Background: TCPTP is a negative regulator of proinflammatory cytokine signaling and may be a therapeutic target for IBD.
Results: Administration of spermidine to intestinal epithelial cells reduced proinflammatory cytokine signaling and subsequent barrier defects in a TCPTP-dependent manner.
Conclusion: Activation of TCPTP by spermidine attenuates inflammatory responses in intestinal epithelial monolayers.
Significance: Protection of epithelial barrier integrity by spermidine in vitro suggests its potential importance for barrier protection in vivo.
Keywords: Epithelial Cell, Interferon, Permeability, STAT Transcription Factor, Tight Junctions
Abstract
The gene locus encoding protein-tyrosine phosphatase non-receptor type 2 (PTPN2) has been associated with inflammatory bowel disease. Expression of the PTPN2 gene product, T cell protein-tyrosine phosphatase (TCPTP), in intestinal epithelial cells has been shown to play an important role in the protection of epithelial barrier function during periods of inflammation by acting as a negative regulator of the proinflammatory cytokine IFN-γ. Therefore, agents that increase the activity of TCPTP are of general interest as modifiers of inflammatory signaling events. A previous study demonstrated that the small molecule spermidine is a selective activator of TCPTP in vitro. The aim of this study was to investigate whether activation of TCPTP by spermidine was capable of alleviating IFN-γ-induced, proinflammatory signaling and barrier dysfunction in human intestinal epithelial cells. Studies revealed that treatment of T84 and HT29/cl.19A colonocytes with spermidine increased both TCPTP protein levels and enzymatic activity, correlating with a decrease in the phosphorylation of the signal transducers and activators of transcription 1 and 3, downstream mediators of IFN-γ signaling, upon coadministration of spermidine to IFN-γ-treated cells. On a functional level, spermidine protected barrier function in the setting of inflammation, restricting the decrease in transepithelial electrical resistance and the increase in epithelial permeability induced by IFN-γ in coincubation experiments. These data implicate spermidine as a potential therapeutic agent to treat conditions associated with elevated IFN-γ signaling and a faulty mucosal barrier.
Introduction
Inflammatory bowel disease (IBD)3 is the umbrella term given to two separate but related conditions, Crohn's disease, and ulcerative colitis, both of which are characterized by chronic inflammation of the intestinal tract. In IBD, proinflammatory cytokines such as IFN-γ and TNF-α are present at high concentrations, with elevated levels of IFN-γ predominating in Crohn's disease (1).
Treatment of intestinal epithelial cell monolayers with IFN-γ has been shown to decrease barrier function (2), resulting in decreases in transepithelial electrical resistance (TER) and increases in epithelial cell permeability (3, 4). These effects are due, in part, to disruption of apical cytoskeletal constituents and the reorganization of tight junction proteins like occludin and zonula occluden 1 (ZO-1) (2). Resolution of the barrier occurs when concentrations of proinflammatory cytokines fall through decreased production or because of increased expression and/or activity of negative regulators of cytokine signaling. One of these negative regulators of IFN-γ cytokine signaling is T cell protein-tyrosine phosphatase (TCPTP).
TCPTP is a member of the protein-tyrosine phosphatase family (PTP) whose members share a highly conserved catalytic motif. PTPs play an integral role in regulating a variety of basic cellular processes, including cell growth and differentiation (5). Among TCPTP substrates are the phosphotyrosine residues on the receptors for epidermal growth factor (6) and insulin (7). Additionally, TCPTP negatively regulates phosphorylation of the IFN-γ receptor (IFNγR) and the IFN-γ signaling molecules signal transducers and activators of transcription (STAT) 1 and 3 (8, 9). Dephosphorylation of STATs by TCPTP leads to their inactivation and termination of STAT-induced transcription (8). TCPTP, therefore, plays an integral role in regulating IFN-γ signaling and maintaining cellular homeostasis.
Genome-wide association studies have revealed that individuals with single nucleotide polymorphic mutations in the locus of the PTPN2 gene that encodes TCPTP have an increased susceptibility to developing Crohn disease and ulcerative colitis (10, 11). Although it has been shown that TCPTP expression and activity is increased in response to IFN-γ, reduced TCPTP expression leads to increased effects of IFN-γ on STAT signaling and a greater reduction in barrier function (12, 13). Therefore, agents that increase the activity of TCPTP are of general interest as modifiers of inflammatory signaling events.
A recent study demonstrated that the small molecule spermidine is a selective activator of TCPTP in vitro (14). Small molecule activation of PTPs provides new insights into the potential for pharmacologic targeting of phosphatases to regulate specific cellular events. By investigating the effects of spermidine on TCPTP in intestinal epithelial cells, our studies serve not only to promote a further understanding of the role of TCPTP in intestinal epithelial barrier maintenance but also to identify a possible therapeutic agent for conditions associated with dysregulated IFN-γ signaling and intestinal barrier defects.
EXPERIMENTAL PROCEDURES
Materials
Human recombinant IFN-γ (Roche, Mannheim, Germany); spermidine trihydrochloride (Sigma); spermine tetrahydrochloride (Sigma); cycloheximide (Sigma); monoclonal mouse anti-TCPTP antibody CF-4, which detects the 45-kD and 48-kD isoforms (Calbiochem, San Diego, CA); anti-phospho-STAT1 (Tyr701), anti-STAT1, anti-phospho-STAT3 (Tyr705), and anti-STAT3 (Cell Signaling Technology, Danvers, MA); and monoclonal mouse anti-β-actin (Sigma) were obtained from the sources noted. Millicell culture plate inserts were purchased from Millipore Corp. (Bedford, MA). All other reagents were of analytical grade and were acquired commercially.
Tissue Culture
Human T84 colonic epithelial cells (passages 20–40) were grown in 1:1 Dulbecco's modified Eagle's medium/Ham's F-12 medium with l-glutamine and 15 mm HEPES (Mediatech Inc., Manassas, VA), supplemented with 5% newborn calf serum (Invitrogen) and 1% penicillin/streptomycin (Cellgro/Mediatech, Herndon, VA) at 37 °C in 5% CO2. Cells were fed twice a week and then seeded onto 30-mm Millicell transwell polycarbonate filters (0.5 × 106 cells) for Western blot analysis. When grown on polycarbonate filters, T84 cells acquire the polarized phenotype of native colonic epithelia (15).
HT29/cl.19A cells (16) were grown to confluence in McCoys's 5A medium with l-glutamine and supplemented with 10% FBS (HyClone Laboratories Inc., Logan, UT) at 37 °C in 5% CO2 (passages 20–40). Cells were fed three times a week, passaged once a week in 75-cm2 flasks, and then seeded onto 30-mm Millicell transwell polycarbonate filters (0.5 × 106 cells) for Western blot analysis.
For barrier function studies, T84 cells were seeded onto 12-mm Millicell-HA culture plate inserts (filter membranes) and grown for 14–21 days before study, at which time they had stable values of transepithelial electrical resistance ≥ 1000 Ω/cm2. IFN-γ (1000 units/ml) was added basolaterally, whereas spermidine (10 μm), spermine (10 μm), and cycloheximide (35.5 μm) were added bilaterally.
RNA Isolation and Real-time Polymerase Chain Reaction
Total RNA was isolated, and DNA was removed from T84 cells using the Direct-zol RNA MiniPrep kit (Zymogen, Irvine, CA) according to the instructions of the manufacturer. RNA purity and concentration were assessed by absorbance at 260 and 280 nm. cDNA synthesis was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative reverse transcriptase polymerase chain reaction was performed using MESA GREEN qPCR MasterMix Plus for SYBR assays (Eurogentec, San Diego, CA) on a StepOnePlus real-time PCR system using Step One software v2.0 (Applied Biosystems). Measurements were performed in triplicate, human GAPDH was used as an endogenous control, and results were analyzed by the ΔΔCT method.
Preparation of Cytoplasmic Lysates
On the day of the experiment, cells from inserts containing T84 and HT29 monolayers were suspended in ice-cold lysis buffer (50 mm Tris, 150 mm NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 20 μm NaF, 1 mm EDTA, 1 μg/ml antipain, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mm NaVO3, and 100 μg/ml phenylmethylsulfonyl fluoride), vortexed thoroughly, and subjected to lysis using a 22-gauge needle. Cells were centrifuged at 10,000 rpm for 10 min to remove insoluble material, and an aliquot was removed from each sample to determine protein content (Bio-Rad protein assay according to the instructions of the manufacturer). Samples were resuspended in loading buffer (50 mm Tris (pH 6.8), 2% SDS, 100 mm dithiothreitol, 0.2% bromphenol blue, and 20% glycerol) and boiled for 5 min.
Western Blotting
Samples suspended in loading buffer were loaded onto a 4–15% gradient polyacrylamide gel to resolve proteins. The proteins were transferred onto a polyvinylidene difluoride membrane (Millipore). The membrane was incubated in blocking buffer (5% bovine serum albumin (BSA) in 0.1% Tris-buffered saline and Tween 20 (TBST)) for 1 h followed by overnight incubation of the membrane in blocking buffer containing primary antibody diluted 1:1000. This was followed by four 15-min washes with 0.1% TBST. After washing, secondary antibody (goat-anti-rabbit, Cell Signaling Technology, or goat-anti-mouse IgG conjugated to horseradish perodixase, BD Biosciences) diluted 1:2000 was added to the membrane and incubated for 30 min. This was followed by four more 15-min washes with wash buffer. The membrane was then treated with chemiluminescent solution according to the directions of the manufacturer (Thermo Scientific, Rockford, IL) and exposed to film. Densitometric analysis of the blot was performed using ImageJ software (National Institutes of Health).
