Mechanisms of aminoacyl-tRNA synthetases: a critical consideration of recent results. Biochemistry 28, 6787–6795 [DOI] [PubMed] [Google Scholar]
- 84. Brown P., Richardson C. M., Mensah L. M., O'Hanlon P. J., Osborne N. F., Pope A. J., Walker G. (1999) Molecular recognition of tyrosinyl adenylate analogues by prokaryotic tyrosyl tRNA synthetases. Bioorg. Med. Chem. 7, 2473–2485 [DOI] [PubMed] [Google Scholar]
- 85. Hall N., Pain A., Berriman M., Churcher C., Harris B., Harris D., Mungall K., Bowman S., Atkin R., Baker S., Barron A., Brooks K., Buckee C. O., Burrows C., Cherevach I., Chillingworth C., Chillingworth T., Christodoulou Z., Clark L., Clark R., Corton C., Cronin A., Davies R., Davis P., Dear P., Dearden F., Doggett J., Feltwell T., Goble A., Goodhead I., Gwilliam R., Hamlin N., Hance Z., Harper D., Hauser H., Hornsby T., Holroyd S., Horrocks P., Humphray S., Jagels K., James K. D., Johnson D., Kerhornou A., Knights A., Konfortov B., Kyes S., Larke N., Lawson D., Lennard N., Line A., Maddison M., McLean J., Mooney P., Moule S., Murphy L., Oliver K., Ormond D., Price C., Quail M. A., Rabbinowitsch E., Rajandream M. A., Rutter S., Rutherford K. M., Sanders M., Simmonds M., Seeger K., Sharp S., Smith R., Squares R., Squares S., Stevens K., Taylor K., Tivey A., Unwin L., Whitehead S., Woodward J., Sulston J. E., Craig A., Newbold C., Barrell B. G. (2002) Sequence of Plasmodium falciparum chromosomes 1, 3–9, and 13. Nature 419, 527–531 [DOI] [PubMed] [Google Scholar]
- 86. van Dooren G. G., Marti M., Tonkin C. J., Stimmler L. M., Cowman A. F., McFadden G. I. (2005) Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol. Microbiol. 57, 405–419 [DOI] [PubMed] [Google Scholar]
- 87. Pedroni M. J., Luu T. N., Lau A. O. (2012) Babesia bovis: a bipartite signal directs the glutamyl-tRNA synthetase to the apicoplast. Exp. Parasitol. 131, 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bhatt T. K., Kapil C., Khan S., Jairajpuri M. A., Sharma V., Santoni D., Silvestrini F., Pizzi E., Sharma A. (2009) A genomic glimpse of aminoacyl-tRNA synthetases in malaria parasite Plasmodium falciparum. BMC Genomics 10, 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ilan J., Ilan J. (1969) Aminoacyl transfer ribonucleic acid synthetases from cell-free extract of Plasmodium berghei. Science 164, 560–562 [DOI] [PubMed] [Google Scholar]
- 90. Istvan E. S., Dharia N. V., Bopp S. E., Gluzman I., Winzeler E. A., Goldberg D. E. (2011) Validation of isoleucine utilization targets in Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 108, 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jackson K. E., Pham J. S., Kwek M., De Silva N. S., Allen S. M., Goodman C. D., McFadden G. I., de Pouplana L. R., Ralph S. A. (2012) Dual targeting of aminoacyl-tRNA synthetases to the apicoplast and cytosol in Plasmodium falciparum. Int. J. Parasitol. 42, 177–186 [DOI] [PubMed] [Google Scholar]
- 92. Sheppard K., Akochy P. M., Salazar J. C., Söll D. (2007) The Helicobacter pylori amidotransferase GatCAB is equally efficient in glutamine-dependent transamidation of Asp-tRNAAsn and Glu-tRNAGln. J. Biol. Chem. 282, 11866–11873 [DOI] [PubMed] [Google Scholar]
- 93. Sheppard K., Akochy P. M., Söll D. (2008) Assays for transfer RNA-dependent amino acid biosynthesis. Methods 44, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lapointe J., Duplain L., Proulx M. (1986) A single glutamyl-tRNA synthetase aminoacylates tRNAGlu and tRNAGln in Bacillus subtilis and efficiently misacylates Escherichia coli tRNAGln1 in vitro. J. Bacteriol. 165, 88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Katz A., Banerjee R., de Armas M., Ibba M., Orellana O. (2010) Redox status affects the catalytic activity of glutamyl-tRNA synthetase. Biochem. Biophys. Res. Commun. 398, 51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen J., Brevet A., Fromant M., Lévêque F., Schmitter J. M., Blanquet S., Plateau P. (1990) Pyrophosphatase is essential for growth of. Escherichia coli. J. Bacteriol. 