Abstract
CXCL12 governs cellular motility, a process deregulated by hematopoietic stem cell oncogenes such as p210-BCR-ABL. A phosphoproteomics approach to the analysis of a hematopoietic progenitor cell line treated with CXCL12 and the Rac 1 and 2 inhibitor NSC23766 has been employed to objectively discover novel mechanisms for regulation of stem cells in normal and malignant hematopoiesis. The proteomic data sets identified new aspects of CXCL12-mediated signaling and novel features of stem cell regulation. We also identified a novel phosphorylation event in hematopoietic progenitor cells that correlated with motile response and governed by the chemotactic factor CXCL12. The novel phosphorylation site on PTPRC/CD45; a protein tyrosine phosphatase, was validated by raising an antibody to the site and also using a mass spectrometry absolute quantification strategy. Site directed mutagenesis and inhibitor studies demonstrated that this single phosphorylation site governs hematopoietic progenitor cell and lymphoid cell motility, lies downstream from Rac proteins and potentiates Src signaling. We have also demonstrated that PTPRC/CD45 is down-regulated in leukemogenic tyrosine kinase expressing cells. The use of discovery proteomics has enabled further understanding of the regulation of PTPRC/CD45 and its important role in cellular motility in progenitor cells.
Localization of the hematopoietic stem cell (HSC)1 in its niche microenvironment is vital for stem cell maintenance (1–3). Important factors in the regulation of stem cell migration, retention or mobilization include tethering and signaling via the Stromal Derived Factor 1 (SDF-1α, CXCL12)/CXCR4 receptor, and Stem Cell Factor (SCF)/c-kit receptor tyrosine kinase, plus integrin mediated attachment (1, 3–6). CXCL12 acts as a chemo attractant for HSC and hematopoietic progenitor cells (HPC) and is expressed by bone marrow stroma (7, 8). It induces integrin-mediated adhesion of HSC/P, thus facilitating transendothelial migration, homing, and bone marrow engraftment and retention (9–12), events also governed by the Rac GTPase family Rac1 and 2 (4, 13). Rac is responsible for regulating pleiotropic signaling events in HPCs, including homing, engraftment, and mobilization, cytoskeleton rearrangement, transcriptional activation, survival, and cell-cycle progression (4, 13, 14). Rac 1 and 2 have differing roles in regulating stem cell movement and retention (5, 6, 15, 16). Downstream features of Rac action remain to be fully elucidated and in part this can be achieved with the use of a Rac inhibitor (17).
The p210-BCR-ABL fusion protein is generated by a (t9; 22) translocation that is both necessary and sufficient for the development of chronic myelogenous leukemia (CML) (18, 19). P210-BCR-ABL induces abnormal adhesion and migration of hematopoietic progenitors and is responsible for a transformed phenotype (20, 21). CML is characterized by myeloproliferation in the bone marrow and egress of leukemic stem and progenitor cells (18, 22, 23), thus motility proteins have been implicated in the transformation of HSC via p210-BCR-ABL. Sengupta et al. (2010) (24) demonstrated that inducible p210-BCR-ABL increased egress of Leukemia stem cells (LSC) in the transgenic mice. Loss of Rac2 has been shown to prolong survival of mice with a p210-BCR-ABL initiated myeloproliferative disease (25). The increased survival was because of lower levels of tumor initiating Lin− Sca+ c-Kit+ (LSK) cells in vivo (24).
Here we have constructed a series of phoshoproteomic experiments to investigate and discover novel regulators of motility in hematopoietic progenitor cells and how this is affected by the presence of the protein tyrosine kinase p210-BCR-ABL. The interpretation of the data sets led to the identification of a phosphorylation event at S962 of PTPRC/CD45. It was subsequently demonstrated that this phosphorylation event was involved in stem cell motility. This pathway is also impacted on and inhibited by the p210-BCR-ABL oncogenic tyrosine kinase.
EXPERIMENTAL PROCEDURES
Enrichment of Hematopoietic Cells and Cell Culture
Ba/F3 cells expressing the leukemic oncogenes p210-BCR-ABL, NPM/ALK, TEL/PDGFRb, PIP1/PDGFRa, Flt3/ITD, and c-Kit D816V were cultured as described previously (26). The FDCP-Mix (Clone A4) cell line was cultured as described (27). Murine bone marrow was collected and prepared from C57Bl/6J mice and PTPRC/CD45 null mice (C57 bl/6J background, Jackson Labs) and lineage marker depleted cells were enriched as previously described (28, 29). Murine Lin- cells were cultured under the same conditions as the FDCP-Mix cells however with the addition 10 ng/ml of SCF and Flt3 ligand. The Jurkat cell line was maintained in RPMI and 10% (v/v) fetal calf serum.
Human CD34+ cells were derived from peripheral blood. Fresh leukapheresis samples obtained from patients with newly diagnosed CP CML with written informed consent. Non CML CD34+ cells were from autologous donors with non-stem cell disorders. After thawing, cells were cultured overnight in growth factors (SCF 0.2 ng/ml, G-CSF 1 ng/ml, GM-CSF 0.2 ng/ml, IL-6 1 ng/ml, LIF 0.05 ng/ml, MIP-α 0.2 ng/ml, Stem Cell Technologies, Vancouver, Canada).
Cells were treated with a range of concentrations of small molecular weight inhibitors, cells were exposed to NSC23766 for 16 h before further experimentation, whereas they were exposed to PTP and SU6656 for 1 h.
Cell Fractionation
Nuclear and cytoplasmic fractions were prepared using the Nuclear Extract kit (Active Motif, Brussels, Belgium) as described previously (30). Membrane proteins were enriched for using sucrose density centrifugation as described previously (31).
Mass Spectrometry
Peptides were identified by RP-LC-MS/MS on a QStar® XL mass spectrometer (Applied Biosystems) as described previously (32).
Heavy Labeled Peptide Quantification of pS962 PTPRC/CD45
One hundred micrograms of CXCL12 treated FDCP-Mix cells was spiked with 250 fmol of heavy labeled peptide N[pS]NVVPYDFNR before being fractionated using high pH reverse phase liquid chromatography. Fractions were loaded onto a 15cmX75 μm PepMap C18 3 μm column (LC Packings) using a standard LC Packings UltiMate pump and FAMOS autosampler. Samples were desalted on line before separation using a micro-precolumn (5mmX300 μm) cartridge. The washing solvent was 0.1% formic acid delivered at a flow rate of 30 μl/mins for 3 mins. Peptides were separated over 60mins solvent gradient at 300 nl/mins, over 25 mins buffer B (Buffer A: 2% Acetonitrile, 0.1% Formic acid; Buffer B: 80% Acetonitrile, 0.1% Formic acid) was increased from an initial 8% to 40%. The content of buffer B was increased to 60% over the next 10mins and finally to 80% over the subsequent 2 mins. Chromatography was performed on line to a 4000 Q-TRAP mass spectrometer (AB Sciex). All voltages and gas settings used are described previously (33). The transitions for MRM analysis are presented in Table I.
