Abstract
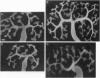
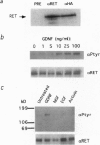
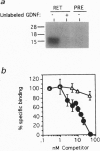
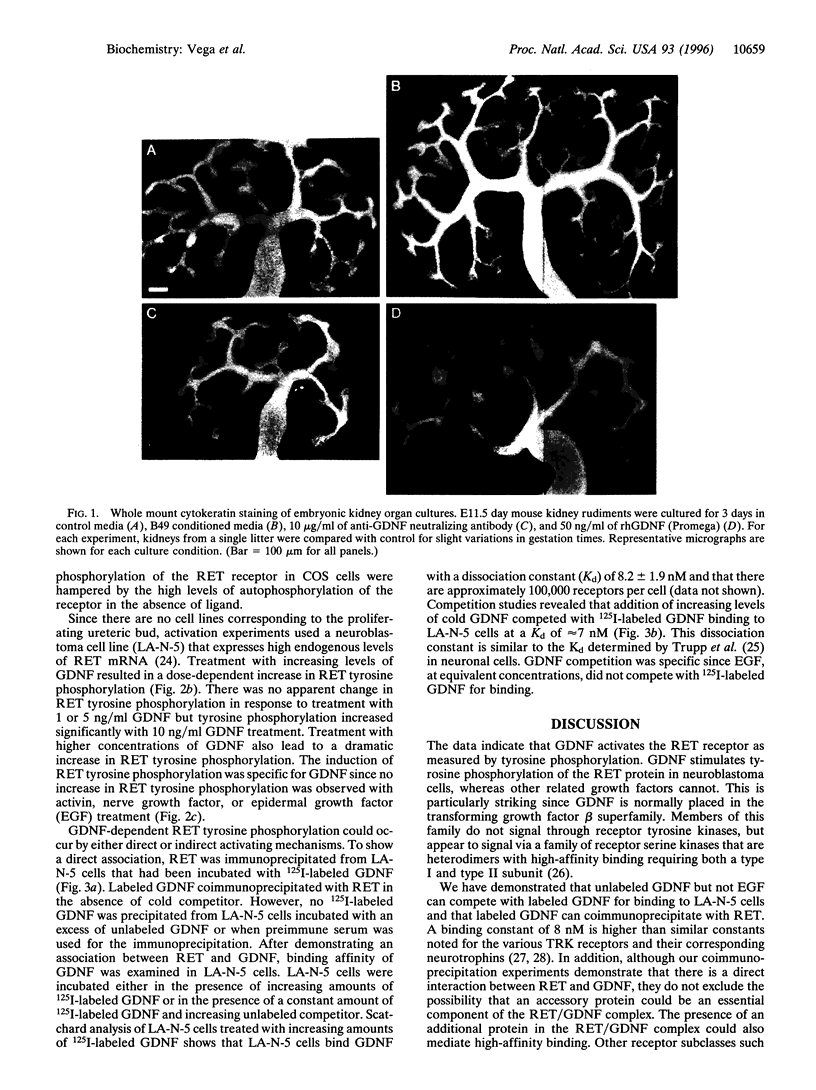
The receptor tyrosine kinase RET functions during the development of the kidney and the enteric nervous system, yet no ligand has been identified to date. This report demonstrates that the glial cell line-derived neurotrophic factor (GDNF) activates RET, as measured by tyrosine phosphorylation of the intracellular catalytic domain. GDNF also binds RET with a dissociation constant of 8 nM, and 125I-labeled GDNF can be coimmunoprecipitated with anti-RET antibodies. In addition, exogenous GDNF stimulates both branching and proliferation of embryonic kidneys in organ culture, whereas neutralizing antibodies against GDNF inhibit branching morphogenesis. These data indicate that RET and GDNF are components of a common signaling pathway and point to a role for GDNF in kidney development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avantaggiato V., Dathan N. A., Grieco M., Fabien N., Lazzaro D., Fusco A., Simeone A., Santoro M. Developmental expression of the RET protooncogene. Cell Growth Differ. 1994 Mar;5(3):305–311. [PubMed] [Google Scholar]
- Baumann H., Symes A. J., Comeau M. R., Morella K. K., Wang Y., Friend D., Ziegler S. F., Fink J. S., Gearing D. P. Multiple regions within the cytoplasmic domains of the leukemia inhibitory factor receptor and gp130 cooperate in signal transduction in hepatic and neuronal cells. Mol Cell Biol. 1994 Jan;14(1):138–146. doi: 10.1128/mcb.14.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995 Aug 31;376(6543):768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Bunone G., Borrello M. G., Picetti R., Bongarzone I., Peverali F. A., de Franciscis V., Della Valle G., Pierotti M. A. Induction of RET proto-oncogene expression in neuroblastoma cells precedes neuronal differentiation and is not mediated by protein synthesis. Exp Cell Res. 1995 Mar;217(1):92–99. doi: 10.1006/excr.1995.1067. [DOI] [PubMed] [Google Scholar]
- GROBSTEIN C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science. 1953 Jul 10;118(3054):52–55. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- Hellmich H. L., Kos L., Cho E. S., Mahon K. A., Zimmer A. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev. 1996 Jan;54(1):95–105. doi: 10.1016/0925-4773(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Hofstra R. M., Landsvater R. M., Ceccherini I., Stulp R. P., Stelwagen T., Luo Y., Pasini B., Höppener J. W., van Amstel H. K., Romeo G. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994 Jan 27;367(6461):375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- Jing S., Wen D., Yu Y., Holst P. L., Luo Y., Fang M., Tamir R., Antonio L., Hu Z., Cupples R. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996 Jun 28;85(7):1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Kuo-Jang K., Ramirez V. D. Prolactin receptors: a comparison between chloramine-T and lactoperoxidase iodination. J Endocrinol Invest. 1978 Jul;1(3):233–238. doi: 10.1007/BF03350386. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993 May 21;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lin L. F., Zhang T. J., Collins F., Armes L. G. Purification and initial characterization of rat B49 glial cell line-derived neurotrophic factor. J Neurochem. 1994 Aug;63(2):758–768. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Liu X., Vega Q. C., Decker R. A., Pandey A., Worby C. A., Dixon J. E. Oncogenic RET receptors display different autophosphorylation sites and substrate binding specificities. J Biol Chem. 1996 Mar 8;271(10):5309–5312. doi: 10.1074/jbc.271.10.5309. [DOI] [PubMed] [Google Scholar]
