Abstract
Simian foamy viruses (SFVs) are ubiquitous in non-human primates (NHPs). As in all retroviruses, reverse transcription of SFV leads to recombination and mutation. Because more humans have been shown to be infected with SFV than with any other simian borne virus, SFV is a potentially powerful model for studying the virology and epidemiology of viruses at the human/NHP interface. In Asia, SFV is likely transmitted to humans through macaque bites and scratches that occur in the context of everyday life. We analyzed multiple proviral sequences from the SFV gag gene from both humans and macaques in order to characterize retroviral transmission at the human/NHP interface in Bangladesh. Here we report evidence that humans can be concurrently infected with multiple SFV strains, with some individuals infected by both an autochthonous SFV strain as well as a strain similar to SFV found in macaques from another geographic area. These data, combined with previous results, suggest that both human-facilitated movement of macaques leading to the introduction of non-resident strains of SFV and retroviral recombination in macaques contribute to SFV diversity among humans in Bangladesh.
Keywords: Bangladesh, emerging infectious diseases, retroviral recombination, simian foamy virus, zoonotic transmission
INTRODUCTION
Foamy viruses (FVs) are a subfamily of retroviruses that are widely distributed in vertebrate hosts, including non-human primates, cats, cows and horses.1 FVs are ancient exogenous viruses that are efficiently transmitted between hosts but are not known to cause disease.2 Although nearly ubiquitous in non-human primates (NHPs), simian foamy virus (SFV) is not endemic in Homo sapiens. Zoonotic infection of humans with SFV has been previously detected in several countries and in various contexts of interspecies contact including NHP laboratory and zoo workers in North America, ‘monkey temple' workers, pet owners and people living around free-ranging macaques in South and Southeast Asia and bushmeat hunters in Central Africa.3
To date, NHP-to-human transmission of SFV has been documented in >100 people, far more than the number of cross-species transmission events for the other known NHP-borne retroviruses: simian immunodeficiency virus, simian retrovirus D or simian T-cell lymphotrophic virus.3,4,5,6,7 Given the high prevalence of SFV in NHP and the diversity and extent of the interface between humans and NHP, it is estimated that globally the number of infected individuals may reach the tens of thousands.8,9 Additionally, the few longitudinal studies of SFV infected humans suggest that SFV is not transmitted from one person to another.10 As such, characterization of the factors that influence zoonotic transmission of SFV may provide the best contemporary model for understanding how and why some NHP viruses are capable of causing sustained human-to-human transmission.
Our group has studied the interface between humans and NHP in Bangladesh over the past 6 years, focusing on cross-species transmission of infectious agents.11,12,13 Bangladesh is one of the world's most densely populated countries, with many urban areas located within or near NHP habitats. Several NHP taxa are native to Bangladesh, with the rhesus macaque (Macaca mulatta) the most numerous and widely distributed.14 As forest habitat continues to dwindle, the small, ecologically flexible rhesus thrives in human-altered habitats, particularly in areas where cultural attitudes encourage their tolerance.15 While contact between humans and rhesus macaques occurs primarily in communities where the macaques are free-ranging, it also occurs at religious shrines that have become refuges for macaques and through macaque pet ownership. In addition, a nomadic people, known variously as Bade, or Qalandar, have traveled the region for centuries, making a livelihood by staging performances with trained macaques. Unlike Africa, hunting of monkeys for bushmeat is rare in Bangladesh, being confined to indigenous peoples in the Chittagong Hill Tracts areas that border Myanmar.16
NHP-to-human transmission of SFV is thought to occur most commonly through bites. SFV replicates actively in the oral mucosae of infected monkeys, achieving high concentrations in saliva, where genomic and mRNAs can readily be detected.17 However, relatively little is known concerning how host or viral characteristics influence SFV transmission. Even severe bites do not reliably result in infection.9 Conversely, some humans with documented infection do not recall significant exposure to NHP or NHP tissue.
Other important questions regarding zoonotic SFV transmission and infection remain unresolved. For example, while proviral DNA is detected persistently over decades in some SFV infected humans; other individuals develop antibodies to SFV without detectable proviral DNA.3 It remains to be discovered whether some individuals clear infection, or whether certain viral strains are more capable of persistence. Further research is needed to determine if, analogous to SIV/HIV, in which recombination between retroviral strains in a novel host was a precursor event to the evolution of pandemic viruses, there is evidence that SFVs recombine in human hosts. Few studies have been conducted to determine whether or not the concentration of SFV in saliva influences zoonotic transmission, or how the human immune response facilitates or prevents infection or continued viremia (Soliven et al. and Matsen et al., unpublished). And while data collected in other geographic areas and other contexts of human–macaque contact have provided insight into how demographic variables and macaque and human behavior contribute to the likelihood of macaque-on-human aggression, relatively little is known about how these variables influence retroviral transmission.18
Here we present data describing human exposures to rhesus macaques and infection with SFV at several sites in Bangladesh and in performing monkey owners. Most other studies have focused on a region of the pol gene (integrase), which is highly conserved and thus may not reveal SFV variation found in infected individuals of a species. We have obtained multiple clones from whole blood and sequenced a portion of the SFV gag gene, chosen over pol to reveal greater SFV genetic variation, and used the sequence data for comparison of the virus found in humans to that found in macaques with which they interact. We show that humans can be concurrently infected by multiple SFV strains; some individuals are infected by both an autochthonous (originating where found) SFV strain as well as a strain similar to SFV found in macaques from other geographic areas. Increasing age was a significant risk factor for zoonotic transmission of SFV in humans. Female sex and Hindu religion may also be associated with increased risk. These data combined with our previous results15 suggest that human-facilitated movement of macaques, multiple SFV infections and retroviral recombination all contribute to retroviral diversity in Bangladesh.
MATERIALS AND METHODS
Study sites and populations
The study population included 209 human subjects sampled at five community sites (Sylhet, Bormi, Dhamrai, Narayanganj and Charmaguria), as well as 13 performing monkey owners belonging to a nomadic group who stage performances featuring their trained macaques. The community sites were established in 2007 in locales where macaques range freely and where residents were willing to participate in longitudinal research on cross-species transmission of infectious agents. We sampled the performing monkey owners opportunistically, when we encountered them en route to or from our community study sites. An analysis of the SFV diversity among monkeys from Bangladesh is presented in our companion study.15 The data from that study also form part of the macaque SFV data set investigated in the present study. Here we present a brief description of the field sites (Figure 1) included in this study.
Figure 1.
