Abstract
Primary objective
Heparin-binding EGF-like growth factor (HB-EGF) protects the intestine from damage in animals. Future clinical trials of HB-EGF may involve administration of repeated doses of HB-EGF. Since HB-EGF activates EGF receptors which have been implicated in tumor development, we examined the effects of HB-EGF overexpression in the intestine.
Research design
We generated transgenic (TG) mice in which the human HB-EGF gene is driven by the villin promoter to overexpress HB-EGF along the crypt-villous axis from the duodenum to the colon.
Results
HB-EGF TG mice have increased enterocyte proliferation balanced by increased enterocyte apoptosis. Despite prolonged overexpression of HB-EGF, no evidence of intestinal hyperplasia or tumor formation occurs. Although HB-EGF TG mice have no significant phenotypic alterations under basal conditions, they have increased resistance to intestinal injury.
Conclusions
Prolonged intestinal HB-EGF overexpression results in no significant phenotypic alterations under basal conditions, but confers protection against intestinal injury.
Keywords: Intestine, villin promoter, villous, HB-EGF, transgenic, injury
Introduction
Heparin-binding EGF-like growth factor (HB-EGF) is a heparin binding (HB) member of the EGF family (Higashiyama et al. 1991) that was originally identified in the conditional medium of cultured human macrophages (Besner and Klagsbrun 1991). HB-EGF is produced as a 208 amino acid transmembrane precursor molecule (proHB-EGF) that undergoes extracellular proteolytic cleavage to yield the mature secreted form of the growth factor (sHB-EGF) (Thompson et al. 1994; Mori et al. 2003; Nanba et al. 2003). Similar to other EGF family members, HB-EGF binds to and activates the EGF receptor (EGFR; ErbB-1). In addition, HB-EGF activates ErbB-4 (Raab and Klagsbrun 1997; Iwamoto and Mekada 2000) as well as the HB-EGF specific receptor nardilysin, leading to increased cell migration (Nishi et al. 2001). Soluble HB-EGF acts as a potent mitogen and chemotactic factor for many cell types, promoting cell proliferation, survival, and regeneration (Krampera et al. 2005; Feng et al.; Khai et al. 2006; Singh et al. 2007). Membrane-anchored forms of HB-EGF act in a juxtacrine manner to exert pro-apoptotic effects (Iwamoto and Mekada 2000; Pan et al. 2002).
We have shown that HB-EGF protects the intestines from multiple forms of injury including ischemia/reperfusion injury (El-Assal and Besner 2004), hemorrhagic shock and resuscitation (HS/R) (El-Assal et al. 2007), and necrotizing enterocolitis (NEC: Feng et al. 2005). Others have shown that HB-EGF protects the heart (Perrella et al. 1994), brain (Sugiura et al. 2005), kidneys (Singh et al. 2007), and liver (Kiso et al. 1995) from similar types of injury. In the intestine, exogenous administration of HB-EGF promotes enterocyte migration and proliferation (Feng and Besner 2007), and enhances endothelial nitric oxide synthase (eNOS)-mediated angiogenesis (Mehta and Besner 2007). In addition, HB-EGF inhibits the expression of inflammatory cytokines (Rocourt et al. 2007), adhesion molecules, and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury (Xia et al. 2003). HB-EGF also prevents intestinal epithelial cell (IEC) apoptosis (El-Assal and Besner 2004; Feng et al. 2006), decreases bacterial translocation (Xia et al. 2002), and preserves gut barrier function (El-Assal et al. 2007) after intestinal injury.
Maintenance of intestinal mucosal integrity depends on an extraordinarily fast-paced and complicated homeostatic system (Ahuja et al. 2006; Schenk and Mueller 2008; Scoville et al. 2008). The intestinal mucosa is composed of five major cell types including absorptive enterocytes, goblet cells, paneth cells, enteroendocrine cells, and stem cells (SCs). A single villus containing ~3000 cells is encircled by 8–10 crypts that each contain ~250 cells in the mouse intestinal epithelium (Gordon and Hermiston 1994). SCs at the base of the crypts generate epithelial cells which migrate upward along a vertical axis toward the villous or downward to the base of the crypt. Upward-moving cells differentiate into absorptive enterocytes (70–80%), goblet cells (20%), or enteroendocrine cells (5%). Downward migrating cells differentiate into antibacterial protein producing Paneth cells (Gordon and Hermiston 1994). During a life span of 48–72 h, the enterocytes proliferate, migrate, and differentiate at an extraordinary rate. Despite this, the small intestine maintains a relatively constant mass with remarkably low incidence of epithelial cancer. This is achieved by the balance between proliferation and programed cell death that removes both senescent and genetically damaged cells. Villi exfoliate up to 30% of their cells daily, mainly at their distal tips. Crypts likewise eradicate cells, particularly when exposed to damaging chemicals or irradiation. An imbalance or interruption of intestinal homeostasis can lead to tumor formation or to intestinal damage after stress.