Immunoprecipitation
6 × 106 T84 and HT29 cells were prepared in 75-cm2 flasks. Following stimulation, cells were scraped and suspended in 1 ml of ice-cold PBS. Cells were centrifuged for 5 min at 5000 rpm. The pellet was fully resuspended and lysed in 500 μl of ice-cold lysis buffer (5% IGEPAL CA-360, 750 mm NaCl, 250 mm Tris-Cl (pH 8.0), 1 μg/ml antipain, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 100 μg/ml phenylmethylsulfonyl fluoride). Lysed cells were centrifuged for 10 min at 10,000 rpm to pellet DNA and small nonsoluble particles. 1 μg of monoclonal mouse anti-TCPTP CF-4 antibody was added per sample and placed on a rotating platform at 4 °C for 1 h. This was followed by the addition of 30 μl of the 50% (v/v) protein A-Sepharose beads to the cell lysate/antibody mix and allowed to incubate on a rotating platform at 4 °C for 1 h. Samples were then centrifuged briefly to pellet the protein A-Sepharose-antibody-antigen complex, and the complex was washed three times with cold lysis buffer followed by three more washes with phosphatase reaction buffer (25 mm HEPES, 50 mm NaCl, and 1 mm ditheothreitol). Beads were resuspended in phosphatase reaction buffer and allowed to warm to room temperature before the initiation of the phosphatase assay.
Phosphatase Activity Assay
Phosphatase activity was assessed using the EnzChek phosphatase assay kit (Molecular Probes, Eugene OR) according to the instructions of the manufacturer using the fluorescent phosphatase substrate 6,8-difluoro-4-methylumbelliferyl phosphate. Upon dephosphorylation, 6,8-difluoro-4-methylumbelliferyl phosphate fluoresces at an excitation/emission wavelength of 360/460. TCPTP was first immunoprecipitated from whole cell lysates using anti-TCPTP antibody (see above). Immunoprecipitates were incubated for 15 min with 6,8-difluoro-4-methylumbelliferyl phosphate, after which fluorescence was detected with a SpectraMax M2 fluorescence microplate reader using SoftMax Pro v. 5 software (Molecular Devices, Sunnyvale, CA). Fluorescence was measured every 15 min for the first hour and every 30 min thereafter. Measurements were performed in triplicate. A sample from each immunoprecipitation was run on SDS-PAGE and probed for TCPTP to account for equal protein loading. To account for any differences in overall phosphatase amounts, fluorescence activity units gathered from each assay were compared with TCPTP densitometric values obtained from Western blotting. Thus, values represent the specific activity of TCPTP rather than total quantities of the enzyme.
Small Interfering RNA Transfection
2 × 106 T84 cells were seeded 3 days before transfection. For TCPTP, 100 pmol of three different annealed Silencer predesigned siRNA oligonucleotides (Applied Biosystems) were transfected into T84 cells using the Amaxa nucleofector system (Amaxa, Gaithersburg, MD). After transfection, cells were cultured on filter membranes for 72 h before treatment. Control siRNA SMARTpool (Upstate Biotechnology/Dharmacon, Chicago, IL) (100 pmol/transfection) was used as a negative control.
Transepithelial Electrical Resistance
TER across T84 monolayers was assessed by voltohmeter (WPI, Sarasota, FL) and companion electrodes (Millipore). Measurements were calculated in ohm/square centimeters and expressed as a percentage of the base-line measurement.
Transepithelial Permeability Studies
Transepithelial permeability was measured as 4-kDa FITC-dextran (Sigma) flux across IEC monolayers. Following spermidine and IFN-γ treatment, cells were washed three times and incubated for 30 min at 37 °C with Ringer's solution to equilibrate. FITC-dextran (1 mg/ml) was added to the apical compartment of the monolayer. Two hours later, 100 μl of the basolateral solution was removed, and fluorescence was detected using a microplate reader (Molecular Devices).
Fluorescence Microscopy
T84 cells (1 × 105) were seeded onto coverslips and allowed to grow for 2 days. On day 3, fresh medium (DMEM) was added and pretreated with SPD (10 μm) for 15 min. After 15 min, the cells were treated with IFN-γ (1000 units/ml) ± SPD for 15 min. After treatment, cells were washed twice with PBS and fixed with 3.7% paraformaldehyde for 20 min at room temperature. Cells were washed with PBS five times and then blocked with 5% normal goat serum in 0.3% Triton X-100 in PBS for 30 min. The cells were incubated with rabbit-anti-phospho-PI3K (1:50, Cell Signaling Technology) overnight at 4 °C. Cells were then washed five times with PBS and incubated with secondary Alexa Fluor 488-conjugated goat anti-rabbit (1:200, Jackson ImmunoResearch Laboratories, Westgrove, PA) for 1 h at room temperature. Cells were washed for 5 min five times, rinsed in water, and mounted with ProLong Gold plus DAPI (Invitrogen). Fluorescence microscopy was performed using an EVOS fluorescent microscope (Life Technologies), and the final image was prepared using Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Confocal Microscopy
1 × 106 T84 cells were seeded onto coverslips and allowed to grow for 3 days. After treatment, cells were washed twice with PBS and fixed with 4% paraformaldehyde for 10 min. After three washes with PBS, cells were blocked with 1% BSA in PBS for 1 h and washed with PBS once. Mouse-anti-ZO-1 antibody (1 μg/ml, Invitrogen) and rabbit-anti-occludin (1 μg/ml, Invitrogen) in 1% BSA in PBS were applied overnight. Cells were washed three times with PBS, and secondary Alexa Fluor 488-conjugated goat anti-mouse, Alexa Fluor 568-conjugated goat anti-rabbit, and Alexa Fluor 647-phalloidin (excitation/emission maxima at 495/519, 579/603, and 650/668, respectively, Molecular Probes) were added in a 1:100 dilution in 1% BSA in PBS for 30 min at room temperature. Cells were washed three times with PBS, incubated with Hoechst 33258 (excitation/emission maxima at 352/461 nm, Molecular Probes) in PBS (1:100) for 5 min at room temperature, and washed three times in PBS. Finally, cells on coverslips were transferred onto glass slides and mounted with ProLong Gold antifade reagent (Molecular Probes). Confocal microscopy was performed using a Zeiss LSM 510 laser-scanning confocal system on a Zeiss Axioscope 2 upright microscope (Zeiss, Jena, Germany). Data analysis was performed using Zeiss LSM 5 Image Examiner software (Zeiss).
Statistical Analysis
All data are mean ± S.E. for a series of experiments. Statistical analysis was performed by Student's unpaired t test or analysis of variance (ANOVA) and Student-Newman-Keuls post-test using Graph Pad Instat 3 software (Graph Pad Software, La Jolla, CA). p < 0.05 was considered significant.
RESULTS
Spermidine Increases TCPTP Expression at the Protein Level
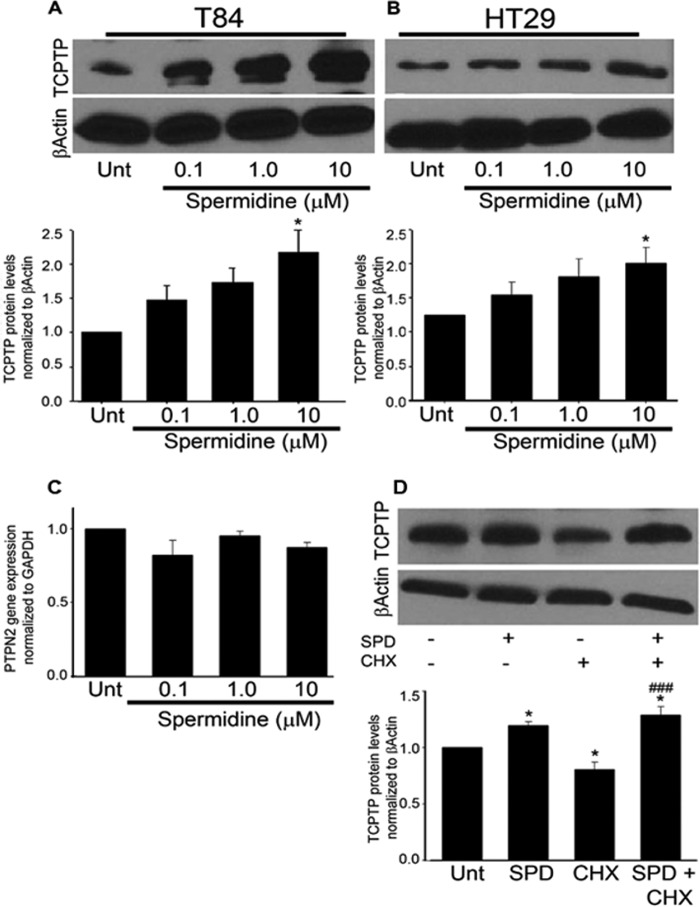
To study whether spermidine had any effect on TCPTP expression in human IECs, T84 and HT29 cells grown as monolayers on permeable supports were treated with increasing concentrations of spermidine (0.1, 1.0, and 10 μm) for 24 h. Spermidine was applied to both the apical and basolateral compartments. Western blotting indicated that TCPTP protein levels were increased in a dose-dependent manner in both T84 (p < 0.05, Fig. 1A) and HT29 (p < 0.05, B) IECs. In T84 cells treated with 10 μm spermidine, TCPTP levels were 2.2 ± 0.3-fold greater than those found in control cells (p < 0.05, Fig. 1A). Spermidine was also administered to T84 and HT29 monolayers at concentrations of 100 μm and 1 mm. However, these concentrations led to significant decreases in TER following 24-hour incubation and were not pursued in further studies. In separate cell viability studies, wells treated with 1 mm spermidine and, to a lesser extent, 100 μm, had higher numbers of dead cells than control wells as determined by trypan blue exclusion (p < 0.001, n = 4, data not shown). 10 μm spermidine did not affect cell viability. Interestingly, RT-PCR analysis revealed no increase in PTPN2 mRNA levels in cells treated with spermidine compared with untreated T84 cells (Fig. 1C). Therefore, in an effort to further elucidate the mechanism by which spermidine increased TCPTP protein levels, the ribosomal inhibitor cycloheximide was used to investigate whether spermidine induced TCPTP protein synthesis. Previous studies employing the T84 cell line used cycloheximide at a final concentration of 35.5 μm to effectively inhibit protein synthesis (17). Therefore, T84 cells grown as monolayers on permeable supports were treated with cycloheximide (35.5 μm) for 30 min followed by the addition of spermidine (10 μm) for 24 h. Western blotting indicated a 19 ± 6% decrease in TCPTP protein levels in T84 cells incubated with cycloheximide compared with the dimethyl sulfoxide vehicle control (p < 0.05, Fig. 1D). Coadministration of spermidine to cycloheximide-treated T84 cells significantly restored the TCPTP decrease (p < 0.001, Fig. 1D). The results of this experiment suggest that the effect of spermidine on TCPTP protein does not involve protein synthesis. Although it cannot be definitively concluded from this particular experiment, the increase in TCPTP protein may be due to spermidine causing posttranslational stabilization of TCPTP, perhaps through direct physical interaction. However, the increase in TCPTP protein elicited by spermidine could also be an indirect result of the polyamine interfering with protein-degradative pathways. The exact nature of this regulation will be the focus of future studies.