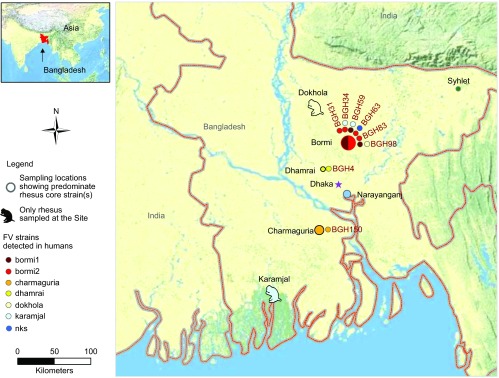
Sampling locations and diversity of SFV strains detected in zoonotically infected human subjects. Human subjects were interviewed and sampled at five urban sites throughout Bangladesh where rhesus macaques were free-ranging. We have recently shown that at each of these sites a core strain(s) of SFV can be detected in the macaques.15 The rhesus SFV core strains are color-coded on this map. For human subjects (denoted by ‘BGH') who were found to be infected with SFV, we obtained multiple clones from whole blood and sequenced a portion of the SFV gag gene. The sequence data were used to compare the virus found in humans to that found in macaques from that site. The color of the circle(s) next to each of the BGH identifiers signifies the SFV strain that was detected in these humans. Note that four of the subjects were concurrently infected by multiple SFV strains and that some individuals are infected by both an autochthonous (originating where found) SFV strain as well as a strain similar to SFV found in macaques from other geographic areas. nks, no known source.
Sylhet
Located in the northeastern part of the country, within 35 km of the border with India's Assam province, Sylhet is the third largest city in Bangladesh. A population of approximately 125 macaques is found at the Shah Jalal shrine in the center of city. Sylhet's macaques range over the shrine site and into the immediately adjacent neighborhood. The macaques of Shah Jalal are regularly provisioned by the shrine staff and often receive food from visitors to the shrine. Fourteen human subjects who live close to the shrine participated in the study.
Bormi
In the town of Bormi, approximately 50 km north of Dhaka, more than 200 rhesus macaques range throughout the village, moving freely between Hindu and Muslim areas, often entering homes in search of food. Although some Bormi residents occasionally offer food to the macaques, there is no organized provisioning. A total of 105 human subjects from Bormi participated in this study.
Dhamrai
The town of Dhamrai is located 40 km southwest of Bormi. Approximately 75–100 macaques range freely through Dhamrai. The macaques favor areas with large shade and fruiting trees, which are more commonly found in Hindu neighborhoods. As in Bormi, there is no organized provisioning of macaques. Nine subjects from Dhamrai were included in this dataset.
Narayanganj
Narayanganj is a densely populated port city approximately 20 km south of Dhaka. The rhesus macaque population continues to decline in this area as urban development displaces green spaces. The estimated 60 macaques in this city are not provisioned and are not well habituated to humans. Thirty-six human subjects from Narayanganj participated in the study.
Charmaguria
Charmaguria is located 150 km south/southwest of Dhaka along the Padma River. The >200 macaques in this area are provisioned by a local organization at a central location, and abundant green areas in this small town provide natural corridors along which the animals range widely. Forty-five human subjects from Charmaguria participated in the study.
Recruitment, informed consent and sampling protocols, including follow-up of SFV positive individuals, have been approved by the University of Washington's Human Subjects Review Board (23055) and an analogous review board at Jahangirnagar University, Bangladesh. Biological and demographic data from human subjects were coded with unique, anonymous identifiers that began with ‘BanGladesh Human' (BGH). At each community site, Bangladeshi team members initiated contact with neighborhood leaders and identified a central location within the macaques' range for data collection. Bangladeshi team members then partnered with community residents and fanned out on foot, requesting the participation of people who identified themselves as having been bitten or injured by macaques. Inclusion criteria for the subjects included age greater than 18, self-reported exposure to NHP and/or long-term (>20 years) residence in the village. Written informed consent was obtained from all subjects. Demographic data and data describing NHP exposures were acquired through a questionnaire administered by a team member in the local language, Bengali. Ten milliliters of whole blood was collected from each subject's antecubital vein in a tube containing ethylene diamine tetraacetic acid, stored on ice for not more than 12 h, then centrifuged. Whole blood and plasma aliquots were stored at −80 °C until analyzed. Whatman Sterile Omni Swabs (Whatman, Piscataway, NJ, USA) were used to acquire buccal mucosal samples. Swabs were immediately placed in 500 µL of Qiagen Buffer RLT (Qiagen, Valencia, CA, USA) cooled on ice for not more than 12 h before being frozen at −80 °C until analyzed. These methods are essentially identical to those we used to acquire and store biological samples derived from macaques.15
Repeat sampling of confirmed SFV positive humans
If a human subject was confirmed SFV positive by polymerase chain reaction (PCR), the research team attempted to contact and resample the subject approximately 1 year after the initial sampling. The biological sample collection protocols for follow-up sampling of the SFV positive subjects were the same used during the initial sampling. In addition, SFV positive subjects received a brief physical exam and an in-depth medical history was collected.
Rhesus macaque sampling
Our companion manuscript for this project15 provides all of the animal sampling and laboratory protocols. SFV gag sequences from a total of 184 rhesus macaques are included in the present study (Figure 2). We also include in this dataset sequences obtained from four rhesus, two from Dhamrai and two from Bormi, that we were able to resample two years after their initial sampling.
Figure 2.
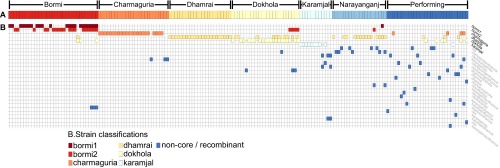
Blood strain classifications for monkeys from sampling populations. For each monkey, a color bar corresponding to the monkeys sampling population is presented in (A), and colored tiles corresponding to strains observed within that monkey's blood sample shown in (B). The non-core strains are labeled according to recombinant relationships established with parental strains. Positive matches are defined by at least 90% likelihood for 200 or more contiguous base pairs in a cBrother analysis. Regions of the genome matching no parental strains above this threshold for at least 200 nt were marked as nks (no known source). This is a simplified version of a figure from the companion paper for this study,15 but with updated strain classifications.
SFV detection
Western blot
Rhesus macaque fibroblast Telo-RF cell line19 was infected with SFV M. mulatta or M. fascicularis, or left uninfected. After 48 h, cells were lysed in 1× sodium dodecyl sulfate sample buffer (50 mM Tris-HCl (pH 6.8), 10% glycerol, 2% sodium dodecyl sulfate, 5% 2-mercaptoethanol, 0.01% bromophenol blue) and samples elec trophoresed through 10% polyacrylamide gels. After transfer of proteins to polyvinylidene difluoride membranes (Millipore Billerica, MA, USA), the membranes were blocked in phosphate-buffered saline+0.05% Tween-20 containing 2% non-fat dry milk, and rinsed with water and left to dry. Strips were made, each containing three lanes (molecular weight marker, uninfected Telo-RF and infected Telo-RF cell lysates) and primary antibody (human plasma) was added at a 1∶20 dilution and incubated overnight at 4 °C. Strips were washed three times in phosphate-buffered saline+0.05% Tween-20 before adding secondary goat anti-human horse radish peroxidase (Thermo Scientific and GE Healthcare Rochester, NY, USA) at a 1∶5000 dilution for 1 h at room temperature. Strips were washed three more times before rinsing with water and applying 3,3′,5,5′-tetramethylbenzidine stabilized substrate for horse radish peroxidase (Promega Madision, WI, USA) to visualize bands. Plasma from BGH4, a known SFV positive human20 was used as a positive control.