Upcoming Phase 1 clinical trials of enteral administration of HB-EGF to very low birth-weight premature babies for the prevention of NEC are being designed. These trials will include administration of multiple daily doses of enteral HB-EGF over several weeks. Since HB-EGF binds to and activates EGFR, and since over-activation of EGFR has been implicated in the development of several tumor types (Nicholson et al. 2001), it is important to examine the effects of prolonged HB-EGF exposure on the intestine. To that end, we have generated several lines of transgenic (TG) mice for preclinical in vivo examination of the effects of HB-EGF overexpression in the intestine. These TG mice have been designed to specifically overexpress the human HB-EGF precursor (proHB-EGF) in the intestine using a 12.4 kb villin regulatory and promoter sequence to drive human proHB-EGF gene expression (Madison et al. 2002). The promoter of the villin gene ensures the constant expression of HB-EGF throughout the entire intestine from the duodenum to the colon, from embryogenesis to adulthood. Furthermore, the villin promoter targets transgene expression throughout the entire crypt-villous axis. Here, we present the effects of continuous expression of HB-EGF on the intestine, with examination of intestinal morphology, IEC proliferation and apoptosis, production of the different intestinal cell lineages in the villi and crypts, and response to intestinal injury.
Methods
Villin-HB-EGF plasmid construction
The pBSII-12.4 kb Vill plasmid containing the 12.4 kb promoter fragment from the villin gene was a generous gift from Dr Deborah Gumucio (University of Michigan, Ann Arbor, MI, USA). To produce the pBS-12.4 kb Vill-HB-EGF construct, a 625 bp full length sequence of human proHB-EGF cDNA was obtained by PCR amplification of a pGEM/proHB-EGF cDNA construct (Mishima et al. 1996). This was cloned into the Mlu and SacII restriction sites at the 3′ end of the 12.4 kb villin promoter/enhancer fragment (Madison et al. 2002) and the 5′ end of the SV40 polyadenylation sequence of the pBS-12.4 kbVill plasmid. DNA sequencing was used to confirm the proper human proHB-EGF cDNA orientation and nucleotide sequence in the pBS-12.4 kbVill plasmid.
Generation of TG mice
These studies were approved by Institutional Animal Care and Use Committee of the Children’s Research Institute (IACUC Protocol # AR-06-00092). The DNA fragment containing the villin regulatory sequences linked to human proHB-EGF was released from the vector backbone by digestion of the pBSII-12.4 KbVill-HB-EGF plasmid with PmeI. The DNA fragment was gel- purified and transgene DNA was injected into the pronuclei of FVB/N fertilized murine ova. Founder mice were identified by PCR amplification of tail DNA with specific primers designed at the junction of the mouse villin promoter and the human proHB-EGF transgene: forward 5′-CCA ATG GAG GGT TCT TTT TGT G-3′ and reverse 5′-ATC TCG AGC CGC GGT CAG TGG GAA TTA GTC-3′. Founder mice were mated to FVB/N mice to obtain F1 progeny for analysis of transgene expression by PCR, RT-PCR, and Southern blotting.
Southern blot analysis of HB-EGF TG founder mice
Genomic DNA (10 μg) isolated from tails of potential HB-EGF TG mice was digested with an Ase I restriction enzyme and subjected to electrophoresis in agarose gels. Gels were blotted onto Nylon membranes, cross-linked by exposure to UV radiation in a UV Stratalinker (Stratagene, La Jolla, CA, USA) and subjected to Southern blot analysis using full-length human proHB-EGF as a hybridization probe. The Southern blot probe was prepared using the Rad-prime DNA labeling system (Invitrogen, Carlsbad, CA, USA).
RT-PCR and quantitative SyBrGreen RT-PCR
Total RNA was isolated from tissues using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To remove contaminating genomic DNA, RNA samples were digested with RNAase-free DNAase I (Invitrogen, Carlsbad, CA, USA) and RNA repurified using phenol/chloroform followed by ethanol precipitation. HB-EGF cDNA was amplified by RT-PCR using the specific primer set designed at the junction of mouse villin and the human HB-EGF transgene as described above. TG mice and FVB/N wild type (WT) littermates were further evaluated for proHB-EGF expression in the stomach, duodenum, jejunum, ileum, and colon using SyBrGreen real time RT-PCR. For quantitative real time RT-PCR, 0.5 μl transgene cDNA was amplified in a 1X SyBrGreen mixture with 1.8 μM human HB-EGF specific primers (forward: 5′TGGAGAATGCAA ATATGT-GAAGGA, reverse: 5′AACCCGGGTGGCA-GATG). For the GAPDH internal control, 0.5 μM mouse GAPDH primers (forward: 5′TTGTCTCCT-GCGACTTCAACA and reverse: 5′CCCCGGCAT-CTGAAGGT) were used. The fluorescent signal for target amplification was detected using an ABI7500 real time thermal cycler (Applied Biosystems, Foster City, CA, USA). The expression of human HB-EGF mRNA was determined by calculating (2−ΔΔCt) as described previously (Livak and Schmittgen 2001).
Analysis of body weight
Female and male HB-EGF TG mice and WT littermate control mice were weighed weekly from 4 to 12 weeks of life.
Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting (IP-WB) were carried out to detect proHB-EGF transgene protein production in the jejunum of TG mice. Jejunal lysates were prepared by grinding tissues in mini-homogenizers with 100 ml/mg RIPA buffer with EDTA (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 5 mM EDTA) (Boston BioProducts, Worcester, MA, USA) supplemented with 1X protease inhibitor cocktails (Pierce, Rockford, IL, USA). The protein concentration was measured using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Protein lysates (~4 mg) were incubated with goat anti-HB-EGF antibody (10 μg; R&D Systems, Minneapolis, MN, USA) for 2 h at 4°C. The binding complex was incubated with immobilized protein G (20 μl; Thermo Scientific, Rockford, IL, USA) and pulled down by centrifugation at 4000 rpm for 2 min. The supernatants were removed and the beads were washed with RIPA buffer with 1X protease inhibitors. The proteins with the beads were boiled with 10 × sample buffer (30 μl) at 95°C for 5 min. Proteins were fractioned by SDS-PAGE using 10–20% gradient acrylamide gels (BioRad Laboratory, Hercules, CA, USA). Specific bands were visualized using the enhanced chemifluorescence (ECF) western blotting reagent pack (Amersham, Pittsburgh, PA, USA) as previously described (Chen et al. 2007). proHB-EGF protein was detected by probing blots with a goat anti-HB-EGF antibody (0.2 μg/ml; R&D Systems, Minneapolis, MN, USA) or a rabbit anti-HB-EGF antibody (1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were washed with PBS + 0.1% Tween 20 three times and incubated with anti-goat (1:500 dilution; Sigma, Saint Luis, MO, USA) or anti-rabbit (1:500 dilution, Amersham, Pittsburgh, PA, USA) secondary antibodies conjugated with alkaline phosphatase. After three washes in PBS + 0.1% Tween 20, colorimetric visualization was developed using alkaline phosphatase substrate.
Intestinal histology and immunohistochemical detection of IEC lineages
Histology
Duodenum, jejunum, and ileum from 1- and 5-month-old TG mice (n = 4–6) and WT littermate control mice (n = 4) were removed and fixed in 4% formaldehyde in PBS (pH 7.4) for 48 h at 4°C. Tissues were then paraffin embedded and subjected to hematoxylin and eosin (H&E) staining. Villous height, crypt depth, and muscle thickness (200 ×) were determined using ImageJ 1.39U software (National Institute of Health, Bethesda, Maryland, USA).
Immunohistochemistry
Immunofluorescent staining was performed for detection of transgene human HB-EGF, cleaved caspase 3 detection of apoptotic cells, E-cadherin detection of enterocytes, chromogranin A detection of neuroendocrine cells, and prominin-1 detection of SCs. After dehydration, tissue sections were blocked with 10% donkey serum/PBS for 20 min at RT. Agoatanti-human HB-EGF antibody (20 ng/ml)(R&D Systems, Minneapolis, MN, USA), a rabbit anti-cleaved caspase 3 antibody (v:v = 1:200) (Cell Signaling Technology, Danvers, MA, USA), a rabbit anti-E-cadherin antibody (v:v = 1:25) (Cell Signaling Technology, Danvers, MA, USA), a rabbit polyclonal anti-chromogranin-A (v:v = 1:500) (ABCAM, Cambridge, MA, USA), or a rat monoclonal anti-prominin-1 antibody (v:v = 1:10) (Miltenyi Biotec, Auburn, CA, USA) were applied to the tissue sections according to the manufacturer’s recommended dilutions in 10% donkey serum/PBS for 1 h at RT or overnight at 4°C. After three 10-min washes with PBS/0.1% Tween 20, tissue sections were incubated with goat anti-rabbit IgG (4 μg/ml) conjugated with Alexa Fluo 594 (Invitrogen, Carlsbad, CA, USA) or with Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in 10% donkey serum/PBS for 1 h at RT. Tissue sections were mounted with ProLong Gold antifade reagent with 4′6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA, USA) after three 10-min washes with PBS + 0.1% Tween 20.
We applied the +4 position rule in identifying SCs labeled with an anti-prominin-1 antibody. The transient amplifying pool (progenitor cells) is located above the + 4 cell level position, whereas SCs are located below the + 4 position cells (Haegebarth and Clevers 2009). Although prominin-1 is expressed in both progenitor cells and SCs, the SCs were easily recognized by applying the +4 position criterion, allowing for their proper identification. Enterocyte density was determined in sections subjected to IHC using fluorescein-isothiocyanate-(FITC) labeled anti-E-cadherin antibodies by counting the number of positively stained cells in the distal 200 μm of the villi. Tissue sections were subjected to periodic-acid-Schiff staining (PAS) for detection of goblet cells, which were quantified by counting PAS-positive cells in well-oriented duodenal, jejunal, and ileal crypt-villous units in at least two non-adjacent sections. Paneth cells were quantified in a similar fashion by counting granule-containing crypt cells in H&E-stained sections. Neuroendocrine cells and SCs were quantified in tissue sections subjected to immunofluorescent staining with antibodies to chromogranin A and prominin-1, respectively. At least 15 villi with complete lymphatic tissues or 15 crypts with complete cryptal junctions were counted for quantification of IEC lineage cells, with quantification performed by observers that were blinded to tissue identity.
BrdU IHC for detection of cell proliferation
Proliferation of enterocytes was evaluated using 5-bromo-2′-deoxyuridine (BrdU) labeling. Mice were injected with (BrdU; 120 mg/g) intraperitoneally 2 h prior to sacrifice. Upon sacrifice, intestines were removed, fixed in 4% paraformaldehyde in PBS, and then paraffin embedded. For IHC, sections were deparaffinized, rehydrated in H2O, and endogenous peroxidase was blocked using 3% hydrogen peroxide (Sigma, St Louis, MO, USA) in PBS for 15 min. Antigen retrieval was performed by boiling in citric acid (10 mM, pH 7) for 20 min. Sections were incubated with a mouse anti-BrdU antibody (20 μg/ml) (BD Pharmingen, San Jose, CA, USA) in 10% donkey serum/PBS and staining was visualized using a Mouse to Mouse HRP ready-to-use kit with AEC chromogen (ScyTek Lab, Logan, UT, USA) according to the manufacturer’s protocol. Tissue sections incubated with rabbit IgG or secondary antibody alone served as negative controls. For SC proliferation, the +4 position rule was also applied. The proliferative index was defined as the percent (%) of BrdU labeled nuclei/total nuclei in each crypt.