Table I. Parameters required for MRM analysis of the heavy labelled pS962 PTPRC/CD45 peptide and the endogenous pS962 PTPRC/CD45 peptide.
| Q1 transition (m/z) | Q3 transition (m/z) | Dwell time (mSec) | Collision energy (mV) |
|---|---|---|---|
| 707.8 | 496.2 | 300 | 35 |
| 707.8 | 821.4 | 300 | 35 |
| 707.8 | 840.4 | 300 | 35 |
| 707.8 | 920.4 | 300 | 35 |
| 702.8 | 496.2 | 300 | 35 |
| 702.8 | 811.4 | 300 | 35 |
| 702.8 | 910.4 | 300 | 35 |
| 717.8 | 840.4 | 300 | 35 |
| 717.8 | 811.4 | 300 | 35 |
Data Analysis
Data was processed by a “Thorough” search against a mouse CDS database (mouse_KBMS5_0_20050302) containing 115,658 protein entries using ProteinPilot v2.0 software with default settings; for which the specificity of trypsin, the number of allowed missed cleavages and mass tolerances are preset. The software also does probability based analysis of the majority of the known modifications, however 8plex iTRAQ and MMTS modifications were specified (Applied Biosystems, Warrington, UK). Within Microsoft Excel, data was filtered on Modification and Theoretical MW then sorted on modification to separate phosphorylated and non-phosphorylated peptides. This process gave the number of unique phosphoentities (different mass spectra, they can be the same peptide “backbone” with the same site but other modifications) and phosphopeptides (unique sequences, does not account for different sites or missed cleavages). Subsequent manual analysis of spectral data was undertaken to confirm phosphorylation sites of interest. False discovery rate (FDR) was calculated using a reverse database and the ProteinPilot v.2.0 software. Ratio values for each phosphoentity were obtained from weighted averages of multiple spectra when appropriate, similarly to the way ProteinPilot™ calculate ratios for proteins. The minimum iTRAQ™ reporter ion area used for quantification was set at 20 (arbitrary units). ProteinPilot™ biases were used to correct for any sampling error so that the median value of log2 (ratios) of the phosphoentity distribution is equal to 0. Data is available to search and visualize at www.scalpl.org/hank/PhosphoHexPage?me=HSC%20Chemotaxis, it has also been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the data set identifier PXD000432.
Antibodies
Rabbit anti-mouse pS962 PTPRC/CD45 specific antibody was generated by Eurogentec and used at a dilution of 1:100 for Western blotting, total PTPRC/CD45 antibody (Becton Dickinson UK Ltd) used at 1:1000, Histone H3, Na+K+ ATPase pump, Src and pY416 Src (Cell Signaling Technologies, Danvers, MA) were used at 1:1000, α-Tubulin and actin (Santa Cruz Biotechnology, Santa Cruz, CA) 1:1000. Anti CD45 PE-Cy7 antibody for flow cytometric analysis (ebiosciences)was used at 1:2000.
Immunoassay Using the NanoPro 1000 Instrument
Cells were lysed on ice with Bicine/CHAPS lysis buffer for 1 h with regular vortexing. Following this, lysates were centrifuged at 20,817 × g for 15mins at 4 °C. A NanoPro 1000 (Protein Simple, USA) instrument similar to that described (34) was used to analyze pS962 PTPRC/CD45 in clinical CD34+ samples. Each cell lysate was buffer exchanged with sample diluent plus DMSO Inhibitor mix using Amicon Ultra 3K centrifuge filters (Merck Millipore, Germany) and mixed in a 1:3 ratio with Premix containing pI standard. Each capillary loaded 400 nL sample mix. Primary anti-pS962 PTPRC/CD45 (Eurogentec) and used at 1:20 dilution, secondary (Goat-anti rabbit-biotin) and tertiary (Streptavadin-HRP) were used at a 1:100 dilution prepared with antibody diluent. XDR-peroxide/luminol was used for chemiluminescent detection. Instrument parameters were 25s sample load (400 nL load volume) with 40mins separation at 21000μW followed by optimized immobilization exposure of 90s. Primary, secondary and tertiary incubation times were 480, 60, and 10mins respectively. Detection profile included 11 exposures from 5–1200 s. Spectra analysis was undertaken using Compass software.
Transwell Migration Assay
The migration of FDCP-Mix cells and the primary hematopoietic cells in response to CXCL12 was performed essentially as previously described (35).
Site Directed Analysis and Transfection
Phospho mutants of S962 of PTPRC/CD45 were generated by site directed mutagenesis as per manufacturer's instructions in the Quikchange II kit (Stratagene, Cambridge, UK). Mutants were subcloned into MSCV-GFP retroviral vectors for transfection of Lin- cells. Transfection of Jurkat cells was undertaken by electroporation using program X-001 of the Amaxa II (Lonza) nucleofector reagent V.
RESULTS
Phosphoproteomic Analysis of CXCL12 Action on Multipotent Cells
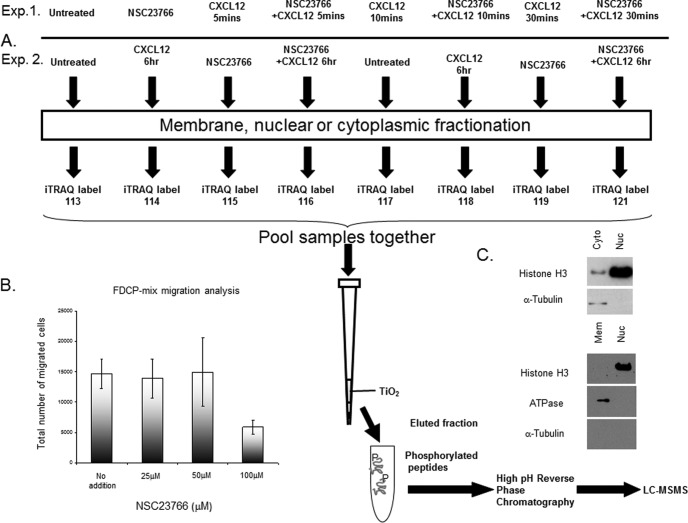
The phosphoproteomic experiment employed the multipotent FDCP-Mix hematopoietic cell line (27) pretreated with or without the Rac1/2 inhibitor NSC23766 and CXCL12 (Fig. 1A). Two experiments were performed to analyze the response of phosphopeptides to CXCL12. The first experiment was performed over a 30min time period in presence or absence of NSC23766 (Fig. 1A, Exp. 1) and the second experiment was a 6 h CXCL12 treatment (Figure 1A, Exp. 2). The concentration of NSC23766 used (100 μm) in this experiment was determined using the Boyden chamber chemotaxis assay and CXCL12, a known chemotactic agent (2) (Fig. 1B). After treatment with CXCL12 and NSC23766, subcellular fractionation, iTRAQ labeling and phosphopeptide enrichment, samples were subjected to relative quantification LC-MSMS. Multipotent FDCP-Mix cells form cobblestones on irradiated long term bone marrow culture stromal cell layers (36, 37) as seen with LSK cells and also respond to CXCL12 (2). CXCL12 treatment with and without NSC23766 in the FDCP-Mix cells enabled the data to be considered in the context of Rac inhibitor sensitive proteins. This is especially pertinent as Rac proteins are key effectors in p210-BCR-ABL mediated transformation (24) and stem cell homing/retention (1, 3, 24, 38, 39). The principal components of the experimental design are shown in Fig. 1A. Cells were fractionated into nuclear, cytosolic or membranous fractions (appropriate marker analyses confirmed this, Fig. 1C) and the enriched phosphopeptides analyzed on a tandem mass spectrometer.