DNA isolation from whole blood
Total genomic DNA was extracted from the whole blood of humans using the QIAamp DNA blood mini kit (Qiagen) according to manufacturer's protocol. We extracted DNA from 200 µL of whole blood. The purified DNA was eluted in 200 µL of buffer AE.
PCR amplification of gag and pol fragments from genomic DNA
A total of 15 µL of the isolated DNA was used for PCR amplification of gag or pol fragments by a two round PCR. First round PCR was a multiplex reaction with both gag and pol primers (primer sequence: G1 (+)(nt 114–134, taken from SFV1 GeneBank sequence NC_001364 NC_001736) 5′-AGG ATG GTG GGG ACC AGC TA-3′ G2 (−) (nt 1451–1433) 5′-CAG GAA GAG GTA CTC GGG G-3′ P1 (+) (nt 5179–5200) 5′-GCC ACC CAA GGA AGT TAT GTG G-3′ P2 (−) (nt 5768–5749) 5′-GCT GCC CCT TGG TCA GAG TG)-3′. The reactions were denatured at 95 °C for 3 min, followed by two cycles of 95 °C for 30 s, 38 °C for 1 min, 72 °C for 1 min, then followed by 25 cycles of 95 °C for 30 s, 52 °C for 1 min and 72 °C for 1 min. Water was used as a negative control. 2 µL of the PCR product was used as template for the second round PCR. Either gag or pol primers (G3 (+) (nt 190–212) 5′-CAA CCT AGA TGG AGA GCT GAA GG-3′ G4 (−) (nt 1425–1406) 5′-GGG GAC AAA CTA CGG CTG GG-3′ P5 (+) (nt 5339–5361) 5′-TAA TCC TCC AGG GTA TCC AAA A-3′ P6 (−) (nt 5728–5707) 5′-ATA CCA TCG ACT ACT ACA AGG A-3′) were used to amplify individual fragments. The reactions were denatured at 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 52 °C for 1 min and 72 °C for 1 min. The expected sizes of the PCR products for gag and pol are 1235 bps and 390 bps, respectively.
Cloning and sequencing of gag fragments
The FV PCR products were gel purified using a QIAquick gel extraction kit (Qiagen) according to manufacturer's protocol. The purified fragments were ligated into the TOPO-TA cloning vector (Invitrogen, Carlsbad, CA, USA) and transformed into Escherichia coli TOP10 competent cells (Invitrogen). Multiple single colonies for each gag sample were selected and plasmid DNA was purified and sequenced. Sequencing was carried out using M13 forward and reverse primers, as well as internal forward and reverse primers for gag by the University of Washington Genomics Facility or GeneWiz Inc. (Seattle, WA, USA). Viral strains are denoted using lowercase letters to distinguish them from the geographic sites where the humans or macaques were sampled (e.g., Dhamrai is a study site where the dhamrai core strain was detected.)
Isolation of RNA from buccal swabs
Frozen buccal swab samples in RLT buffer (Qiagen) were thawed at 37 °C for 15 min with 40 U of the RNaseOUT ribonuclease inhibitor (Invitrogen) and 1 U of RNase-free DNase I (Promega) immediately prior to RNA extraction. Total RNA was extracted according to the standard protocol using the RNeasy Mini Kit (Qiagen). RNA was treated again with 40 U of RNaseOUT and 1 U of RNase-free DNase I at 37 °C for 1 h. Samples were then incubated at 65 °C for 15 min to inactivate enzymes. RNA was stored at −20 °C for subsequent use.
Reverse transcription-PCR (RT-PCR) of buccal swab RNA
RT-PCR was performed to clone partial gag sequences (1235 bp) from buccal swab RNA. First-strand cDNA was synthesized using the Thermoscript RT-PCR system (Invitrogen) according to the manufacturer's protocol using an Oligo(dT)18 primer. The RT reactions were inactivated at 85 °C for 5 min. The resulting cDNAs were used as template for two round PCR as described above for whole blood FV gag amplification.
Analysis
Clustering methods and hypermutation analysis
Sequences under examination in this study exhibited evidence of APOBEC3-mediated hypermutation. A thorough analysis of this activity is presented in Matsen et al. (unpublished). This analysis produced putatively hypermutation negative sequence alignments by removing columns suspected of hypermutation. These alignments were used for the remainder of the analyses in this study, in order to avoid conflation of hypermutation and genuine phylogenetic signal.
Phylogenetic analyses
A phylogenetic tree was constructed from the putatively hypermutation-free alignment using FastTree 2.1.7.21,22 This tree was visualized using Archeopteryx (https://sites.google.com/site/cmzmasek/home/software/archaeopteryx). From the same alignment, SplitsTree 4.12.823 was used to produce a splits network.
Strain analysis
Strains were identified by passing the hypermutation-negative alignment through an iterative recentering clustering algorithm. It was found that a single application of UCLUST v1.124 produced poor clustering results due to the greedy nature of the algorithm. An iterative recentering algorithm, suggested by the UCLUST author at http://drive5.com/usearch/manual/recenter.html, was implemented which dealt well with this issue. For each round of clustering, consensus sequences from the prior round were added to the top of an ungapped alignment, in order of cluster size from greatest to least. For this iteration, clustering was carried out using cluster_smallmem, producing new consensus sequences and clusters. This process was repeated twice. Cluster assignments were further fine-tuned by a script that found the true centroid of each cluster, as defined by the sequence with minimal average normalized Hamming distance to every other sequence in the cluster. The script was then checked to make sure that each sequence clustered with the centroid to which it was closest (again, as defined by normalized Hamming distance), making reassignments as necessary.
This iterative algorithm was applied using a threshold of 97.77%. This threshold, while slightly higher than that used in our companion study,15 better reflects the somewhat lower amount of sequence diversity as a result of removing columns suspected of hypermutation. As mentioned above, viral strains are denoted using lowercase letters to distinguish them from the geographic sites where the humans or macaques were sampled (e.g., Dhamrai is a study site where the dhamrai core strain was primarily found.)