FIGURE 1.
Spermidine increases TCPTP expression at the protein level in T84 and HT29 intestinal epithelial cells. A, SPD (0.1, 1.0, and 10 μm) increased TCPTP protein levels in T84 (A) and HT29 cells (B) in a dose-dependent manner following 24 h treatment (n = 4). Unt, untreated. C, SPD (0.1, 1.0, 10 μm) showed no effect on PTPN2 mRNA expression in T84 cells following 24-h treatment (n = 6). PTPN2 mRNA expression was normalized to the housekeeping gene GAPDH. D, cycloheximide (CHX, 35.5 μm) significantly reduced TCPTP protein levels following 24 h treatment. Coadministration of SPD (10 μm) reversed this effect (n = 4). Whole cell lysates from SPD and CHX-treated cells were obtained, and TCPTP protein levels were assessed by Western blot analysis. β-Actin was used throughout as a loading control, and the relative protein levels were assessed by densitometry. All data are expressed as a percentage of the control ± S.E. *, p < 0.05 compared with untreated control; ###, p < 0.001 compared with CHX-treated cells; Student's unpaired t test or ANOVA and Student-Newman-Keuls post hoc test.
Spermidine Increases TCPTP Activity in Intestinal Epithelial Cells
Next, we investigated whether spermidine altered TCPTP enzymatic activity in T84 and HT29 cells. Following incubation with or without spermidine (10 μm) for 30 min, TCPTP was immunoprecipitated from T84 and HT29 whole cell lysates. Data acquired from the activity assays demonstrated that TCPTP activity in immunoprecipitates incubated with spermidine peaked at 45 min and was 1.9 ± 0.2-fold greater than TCPTP activity in immunoprecipitates from unstimulated T84 cells (p < 0.01, Fig. 2A). Similar results were observed in HT29 cells (p < 0.05, Fig. 2B). These data implicate spermidine as a bona fide stimulus of TCPTP activity in vitro in intestinal epithelial cell lines.
FIGURE 2.
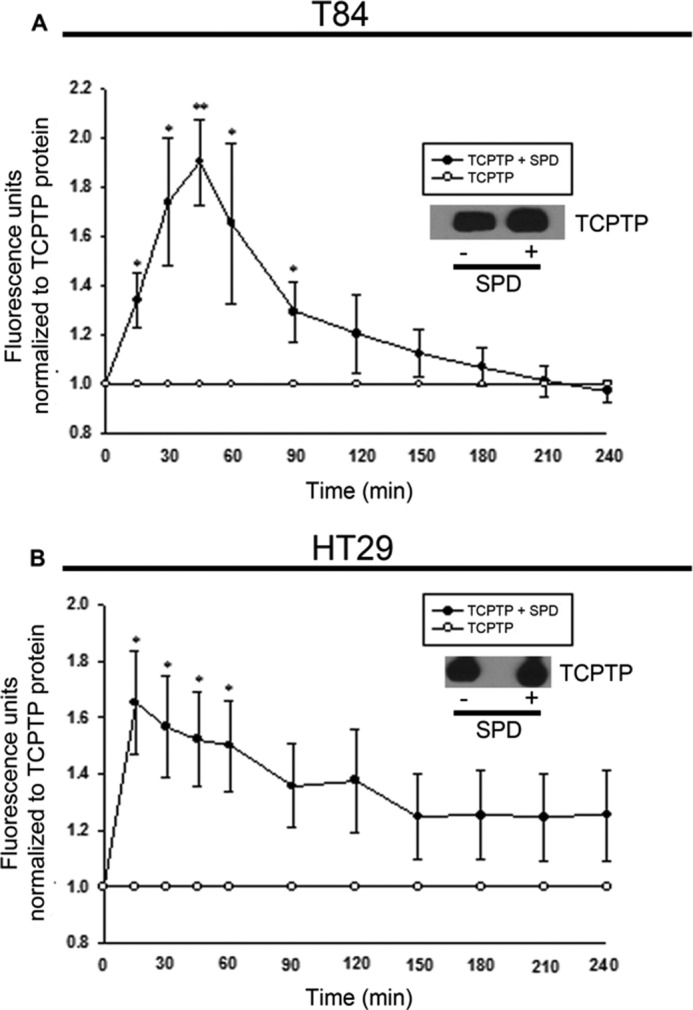
Spermidine increases TCPTP enzymatic activity in T84 and HT29 intestinal epithelial cells. T84 (A) and HT29 (B) cells were treated with SPD (10 μm) for 30 min. TCPTP was immunoprecipitated from whole cell lysates, and TCPTP activity was assessed (n = 3). A sample from each immunoprecipitation was probed for TCPTP by Western blotting to confirm equal protein loading. Fluorescence activity units were compared with TCPTP protein levels to account for any differences in overall phosphatase amounts. All data are expressed as a percentage of the control ± S.E. *, p < 0.05; **, p < 0.01 compared with the respective untreated time point; Student's unpaired t test.
Spermidine Attenuates IFN-γ Signaling in Intestinal Epithelial Cells
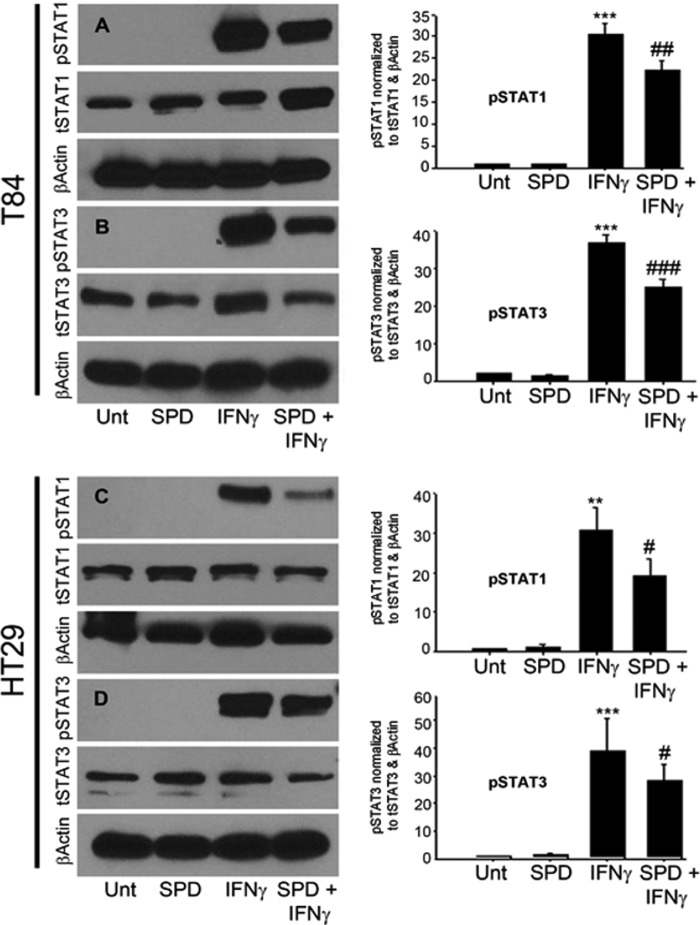
Having determined that spermidine stimulates TCPTP activity, and given our interest in TCPTP regulation of IFN-γ signaling, it was of interest to establish whether spermidine had any effect on IFN-γ signaling and the phosphorylation state of the IFN-γ signaling molecules STAT1 and 3. A pathophysiologically relevant dose of IFN-γ (1000 units/ml) was added basolaterally to T84 monolayers (12). Western blot analysis revealed that IFN-γ induced STAT1 and 3 phosphorylation following 30 min of treatment compared with untreated cells in both T84 (p < 0.001, Fig. 3, A and B) and HT29 (p < 0.01, C and D) cells, respectively. Coadministration of spermidine (10 μm) to IFN-γ-treated T84 cells for this time significantly attenuated IFN-γ-induced phosphorylation of STAT1 (p < 0.01, Fig. 3A) and STAT3 by 26 ± 7% (p < 0.001, B). Likewise, coadministration of spermidine (10 μm) to IFN-γ-treated HT29 cells for this time significantly attenuated IFN-γ-induced phosphorylation of STAT1 by 38 ± 5% (p < 0.05, Fig. 3C) and STAT3 by 19 ± 9% (p < 0.05, D). The decrease in STAT1 and 3 phosphorylation observed in cells coadministered spermidine with IFN-γ, coupled with the observed spermidine-induced increase in TCPTP phosphatase activity, suggests that the increase in TCPTP activity results in down-regulation of IFN-γ signaling through a decrease in STAT1 and 3 phosphorylation.