Fig. 1.
Phosphopeptide analysis of FDCP-Mix cells treated with NSC23766 and/or CXCL12. A, CXCL12 (200 ng/ml) and the effective dose of NSC23766 (100 μm) as assessed in B was used to treat FDCP-Mix cells, different subcellular fractions were isolated (C) for phosphopeptide analysis. Two analyses were performed, Exp. 1. 30mins 200 ng/ml CXCL12 time course with and without NSC23766, Exp. 2. FDCP-Mix cells were treated with 200 ng/ml CXCL12 for 6 h with or without NSC23766. B, FDCP-Mix cells were treated with increasing concentrations of NSC23766 (Rac1/2 inhibitor) for 16 h. The motility of these treated cells was then assessed using a Boyden chamber transwell migration assay. C, Western blot analysis of subcellular fractions of known markers of each fraction; Histone H3 for the nucleus (Nuc), α-Tubulin for the cytosolic fraction (Cyto) and ATPase for the membranous fraction (Mem).
In the experiments to determine the role of CXCL12 in multipotent cell signaling mechanisms we identified 747 phosphopeptide observations in the cytosolic fraction, 92 in the membranous fraction and 557 in the nuclear fraction (supplemental Table S1A). In the 6 h CXCL12 analysis we identified 28 phosphopeptides in the membranous fraction and 250 in the nuclear fraction (supplemental Table S1B). There is no apparent and immediately obvious reason for the difference in the number of phosphopeptides identified between the 2 experiments; this could be because of sample preparation or the analysis. From the analyses that have been performed it can be seen that a number of phosphoevents appear to be differentially regulated by treatment with CXCL12 and NSC23766. From the data accumulated we were interested in selecting and taking forward phosphoevents and demonstrating their importance in motility. We next took observations and validated their role in primitive hematopoietic homing.
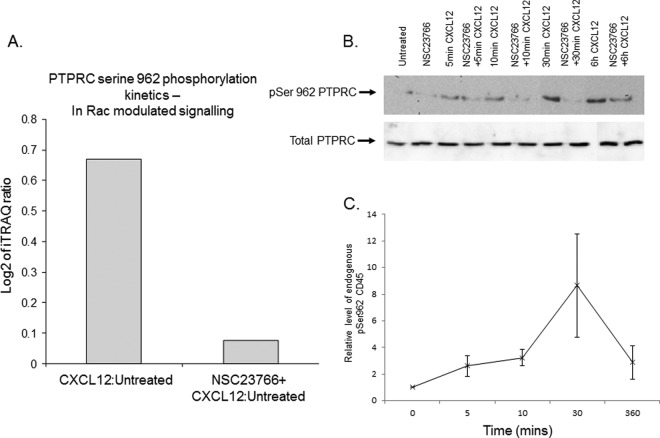
Characterization of a Novel Phosphosite on Protein Tyrosine Phosphatase Receptor Type C
A major aim of our study was to find CXCL12-stimulated phosphorylation events that are NSC23766 sensitive. pS962 of PTPRC/CD45 (NRN[pS]NVVPYDFNR) was seen to be increased in CXCL12-treated cells in an NSC23766 sensitive fashion over a 6 h period (Fig. 2A and supplemental Table S1B).
Fig. 2.
pS962 PTPRC/CD45, a novel phosphorylation event identified as a possible candidate to control cell motility. A, Histogram demonstrating the difference in expression level of the pS962 PTPRC/CD45 site in 100 μm NSC23766 and 200 ng/ml CXCL12 treated FDCP-Mix cells as assessed by iTRAQ based relative quantitation and mass spectrometry. FDCP-Mix cells were treated with NSC23766 or the carrier for 16 h before being exposed to CXCL12 for 6 h. B, A Cell lysates from cells treated as shown were subject to Western blot analysis using a phospho specific antibody to pS962 PTPRC/CD45 was made and used to validate the iTRAQ data. The pS962 and total blots shown are from separate blots, they are representative of the results from three separate biological experiments. C, 250fmol of a heavy labeled isotopomer of the PTPRC/CD45 phosphopeptide was spiked into 100 μg of CXCL12 treated FDCP-Mix cell lysate. The endogenous and heavy labeled peptide were then identified and quantified using multiple reaction monitoring. The graph is plotted from the area under the curve of the 3 Q3 transitions from at least 3 biological replicate experiments, the error bars are standard error of the mean.
CXCL12 induced phosphorylation and NSC23766 sensitivity of the pS962 PTPRC/CD45 seen by the iTRAQ analyses were confirmed using a phosphopeptide specific antibody and Western blotting (Fig. 2B). Interestingly this activation seems to be sustained over a 6 h period (Figs. 2A and 2B). Further verification of this phosphorylation event in CXCL12-treated FDCP-Mix cells was performed using a mass spectrometric technique that not only verifies the presence of a specific peptide species but also gives quantification. This is achieved by adding known amounts of an isotopomeric or heavy labeled pS962 PTPRC/CD45 phosphopeptide and analysis using MRM methodologies on a triple quadrupole mass spectrometer (see Table I for list of transitions). The isotopomeric standard phosphopeptide was spiked into FDCP-Mix cells treated with or without CXCL12. The endogenous peptide from the biological material and the heavy labeled standard co-elute in liquid chromatography but are discerned by the differential mass to charge ratio (m/z). Area under the curve calculations were used for quantification and referenced to the known molar quantity of the heavier standard (Fig. 2C). The fold increase observed in the phosphopeptide within tryptic digests of these cells is mirrored in the Western blot analysis with pS962 PTPRC/CD45 antibody. It is important to note, separate biological material was produced for the validation analysis. Using 3 techniques; iTRAQ mass spectrometry, Western blotting and MRM using an AQUA peptide and at least seven biological replicates we can conclude that the pS962 PTPRC/CD45 is activated by CXCL12 in a Rac 1/2 dependent manner.