Statistical methods
Statistical analysis of demographic variables was performed using the JMP (SAS Institute, Cary, NC, USA) statistical software package; analysis of contingency tables was performed with the R fisher.test function.25
RESULTS
Demographic data including NHP exposures
Over a 5-year period, 2007–2011, biological samples were collected from 222 people. The majority of the subjects (n=209) were sampled from five urban sites and a sixth group (n=13), comprised of nomadic people who travel the country with their performing monkeys, were sampled opportunistically as the research team traveled between sites (Figure 1). Data summarizing the demographic characteristics of the study population and reported bites are presented in Table 1. Overall, 54.2% of male and 54.0% of female subjects reported having been bitten at least once by a rhesus macaque, but the percentage of subjects reporting having been bitten at each site varied significantly by subjects' sex, age class and religion. For example, while >75% of Bormi women reported a bite, only 5.9% of Narayanganj women did.
Table 1. Demographic and bite exposure characteristics of the sample population.
| Site | n | Sex | Age | Bittena by sex | Bitten by age classb | NO of bitesc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | 2 | 3 | 4 | 5 | 6 | 7 | 0 | 1 | >1 | |||
| Bormi | 105 | 40 | 65 | 47.1±17.3 | 24/40 | 50/65 | ND | 11/15 | 20/24 | 14/19 | 11/23 | 18/24 | 31/105 | 55/105 | 19/105 |
| (60.0%) | (76.9%) | (73.3%) | (83.3%) | (73.7%) | (47.8%) | (75%) | (29.5%) | (52.4%) | (18.1%) | ||||||
| Charmaguria | 45 | 25 | 20 | 36.9±16.1 | 10/25 | 10/20 | 2/5 | 8/16 | 1/7 | 3/5 | 3/3 | 3/6 | 25/45 | 16/45 | 4/45 |
| (40.0%) | (50%) | (40%) | (50%) | (14.3%) | (60%) | (100%) | (50%) | (55.6%) | (35.6%) | (8.9%) | |||||
| Dhamrai | 9 | 5 | 4 | 30.2±10.9 | 4/5 | 4/4 | 2/2 | 3/3 | 1/2 | 1/1 | 1/1 | ND | 1/9 | 6/9 | 2/9 |
| (80%) | (100%) | (100%) | (100%) | (50%) | (100%) | (100%) | (11.1%) | (66.7%) | (22.2%) | ||||||
| Narayanganj | 36 | 2 | 34 | 40.0±14.6 | 0/2 | 2/34 | ND | 0/8 | 1/10 | 1/11 | 0/2 | 0/5 | 34/36 | 2/36 | 0/36 |
| (0%) | (5.9%) | (0%) | (10%) | (9.1%) | (0%) | (0%) | (94.4%) | (5.6%) | (0%) | ||||||
| Syhlet | 14 | 12 | 2 | 33.3±7.9 | 2/12 | 1/2 | ND | 0/16 | 3/4 | 0/4 | ND | ND | 11/14 | 2/14 | 1/14 |
| (16.7%) | (50%) | (0%) | (75%) | (0%) | (78.6%) | (14.3%) | (7.1%) | ||||||||
| Performing | 13 | 12 | 1 | 26.8±7.3 | 12/12 | 1/1 | 2/2 | 8/8 | 2/2 | 1/1 | ND | ND | 0/13 | 1/13 | 12/13 |
| (100%) | (100%) | (100%) | (100%) | (100%) | (100%) | (0%) | (7.7%) | (92.3%) | |||||||
| Total | 222 | 96 | 126 | 41.1±16.7 | 52/96 | 68/126 | 6/9 | 30/56 | 28/49 | 20/41 | 15/32 | 21/35 | 102/222 | 82/222 | 38/222 |
| (54.2%) | (54.0%) | (66.7%) | (53.6%) | (57.1%) | (48.8%) | (46.9%) | (60%) | (45.8%) | (36.9%) | (17.1%) | |||||
Bites were self-reported.
Age class 2 (18–19 years old); 3 (20–29 years old); 4 (30–39 years old); 5 (40–49 years old); 6 (50–59 years old); 7 (>60 years old).
Subjects reported whether they had never been bitten (0); bitten once (1); or bitten more than one (>1).
SFV infected humans
Samples from 18 subjects were reactive on Western blot (data not shown). Eleven humans were confirmed to be infected by PCR using pol primers (molecular evidence of proviral DNA) with SFV (Table 2). We were able to isolate and sequence multiple individual SFV gag clones from eight of these subjects. Four of the SFV gag-positive subjects consented to follow-up sampling approximately 1 year after their initial sampling. The remaining SFV positive subjects were either not located or refused follow-up sampling. None of the spouses or children of the SFV positive subjects consented to take part in this study.
Table 2. Characterization of SFV PCR+ subjects.
| Human subject | PCR+ | NO of clones/strain type at initial sampling | NO of clones/strain type at repeat sampling <14 months later | Sex/age class | Site | Context | Reported parenteral exposures | Scar | Location of wound |
|---|---|---|---|---|---|---|---|---|---|
| BGH4 | gag | 4/dhamrai | N/A | Female/2 | Dhamrhai | Lifelong village resident | Severe bite; 15 years prior to sampling | Yes | Lower extremity |
| BGH31 | gag | 6/bormi2 | N/A | Female/3 | Bormi | Lifelong village resident | 2 severe bites; 11 years prior to sampling | Yes | Upper and Lower extremities |
| BGH34 | gag | 5/bormi2 | 3/karamjal | Female/5 | Bormi | Lifelong village resident | 3 severe bites; frst bite 46 years prior to sampling. Most recent 2 years prior to sampling | Yes | Lower extremity and torso |
| BGH59 | gag | 13/bomi14/karamjal | 2/bormi1 | Female/7 | Bormi | Village resident for 50 years | Severe bite; 2 years prior to sampling. Scratched | Yes | Upper extremity |
| BGH63 | gag | 4/bormi22/nks.bgh63 | 9/nks.bgh63 | Female/5 | Bormi | Lifelong village resident | Bite 1 year ago | No | Upper extremity |
| BGH83 | gag | 6/bormi2 | 10/bormi2 | Female/4 | Bormi | Village resident for 21 years | 3 severe bites; first bite 12 years prior to sampling. Most recent 1 year prior to sampling | Yes | Upper extremity and torso |
| BGH98 | gag | 2/bormi12/dokhola | N/A | Female/7 | Bormi | Village resident for 30 years | Severe bite; 4 years prior to sampling | Yes | Upper extremity |
| BGH150 | gag | 5/charmaguria | N/A | Male/ 6 | Charmaguria | Lifelong village resident | 2 severe bites; 1 year prior to sampling | Yes | Upper extremity |
| BGH100 | pol | 3 | N/A | Male/7 | Bormi | Village resident for 8 years | No reported exposure. | No | Not applicable |
| BGH101 | pol | 6 | 4 | Female/5 | Bormi | Village resident for 45 years | 3 severe bites, first bite 40 years prior to sampling. Most recent 1 year prior to sampling | Yes | Lower extremity and torso |
| BGH 209 | pol | 5 | N/A | Female/5 | Narayanganj | Village resident for 30 years | No reported exposure. 30 years in Narayanganj | No | Not applicable |
Abbreviations: PCR, polymerase chain reaction; SFV, simian foamy virus.