172, 5686–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Giaever G., Chu A. M., Ni L., Connelly C., Riles L., Véronneau S., Dow S., Lucau-Danila A., Anderson K., André B., Arkin A. P., Astromoff A., El-Bakkoury M., Bangham R., Benito R., Brachat S., Campanaro S., Curtiss M., Davis K., Deutschbauer A., Entian K. D., Flaherty P., Foury F., Garfinkel D. J., Gerstein M., Gotte D., Güldener U., Hegemann J. H., Hempel S., Herman Z., Jaramillo D. F., Kelly D. E., Kelly S. L., Kötter P., LaBonte D., Lamb D. C., Lan N., Liang H., Liao H., Liu L., Luo C., Lussier M., Mao R., Menard P., Ooi S. L., Revuelta J. L., Roberts C. J., Rose M., Ross-Macdonald P., Scherens B., Schimmack G., Shafer B., Shoemaker D. D., Sookhai-Mahadeo S., Storms R. K., Strathern J. N., Valle G., Voet M., Volckaert G., Wang C. Y., Ward T. R., Wilhelmy J., Winzeler E. A., Yang Y., Yen G., Youngman E., Yu K., Bussey H., Boeke J. D., Snyder M., Philippsen P., Davis R. W., Johnston M. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391 [DOI] [PubMed] [Google Scholar]
- 98. George G. M., van der Merwe M. J., Nunes-Nesi A., Bauer R., Fernie A. R., Kossmann J., Lloyd J. R. (2010) Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana. Plant Physiol. 154, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bowman S., Lawson D., Basham D., Brown D., Chillingworth T., Churcher C. M., Craig A., Davies R. M., Devlin K., Feltwell T., Gentles S., Gwilliam R., Hamlin N., Harris D., Holroyd S., Hornsby T., Horrocks P., Jagels K., Jassal B., Kyes S., McLean J., Moule S., Mungall K., Murphy L., Oliver K., Quail M. A., Rajandream M. A., Rutter S., Skelton J., Squares R., Squares S., Sulston J. E., Whitehead S., Woodward J. R., Newbold C., Barrell B. G. (1999) The complete nucleotide sequence of chromosome 3 of Plasmodium falciparum. Nature 400, 532–538 [DOI] [PubMed] [Google Scholar]
- 100. Young J. A., Fivelman Q. L., Blair P. L., de la Vega P., Le Roch K. G., Zhou Y., Carucci D. J., Baker D. A., Winzeler E. A. (2005) The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol. Biochem. Parasitol. 143, 67–79 [DOI] [PubMed] [Google Scholar]
- 101. Hopkins J., Fowler R., Krishna S., Wilson I., Mitchell G., Bannister L. (1999) The plastid in Plasmodium falciparum asexual blood stages: a three-dimensional ultrastructural analysis. Protist. 150, 283–295 [DOI] [PubMed] [Google Scholar]
- 102. Duchêne A. M., Giritch A., Hoffmann B., Cognat V., Lancelin D., Peeters N. M., Zaepfel M., Maréchal-Drouard L., Small I. D. (2005) Dual targeting is the rule for organellar aminoacyl-tRNA synthetases in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 102, 16484–16489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Curnow A. W., Hong Kw., Yuan R., Kim Si., Martins O., Winkler W., Henkin T. M., Söll D. (1997) Glu-tRNAGln amidotransferase: a novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. U.S.A. 94, 11819–11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bailly M., Blaise M., Lorber B., Becker H. D., Kern D. (2007) The transamidosome: a dynamic ribonucleoprotein particle dedicated to prokaryotic tRNA-dependent asparagine biosynthesis. Mol. Cell 28, 228–239 [DOI] [PubMed] [Google Scholar]
- 105. Ito T., Yokoyama S. (2010) Two enzymes bound to one transfer RNA assume alternative conformations for consecutive reactions. Nature 467, 612–616 [DOI] [PubMed] [Google Scholar]
- 106. Huot J. L., Fischer F., Corbeil J., Madore E., Lorber B., Diss G., Hendrickson T. L., Kern D., Lapointe J. (2011) Gln-tRNAGln synthesis in a dynamic transamidosome from Helicobacter pylori, where GluRS2 hydrolyzes excess Glu-tRNAGln. Nucleic Acids Res. 39, 9306–9315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liu J., Gagnon Y., Gauthier J., Furenlid L., L'Heureux P. J., Auger M., Nureki O., Yokoyama S., Lapointe J. (1995) The zinc-binding site of Escherichia coli glutamyl-tRNA synthetase is located in the acceptor-binding domain. Studies by extended x-ray absorption fine structure, molecular modeling, and site-directed mutagenesis. J. Biol. Chem. 270, 15162–15169 [DOI] [PubMed] [Google Scholar]
- 108. Labaied M., Camargo N., Kappe S. H. (2007) Depletion of the Plasmodium berghei thrombospondin-related sporozoite protein reveals a role in host cell entry by sporozoites. Mol. Biochem. Parasitol. 153, 158–166 [DOI] [PubMed] [Google Scholar]