PTPRC/CD45 Biochemical Analysis
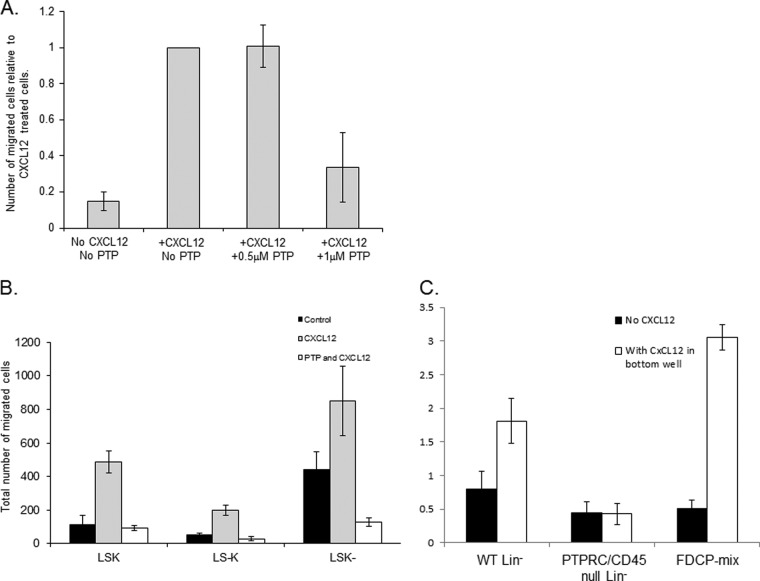
We wanted to determine if an inhibitor of PTPRC/CD45, PTP inhibitor (N-(9,10-Dioxo-9,10-dihydro-phenanthren-2-yl)-2,2-dimethyl-propionamide), could affect CXCL12 induced chemotaxis of FDCP-Mix cells (Fig. 3A). We observed that increasing the concentration of PTP from 0.5 to 1 μm caused a threefold decrease in chemotaxis. Further evidence of PTPRC/CD45 involvement in motility was seen when primary murine hematopoietic progenitor cells LSK, LS−K, and LSK− cells were used in a chemotactic assay with and without PTP (Fig. 3B). When the LSK, LS−K, and LSK− cells were pretreated with PTP; chemotaxis in response to CXCL12 was inhibited. In Fig. 3C it can also clearly be seen that the chemotactic migration in response to CXCL12 is dramatically inhibited in the PTPRC/CD45−/− Lin− cells compared with WT Lin− and FDCP-Mix cells. Thus the protein per se is required for motility. Site directed mutagenesis on the protein would then determine if the phosphosite is important in motile response (see below).
Fig. 3.
The role of PTPRC/CD45 in hematopoietic progenitor chemotaxis. A, The involvement of PTPRC/CD45 in CXCL12 (200 ng/ml) induced motility of FDCP-Mix cells was demonstrated using the transwell motility assay and increasing concentrations of the PTPRC/CD45 inhibitor PTP. B, LSK, LS−K and LSK− sorted cell's motility was assessed in the presence or absence of CXCL12 and PTP. C, The chemotactic ability of PTPRC/CD45−/− Lin− cells was assessed using the transwell assay and compared with wild type Lin− cells (WT Lin−) and FDCP-Mix cells.
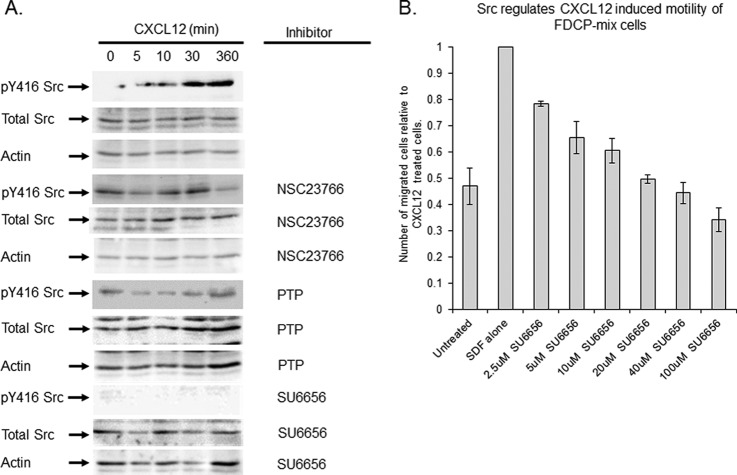
It has been postulated that PTPRC/CD45 activates Src kinase (40) by inducing tyrosine phosphorylation within the catalytic domain (Y416). When FDCP-Mix cells were treated with CXCL12, Y416 Src was phosphorylated and this stimulation was sensitive to NSC23766 and PTP (Rac and PTPRC/CD45 inhibitors respectively) (Fig. 4A), indicating that Src activation is indeed regulated by PTPRC/CD45 which is phosphorylated via a Rac inhibitor sensitive pathway. It has been demonstrated previously that pY416 Src is decreased in PTPRC/CD45−/− Lin− cells compared with wild type cells (41). To demonstrate that Src is involved in the signaling pathway linked to HPC cell migration, FDCP-Mix cells were treated with SU6656 (Src inhibitor) for 1 h before analysis of the cells chemotactic ability using the transwell migration assay. Previous work has demonstrated that an effective dose of SU6656 is 10 μm (42–44), for this reason the range of concentration used was from 2.5–100 μm. A concentration dependent decrease in the ability of the cells to migrate in response to CXCL12 was observed when the cells were treated with SU6656 (Fig. 4B).
Fig. 4.
PTPRC/CD45 regulates Src activity in response to CXCL12 induced motility. A, Further analysis of PTPRC/CD45 biochemistry identified Src downstream of PTPRC/CD45 and Rac1/2. Cells were treated with 100 μm NSC23766 or carrier for 16 h, or 1 μm PTP or 20 μm SU6656 for 1 h before being exposed to CXCL12 over a 6 h period of time. Cells were lysed and blotted for pY416 Src, Total Src and Actin. B, The involvement of Src in CXCL12 induced motility. FDCP-Mix cells were exposed to increasing concentrations of SU6656 for 1 h before their chemotactic ability being assessed using a transwell migration assay.