Although most of the SFV positive subjects reported a severe bite in the past, two did not recall ever having been bitten or even scratched by a monkey. They did, however, indicate that they had fed monkeys and that monkeys would come into their compounds. None of the SFV positive subjects reported owning a pet monkey, hunting monkeys or butchering or consuming monkey meat. These data are summarized in Table 2. Fisher tests, not corrected for five multiple comparisons, suggest that in this opportunistically sampled group, SFV positive subjects were more likely to be female (P=0.024) and Hindu (P=0.023). However, these differences were not significant after Bonferroni correction (P=0.12 for both). Logistic regression analysis revealed a positive correlation between age and PCR-positive status (P=0.0085). No other significant associations were found between persistent SFV infection and other factors under this regression analysis. None of the performing monkey owners or the subjects from Syhlet were PCR-positive. Although the majority (8 of 11) of confirmed SFV positive subjects were from Bormi, after controlling for bite frequency, any association between geographic location and infection was not significant (P=0.165). These data are summarized in Table 3.
Table 3. Demographic characteristics of SFV prevalence in human subjects.
| Number of human subjects | PCR neg | PCR pos | PCR pos% | |
|---|---|---|---|---|
| Field site | ||||
| Bormi | 105 | 97 | 8 | 7.6% |
| Charmaguria | 45 | 44 | 1 | 2.2% |
| Dhamrai | 9 | 8 | 1 | 11.1% |
| Narayanganj | 36 | 35 | 1 | 2.8% |
| Syhlet | 14 | 14 | 0 | 0% |
| Performing | 13 | 13 | 0 | 0% |
| Age classa | ||||
| 18–19 (2) | 9 | 8 | 1 | 11.1% |
| 20–29 (3) | 56 | 56 | 0 | 0% |
| 30–39 (4) | 49 | 48 | 1 | 2.0% |
| 40–49 (5) | 41 | 40 | 1 | 2.4% |
| 50–59 (6) | 32 | 28 | 4 | 12.5% |
| >60 (7) | 35 | 31 | 4 | 11.4% |
| Sex | ||||
| Male | 96 | 94 | 2 | 2.1% |
| Female | 126 | 117 | 9 | 7.1% |
| Bleeding | ||||
| Yes | 118 | 109 | 9 | 7.6% |
| No | 103 | 101 | 2 | 2.0% |
| Bite location | ||||
| Upper extremity | 74 | 67 | 7 | 9.5% |
| Lower extremity | 36 | 33 | 3 | 8.3% |
| Torso | 42 | 39 | 3 | 7.7% |
| Ever bitten | ||||
| Yes | 120 | 111 | 9 | 7.5% |
| No | 102 | 100 | 2 | 2.0% |
| Religion | ||||
| Hindu | 80 | 73 | 7 | 8.8% |
| Muslim | 142 | 138 | 4 | 2.8% |
Abbreviations: PCR, polymerase chain reaction; SFV, simian foamy virus.
Number in () refers to the age class.
SFV gag sequence diversity in infected humans
We have recently shown that 1235 nucleotides of the SFV gag gene can differentiate SFV strains in Bangladeshi rhesus macaques from our sampling sites (Figure 2).15 In that study, the overall SFV gag sequence diversity was investigated using phylogenetics and formalized into strain definitions through a clustering algorithm. Among these strains, six were found to account for the majority of the sequence diversity found and strongly cosegregated with the monkey populations. These were identified as core strains.
In the present study, DNA was extracted from blood of SFV positive humans and the same portion of SFV gag was PCR-amplified, cloned and sequenced. Table 2 indicates the number of clones obtained and sequenced from each subject. With the exception of one subject (BGH63 described below), all sequences obtained from humans matched one of the six core strains (bormi1, bormi2, dokhola, dhamrai, charmaguria, karamjal) that we had previously characterized in the monkeys (Figures 3 and 4).
Figure 3.
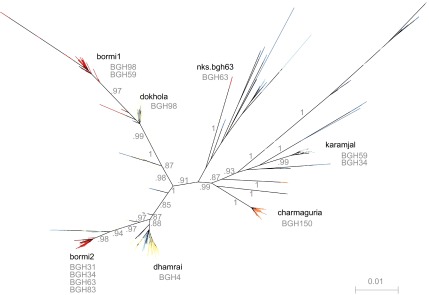
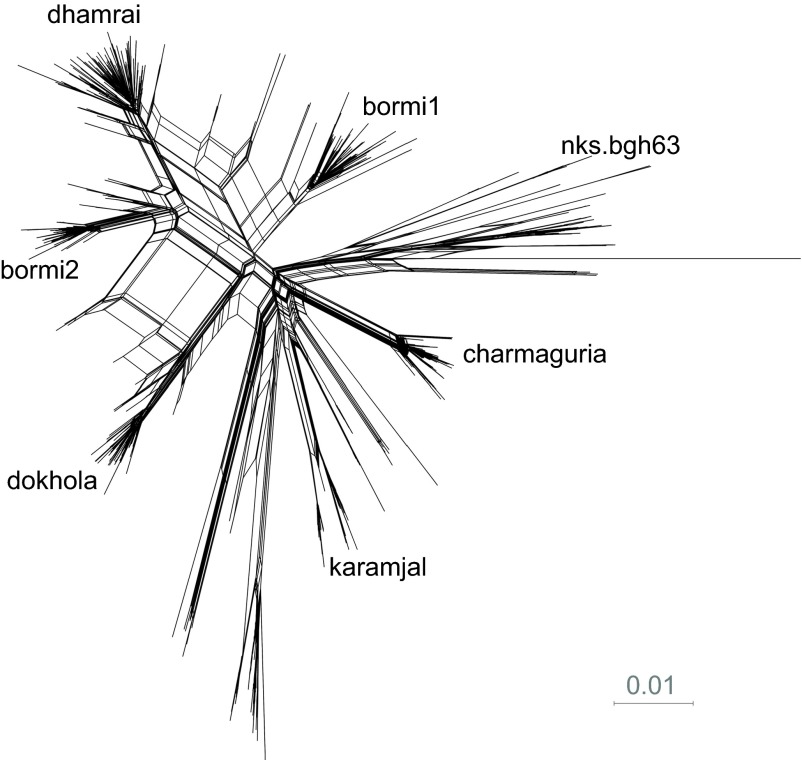
Foamy virus sequence diversity overview of infected human subjects. Phylogenetic tree built from gag nucleotide clones using FastTree. Due to recombination, this tree should not be interpreted as an evolutionary history, but as an indication of the tight clustering seen in the data. Edge labels show SH-like support values. IDs of SFV positive humans are displayed beneath strain labels of the strains with which they were infected. ID, identity; SFV, simian foamy virus; SH, Shimodaira–Hasegawa.