TUNEL and caspase 3 immunostaining for detection of apoptosis
Apoptotic cells in the intestine were identified by terminal deoxynucleotidyl transferase dUTP nick end labeling using an ApopTag Red In Situ apoptosis detection kit (Chemicon International, Temecula, CA, USA) following the manufacturer’s protocol. Sections were blocked with 10% donkey serum/PBS for 20 min at RT. Since cell death involving DNA fragmentation may not always be due to apoptosis, cleaved caspase 3 immunostaining was also performed by double staining the sections with a rabbit anti-cleaved caspase 3 antibody (1:25) (Cell Signaling Technology, Danvers, MA, USA).
Analysis of gut associated lymphoid tissue (GALT)
Isolation of Peyer’s patches
Lymphocyte isolation from Peyer’s patches was performed as described previously (Deitch et al. 1990). Peyer’s patches were excised from the serosal side of the intestine and teased apart using 18-gauge needles. Fragments were treated with type 1 collagenase (50 units/ml) (Sigma, St Louis, MO, USA) in modified essential medium for 60 min at 37°C with constant rocking. After collagenase digestion, cell suspensions were passed through nylon filters and centrifuged at 1500 rpm for 10 min at 4°C. Pellets were resuspended in 1 ml RPMI medium with 25% FBS and kept on ice until assayed.
Flow cytometry
To determine the phenotypes of the lymphocytes isolated from the Peyer’s patches, 105 cells were suspended in 50 μL HBSS containing either fluorescein-conjugated (FITC) anti-CD3 (clone 145-2C11; R&D Pharmigen, San Diego, CA, USA) or phycoerythrin-conjugated (PE) goat anti-mouse immunoglobulin (Southern Biotechnology Associates, Birmingham, AL, USA) to identify T cells and B cells, respectively, or FITC-anti-CD4 (clone RM4-5) and PE-anti-CD8 (clone 53–67; R&D Pharmigen, San Diego, CA, USA) to identify T helper cells and T killer cells, respectively. All antibodies were diluted to 2.5 μg/ml in hepes-buffered saline (HBSS) containing 0.1% azide for 30 min on ice. After staining, cells were washed twice in HBSS and were fixed in 1% paraformaldehyde (Sigma, St Louis, MO, USA). Flow cytometric analysis was performed using a Profile I counter (Coulter, Hileah, IL, USA).
Dendritic cell IHC
Briefly, deparaffinized rehydrated sections were treated in 0.1% trypsin (Sigma Chemical Company, St Louis, MO, USA) for 30 min at room temperature. Staining for dendritic cells in mice intestine was obtained by rat anti-mouse dendritic cell antibody (BD Pharmingen, San Jose, CA, USA). The incubation time for the first antibody was 1 h at room temperature. The steps of immunohistochemistry (IHC) were conducted using Mouse to Mouse HRP staining kit (ScyTek Laboratories, Logan, UT, USA) according to the recommendations of the manufacturer. Dendritic cell antibody staining was labeled using AEC, and was counter stained using H&E stain. After staining, slides were screened for positive staining cells that were mainly detected in and close to the intestinal lymph follicles. The number of dendritic cells was counted in 5, 400 × microscopic fields.
Hemorrhagic shock and resuscitation model
The animal procedure was approved by the Institutional Animal Care and Use Committee of The Research Institute at Nationwide Children’s Hospital (Protocol #00903AR). HB-EGF TG and WT mice were randomly assigned to the following groups: (1) experimental group (n = 12): animals were subjected to Hemorrhagic shock and resuscitation (HS/R) and sacrificed 3 h after the initiation of resuscitation; (2) control group (n = 6): animals were fasted for 16–18 h with access to water only before being sacrificed. Eight-to twelve-week-old male HB-EGF TG or WT mice weighing 25–30 g were fasted for 16–18 h with access to water only before experimentation. Under inhalation anesthesia using 2% isoflurane, the right and left femoral arteries were cannulated with PE10 tubing (Becton Dickinson, Sparks, MD, USA). The right arterial catheter was connected to a pressure monitor (Grass, West Warwick, RI, USA) to follow mean arterial pressure (MAP). Blood was withdrawn over 15 min via the left femoral artery catheter to reduce the MAP to 30 mmHg. Blood was withdrawn and returned to the animal as needed to maintain a MAP of 30–35 mmHg. At the end of the shock period (90 min) mice were resuscitated with the shed blood plus two times that volume of Ringer’s lactate solution infused slowly over 30 min.