FIGURE 3.
Spermidine attenuates STAT1 and 3 phosphorylation in IFN-γ-treated T84 and HT29 intestinal epithelial cells. IFN-γ (1000 units/ml) induced phosphorylation of STAT1 and 3 in T84 (A and B) and HT29 (C and D) cells following 30-min treatment. Coadministration of SPD (10 μm) for this time significantly attenuated IFN-γ-induced phosphorylation of STAT1 and 3 in both T84 and HT29 cells (n = 3–5). Whole cell lysates from IFN-γ and/or SPD-treated T84 and HT29 cells were obtained, and STAT1 and 3 protein levels were assessed by Western blot analysis. β-Actin was used throughout as a loading control, and the relative protein levels were assessed by densitometry. All data are expressed as a percentage of the control ± S.E. **, p < 0.01; ***, p < 0.001 compared with untreated control; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 compared with IFN-γ-treated cells; ANOVA and Student-Newman-Keuls post hoc test. Unt, untreated.
Spermidine Inhibits PI3K Activation and Protects Intestinal Epithelial Barrier Function from Inflammatory Cytokine Exposure
IFN-γ is known to partially mediate its effects on barrier function through an increase in PI3K activation (18, 19). To assess whether spermidine interrupted this signaling pathway, we gauged the level of IFN-γ induction of PI3K activation in the presence or absence of SPD by examining phosphorylation of the p85/p55 subunits of PI3K under these conditions. IFN-γ increased phosphorylation of PI3K in T84 cells, and this was restricted in the presence of SPD, suggesting that SPD can reduce activation of this key mediator of IFN-γ-induced barrier defects (Fig. 4A). IFN-γ has been reported to decrease TER in T84 cells by as much as 40% as early as 24 h after treatment (4). Therefore, we next wanted to determine whether spermidine exhibited a protective effect on IEC barrier function following challenge with IFN-γ. IFN-γ (1000 units/ml) was added basolaterally to T84 cells when they had established a stable monolayer in which the base-line TER before treatment was ≥ 1000 Ω/cm2. Following 24-hour treatment with IFN-γ, TER decreased on average by 35 ± 5% (p < 0.001, Fig. 4B). Coadministration of spermidine (10 μm) for this time significantly lessened the effects of IFN-γ. Following a 24-hour coincubation, spermidine reduced the effect of IFN-γ treatment alone by 44 ± 7% (p < 0.05, Fig. 4B). IFN-γ also significantly increased epithelial cell permeability following 24 h of treatment as measured by 4-kDa FITC-dextran flux (p < 0.05, Fig. 4C). Coadministration of spermidine (10 μm) significantly reduced the flux of FITC-dextran across T84 cell monolayers (p < 0.05, Fig. 4C). These studies revealed a protective role for spermidine in the regulation of epithelial barrier function.
FIGURE 4.
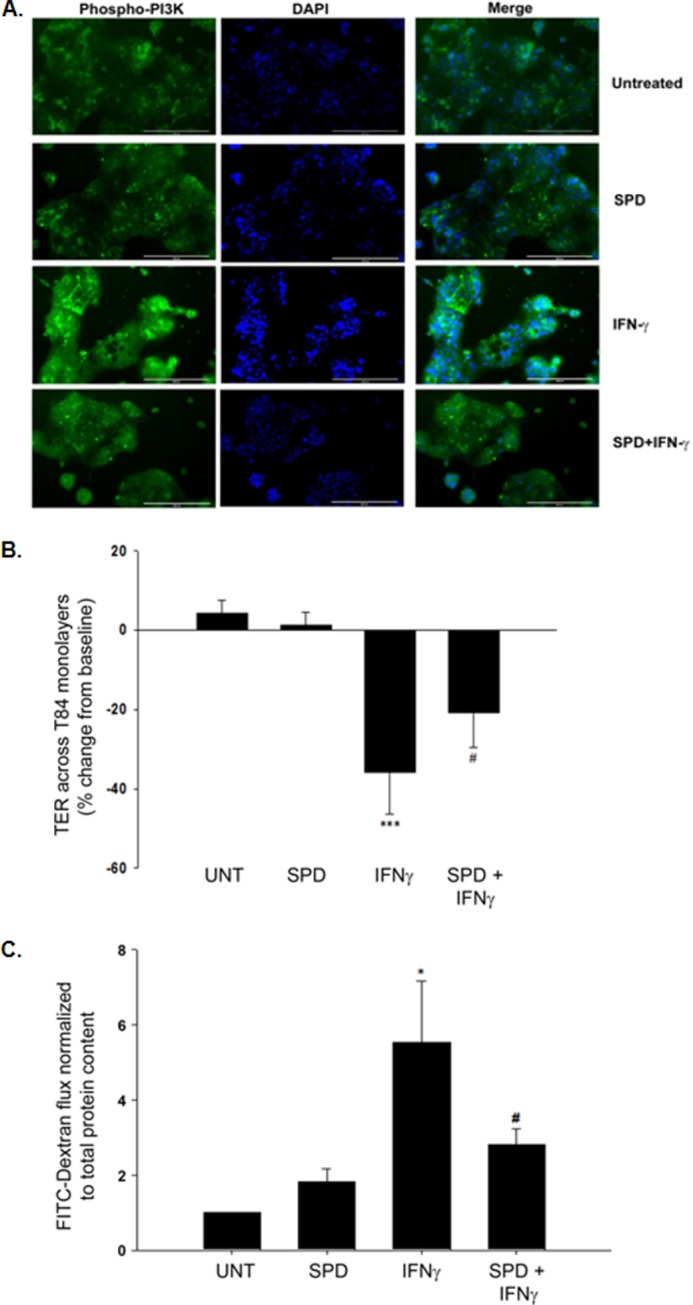
Spermidine inhibits PI3K activation by IFN-γ and protects intestinal epithelial barrier function from inflammatory cytokine treatment. A, T84 cells (1 × 105) were seeded onto coverslips and allowed to grow for 2 days. On day 3, fresh medium (DMEM) was added, and cells were pretreated ± SPD (10 μm) for 15 min. After 15 min, the cells were treated with IFN-γ (1000 units/ml) for 15 min. PI3K activation was detected with a rabbit anti-phospho-PI3K antibody (phospho-p85Tyr-458/p55Tyr-199; 1:50 dilution; green). DNA was stained with DAPI (blue). IFN-γ treatment increased PI3K phosphorylation, and this was reduced in the presence of SPD (representative image from five fields of view, ×20, n = 2, scale bar = 200 μm). B, IFN-γ (1000 units/ml) was added basolaterally to T84 monolayers that had stable TER values of ≥ 1000 Ω/cm2. Following 24-h treatment, TER values decreased by 35% of their initial base-line measurements. Coadministration of SPD (10 μm) for this time significantly reduced the effects of IFN-γ on barrier resistance (n = 4). C, following 24-h treatment, IFN-γ (1000 units/ml) also induced increases in epithelial permeability as measured by FITC-dextran flux. Coadministration of SPD (10 μm) for this time significantly reduced the effects of IFN-γ on barrier permeability (n = 4). Data are expressed as a percentage of the untreated control ± S.E. *, p < 0.05; ***, p < 0.001 compared with untreated cells; #, p < 0.05 compared with IFN-γ-treated cells; ANOVA and Student-Newman-Keuls post hoc test. UNT, untreated.