Figure 4.
Splits network giving an overview of sequence diversity. Neighbor-net splits network built by SplitsTree4 showing the tight clustering and recombination observed in the sequence data. In a splits network, sets of parallel edges represent ‘splits': an aspect of the data that separates one collection of sequences from another. Pairs of splits that contradict each other, as is expected when recombinant sequences are present, are displayed as paralleograms.
Each of the SFV positive humans harbored a core strain that was abundant in the free-ranging macaque population associated with their community (Figures 2 and 3). For example, the SFV gag from BGH150, a man born and living in Charmaguria who reported two severe bites within a year of being sampled, has a virus with SFV gag similar to that sequenced from Charmaguria rhesus macaques. The SFV gag of virus from BGH4, a woman bitten as a young child in Dhamrai, was most similar to the dhamrai SFV gag core strain. BGH83 and BGH31 had each been bitten multiple times by rhesus macaques in their village of Bormi and were infected with the bormi1 core strain that was detected in these macaques.
The analysis of 15 clones obtained from BGH63, a lifelong resident of Bormi, revealed infection by two strains, one of which clustered with the bormi2 core strain and the other separately from all other sequences. A cBrother26 recombination analysis was not able to establish a relationship between this strain and any of the strains circulating in the rhesus populations that we have sampled in Bangladesh; this sequence also sits far from other strains in the phylogenetic tree. As such, this strain was labeled nks.bgh63 (no known source).
There were three other subjects, BGH34, BGH59 and BGH98, all from Bormi, who were dually infected and had an SFV strain not found in their local macaque population. BGH59 and BGH34 were infected with bormi1 and bormi2, respectively, and co-infected with the karamjal strain. BGH98 was infected with both the bormi1 strain and with the dokhola strain. And as noted above BGH63 was infected with the bormi2 and nks.bgh63 strains. All four of these multiply infected women reported being long-term residents of Bormi, and bitten only by the monkeys ranging in their village.
Serial sampling of SFV infected humans
Four of the SFV positive subjects consented to follow-up blood sampling within 14 months of their initial collection. BGH83 had been bitten multiple times during the >20 years she had lived in Bormi. SFV gag sequences from both time points (separated by 12 months) for BGH83 clustered with the bormi2 core strain. BGH34, born in Bormi, was bitten in 1961, 2005 and 2006. All of these bites left scars. SFV gag sequences from the initial time point sampling of BGH34 in 2007 showed that all five clones were classified as bormi2. When BGH34 was interviewed and resampled 14 months later, she did not report any new bites or scratches; however, the SFV gag sequences from all three clones were classified as karamjal. BGH59 reported a severe bite from a Bormi monkey in 2006. When she was sampled in 2008, 13 of her 17 clones were classified as bormi1 and four clones were classified as karamjal. Twelve months later, BGH59 was resampled. Only two clones were successfully sequenced from her at this time point and both were classified as bormi1. Six clones were sequenced from BGH63 following her initial sampling in 2008. The bormi2 core strain was detected from four of the clones and the remaining two clones were of the nks.bgh63 strain. When BGH63 was sampled 12 months later, all nine clones were identified as the divergent nks.bgh63 strain.
No SFV replication detected in buccal samples from infected humans
We have described17 a quantitative RT-PCR assay for FV RNA, and used this to measure FV RNA levels in Bangladeshi macaques (Soliven et al., unpublished). Using this assay, the limit of detection of FV RNA was 0.5 copies per cell equivalent determined by 18S ribosomal RNA content. We used this assay to determine whether we could detect FV RNA in buccal swabs obtained from infected humans. Although most infected macaques have detectable levels of FV RNA from buccal swabs, we could not detect FV RNA in any of the human buccal swab samples.
DISCUSSION
Interest in studying simian-borne retroviruses derives largely from the enormous impact HIV has had on human health. There is much to learn about how retroviruses are transmitted across species barriers, as well as how these retroviruses and their hosts interact and adapt after infection has taken place.27,28,29,30 At present, SFV is the best natural model for studying cross-species transmission of retroviruses from NHP to humans, in part because zoonotic transmission of SFV has been documented more frequently than any other known simian-borne virus.3
Human/NHP interface in Asia differs from that in Africa
The data presented here expand our knowledge of cross-species transmission of SFV in Asia. Human subjects in the present study all came into contact with rhesus macaques in the context of their daily lives, sharing a geographical area that is the ‘home range' of both primate populations. The towns and villages that constituted research sites for our study are likely similar to hundreds or even thousands of sites throughout Asia, where humans live alongside synanthropic macaques. In these urban locations, where human and macaque populations overlap, conflicts over access to resources, particularly food, often lead to interspecific aggression. However, these interactions are generally tempered by cultural attitudes, as well as environmental and economic realities.31,32,33 In contrast to Africa, where bushmeat hunting and habitat degradation threaten to deplete NHP populations over the next decades,34,35 some synanthropic primate populations in Asia have actually thrived, and continue to be sustainable, in spite of the pressures presented by increasing human populations.36,37,38,39
Humans who hunt apes for bushmeat can receive bites that are dramatic, disfiguring and thus difficult to forget. In contrast, exposure to macaques in the Asian village setting may be more subtle. When monkeys are a ‘normal' part of the urban fauna, a monkey bite that barely breaks the skin, occurring in the context of decades of close proximity to monkeys, may be dismissed as inconsequential. Macaque aggression is often directed at children, likely because children, by virtue of their smaller size, are perceived as less threatening by macaques. Adults may not remember an exposure suffered as a child. Recall bias may thus explain why two of the SFV positive subjects in this study reported that they had never been bitten, or even scratched, by a monkey. Contact between macaque saliva and mucous membranes (e.g., through grooming) is another possible explanation for infection of SFV positive individuals who deny a previous bite or scratch. Other routes of infection, including human-to-human transmission of SFV, have never been shown and are unlikely.
Our recruitment methods (recruiting volunteer subjects who lived in areas where human/NHP contact was the greatest) prevent us from measuring, comparing and extrapolating risk of bite injury and SFV infection to the general population of the areas sampled in Bangladesh. However, within our sampled population, increasing age was found to be associated with increased risk of persistent SFV infection. While this can be explained by cumulative risk over time, it is also possible that immune factors may contribute to this relationship. Sampling bias could also explain this observation. Further research with different recruitment strategies and larger sample sizes are needed to determine which viral and host and exposure characteristics most influence the likelihood of cross-species transmission.