Intestinal barrier function determination
Gut barrier function after exposure to HS/R was used to determine the biological function of intestinal overexpression of HB-EGF upon exposure to injury. A 6 cm segment of distal ileum from animals in each group was obtained 3 h after resuscitation from hemorrhagic shock, and was used to determine intestinal permeability. Mucosal barrier function was assessed using the ex vivo isolated everted sac method as described (Liaudet et al. 2000) with some modifications. The distal ileal segment was used to make the everted gut sac, and was prepared in ice-cold modified Krebs–Henseleit bicarbonate buffer (KHBB, pH 7.4, 10 mM Hepes/137 mM NaCl/5.5 mM KCl/4.2 mM NaHCO3/0.3 mM Na2-HPO4/0.4 mMKH2PO4/0.4 mM MgSO4/0.5 mM MgCl2/1.3 mM CaCl2/19.5 mM glucose). FITC dextran (Mr 4000 Da; FD4) was used as a permeability probe. The everted gut sacs were gently distended by injecting 0.4 ml of KHBB and suspending the sacs in a 50 ml-beaker containing 40 ml of KHBB with added FD4 (60 μg/ml) for 30 min. The incubation medium in the beaker was maintained at a temperature of 37°C and was continuously bubbled with a gas mixture containing 95% O2 and 5% CO2. A 0.5 ml sample was taken from the beaker at the beginning of the incubation to determine the initial FD4 concentration of the mucosal side. After the 30 min incubation, the fluid was aspirated from the inside of the sac to determine the FD4 concentration of the serosal side. The length and diameter of each gut sac was measured. Serosal and mucosal samples were centrifuged for 10 min at1000g at 4°C. Fluorescence of 100 μl of supernatant was measured using a fluorescence spectrophotometer (SpectraMax Plus, Molecular Devices, CA, USA) set at an excitation wavelength of 492 nm (slit width, 2.5 nm) and an emission wavelength of 515 nm (slit width, 10 nm). Gut permeability was expressed as the mucosal-to-serosal clearance of FD4 as follows: (Liaudet et al. 2000)
Statistical analyses
Data are represented as mean ± SD. Statistical analyses for all experiments were performed using one-way ANOVA (repeated measures), with the exception of the intestinal permeability studies which were analysed using the Student t-test. p values < 0.05 were defined as statistically significant.
Results
Generation of HB-EGF TG mice under the control of the villin promoter
We constructed TG mice in which the expression of proHB-EGF was under the control of the mouse 12.4 kb villin promoter (Figure 1A,B). Integration of Vill-HB-EGF into the genome was demonstrated by PCR (Figure 1C) and Southern blot analysis (Figure 1D) of tail DNA using Vill-HB-EGF specific primers and probes. Of eight progeny screened as shown, two were positive for the Vill-HB-EGF transgene. In total, three TG founders were obtained. These founders were backcrossed to FVB mice to establish stable TG HB-EGF mouse lines.
Figure 1.
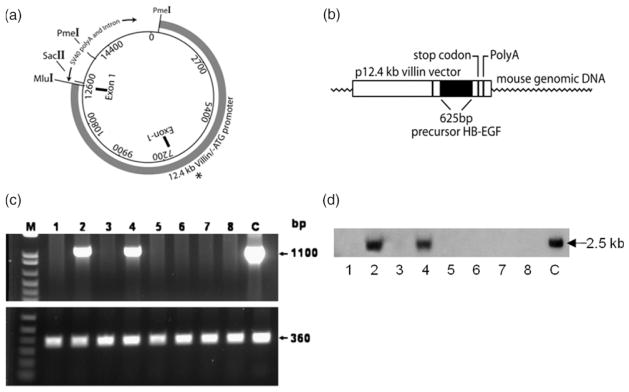
Expression of proHB-EGF under the control of the villin promoter. (A) Villin vector. The vector consists of the 12.4 kb villin promoter sequence and the SV40 polyA signals and intron. The Mlu I and SacII sites where the HB-EGF sequence has been inserted are shown. The two PmeI cut sites for release of the entire construct are also labeled. The asterisk represents the initiation site for transcription of the villin sequences. (B) Villin-proHB-EGF construct. Human precursor HB-EGF cDNA (625 bp) was inserted into the Mlu I and SacII sites at the 3′ end of the villin promoter. (C) PCR of potential HB-EGF TG founder mice. Eight potential founder mice derived from Vill-HB-EGF-injected oocytes were screened for genomic integration of the HB-EGF transgene by PCR of tail DNA using villin promoter and HB-EGF sequence specific primers (top panel). Mouse specific β-actin primers were used as an internal control for amplification (bottom panel). Two HB-EGF TG mice were identified (lanes 2 and 4). c, control DNA (Villin-proHB-EGF). (D) Southern blot analysis of potential HB-EGF TG founder mice. DNA was isolated from the tails of the same eight potential founder mice and subjected to Southern blot analysis using HB-EGF specific probes. The positive bands in lanes 2 and 4 correlated with the PCR results, confirming the identification of two HB-EGF TG mice. C, FVB control DNA.