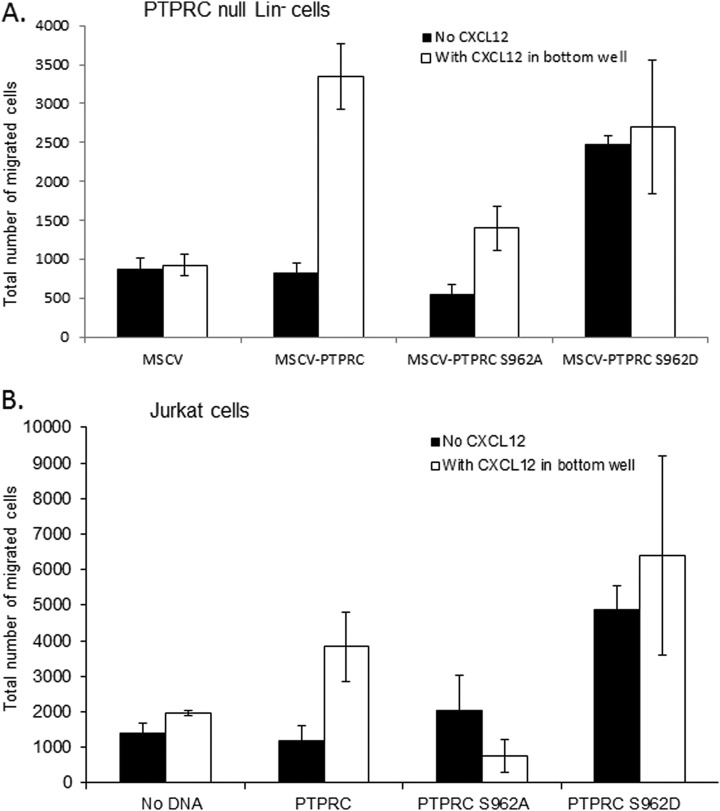
Phosphorylation of S962 Regulates PTPRC/CD45 Mediated Motile Response
To assess the relevance of the observed S962 phosphorylation we undertook site directed mutagenesis of this site. S962 was mutated to an aspartic acid (S962D) to replicate a constitutively phosphorylated site. S962 was also mutated to Alanine (S962A) to inactivate the site. The PTPRC/CD45 null Lin− cells were transfected with MSCV (empty vector, negative control), MSCV-PTPRC/CD45, MSCV-PTPRC/CD45 S962A, and MSCV-PTPRC/CD45 S962D and the resultant cells were used in a transwell motility assay. Lin− cells were used for this experiment because of the low number of LSK cells obtained, also post infection the cells are cultured and will undergo further differentiation. Expression of PTPRC/CD45 in the null Lin− cells sensitizes the cells to CXCL12 induced motility (Fig. 5A). Mutating the S962 to an alanine inhibited the PTPRC/CD45 induced chemotactic ability of the cells; the S962D cells demonstrated increased chemokinesis, however no additional motility of the cells was seen in the presence of CXCL12. Further clarification of this observation was seen when the T lymphocyte cell line Jurkat were transfected with the PTPRC/CD45 clones. Jurkat cells express CXCR4 and are known demonstrate chemotaxis in response to CXCL12. This motility is also known to require the Src family of proteins (45). The PTPRC/CD45 clones were expressed from the proximal lck promoter (wild type PTPRC/CD45, PTPRC/CD45 S962A, and PTPRC/CD45 S962D). We demonstrate once again that mutation of the S962 site to an alanine inhibits chemotaxis, whereas mutation to aspartic acid increased chemokinesis (Fig. 5B).
Fig. 5.
pS962 PTPRC/CD45 is involved in CXCL12 induced motility. A and B, S962 PTPRC/CD45 was mutated to an alanine or an aspartate and transfected into PTPRC/CD45 null lin− (A) and jurkat cells (B), the motility of the resultant cells was assessed using the transwell migration assay.
PTPRC/CD45 in Leukemia
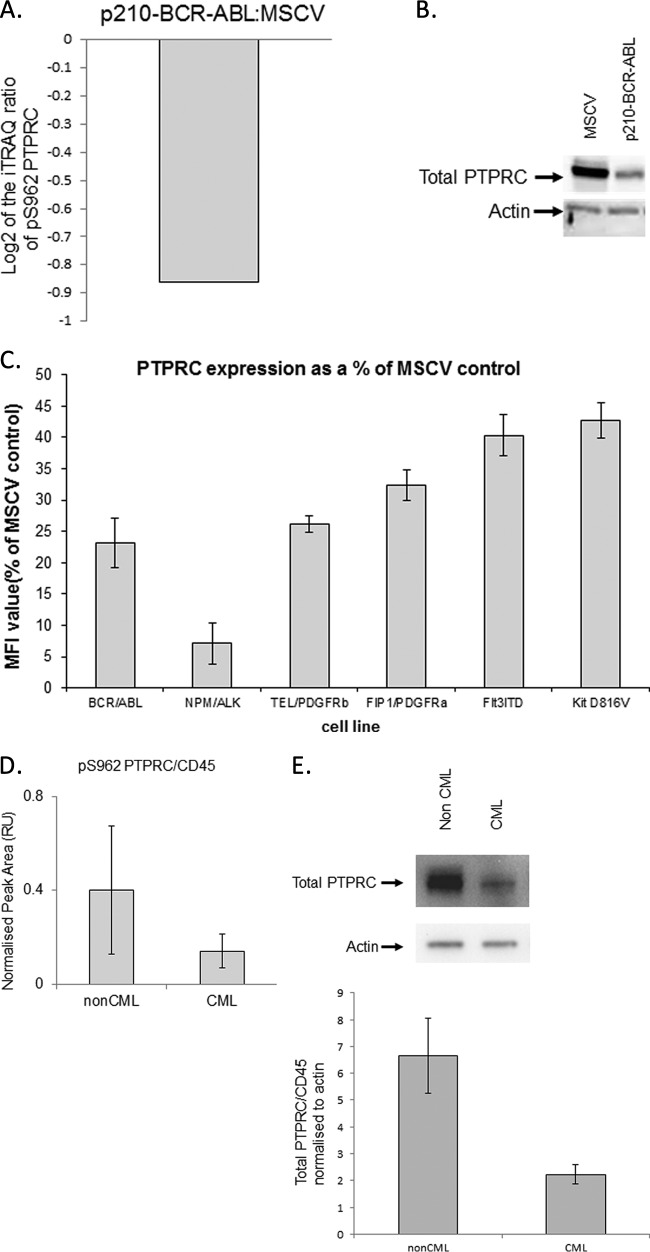
P210-BCR-ABL has been demonstrated to induce abnormal adhesion and migration of hematopoietic progenitors (20, 21). Interestingly a phosphoproteomic analysis of p210-BCR-ABL expressing Ba/F3 cells demonstrated that pS962 PTPRC/CD45 was modulated (see Supplemental Table 2A and Fig. 6A). The relative quantification mass spectrometry experiment undertaken also revealed that we have a decrease in the level of pS962 PTPRC/CD45 in p210-BCR-ABL expressing Ba/F3 cells compared with control Ba/F3 cells (Fig. 6A). However a marked reduction in total PTPRC/CD45 expressed in p210-BCR-ABL expressing Ba/F3 cells compared with the MSCV controls was seen on Western blotting (Fig. 6B). The decrease in total protein expression is the reason for observed decrease in pS962 PTPRC/CD45 level. To investigate this observation further; flow cytometric analysis of total PTPRC/CD45 expression in Ba/F3 cells expressing a range of oncogenic tyrosine kinases was undertaken. P210-BCR-ABL, NPM/ALK, TEL/PDGFRb, FIP1/PDGFRa, Flt3ITD, and Kit D816V are all linked to myeloid leukemias. PTPRC/CD45 is greatly decreased (by more than 50%) in cells expressing this panel of oncogenic tyrosine kinases (Fig. 6C). CD34+ cells from three different patients suffering from CML and CD34+ cells isolated from a control group (three non-CML patients) were compared. Using the NanoPro 1000 instrument we have demonstrated a decrease in the level of pS962 PTPRC/CD45 in CD34+ cells expressing BCR-ABL from 3 patients with CML compared with a non-CML control (n = 3) (Fig. 6D). A similar level of decrease was also identified in total PTPRC/CD45 using densitometry analysis (total PTPRC/CD45 - 2.99-fold decrease in CML compared with nonCML, pS962 PTPRC/CD45 - 2.84-fold decrease in CML compared with nonCML) of Western blots looking at PTPRC/CD45 in CML and non CML CD34+ cells (Fig. 6E). This corroborates the data we saw in the initial observations from cell line analyses to actual clinical material. Further work is underway to determine the possible links that PTPRC/CD45 and its phosphorylation on the S962 site have to myeloid leukemias.