Detection of multiple strains of SFV in infected humans and the role of macaque translocation
Four of the Bormi human subjects were found to be infected with two distinct strains of SFV. While our previously published research showed that infection with two strains of SFV occurs in about one-fourth of adult rhesus macaques sampled in Bangladesh,15 this is the first such demonstration in infected humans. It is possible that these individuals were infected simultaneously from exposure to a macaque with a dual infection, or, alternatively, that two separate exposures occurred. We note that three of the four multiply infected humans were infected with pairs of viruses that were not seen in any of our multiply infected monkeys (Figure 2).
The finding that primates, both human and non-human, can be infected with more than one strain of SFV is significant because co-infection may lead to viral recombination. Indeed, our group has previously reported finding recombination among viruses in Bangladeshi macaques.15 Our discovery of human APOBEC3-mediated hypermutation activity in humans against SFV in vivo suggests that replication, and consequently recombination, may be possible in humans (Matsen et al., unpublished).
The data we present also emphasize the importance of human actions in shaping the population genetics of both macaques and the viruses they harbor and transmit to humans. Out of the six SFV positive humans from Bormi, four had SFV strains which were not reflective of the two dominant strains found in the local monkey population (Figure 2). Of particular interest are two individuals infected with the karamjal SFV circulating among rhesus macaques in Karamjal, a site more than 200 km south of Bormi in the Sundarbans mangrove forest (Figure 1). Outside of Karamjal, this strain was only found in the performing monkeys, sampled hundreds of kilometers away from Karamjal.15 There is no contiguous NHP habitat bridging these distant locations. However, the performing monkey owners reported that they often trapped monkeys in the Sundarbans and occasionally, a performing monkey would escape or be released in a village where they were performing. Collectively, these findings suggest that human activity is responsible for the presence of this Karamjal strain in both humans from Bormi.
There has been no report to date of free-ranging African NHPs or humans concurrently infected with more than one strain of SFV. A possible explanation for this observation may lie in the differing ecologies of Asian macaques and African apes. While translocation via humans has shaped the population structures of many of the macaque groups that we have studied in Bangladesh, it is unlikely that analogous forces are acting to bring together African ape populations, whereby SFV strains from one population can be introduced into another. Additionally, fragmentation of NHP habitat in Africa has increasingly left groups of NHP isolated from one another. Recent data characterizing population genetics in African chimpanzees provide evidence that habitat loss has resulted in high within-group SFV homogeneity.40 As a result, African NHP may provide an inefficient reservoir for SFV recombination, whereas in Asia, frequent translocation may result in retroviral recombination between very distinct strains.
SFV infected subjects sampled at two time points
Data from serial samples of the SFV positive subjects, both human and macaque, resampled at a second time point, suggested that the relative prevalence of the strains may change over time in individuals infected with more than one viral strain. For example, when BGH63 was first sampled, two of the six clones obtained were of the nks.bgh63 strain. At the second time point, a year later, all nine clones clustered with nks.bgh63. In BGH34, all five clones at initial sampling were grouped with the bormi2 core strain, but 14 months later, the three SFV clones from her blood clustered with the karamjal core strain. It is likely that our experimental methods and relatively small sample sizes may have led to an incomplete appreciation of the SFV strains present in a given individual. Deep sequencing of samples taken at different time points will be required to determine whether certain strains can be cleared by the host.
Conclusion
Our results add new considerations to what is known about zoonotic transmission of SFV in South Asia and highlight the rich complexity of the human/NHP interface in this region. Human agency is often responsible for the translocation of macaques from region to region in South Asia; evidence of this movement is seen in both the genetic makeup of the macaques and the viral strains that they harbor and transmit to humans. Nearly all of the SFV infected humans in this study exhibited evidence of APOBEC3-mediated hypermutation. These results, detailed in Matsen et al., unpublished, confirm that at least one round of SFV replication occur in infected humans, making them potential mixing vessels for viral recombination when infected with more than one strain. SFV has co-evolved with NHP for at least 30 million years, and the presumed lack of SFV pathogenicity in NHP has been attributed to this long term co-speciation of virus and host.2 However, the past decades have witnessed massive increases in the numbers of and mobility of humans in Asia, and these forces are now bringing NHP-borne infectious agents together at an increased rate. It is not known whether these forces will lead to the appearance of SFV strains with new phenotypic characteristics, including the ability to be transmitted from human to human and to cause disease. However, the possibility suggests that continued monitoring of the virus at the human/NHP interface is warranted. Regardless of whether SFV becomes a significant pathogen, it remains our best natural model of how infectious agents are transmitted across the human/NHP interface.
Acknowledgments
The authors wish to thank the villagers throughout our study sites who have graciously allowed us into their homes and who continue to support this research. This work would not be possible without the efforts of our student assistants and faculty in the Wildlife Branch of the Department of Zoology at Jahangirnagar University, Bangladesh. We also thank the Bangladesh Forest Department for their permission and constant support of our research program. Dr John Heidrich (Ventana Animal Clinic, Albuquerque, NM, USA) and Dr Michael Schillaci (University of Toronto Scarborough, Toronto, Ont., Canada), as well as Hanna and Leah Engel (Team monkey, Seattle, WA, USA) and Lynn Johnson (National Geographic Magazine, Washington, DC, USA), were outstanding assistants during multiple field seasons. We thank Robin Watanabe (University of Washington, Seattle, WA, USA), Connor McCoy, Leah Engel and Brandon Duffy (Fred Hutchinson Cancer Research Center, Seattle, WA, USA), for their laboratory technical assistance and discussions and Amanda Zeller (University of Washington, Seattle) for administrative support. We are grateful to Dr Harmit Malik and Dr Michael Emerman (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) for their insightful suggestions and comments. This research was supported by funding from NIH-NIAID grants R01 AI078229, R01AI078229-03S1, R03 AI064865, NIH-NCI grant CA18282, NIH-NCRR grant P51 RR000166 and New Development Institutional Support from the Fred Hutchinson Cancer Research Center.