Activation of TCPTP Is Specific for the Chemical Nature of Spermidine
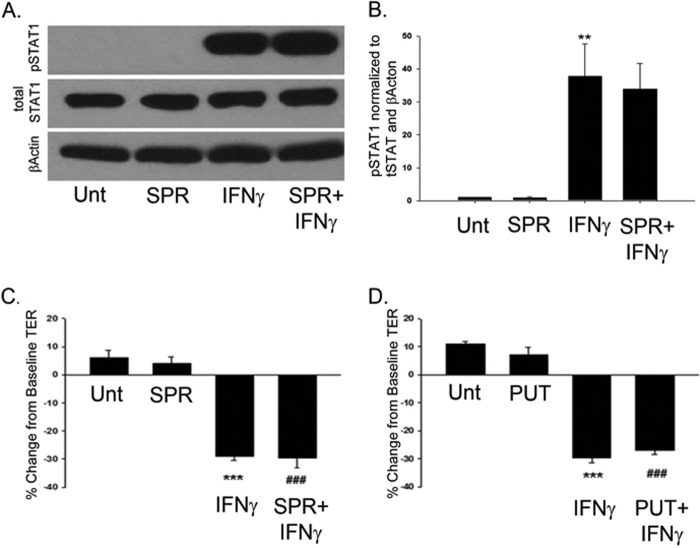
There are other polyamines that have similar chemical structures to spermidine. Therefore, it was of interest to determine whether or not other structurally similar polyamines had any effect on IFN-γ signaling events in T84 cells. One of these chemically relevant polyamines is spermine, which is produced by the addition of a propylamine group to spermidine, facilitated by the enzyme spermine transferase (20). To study the effects of spermine on IFN-γ signaling, IFN-γ (1000 units/ml) was added basolaterally to T84 monolayers. Western blot analysis revealed that IFN-γ induced STAT1 phosphorylation following 30 min of treatment (p < 0.01, Fig. 5). Bilateral coadministration of spermine showed no significant reduction of IFN-γ-induced STAT1 phosphorylation, revealing that activation of TCPTP seems to be specific for the chemical nature of spermidine. On a functional level, IFN-γ (1000 units/ml) was added basolaterally to T84 monolayers that had stable TER values of ≥ 1000 Ω/cm2. Following 24-h treatment, TER values were significantly decreased from their initial base-line measurements (p < 0.001, Fig. 5, C and D). Coadministration of either spermine (SPR, 10 μm) or putrescine (PUT, 10 μm), another structurally similar polyamine, with IFN-γ showed no protective effect against IFN-γ-induced barrier resistance (n = 3, Fig. 5, C and D).
FIGURE 5.
Attenuation of IFN-γ signaling and barrier defects is specific for the chemical nature of spermidine. A, IFN-γ (1000 units/ml) induced STAT1 phosphorylation following 30 min of treatment. Coadministration of spermine (SPR, 10 μm), a structurally similar polyamine, for this time showed no significant decrease in IFN-γ-induced phosphorylation of STAT1 as shown in the densitometric analysis (B). Whole cell lysates from IFN-γ and/or SPD-treated T84 cells were obtained, and STAT1 protein levels were assessed by Western blot analysis. β-Actin was used throughout as a loading control, and the relative protein levels were assessed by densitometry (n = 4). C and D, IFN-γ (1000 units/ml) was added basolaterally to T84 monolayers that had stable TER values of ≥ 1000 Ω/cm2. Following 24-h treatment, TER values were significantly decreased from their initial base-line measurements. Coadministration of SPR (10 μm) (B) and putrescine (PUT, 10 μm) (C) showed no protective effect against IFN-γ-induced barrier resistance (n = 4). Data are expressed as a percentage of the untreated control ± S.E. **, p < 0.01; ***, p < 0.001 compared with untreated cells; ###, p < 0.001 compared with SPR- or PUT-treated cells alone; ANOVA and Student-Newman-Keuls post-hoc test. Unt, untreated.
Spermidine Attenuates IFN-γ Signaling in a TCPTP-dependent Manner
Next, it was of interest to investigate whether the attenuation of STAT1 phosphorylation observed in T84 cells coadministered spermidine with IFN-γ was actually mediated by TCPTP. T84 cells were transfected with either TCPTP-specific siRNA or nonspecific control siRNA, seeded on permeable supports, and allowed to grow for 72 h. Nonspecific effects of the siRNA transfections were not observed, as shown by equivalent levels of the loading control, β-actin, and total STAT1 in each experiment. Following the addition of IFN-γ (1000 units/ml) to cell monolayers, Western blotting revealed that IFN-γ induced STAT1 phosphorylation following 30 min of treatment and that this level was increased in the TCPTP knockdown samples (n = 3, Fig. 6). As expected, a decrease in STAT1 phosphorylation was observed in control siRNA-transfected cells coadministered spermidine (10 μm) with IFN-γ (p < 0.05, Fig. 6). Interestingly, the decrease in STAT1 phosphorylation caused by spermidine was not observed in TCPTP knockdown cells compared with control siRNA-treated cells (p < 0.001, Fig. 6). Together, these data indicate that TCPTP is the likely mediator through which spermidine reduces IFN-γ-induced STAT1 phosphorylation.
FIGURE 6.
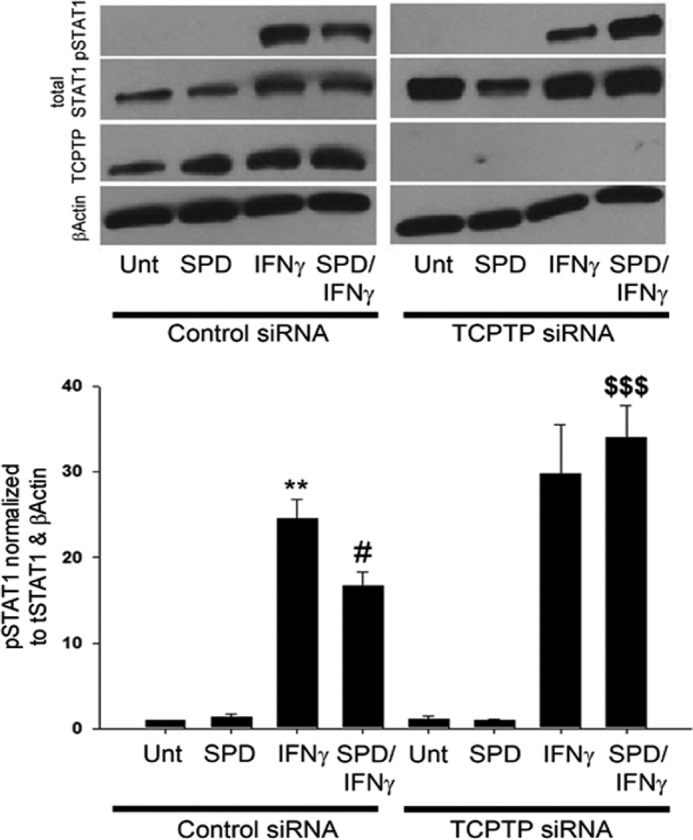
Spermidine attenuates IFN-γ signaling in a TCPTP-dependent manner. T84 cells were transfected with either TCPTP-specific siRNA or nonspecific control siRNA. IFN-γ (1000 units/ml) induced STAT1 phosphorylation following 30 min of treatment. A decrease in STAT1 phosphorylation was observed in control siRNA-transfected cells coadministered SPD (10 μm) with IFN-γ for this time. The decrease in STAT1 phosphorylation by SPD was not realized in coadministration studies in TCPTP knockdown cells (n = 3). Whole cell lysates from IFN-γ- and/or SPD-treated T84 cells were obtained, and STAT1 protein levels were assessed by Western blot analysis. β-Actin was used throughout as a loading control, and the relative protein levels were assessed by densitometry. All data are expressed as a percentage of the control ± S.E. **, p < 0.01 compared with untreated control siRNA-transfected cells; #, p < 0.05 compared with IFNγ treatment of control siRNA-transfected cells; $$$, p < 0.001 compared with IFNγ/SPD treatment of control siRNA-transfected cells. Unt, untreated.
Spermidine Protects Intestinal Epithelial Barrier Function in a TCPTP-dependent Manner
Having determined that spermidine plays a protective role in epithelial barrier function following challenge with IFN-γ, it was of interest to determine whether this effect was mediated through TCPTP. T84 cells were transfected with either TCPTP-specific siRNA or nonspecific control siRNA, seeded on permeable supports, and allowed to grow for 48 h before treatment. Nonspecific effects of the siRNA transfections were not observed, as shown by equivalent levels of the loading control, β-actin, in each experiment. Following the addition of IFN-γ (1000 units/ml) to cell monolayers, TER decreased on average by 8 ± 1% following 24 h of treatment (p < 0.01, Fig. 7). As expected, coadministration of spermidine (10 μm) for this time lessened the effects of IFN-γ on barrier function, resulting in a TER decrease of only 2 ± 4% (p < 0.05, Fig. 7). In TCPTP knockdown cells, the decrease in TER was further exacerbated in the presence of IFN-γ (p < 0.001, Fig. 7). Interestingly, coadministration of spermidine to IFN-γ-treated TCPTP-deficient cells did not result in a restoration of TER. The results of these experiments indicate that the protective role for spermidine in the regulation of intestinal epithelial barrier function is mediated, at least in part, by TCPTP.
FIGURE 7.