Fig. 6.
pSer962 PTPRC/CD45 status in p210-BCR-ABL expressing cells. A, A graphical representation of the iTRAQ result from the mass spectrometry experiment. B, Using the total PTPRC/CD45 antibodies, we probed Ba/F3 whole cell lysates expressing MSCV and p210-BCR-ABL. C, Flow cytometric analysis of PTPRC/CD45 expression on cells expressing different oncogenic tyrosine kinases linked to various myeloid leukemias was performed using a PTPRC/CD45 PE-Cy7 antibody on a FACS Caliber (BD Biosciences).The analysis is based on the mean fluorescent intensity (MFI) of the cells. D, PS962 PTPRC/CD45 antibody and the NanoPro 1000 instrument was used to determine levels of the pS962 PTPRC/CD45 in human clinical CD34+ cells derived from CML (n = 3 patients) and non CML (n = 3 patients) patients. Bar chart represents area for phosphorylated PTPRC/CD45 peak (pI 4.91) acquired using cIEF immunoassay. Error bars represent standard error of the mean of 3 biological replicate experiments. E, Total PTPRC/CD45 was also identified in CML and nonCML patients via Western blot. The Western blot is representative of three CML and 3 nonCML samples. The histogram was calculated using densitometry and the amount of PTPRC/CD45 was normalized by the intensity of the actin. The error bars depict the standard error of the mean from the three biological replicates.
DISCUSSION
Using the hematopoietic progenitor cell line FDCP-Mix; we sought to uncover a mechanistic event that is relevant in primitive hematopoietic cell motile responses. The understanding of hematopoietic stem cell motility and directed movement is an essential aspect of understanding stem cell homeostasis. The hematopoietic progenitor cell line FDCP-Mix cells chemotactically move in response to CXCL12 and this response is inhibited when the cells are exposed to the Rac1/2 inhibitor NSC23766. CML is a disease that is closely associated with HSC (46) and requires Rac signaling (24, 25). Phosphoproteomic differences in p210-BCR-ABL expressing lines compared with control are interesting in the context of oncogenesis with the recent publication from Pierce et al. 2012 (26) investigating this aspect further. It is also interesting the fact that p210-BCR-ABL expressing cells have less chemotactic ability (21).
Recent work from Lapidot (47, 48) has demonstrated the requirement for PTPRC/CD45 for CD34+ cell motility and also a hyperadhesive phenotype within the bone marrow, partly because of PTPRC/CD45's function in regulating the expression of β1-integrins. Blocking the PTPRC/CD45 activity reduced the homing activity of the HSC and LSC and this regulation appeared to involve Src. Previously a number of observations have demonstrated the importance of Src and the Src family of kinases in regulating stem cell migration (49–51). The best known function of PTPRC/CD45 is its ability to regulate Src and the Src family kinase activity (52). The major substrates of PTPRC/CD45 appear to be Lck and Fyn in T cells and Lyn, Fyn, and Blk in B cells (53, 54). Although Rac 1/2, PTPRC/CD45, and Src have all been implicated in motility these proteins have never been linked previously.
Little is known about how PTPRC/CD45 regulates signaling and even less is known about the mechanism of PTPRC/CD45 activation. There is a lack of evidence for ligand-induced activation of PTPRC/CD45 (a common mechanism for receptor tyrosine kinases). Surprisingly the post translational mechanisms involved in PTPRC/CD45 activity are also unknown. From the work that we have reported here we have demonstrated a link between CXCL12, CXCR4, Rac, PTPRC/CD45, and Src in regulating HSC/P motility (see Figs. 1 - 5). From the data we demonstrated that the kinetics of pS962 PTPRC/CD45 was modulated in response to CXCL12, also the PTPRC/CD45 protein is decreased in to the presence of p210-BCR-ABL. It has not yet been demonstrated that Rac is an upstream activator of PTPRC/CD45 stimulated by CXCR4 and CXCL12. However we have demonstrated that pretreatment of the HPC with NSC23766 inhibited activating phosphorylation of PTPRC/CD45 and Src, likewise pretreatment with PTP inhibited Src phosphorylation. In all cases pre-treatment of the cells with the small molecule inhibitors decreased CXCL12 induced migration, indicating their involvement in regulation of migration. Further validation of the involvement of PTPRC/CD45 in regulating HSC/P chemotaxis was demonstrated by the lack of motility of PTPRC/CD45 null Lin− cells. We have demonstrated that PTPRC/CD45 is activated via Rac modulated pathways after exposure to CXCL12.
Supplementary Material
Acknowledgments
We thank Dr David Williams (Harvard) for provision of NSC23766.
Footnotes
* This work is supported by Leukemia Lymphoma Research (UK) with support also from Cancer Research UK and by the Glasgow and Manchester Experimental Cancer Medicine Centres (ECMCs), which is funded by Cancer Research UK and the Chief Scientist's Office (Scotland). This work was also made possible using patient samples donated as part of the UK SPIRIT2 Trial.
 This article contains supplemental Tables S1 and S2.
This article contains supplemental Tables S1 and S2.
Contributions: AJKW, AP, ADW and ES designed the study. AJKW, AP, EJ, MAO, RDU, MW and SAA performed the experiments. AJKW, ADW, CZ, LL and ES analyzed the data. AJKW, TLH, and ADW, wrote the manuscript.
Conflicts of interest: The authors declare they have no conflicting interests.
1 The abbreviations used are:
- HSC/P
- hematopoietic stem and progenitor cells
- LC
- liquid chromatography
- LSK
- Lin− Sca+ c-kit+ cells
- [pS]
- phosphorylated serine.