References
- Meiering CD, Linial ML. Historical perspective of foamy virus epidemiology and infection. Clin Microbiol Rev. 2001;14:165–176. doi: 10.1128/CMR.14.1.165-176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer WM, Salemi M, Shanmugam V, et al. Ancient co-speciation of simian foamy viruses and primates. Nature. 2005;434:376–380. doi: 10.1038/nature03341. [DOI] [PubMed] [Google Scholar]
- Gessain A, Rua R, Betsem E, Turpin J, Mahieux R. HTLV-3/4 and simian foamy retroviruses in humans: discovery, epidemiology, cross-species transmission and molecular virology. Virology. 2013;435:187–199. doi: 10.1016/j.virol.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabbaz RF, Heneine W, George JR, et al. Infection of a laboratory worker with simian immunodeficiency virus. N Engl J Med. 1994;330:172–177. doi: 10.1056/NEJM199401203300304. [DOI] [PubMed] [Google Scholar]
- Lerche NW, Switzer WM, Yee JL, et al. Evidence of infection with simian type D retrovirus in persons occupationally exposed to nonhuman primates. J Virol. 2001;75:1783–1789. doi: 10.1128/JVI.75.4.1783-1789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer WM, Bhullar V, Shanmugam V, et al. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J Virol. 2004;78:2780–2789. doi: 10.1128/JVI.78.6.2780-2789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish ML, Wolfe ND, Ndongmo CB, et al. Central African hunters exposed to simian immunodeficiency virus. Emerg Infect Dis. 2005;11:1928–1930. doi: 10.3201/eid1112.050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel G, Hungerford LL, Jones-Engel L, et al. Risk assessment: a model for predicting cross-species transmission of simian foamy virus from macaques (M. fascicularis) to humans at a monkey temple in Bali, Indonesia. Am J Primatol. 2006;68:934–948. doi: 10.1002/ajp.20299. [DOI] [PubMed] [Google Scholar]
- Switzer WM, Shaohua T, Ahuka-Mundeke S, et al. Novel simian foamy virus infections from multiple monkey species in women from the Democratic Republic of Congo. Retrovirology. 2012;9:100. doi: 10.1186/1742-4690-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boneva RS, Switzer WM, Spira TJ, et al. Clinical and virological characterization of persistent human infection with simian foamy viruses. AIDS Res Hum Retroviruses. 2007;23:1330–1337. doi: 10.1089/aid.2007.0104. [DOI] [PubMed] [Google Scholar]
- Oberste MS, Feeroz MM, Maher K, et al. Characterizing the picornavirus landscape among synanthropic nonhuman primates in Bangladesh, 2007–2008. J Virol. 2013;87:558–571. doi: 10.1128/JVI.00837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Feeroz MM, Maher K, et al. Naturally acquired picornavirus infections in nonhuman primates at the Dhaka Zoo. J Virol. 2013;87:572–580. doi: 10.1128/JVI.00838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EA, Engel GA, Feeroz MM, et al. Influenza virus infection in nonhuman primates. Emerg Infect Dis. 2012;18:1672–1675. doi: 10.3201/eid1810.120214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K, Aziz MA, Alam SM, et al. Distribution of rhesus macaques (Macaca mulatta) in Bangladesh: inter-population variation in group size and composition. Primate Conserv. 2013;26:115–124. [Google Scholar]
- Feeroz MM, Soliven K, Small CT, et al. Population dynamics of rhesus macaques and associated foamy virus in Bangladesh. Emerg Microbes Infect. 2013;2:e29. doi: 10.1038/emi.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury MSH, Halim M, Miah M, et al. Research communication: biodiversity use through harvesting faunal resources from forests by the Mro tribe in the Chittagong Hill Tracts, Bangladesh. Int J Biodiversity Sci Manage. 2007;3:56–62. [Google Scholar]
- Murray SM, Picker LJ, Axthelm MK, Linial ML. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. J Virol. 2006;80:663–670. doi: 10.1128/JVI.80.2.663-670.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes A, Gamerl S. Disproportionate participation by age/sex classes in aggressive interactions between long-tailed macaques (Macaca fascicularis) and human tourists at Padangtegal monkey forest, Bali, Indonesia. Am J Primatol. 2005;66:197–204. doi: 10.1002/ajp.20138. [DOI] [PubMed] [Google Scholar]
- Kirchoff V, Wong S, St JS, Pari GS. Generation of a life-expanded rhesus monkey fibroblast cell line for the growth of rhesus rhadinovirus (RRV) Arch Virol. 2002;147:321–333. doi: 10.1007/s705-002-8322-9. [DOI] [PubMed] [Google Scholar]
- Jones-Engel L, May CC, Engel GA, et al. Diverse contexts of zoonotic transmission of simian foamy viruses in Asia. Emerg Infect Dis. 2008;14:1200–1208. doi: 10.3201/eid1408.071430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN. DPAAP FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. Vienna; R Foundation for Statistical Computing; 2012. [Google Scholar]
- Fang F, Ding J, Minin VN, Suchard MA, Dorman KS. cBrother: relaxing parental tree assumptions for Bayesian recombination detection. Bioinformatics. 2007;23:507–508. doi: 10.1093/bioinformatics/btl613. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith JO, George D, Pepin KM, et al. Epidemic dynamics at the human–animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engering A, Hogerwerf L, Slingenbergh J. Pathogen–host–environment interplay and disease emergence. Emerg Microbes Infect. 2013;2:e5. doi: 10.1038/emi.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli S, Peeters M. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS. 2012;26:659–673. doi: 10.1097/QAD.0b013e328350fb68. [DOI] [PubMed] [Google Scholar]
- Jones-Engel L, Engel GA, Heidrich J, et al. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg Infect Dis. 2006;12:900–906. doi: 10.3201/eid1206.060030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes A. Human culture and monkey behavior: assessing the contexts of potential pathogen transmission between macaques and humans. Am J Primatol. 2006;68:880–896. doi: 10.1002/ajp.20295. [DOI] [PubMed] [Google Scholar]
- Fuentes An, Kalchik S, Gettler L, Kwiatt A, Konecki M, Jones-Engel L. Characterizing human–macaque interactions in Singapore. Am J Primatol. 2008;70:879–883. doi: 10.1002/ajp.20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquilli S, Abedi-Lartey M, Amsini FL, et al. Lack of conservation effort rapidly increases African great ape extinction risk. Conserv Lett. 2012;5:48–55. [Google Scholar]
- Walsh PD, Abernethy KA, Bermejo M, et al. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422:611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- Southwick CH, Siddiqi MF. Population status of nonhuman primates in Asia, with emphasis on rhesus macaques in India. Am J Primatol. 1994;34:51–59. doi: 10.1002/ajp.1350340110. [DOI] [PubMed] [Google Scholar]
- Southwick C, Siddiqi MF.Rhesus commensalism in India: problems and prospectsIn: PattersonJD, Wallis J (eds.)Commensalism and conflict: human–primate interface Norman, OK; American Society of Primatologists; 2005240–257. [Google Scholar]
- Malaivijitnond S, Hamada Y. Current situation and status of Long-tailed macques (Macaca fascicularis) in Thailand. Nat Hist J Chulalongkom Univers. 2008;8:185–204. [Google Scholar]
- Wong CL, Ni IH. Population dynamics of the feral macaques in the Kowloon Hills of Hong Kong. Am J Primatol. 2000;50:53–66. doi: 10.1002/(SICI)1098-2345(200001)50:1<53::AID-AJP5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Blasse A, Calvignac-Spencer S, Merkel K, et al. Mother-offspring transmission and age-dependent accumulation of simian foamy virus in wild chimpanzees. J Virol. 2013;87:5193–5204. doi: 10.1128/JVI.02743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]