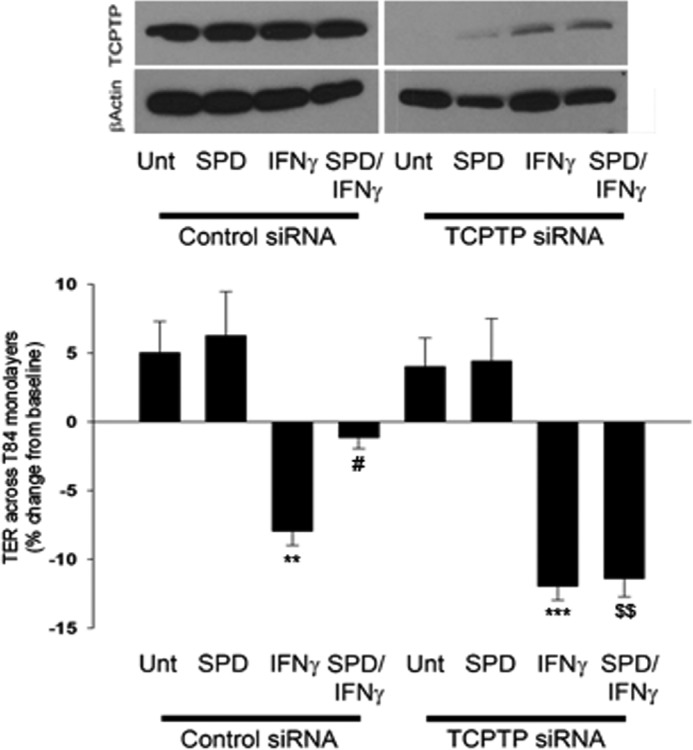
Spermidine protects intestinal epithelial barrier function in a TCPTP-dependent manner. T84 cells were transfected with either TCPTP-specific or nonspecific control siRNA and allowed to grow for 48 h, at which point IFN-γ (1000 units/ml) was added basolaterally to the monolayers. Following 24 h treatment, TER values decreased on average by 8% of their initial base-line measurements. Coadministration of SPD (10 μm) for this time significantly reduced the effects of IFN-γ on barrier resistance (n = 5). The protective effect of SPD on IFN-γ-induced decreases in TER was not realized in TCPTP knockdown cells (n = 5). Whole cell lysates from IFN-γ- and/or SPD-treated T84 cells were obtained, and TCPTP protein levels were assessed by Western blot analysis. **, p < 0.01; ***, p < 0.001 compared with untreated cells; #, p < 0.05 compared with IFN-γ treatment of control siRNA-transfected cells; $$, p < 0.01 compared with IFN-γ/SPD treatment of control siRNA-transfected cells. Unt, untreated.
Spermidine Protects IFN-γ-induced Redistribution of Tight Junction Proteins
Finally, it was of interest to investigate whether spermidine protected against IFN-γ-induced occludin and ZO-1 tight junction protein reorganization, a key step in IFN-γ-induced barrier defects. Confocal microscopy revealed occludin (OCLN) and ZO-1 localization at apical tight junctions in untreated (Fig. 8A, Unt) and spermidine-treated (B) monolayers, yielding a typical cobblestone-like appearance (21). However, when cells were treated with IFN-γ (1000 units/ml, 24 h, Fig. 8C), OCLN appeared substantially diminished at apical tight junctions when compared with both the untreated and SPD-treated cells. Coadministration of spermidine (10 μm, 24 h, Fig. 8D) with IFN-γ resulted in partial retention of the cobblestone appearance of OCLN localization, indicating the preservation of tight junctions in the presence of spermidine. Additionally, untreated (Fig. 8E) and SPD-treated cells (F) showed sharp localization of ZO-1 at the tight junctions between adjacent cells. IFN-γ treatment (Fig. 8G) resulted in large gaps between adjacent cells delineated by the ZO-1-enriched membrane. Cells coincubated with IFN-γ and SPD (Fig. 8H) showed an absence of gaps between adjacent cells, thus supporting observations from OCLN localization that indicate a protective effect of spermidine against IFN-γ-mediated barrier dysfunction.
FIGURE 8.
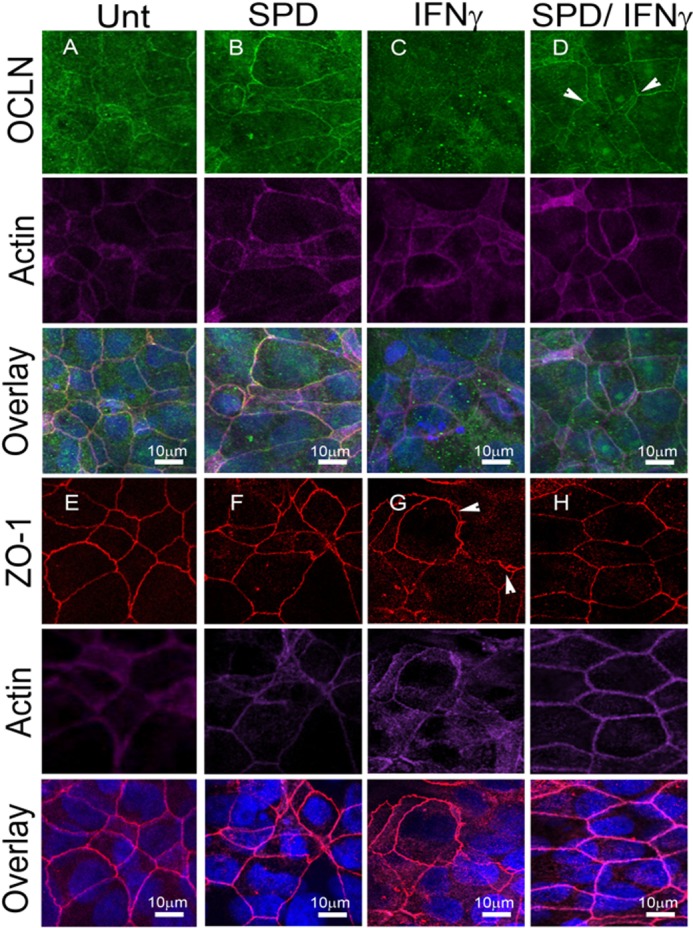
Spermidine protects against IFN-γ-induced redistribution of tight junction proteins. Confocal images of untreated (Unt) T84 (A), spermidine-treated (B, 10 μm, 24 h), IFN-γ-treated (C, 1000 units/ml, 24 h), and spermidine and IFN-γ (D) cotreatment reveal occludin (green) enrichment at the apical tight junction (arrow) in untreated and SPD cells when compared with the reduction in OCLN localization in IFN-γ-treated cells. D, retention of OCLN at the apical tight junction (arrow) in SPD/IFN-γ cotreatment. ZO-1 (red) shows sharp enrichment (arrows) between adjacent cells in both untreated and SPD-treated cells (E and F, respectively). IFN-γ treatment (G) alone shows gaps between adjacent cells (arrowhead) when compared with SPD and IFN-γ cotreatment, where gaps are not present (H). Images were taken at ×63 magnification with an additional ×2 digital zoom. Nuclear staining is blue, and actin staining is purple.
DISCUSSION
TCPTP has been shown to play an essential role in regulating inflammatory cytokine signaling by inactivating the IFN-γ signaling molecules STAT1 and STAT3. This view is supported by the fact that Ptpn2 knockout mice die within 3–5 weeks from systemic inflammation characterized by elevated levels of IFN-γ and TNF-α (22). Single nucleotide polymorphisms (SNPs) in the PTPN2 gene locus that encodes TCPTP have been associated with inflammatory bowel disease, and it is thought that these mutations could lead to a loss of function (10, 11). Although this has not been formally proven, it is thought that loss of TCPTP function may contribute to IBD progression, in part because of prolonged IFN-γ-induced STAT activation of transcription and subsequent increases in intestinal permeability (12, 13). Therefore, agents like spermidine that increase TCPTP activity are of general interest as modifiers of inflammatory signaling events. Our experiments with spermidine were designed to examine its effects on TCPTP expression and enzymatic activity as well as inflammatory cytokine signaling.
Spermidine belongs to a class of molecules called polyamines that are involved in many physiological processes, including cell growth and proliferation, immunity, protein and nucleic acid synthesis, and certain cellular signaling processes (19). Spermidine is a low molecular weight amine that is water-soluble and fully protonated at physiological pH (23), thereby exhibiting polycationic character. It is the charged chemical nature of spermidine that is thought to play a critical role in the biological activity of the compound, including its activating effect on various enzymes like TCPTP (14, 24, 25). Although the proposed mechanism by which spermidine increases TCPTP activity has not been clearly defined, it is known that spermidine interacts with TCPTP at the same site as the α1-integrin cytoplasmic tail residues 1164–1179 identified previously to activate TCPTP (6, 14). One current hypothesis suggests that the polycationic character of spermidine is responsible for breaking an autoregulatory intramolecular bond between the carboxy-terminal and catalytic domain in TCPTP (14). The idea that electrostatic interactions may mediate TCPTP activity is supported by previous work establishing activation of TCPTP in response to stimulation with the positively charged cytoskeletal protein α1-integrin (6) as well as in response to increases in ionic strength (26). By applying the approach of pharmacological activation of TCPTP by spermidine to a model of inflammation, we have shown that this small polyamine is capable of attenuating IFN-γ-induced cell signaling and barrier defects. In our studies, coadministration of spermidine to IFN-γ-treated cells attenuated IFN-γ-induced phosphorylation of STAT1 and STAT3 as well as redistribution of the tight junctional proteins occludin and ZO-1. However, we acknowledge that the involvement of STAT1 in IFN-γ-induced barrier dysfunction is controversial. Therefore, although inhibition of STAT1 activation may be partially involved in the protective effects of spermidine on barrier function, other pathways may also be targeted by spermidine. Effects of spermidine on multiple pathways would likely lead to a greater overall protective effect on barrier function than contributed by the partial inhibition of STAT protein phosphorylation alone. With this in mind, we investigated the effect of spermidine on a well established mediator of IFN-γ-induced barrier defects, PI3K. Our observation that spermidine reduced IFN-γ-stimulated phosphorylation of the p85 subunit of PI3K (cf. Fig. 4A) indicates that the protective effect of spermidine against IFN-γ disruption of the epithelial barrier may also be mediated in part through inhibition of PI3K activity (18, 19).