REFERENCES
- 1. Cancelas J. A., Lee A. W., Prabhakar R., Stringer K. F., Zheng Y., Williams D. A. (2005) Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat. Med. 11, 886–891 [DOI] [PubMed] [Google Scholar]
- 2. Whetton A. D., Lu Y., Pierce A., Carney L., Spooncer E. (2003) Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav 1. Blood 102, 2798–2802 [DOI] [PubMed] [Google Scholar]
- 3. Williams D. A., Zheng Y., Cancelas J. A. (2008) Rho GTPases and regulation of hematopoietic stem cell localization. Methods Enzymol. 439, 365–393 [DOI] [PubMed] [Google Scholar]
- 4. Etienne-Manneville S., Hall A. (2002) Rho GTPases in cell biology. Nature 420, 629–635 [DOI] [PubMed] [Google Scholar]
- 5. Gu Y., Filippi M. D., Cancelas J. A., Siefring J. E., Williams E. P., Jasti A. C., Harris C. E., Lee A. W., Prabhakar R., Atkinson S. J., Kwiatkowski D. J., Williams D. A. (2003) Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302, 445–449 [DOI] [PubMed] [Google Scholar]
- 6. Yang F. C., Atkinson S. J., Gu Y., Borneo J. B., Roberts A. W., Zheng Y., Pennington J., Williams D. A. (2001) Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc. Natl. Acad. Sci. U.S.A. 98, 5614–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagasawa T., Tachibana K., Kishimoto T. (1998) A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin. Immunol. 10, 179–185 [DOI] [PubMed] [Google Scholar]
- 8. Peled A., Grabovsky V., Habler L., Sandbank J., Arenzana-Seisdedos F., Petit I., Ben-Hur H., Lapidot T., Alon R. (1999) The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J. Clin. Invest. 104, 1199–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kollet O., Spiegel A., Peled A., Petit I., Byk T., Hershkoviz R., Guetta E., Barkai G., Nagler A., Lapidot T. (2001) Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood 97, 3283–3291 [DOI] [PubMed] [Google Scholar]
- 10. Ma Q., Jones D., Springer T. A. (1999) The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 10, 463–471 [DOI] [PubMed] [Google Scholar]
- 11. Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S., Kitamura Y., Yoshida N., Kikutani H., Kishimoto T. (1996) Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 [DOI] [PubMed] [Google Scholar]
- 12. Peled A., Kollet O., Ponomaryov T., Petit I., Franitza S., Grabovsky V., Slav M. M., Nagler A., Lider O., Alon R., Zipori D., Lapidot T. (2000) The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95, 3289–3296 [PubMed] [Google Scholar]
- 13. Bishop A. L., Hall A. (2000) Rho GTPases and their effector proteins. Biochem. J. 348 Pt 2, 241–255 [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L., Zheng Y. (2007) Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 17, 58–64 [DOI] [PubMed] [Google Scholar]
- 15. Filippi M. D., Harris C. E., Meller J., Gu Y., Zheng Y., Williams D. A. (2004) Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat. Immunol. 5, 744–751 [DOI] [PubMed] [Google Scholar]
- 16. Gu Y., Jia B., Yang F. C., D'Souza M., Harris C. E., Derrow C. W., Zheng Y., Williams D. A. (2001) Biochemical and biological characterization of a human Rac2 GTPase mutant associated with phagocytic immunodeficiency. J. Biol. Chem. 276, 15929–15938 [DOI] [PubMed] [Google Scholar]
- 17. Gao Y., Dickerson J. B., Guo F., Zheng J., Zheng Y. (2004) Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. U.S.A. 101, 7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daley G. Q., Van Etten R. A., Baltimore D. (1990) Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247, 824–830 [DOI] [PubMed] [Google Scholar]
- 19. Michor F., Iwasa Y., Nowak M. A. (2006) The age incidence of chronic myeloid leukemia can be explained by a one-mutation model. Proc. Natl. Acad. Sci. U.S.A. 103, 14931–14934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren R. (2005) Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat. Rev. Cancer 5, 172–183 [DOI] [PubMed] [Google Scholar]
- 21. Salgia R., Quackenbush E., Lin J., Souchkova N., Sattler M., Ewaniuk D. S., Klucher K. M., Daley G. Q., Kraeft S. K., Sackstein R., Alyea E. P., von Andrian U. H., Chen L. B., Gutierrez-Ramos J. C., Pendergast A. M., Griffin J. D. (1999) The BCR/ABL oncogene alters the chemotactic response to stromal-derived factor-1alpha. Blood 94, 4233–4246 [PubMed] [Google Scholar]
- 22. Druker B. J., O'Brien S. G., Cortes J., Radich J. (2002) Chronic myelogenous leukemia. Hematology Am. Soc. Hematol. Educ. Program 1, 111–135 [DOI] [PubMed] [Google Scholar]
- 23. Kogan S. C., Ward J. M., Anver M. R., Berman J. J., Brayton C., Cardiff R. D., Carter J. S., de Coronado S., Downing J. R., Fredrickson T. N., Haines D. C., Harris A. W., Harris N. L., Hiai H., Jaffe E. S., MacLennan I. C., Pandolfi P. P., Pattengale P. K., Perkins A. S., Simpson R. M., Tuttle M. S., Wong J. F., Morse H. C., 3rd (2002) Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood 100, 238–245 [DOI] [PubMed] [Google Scholar]
- 24. Sengupta A., Arnett J., Dunn S., Williams D. A., Cancelas J. A. (2010) Rac2 GTPase deficiency depletes BCR-ABL+ leukemic stem cells and progenitors in vivo. Blood 116, 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas E. K., Cancelas J. A., Chae H. D., Cox A. D., Keller P. J., Perrotti D., Neviani P., Druker B. J., Setchell K. D., Zheng Y., Harris C. E., Williams D. A. (2007) Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell 12, 467–478 [DOI] [PubMed] [Google Scholar]
- 26. Pierce A., Williamson A., Jaworska E., Griffiths J. R., Taylor S., Walker M., O'Dea M. A., Spooncer E., Unwin R. D., Poolman T., Ray D., Whetton A. D. (2012) Identification of nuclear protein targets for six leukemogenic tyrosine kinases governed by post-translational regulation. PLoS One 7, e38928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spooncer E., Heyworth C. M., Dunn A., Dexter T. M. (1986) Self-renewal and differentiation of interleukin-3-dependent multipotent stem cells are modulated by stromal cells and serum factors. Differentiation 31, 111–118 [DOI] [PubMed] [Google Scholar]
- 28. Nilsson S. K., Johnston H. M., Coverdale J. A. (2001) Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 97, 2293–2299 [DOI] [PubMed] [Google Scholar]
- 29. Unwin R. D., Smith D. L., Blinco D., Wilson C. L., Miller C. J., Evans C. A., Jaworska E., Baldwin S. A., Barnes K., Pierce A., Spooncer E., Whetton A. D. (2006) Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood 107, 4687–4694 [DOI] [PubMed] [Google Scholar]
- 30. Williamson A. J., Smith D. L., Blinco D., Unwin R. D., Pearson S., Wilson C., Miller C., Lancashire L., Lacaud G., Kouskoff V., Whetton A. D. (2008) Quantitative proteomics analysis demonstrates post-transcriptional regulation of embryonic stem cell differentiation to hematopoiesis. Mol. Cell.. Proteomics 7, 459–472 [DOI] [PubMed] [Google Scholar]