- Mathews L. S. Activin receptors and cellular signaling by the receptor serine kinase family. Endocr Rev. 1994 Jun;15(3):310–325. doi: 10.1210/edrv-15-3-310. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Hendrickson W. A. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993 May 7;73(3):421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Klein R. D., Fariñas I., Sauer H., Armanini M., Phillips H., Reichardt L. F., Ryan A. M., Carver-Moore K., Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996 Jul 4;382(6586):76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Eng C., Healey C. S., Clayton D., Kwok J. B., Gardner E., Ponder M. A., Frilling A., Jackson C. E., Lehnert H. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet. 1994 Jan;6(1):70–74. doi: 10.1038/ng0194-70. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Kwok J. B., Healey C. S., Elsdon M. J., Eng C., Gardner E., Love D. R., Mole S. E., Moore J. K., Papi L. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993 Jun 3;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Houenou L. J., Johnson J. E., Lin L. F., Li L., Lo A. C., Newsome A. L., Prevette D. M., Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995 Jan 26;373(6512):344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Pachnis V., Mankoo B., Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993 Dec;119(4):1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Pichel J. G., Shen L., Sheng H. Z., Granholm A. C., Drago J., Grinberg A., Lee E. J., Huang S. P., Saarma M., Hoffer B. J. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996 Jul 4;382(6586):73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Rogers S. A., Ryan G., Hammerman M. R. Insulin-like growth factors I and II are produced in the metanephros and are required for growth and development in vitro. J Cell Biol. 1991 Jun;113(6):1447–1453. doi: 10.1083/jcb.113.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. A., Ryan G., Purchio A. F., Hammerman M. R. Metanephric transforming growth factor-beta 1 regulates nephrogenesis in vitro. Am J Physiol. 1993 Jun;264(6 Pt 2):F996–1002. doi: 10.1152/ajprenal.1993.264.6.F996. [DOI] [PubMed] [Google Scholar]
- Romeo G., Ronchetto P., Luo Y., Barone V., Seri M., Ceccherini I., Pasini B., Bocciardi R., Lerone M., Käriäinen H. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994 Jan 27;367(6461):377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- Rothenpieler U. W., Dressler G. R. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993 Nov;119(3):711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994 Jan 27;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Sánchez M. P., Silos-Santiago I., Frisén J., He B., Lira S. A., Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996 Jul 4;382(6586):70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Cooper G. M. ret transforming gene encodes a fusion protein homologous to tyrosine kinases. Mol Cell Biol. 1987 Apr;7(4):1378–1385. doi: 10.1128/mcb.7.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A., Lindqvist E., Lin L. F., Ogren S. O., Young D., Hoffer B. J., Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995 Jan 26;373(6512):335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Trupp M., Arenas E., Fainzilber M., Nilsson A. S., Sieber B. A., Grigoriou M., Kilkenny C., Salazar-Grueso E., Pachnis V., Arumäe U. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996 Jun 27;381(6585):785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Trupp M., Rydén M., Jörnvall H., Funakoshi H., Timmusk T., Arenas E., Ibáez C. F. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995 Jul;130(1):137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Woolf A. S., Kolatsi-Joannou M., Hardman P., Andermarcher E., Moorby C., Fine L. G., Jat P. S., Noble M. D., Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995 Jan;128(1-2):171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Matheson C., Lopez O. T. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995 Jan 26;373(6512):341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]