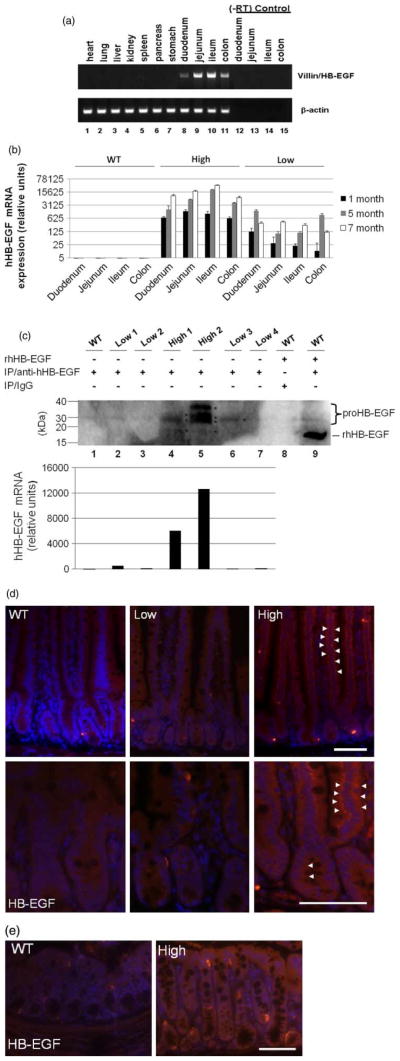
Vill-HB-EGF is selectively expressed in the intestine
To assess the selectivity of expression of the HB-EGF transgene mRNA in the intestine, mRNA from 11 different tissues of a TG mouse was subjected to RT-PCR using Vill-HB-EGF specific primers. We found that HB-EGF was expressed in duodenum, jejunum, ileum, and colon only. Other organs including the heart, lung, liver, kidney, spleen, pancreas, and stomach did not express proHB-EGF mRNA (Figure 2A). Intestine from two lines of TG mice and FVB WT littermates were further analysed for proHB-EGF mRNA expression using real time RT-PCR. proHB-EGF was expressed in all regions of the intestine in mice at 1 month of age, with continued and even increased expression at 5 and 7 months of age (Figure 2B). Vill-HB-EGF mRNA was not detected in WT littermate control mice (Figure 2B). Based on HB-EGF expression levels, two lines of TG mice were labeled as the “high expression” (high) and the “low expression” (low) TG mice, respectively. Approximately, 611–1485 and 1807–20520 fold expression of HB-EGF mRNA was observed in the intestine of high expression TG mice in the first and fifth months of life respectively, compared to the expression of HB-EGF mRNA in the intestine of WT littermates (which was arbitrarily set at 1). The low expression TG line displayed less intensive proHB-EGF mRNA expression, ranging from 12–124 and 99–1597 fold increase compared to 1- and 5-month-old WT mice respectively.
Figure 2.
Overexpression of HB-EGF in TG mouse intestine. (A) human HB-EGF mRNA expression as determined by RT-PCR. Tissues from nine different organs were harvested from 1-month-old TG and WT mice and RNA was isolated. RT-PCR using villin- and HB-EGF-specific primers was performed (upper panel). RT-PCR for β-actin was performed in parallel as an internal control of the amplification signal (lower panel). Lanes 12–15, (−)RT controls. (B) human HB-EGF mRNA expression determined by real time RT-PCR. HB-EGF expression in TG mice was determined using real time RT-PCR with specific primers to hHB-EGF that do not amplify murine HB-EGF. Note that the Y-axis is shown in a log scale. (C) HB-EGF protein production as determined by immunoprecipitation and Western blotting. Transgene protein product from jejunal lysates (2 mg) was immunoprecipitated with a goat anti-HB-EGF polyclonal antibody (20 μg) and subsequently detected by Western blotting. WT jejunum served as a negative control (Lane 1). Recombinant mature HB-EGF protein (19 kD) spiked into WT jejunal lysates was immunoprecipitated with goat IgG to serve as an additional negative control (Lane 8) or with goat anti-HB-EGF antibodies to serve as a positive control (Lane 9). RT-PCR demonstrating the corresponding human HB-EGF transgene expression for the lanes in Panel C. WT, wild type; low, 1–low 4, intestine from four individual low expression TG mice; high 1 and high 2, intestine from two individual high expression TG mice. (D) Immunofluorescent staining of transgene human HB-EGF protein in the jejunum using anti-human-HB-EGF antibodies. Positively stained cells were stained red (Cy3). Tissues were counterstained with DAPI (blue). White arrowheads indicate perinuclear HB-EGF staining. Upper panels, magnification 200 ×; lower panels, magnification 400 ×. (E) Immunofluorescent staining of transgene human HB-EGF protein in the colon. WT, wild type; low, low expression HB-EGF TG mice; high, high expression HB-EGF TG mice. Scale bars represent 50 μm.
Protein production of proHB-EGF positively correlated with HB-EGF mRNA expression in HB-EGF TG mice (Figure 2C). IP-WB detected proHB-EGF protein products in the jejunal lysates of TG mice, with increased HB-EGF protein detected in the jejunum of the high expression TG line compared to the low expression TG line. The multiple bands detected likely contain the three human precursor HB-EGF protein species previously described due to glycosylation (upper bands between 35–25 kD) (Davis et al. 1996) and the mature form of HB-EGF (19 kDa, lower band). Densitometric analysis indicated that the majority (>90%) of transgene protein is in the precursor (proHB-EGF) form (data not shown). IP-WB showed that the proportion of sHB-EGF: proHB-EGF decreases as the overexpression of HB-EGF increases (high 1 [lane 4] compared to high 2 [lane 5], Figure 2C). The mature, soluble form of HB-EGF is released from the cell surface after being processed by proteases. In our studies, we harvested intestinal tissues that were thoroughly washed in PBS prior to lysis for IP-WB. Therefore, we expected very low levels of sHB-EGF in our IP-WB samples. Immunostaining for human pro-HB-EGF indicated that transgene HB-EGF was expressed throughout the entire crypt-villus axis of the small bowel (jejunum) (Figure 2D) and colon (Figure 2E). The majority of transgene expression was located perinuclearly.