- 31. Holland M., Castro F. V., Alexander S., Smith D., Liu J., Walker M., Bitton D., Mulryan K., Ashton G., Blaylock M., Bagley S., Connolly Y., Bridgeman J., Miller C., Krishnan S., Dempsey C., Masurekar A., Stern P., Whetton A., Saha V. (2011) RAC2, AEP, and ICAM1 expression are associated with CNS disease in a mouse model of pre-B childhood acute lymphoblastic leukemia. Blood 118, 638–649 [DOI] [PubMed] [Google Scholar]
- 32. Griaud F., Williamson A. J., Taylor S., Potier D. N., Spooncer E., Pierce A., Whetton A. D. (2012) BCR/ABL modulates protein phosphorylation associated with the etoposide-induced DNA damage response. J. Proteomics 77, 14–26 [DOI] [PubMed] [Google Scholar]
- 33. Unwin R. D., Griffiths J. R., Leverentz M. K., Grallert A., Hagan I. M., Whetton A. D. (2005) Multiple reaction monitoring to identify sites of protein phosphorylation with high sensitivity. Mol. Cell. Proteomics 4, 1134–1144 [DOI] [PubMed] [Google Scholar]
- 34. O'Neill R. A., Bhamidipati A., Bi X., Deb-Basu D., Cahill L., Ferrante J., Gentalen E., Glazer M., Gossett J., Hacker K., Kirby C., Knittle J., Loder R., Mastroieni C., Maclaren M., Mills T., Nguyen U., Parker N., Rice A., Roach D., Suich D., Voehringer D., Voss K., Yang J., Yang T., Vander Horn P. B. (2006) Isoelectric focusing technology quantifies protein signaling in 25 cells. Proc. Natl. Acad. Sci. U.S.A. 103, 16153–16158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pierce A., Lu Y., Hamzah H. G., Thompson S., Owen-Lynch P. J., Whetton A. D., Spooncer E. (2006) Differential effect of leukaemogenic tyrosine kinases on cell motility is governed by subcellular localisation. Br. J. Haematol. 133, 345–352 [DOI] [PubMed] [Google Scholar]
- 36. Heyworth C. M., Alauldin M., Cross M. A., Fairbairn L. J., Dexter T. M., Whetton A. D. (1995) Erythroid development of the FDCP-Mix A4 multipotent cell line is governed by the relative concentrations of erythropoietin and interleukin 3. Br. J. Haematol. 91, 15–22 [DOI] [PubMed] [Google Scholar]
- 37. Choong M. L., Luo B., Lodish H. F. (2004) Microenvironment-driven changes in the expression profile of hematopoietic cobblestone area-forming cells. Ann. Hematol. 83, 160–169 [DOI] [PubMed] [Google Scholar]
- 38. Sanchez-Aguilera A., Lee Y. J., Lo Celso C., Ferraro F., Brumme K., Mondal S., Kim C., Dorrance A., Luo H. R., Scadden D. T., Williams D. A. (2011) Guanine nucleotide exchange factor Vav1 regulates perivascular homing and bone marrow retention of hematopoietic stem and progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 108, 9607–9612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shang X., Cancelas J. A., Li L., Guo F., Liu W., Johnson J. F., Ficker A., Daria D., Geiger H., Ratner N., Zheng Y. (2011) R-Ras and Rac GTPase cross-talk regulates hematopoietic progenitor cell migration, homing, and mobilization. J. Biol. Chem. 286, 24068–24078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alexander D. R. (2000) The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Semin. Immunol. 12, 349–359 [DOI] [PubMed] [Google Scholar]
- 41. Griaud F., Pierce A., Gonzalez Sanchez M. B., Scott M., Abraham S. A., Holyoake T. L., Tran D. D., Tamura T., Whetton A. D. (2012) A pathway from leukemogenic oncogenes and stem cell chemokines to RNA processing via THOC5. Leukemia 27(4), 932–940 [DOI] [PubMed] [Google Scholar]
- 42. Bartscht T., Lehnert H., Gieseler F., Ungefroren H. (2012) The Src family kinase inhibitors PP2 and PP1 effectively block TGF-beta1-induced cell migration and invasion in both established and primary carcinoma cells. Cancer Chemother. Pharmacol. 70, 221–230 [DOI] [PubMed] [Google Scholar]
- 43. Kim A. Y., Lee C. G., Lee da Y., Li H., Jeon R., Ryu J. H., Kim S. G., Bartscht T., Lehnert H., Gieseler F., Ungefroren H., Robak T., Robak E. (2012) Enhanced antioxidant effect of prenylated polyphenols as Fyn inhibitor The Src family kinase inhibitors PP2 and PP1 effectively block TGF-beta1-induced cell migration and invasion in both established and primary carcinoma cells Tyrosine kinase inhibitors as potential drugs for B-cell lymphoid malignancies and autoimmune disorders. Free Radic. Biol. Med. 53, 1198–120822771471 [Google Scholar]
- 44. Robak T., Robak E. (2012) Tyrosine kinase inhibitors as potential drugs for B-cell lymphoid malignancies and autoimmune disorders. Expert Opin. Investig. Drugs 21, 921–947 [DOI] [PubMed] [Google Scholar]
- 45. Zaman S. N., Resek M. E., Robbins S. M. (2008) Dual acylation and lipid raft association of Src-family protein tyrosine kinases are required for SDF-1/CXCL12-mediated chemotaxis in the Jurkat human T cell lymphoma cell line. J. Leukoc. Biol. 84, 1082–1091 [DOI] [PubMed] [Google Scholar]
- 46. Sloma I., Jiang X., Eaves A. C., Eaves C. J. (2010) Insights into the stem cells of chronic myeloid leukemia. Leukemia 24, 1823–1833 [DOI] [PubMed] [Google Scholar]
- 47. Shivtiel S., Kollet O., Lapid K., Schajnovitz A., Goichberg P., Kalinkovich A., Shezen E., Tesio M., Netzer N., Petit I., Sharir A., Lapidot T. (2008) CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J. Exp. Med. 205, 2381–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shivtiel S., Lapid K., Kalchenko V., Avigdor A., Goichberg P., Kalinkovich A., Nagler A., Kollet O., Lapidot T. (2011) CD45 regulates homing and engraftment of immature normal and leukemic human cells in transplanted immunodeficient mice. Exp. Hematol. 39, 1161–1170 e1161 [DOI] [PubMed] [Google Scholar]
- 49. Orschell C. M., Borneo J., Munugalavadla V., Ma P., Sims E., Ramdas B., Yoder M. C., Kapur R. (2008) Deficiency of Src family kinases compromises the repopulating ability of hematopoietic stem cells. Exp. Hematol. 36, 655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roach T., Slater S., Koval M., White L., Cahir McFarland E. D., Okumura M., Thomas M., Brown E. (1997) CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr. Biol. 7, 408–417 [DOI] [PubMed] [Google Scholar]
- 51. Thomas R. M., Schmedt C., Novelli M., Choi B. K., Skok J., Tarakhovsky A., Roes J. (2004) C-terminal SRC kinase controls acute inflammation and granulocyte adhesion. Immunity 20, 181–191 [DOI] [PubMed] [Google Scholar]
- 52. Lai J. C., Wlodarska M., Liu D. J., Abraham N., Johnson P. (2010) CD45 regulates migration, proliferation, and progression of double negative 1 thymocytes. J. Immunol. 185, 2059–2070 [DOI] [PubMed] [Google Scholar]
- 53. DeFranco A. L. (2000) B-cell activation 2000. Immunol. Rev. 176, 5–9 [PubMed] [Google Scholar]
- 54. Hermiston M. L., Xu Z., Weiss A. (2003) CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 21, 107–137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.