Together our data suggest that spermidine is effectively taken up by T84 and HT29 cells, either passively through diffusion and/or via a transport system, and is capable of interacting with and activating TCPTP, leading to increased dephosphorylation events. It is important, though, not to rule out the possibility that the effect of spermidine on TCPTP activity may be due to a spermidine metabolite because spermidine can be oxidized in serum-containing medium (27).
In our siRNA knockdown studies, it was revealed that the reduction of IFN-γ-induced STAT1 and STAT3 phosphorylation by spermidine was mediated through TCPTP. Cells lacking TCPTP showed no decrease in IFN-γ signaling when treated with spermidine, indicating that spermidine limits IFN-γ signaling, likely through activation of this cellular phosphatase. It was also interesting that other structurally similar polyamines, spermine and putrescine, had no significant effect on IFN-γ-induced STAT1 phosphorylation or change in TER, respectively. To a certain degree, the results of this experiment were surprising because the structures of both spermidine and spermine are so similar. However, the results of this experiment are encouraging as to the possible specificity of spermidine for in vivo use with respect to other polyamines. Additionally, they shed light onto the chemical nature of TCPTP activation and may serve as a starting point for the pharmacological design of more potent activators of this phosphatase. This may be of more practical use with respect to in vivo targeting of spermidine-activated pathways in intestinal epithelial cells because spermidine itself is likely to have effects on many cell types.
The effects of IFN-γ on barrier function have been well established both at the biochemical and functional levels. The compromising effect of IFN-γ on the epithelial barrier is characterized by alterations in expression of the tight junction proteins occludin and ZO-1, resulting in increased transepithelial permeability and decreased TER (3, 4, 28, 29, 31–34). We have shown that coadministration of spermidine with IFN-γ to epithelial monolayers significantly attenuated the IFN-γ-induced drop in TER and concurrent increases in epithelial permeability. Moreover, the protective effect of spermidine on barrier function appeared to be TCPTP-mediated because the restoration of TER was no longer observed in TCPTP knockdown cells treated with IFN-γ and coadministered spermidine. One issue we were unable to resolve was whether the acute early activation of TCPTP or the later increase in TCPTP protein levels were responsible for the protective effect of SPD on TER. We attempted to answer this using a TCPTP inhibitor (a gift from Dr. Zhong-Yin Zhang) but could not obtain consistent findings (data not shown). However, given that activation of two other signaling mediators, PI3K and adenosine monophosphate-activated protein kinase, up to 6 h after IFN-γ exposure remains critical for the subsequent effects of IFN-γ on barrier function and that these barrier effects do not fully manifest in T84 intestinal epithelial cells until 48–72 h after IFN-γ treatment, it is not unreasonable to suggest that both acute activity and increased overall levels of TCPTP are likely involved in the protective effect of SPD (18, 35).
Our studies employing confocal microscopy suggest that the effect of spermidine in the presence of IFN-γ reduces IFN-γ-induced redistribution of occludin and ZO-1. Treatment of T84 cells with IFN-γ led to the formation of large paracellular gaps between adjacent cells, much like what was observed in previous studies in response to ethanol treatment of intestinal epithelial monolayers (36). The addition of spermidine to IFN-γ-treated monolayers led to an amelioration of paracellular openings, providing a plausible mechanism for the observed effects of spermidine in the protection of barrier function. Although our biochemical and barrier function data suggest that this phenomenon is TCPTP-mediated, it is also reasonable that other pathways are involved. Our data suggest that spermidine inhibits IFN-γ-induced activation of PI3K and that this may also contribute to the protective effect of spermidine on epithelial barrier maintenance, perhaps in addition to the spermidine-stimulated, TCPTP-mediated pathway. On the basis of studies showing that TCPTP inhibits EGF-induced PI3K signaling by dephosphorylating the EGF receptor, we suspect that the reduction in IFN-γ-induced PI3K phosphorylation by spermidine occurs via TCPTP dephosphorylation of targets upstream of PI3K. However, this remains to be confirmed (37). Additionally, previous reports have suggested an important role for polyamines in the regulation of tight junction protein expression as well as intestinal epithelial barrier integrity under various physiological conditions. Work by Guo et al. (38, 39) demonstrated that depletion of cellular polyamines by difluoromethylornithine (DFMO), a specific inhibitor of polyamine synthesis, dramatically decreased levels of occludin and ZO-1 and led to increases in paracellular permeability in IEC-6 cells . Furthermore, the addition of exogenous spermidine to DFMO-treated IEC-6 cells not only led to a restoration of occludin and ZO-1 protein levels but also restored monolayer permeability to normal levels (39). The results of these studies imply an important role for spermidine in tight junction expression and barrier maintenance. Moreover, these findings reinforce our microscopy data that depict a protective effect of spermidine on occludin and ZO-1 distribution following challenge with IFN-γ. Our work expands upon these findings by identifying that spermidine acts via a clinically relevant phosphatase whose activity is believed to be compromised in at least a subset of IBD, celiac disease, and type 1 diabetes patients harboring SNPs in the PTPN2 gene (10, 11, 30, 40).
In summary, agents such as spermidine that increase TCPTP protein levels and/or activity are of general interest as modifiers of inflammatory cytokine signaling. Our work here represents the first steps in understanding the effects of spermidine on TCPTP in intestinal epithelial cells. Our data implicate spermidine as a bona fide stimulus of TCPTP in intestinal epithelial cells, and activation of TCPTP mediates, at least in part, the protective effects of spermidine on intestinal epithelial barrier function. Attenuation of IFN-γ signaling by spermidine and resolution of barrier dysfunction in an in vitro cell model validate further investigation of the use of spermidine in vivo to mitigate barrier defects in a murine model of intestinal inflammation. Alleviation of proinflammatory cytokine signaling and the protection of tight junction integrity, with the presumed consequence of an enhancement of barrier function, would be extremely desirable in the context of inflammation. These data suggest that spermidine, and/or spermidine-activated signaling pathways, may have therapeutic value in treating conditions associated with intestinal epithelial barrier defects.
Acknowledgments
We thank Dr. Leo Hawel, III and Dr. Craig Byus (University of California, Riverside) for helpful discussions.
This research was supported, in whole or in part, by National Institutes of Health Grant R01DK091281 (to D. F. M.).
- IBD
- inflammatory bowel disease
- TER
- transepithelial electrical resistance
- TCPTP
- T cell protein-tyrosine phosphatase
- STAT
- signal transducer and activator of transcription
- IEC
- intestinal epithelial cell
- ANOVA
- analysis of variance
- SPD
- spermidine
- OCLN
- occludin
- Ω
- ohm.