Body weight of HB-EGF TG mice
Vill-HB-EGF TG and WT littermate mice were weighed weekly to determine the effects of hHB-EGF transgene expression on body weight. No difference in body weight was found in high expression TG mice compared to WT littermate control mice (Figure 3).
Figure 3.
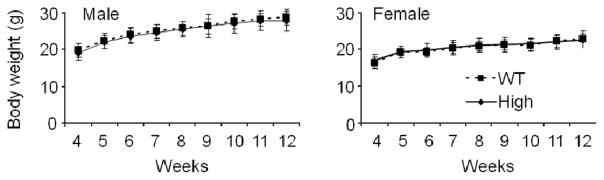
Body weight determinations of HB-EGF TG and WT mice. Body weights of male and female TG mice and WT littermates were determined weekly from 4–12 weeks of life. N = 35 animals per group.
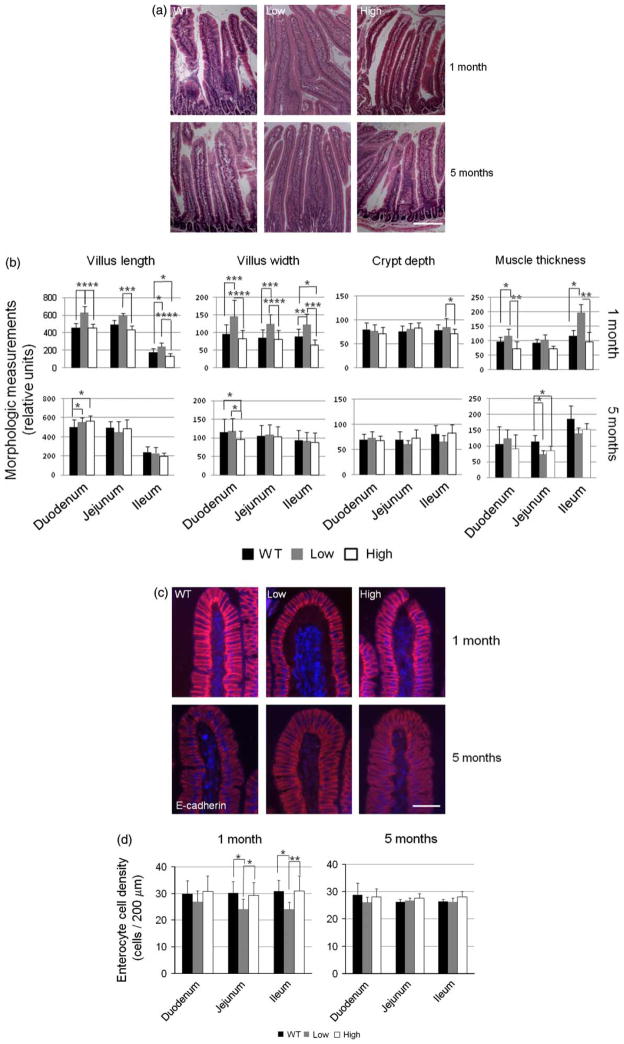
Morphologic changes in the intestine of HB-EGF TG mice
Morphologic analyses of mice at 1, 3, 5, and 7 months revealed some morphologic differences between WT and TG mice at 1 month of age that became insignificant as mice grew older. Therefore, only representative morphologic results of mice at 1 month, and 5 months of age are shown. Intestinal morphology was determined by examination of histologic sections of TG and WT mouse intestine stained with H&E (Figure 4A), with morphometric quantification performed using ImageJ 1.39U software (Figure 4B). There were small but statistically significant increases in villous length (L) and villous width (W) in low expression TG mice compared to WT mice at 1 month of age in the duodenum (L: 623 ± 77 vs. 459 ± 11, p < 0.001; W: 144 ± 46 vs. 95 ± 26, p < 0.005), jejunum (L: 598 ± 27 vs. 490 ± 52, p < 0.005; W: 125 ± 27 vs. 85 ± 23, p < 0.005), and ileum (L: 241 ± 46 vs. 181 ± 41, p < 0.05; W: 122 ± 31 vs. 88 ± 22, p < 0.05) (Figure 4B). Interestingly, the villous length and width in the high expression TG mice at 1 month of age were not statistically different from that of WT mice (Figure 4B). By 5 months of age, there were no differences in villous height or villous width in any of the groups of mice except for slight differences in the duodenum. There were no differences in crypt depth between any of the groups of mice at either 1 or 5 months of age with the exception of the ileum of low expression and high expression TG mice at 1 month of age (Figure 4B). Overexpression of HB-EGF was associated with increased duodenal and ileal muscularis externa thickness in mice at 1 month of age (Figure 4B). Low expression TG mice had the thickest muscular layers. This effect was no longer observed at 5 months of age, where WT mice had thicker muscle layers compared to TG mice.
Figure 4.