REFERENCES
- 1. Fuss I. J., Neurath M., Boirivant M., Klein J. S., de la Motte C., Strong S. A., Fiocchi C., Strober W. (1996) Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 157, 1261–1270 [PubMed] [Google Scholar]
- 2. Turner J. R. (2009) Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 [DOI] [PubMed] [Google Scholar]
- 3. Madara J. L., Stafford J. (1989) Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest. 83, 724–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Youakim A., Ahdieh M. (1999) Interferon-γ decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am. J. Physiol. 276, G1279–1288 [DOI] [PubMed] [Google Scholar]
- 5. Tonks N. K., Neel B. G. (2001) Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13, 182–195 [DOI] [PubMed] [Google Scholar]
- 6. Mattila E., Pellinen T., Nevo J., Vuoriluoto K., Arjonen A., Ivaska J. (2005) Negative regulation of EGFR signalling through integrin-α1β1-mediated activation of protein tyrosine phosphatase TCPTP. Nat. Cell Biol. 7, 78–85 [DOI] [PubMed] [Google Scholar]
- 7. Galic S., Klingler-Hoffmann M., Fodero-Tavoletti M. T., Puryer M. A., Meng T. C., Tonks N. K., Tiganis T. (2003) Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Mol. Cell Biol. 23, 2096–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ten Hoeve J., de Jesus Ibarra-Sanchez M., Fu Y., Zhu W., Tremblay M., David M., Shuai K. (2002) Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol. Cell Biol. 22, 5662–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamoto T., Sekine Y., Kashima K., Kubota A., Sato N., Aoki N., Matsuda T. (2002) The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem. Biophys. Res. Commun. 297, 811–817 [DOI] [PubMed] [Google Scholar]
- 10. Franke A., McGovern D. P., Barrett J. C., Wang K., Radford-Smith G. L., Ahmad T., Lees C. W., Balschun T., Lee J., Roberts R., Anderson C. A., Bis J. C., Bumpstead S., Ellinghaus D., Festen E. M., Georges M., Green T., Haritunians T., Jostins L., Latiano A., Mathew C. G., Montgomery G. W., Prescott N. J., Raychaudhuri S., Rotter J. I., Schumm P., Sharma Y., Simms L. A., Taylor K. D., Whiteman D., Wijmenga C., Baldassano R. N., Barclay M., Bayless T. M., Brand S., Buning C., Cohen A., Colombel J. F., Cottone M., Stronati L., Denson T., De Vos M., D'Inca R., Dubinsky M., Edwards C., Florin T., Franchimont D., Gearry R., Glas J., Van Gossum A., Guthery S. L., Halfvarson J., Verspaget H. W., Hugot J. P., Karban A., Laukens D., Lawrance I., Lemann M., Levine A., Libioulle C., Louis E., Mowat C., Newman W., Panes J., Phillips A., Proctor D. D., Regueiro M., Russell R., Rutgeerts P., Sanderson J., Sans M., Seibold F., Steinhart A. H., Stokkers P. C., Torkvist L., Kullak-Ublick G., Wilson D., Walters T., Targan S. R., Brant S. R., Rioux J. D., D'Amato M., Weersma R. K., Kugathasan S., Griffiths A. M., Mansfield J. C., Vermeire S., Duerr R. H., Silverberg M. S., Satsangi J., Schreiber S., Cho J. H., Annese V., Hakonarson H., Daly M. J., Parkes M. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parkes M., Barrett J. C., Prescott N. J., Tremelling M., Anderson C. A., Fisher S. A., Roberts R. G., Nimmo E. R., Cummings F. R., Soars D., Drummond H., Lees C. W., Khawaja S. A., Bagnall R., Burke D. A., Todhunter C. E., Ahmad T., Onnie C. M., McArdle W., Strachan D., Bethel G., Bryan C., Lewis C. M., Deloukas P., Forbes A., Sanderson J., Jewell D. P., Satsangi J., Mansfield J. C., Wellcome Trust Case Control Consortium, Cardon L., Mathew C. G. (2007) Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat. Genet. 39, 830–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scharl M., Paul G., Weber A., Jung B. C., Docherty M. J., Hausmann M., Rogler G., Barrett K. E., McCole D. F. (2009) Protection of epithelial barrier function by the Crohn's disease associated gene protein tyrosine phosphatase n2. Gastroenterology 137, 2030–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scharl M., Hruz P., McCole D. F. (2010) Protein tyrosine phosphatase non-receptor Type 2 regulates IFN-γ-induced cytokine signaling in THP-1 monocytes. Inflamm. Bowel Dis. 16, 2055–2064 [DOI] [PubMed] [Google Scholar]
- 14. Mattila E., Marttila H., Sahlberg N., Kohonen P., Tähtinen S., Halonen P., Perälä M., Ivaska J. (2010) Inhibition of receptor tyrosine kinase signalling by small molecule agonist of T-cell protein tyrosine phosphatase. BMC Cancer 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. (1984) A human colonic tumor cell line that maintains vectorial electrolyte transport. Am. J. Physiol. 246, G204–208 [DOI] [PubMed] [Google Scholar]
- 16. Augeron C., Laboisse C. L. (1984) Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 44, 2961–3969 [PubMed] [Google Scholar]
- 17. Cafferata E. G., González-Guerrico A. M., Giordano L., Pivetta O. H., Santa-Coloma T. A. (2000) Interleukin-1β regulates CFTR expression in human intestinal T84 cells. Biochim. Biophys. Acta 1500, 241–248 [DOI] [PubMed] [Google Scholar]
- 18. McKay D. M., Watson J. L., Wang A., Caldwell J., Prescott D., Ceponis P. M., Di Leo V., Lu J. (2007) Phosphatidylinositol 3′-kinase is a critical mediator of interferon-γ-induced increases in enteric epithelial permeability. J. Pharmacol. Exp. Ther. 320, 1013–1022 [DOI] [PubMed] [Google Scholar]
- 19. Boivin M. A., Roy P. K., Bradley A., Kennedy J. C., Rihani T., Ma T. Y. (2009) Mechanism of interferon-γ-induced increase in T84 intestinal epithelial tight junction. J. Interferon Cytokine Res. 29, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moinard C., Cynober L., de Bandt J. P. (2005) Polyamines. Metabolism and implications in human diseases. Clin. Nutr. 24, 184–197 [DOI] [PubMed] [Google Scholar]
- 21. Fanning A. S., Jameson B. J., Jesaitis L. A., Anderson J. M. (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273, 29745–29753 [DOI] [PubMed] [Google Scholar]
- 22. Heinonen K. M., Nestel F. P., Newell E. W., Charette G., Seemayer T. A., Tremblay M. L., Lapp W. S. (2004) T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood 103, 3457–3464 [DOI] [PubMed] [Google Scholar]
- 23. Seiler N., Delcros J. G., Moulinoux J. P. (1996) Polyamine transport in mammalian cells. An update. Int. J. Biochem. Cell Biol. 28, 843–861 [DOI] [PubMed] [Google Scholar]
- 24. Hoet P. H., Nemery B. (2000) Polyamines in the lung. Polyamine uptake and polyamine-linked pathological or toxicological conditions. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L417–433 [DOI] [PubMed] [Google Scholar]
- 25. Johnson L. R., McCormack S. A. (1999) Healing of gastrointestinal mucosa. Involvement of polyamines. News Physiol. Sci. 14, 12–17 [DOI] [PubMed] [Google Scholar]
- 26. Hao L., Tiganis T., Tonks N. K., Charbonneau H., (1997) The noncatalytic C-terminal segment of the T cell protein tyrosine phosphatase regulates activity via an intramolecular mechanism. J. Biol. Chem. 272, 29322–29329 [DOI] [PubMed] [Google Scholar]
- 27. Otsuka H. (1971) The toxic effect of spermidine on normal and transformed cells. J. Cell Sci. 9, 71–84 [DOI] [PubMed] [Google Scholar]
- 28. Watson C. J., Hoare C. J., Garrod D. R., Carlson G. L., Warhurst G. (2005) Interferon-γ selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J. Cell Sci. 118, 5221–5230 [DOI] [PubMed] [Google Scholar]
- 29. Utech M., Ivanov A. I., Samarin S. N., Bruewer M., Turner J. R., Mrsny R. J., Parkos C. A., Nusrat A. (2005) Mechanism of IFN-γ-induced endocytosis of tight junction proteins. Myosin II-dependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell 16, 5040–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Festen E. A., Goyette P., Green T., Boucher G., Beauchamp C., Trynka G., Dubois P. C., Lagacé C., Stokkers P. C., Hommes D. W., Barisani D., Palmieri O., Annese V., van Heel D. A., Weersma R. K., Daly M. J., Wijmenga C., Rioux J. D. (2011) A meta-analysis of genome-wide association scans identifies IL18RAP, PTPN2, TAGAP, and PUS10 as shared risk loci for Crohn's disease and celiac disease. PLoS Genet. 7, e1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruewer M., Utech M., Ivanov A. I., Hopkins A. M., Parkos C. A., Nusrat A. (2005) Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 19, 923–933 [DOI] [PubMed] [Google Scholar]
- 32. Wang F., Graham W. V., Wang Y., Witkowski E. D., Schwarz B. T., Turner J. R. (2005) Interferon-γ and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 166, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beaurepaire C., Smyth D., McKay D. M. (2009) Interferon-γ regulation of intestinal epithelial permeability. J. Interferon Cytokine Res. 29, 133–144 [DOI] [PubMed] [Google Scholar]
- 34. Adams R. B., Planchon S. M., Roche J. K. (1993) IFN-γ modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J. Immunol. 150, 2356–2363 [PubMed] [Google Scholar]
- 35. Scharl M., Paul G., Barrett K. E., McCole D. F. (2009) AMP-activated protein kinase mediates the interferon-γ-induced decrease in intestinal epithelial barrier function. J. Biol. Chem. 284, 27952–27963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma T. Y., Nguyen D., Bui V., Nguyen H., Hoa N. (1999) Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 276, G965-G974 [DOI] [PubMed] [Google Scholar]
- 37. Tiganis T., Kemp B. E., Tonks N. K. (1999) The protein-tyrosine phosphatase TCPTP regulates epidermal growth factor receptor-mediated and phosphatidylinositol 3-kinase-dependent signaling. J. Biol. Chem. 274, 27768–27775 [DOI] [PubMed] [Google Scholar]
- 38. Guo X., Rao J. N., Liu L., Zou T. T., Turner D. J., Bass B. L., Wang J. Y. (2003) Regulation of adherens junctions and epithelial paracellular permeability. A novel function for polyamines. Am. J. Physiol. Cell Physiol. 285, C1174-C1187 [DOI] [PubMed] [Google Scholar]
- 39. Guo X., Rao J. N., Liu L., Zou T., Keledjian K. M., Boneva D., Marasa B. S., Wang J. Y. (2005) Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1159–1169 [DOI] [PubMed] [Google Scholar]
- 40. Todd J. A., Walker N. M., Cooper J. D., Smyth D. J., Downes K., Plagnol V., Bailey R., Nejentsev S., Field S. F., Payne F., Lowe C. E., Szeszko J. S., Hafler J. P., Zeitels L., Yang J. H., Vella A., Nutland S., Stevens H. E., Schuilenburg H., Coleman G., Maisuria M., Meadows W., Smink L. J., Healy B., Burren O. S., Lam A. A., Ovington N. R., Allen J., Adlem E., Leung H. T., Wallace C., Howson J. M., Guja C., Ionescu-Tîrgovite C., Genetics of Type 1 Diabetes in Finland, Simmonds M. J., Heward J. M., Gough S. C., Wellcome Trust Case Control Consortium, Dunger D. B., Wicker L. S., Clayton D. G. (2007) Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 39, 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]