Intestinal histology and morphometric analysis of HB-EGF TG and WT mice. (A) Intestinal histology. Shown are representative H&E-stained sections of duodenum from WT, high expression TG mice, and low expression TG mice at 1 month and 5 months of age. The scale bar represents 100 μm. (B) Intestinal morphometric analysis. Villous length, villous width, crypt depth, and muscle thickness in H&E-stained sections of duodenum, jejunum, and ileum from WT, high expression TG mice, and low expression TG mice were quantified using ImageJ 1.39U software. (C) Enterocyte immunostaining. Shown are jejunal sections from WT, high expression TG, and low expression TG mice immunostained with rabbit anti-E-cadherin and secondary goat anti-rabbit IgG conjugated with Alexa 560 (red). The nulei were conterstained with DAPI (blue). The scale bar represents 50 μm. (D) Enterocyte cell density. Columnar enterocyte cell densities were quantified by counting the number of cells in the distal 200 μm from the distal tips of the villi. Each graph bar represents quantification of 15 villous tips. All values shown represent mean ± SD. Statistical significance was determined by one-way ANOVA (repeated measures). * p < 0.05; ** p < 0.01; *** p < 0.005; **** p < 0.001; WT, wild type; low, low expression HB-EGF TG mice; high, high expression HB-EGF TG mice.
In the low expression TG mice, enterocyte cell and nuclear volumes in the jejunum and ileum of 1 month old TG mice were mildly increased compared to WT mice (Figure 4C), resulting in lower enterocyte density (jejunum: 24.2 ± 3.7 vs. 30.2 ± 4.3 cells/10 μm, p < 0.05; ileum: 24.1 ± 2.7 vs. 30.8 ± 4.1 cells/10 μm, p < 0.01, (Figure 4D). There were no differences in enterocyte density between high expression TG mice and WT mice.
Due to the theoretical concern of whether long-term overexpression of HB-EGF could cause hyperplasia or tumor formation in mouse intestine, we examined the small and large intestine of older age low expression and high expression HB-EGF TG mice. There was no evidence of hyperplasia, polyps, or tumor growth observed in any TG mice at either 1 year (low, n = 2; high, n = 4) or 1.5 years (high, n = 8) of age (data not shown).
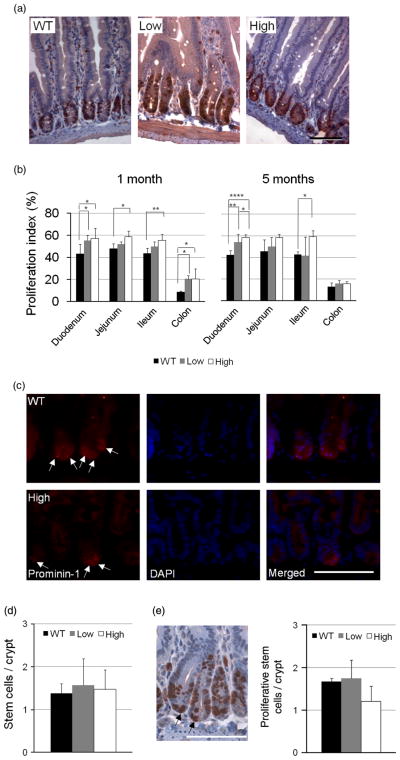
Cell proliferation in HB-EGF TG mice
BrdU IHC was used to identify proliferating cells (Figure 5A). Crypt cell proliferative activity in low expression and high expression HB-EGF TG mice [duodenum (55.3 ± 4.8%; 57.2 ± 9.3%), jejunum (52.2 ± 2.1%; 58.7 ± 5.3%), ileum (49.8 ± 4.6%; 55.6 ± 5.3%), and colon (20.5 ± 3.2%; 20.7 ± 8.9%)] was higher than that of WT control mice [duodenum (43 ± 9.0%), jejunum (48.1 ± 4.3%), ileum (43.6 ± 5.0%), and colon (8.7 ± 0.8%)] at 1 month of age (Figure 5B). The differences in proliferative activity between high expression TG mice and WT mice persisted at 5 months of age. However, the proliferation indices in low expression TG mice showed no differences compared to WT mice in the jejunum and ileum at 5 months of age. Since proliferative cells are derived from SCs, we next examined the effect of HB-EGF overexpression on SCs. SCs below cell +4 level in the jejunum of WT, low expression TG mice, and high expression TG mice at 1 month of age were identified by anti-prominin-1 antibody immunostaining (Figure 5C). There were no significant differences in the number of SCs per crypt (Figure 5D) or in the number of proliferating SCs per crypt (Figure 5E) between WT mice and HB-EGF TG mice.
Figure 5.
Crypt cell proliferation in HB-EGF TG and WT mice. (A) BrdU immunostaining. Shown are representative jejunual sections from 1-month-old WT, high expression TG, and low expression TG mice subjected to BrdU immunostaining. Proliferating cells are stained red-brown. (B) Quantification of crypt cell proliferation. Proliferation indices were calculated as the percent (%) of BrdU positive nuclei/total crypt nuclei. Nuclei in at least 15 crypts from the duodenum, jejunum, and ileum were measured to determine proliferation indices. (C) SC IHC. SCs in jejunal crypts of 1-month-old WT mice are shown. SCs were labeled with rat anti-prominin-1 monoclonal antibody (red staining). (D) Quantification of SCs. Prominin-1 positive cells beneath cell +4 level in 15 crypts were counted. (E) Proliferative SCs in crypts (arrows). BrdU positive cells beneath cell +4 position were scored. The scale bars in A, C, and E represent 50 μm. Values shown are mean ± SD. Statistical significance was determined by one-way ANOVA (repeated measures). * p < 0.05; ** p < 0.01; **** p < 0.001; WT, wild type; low, low expression HB-EGF TG mice; high, high expression HB-EGF TG mice.
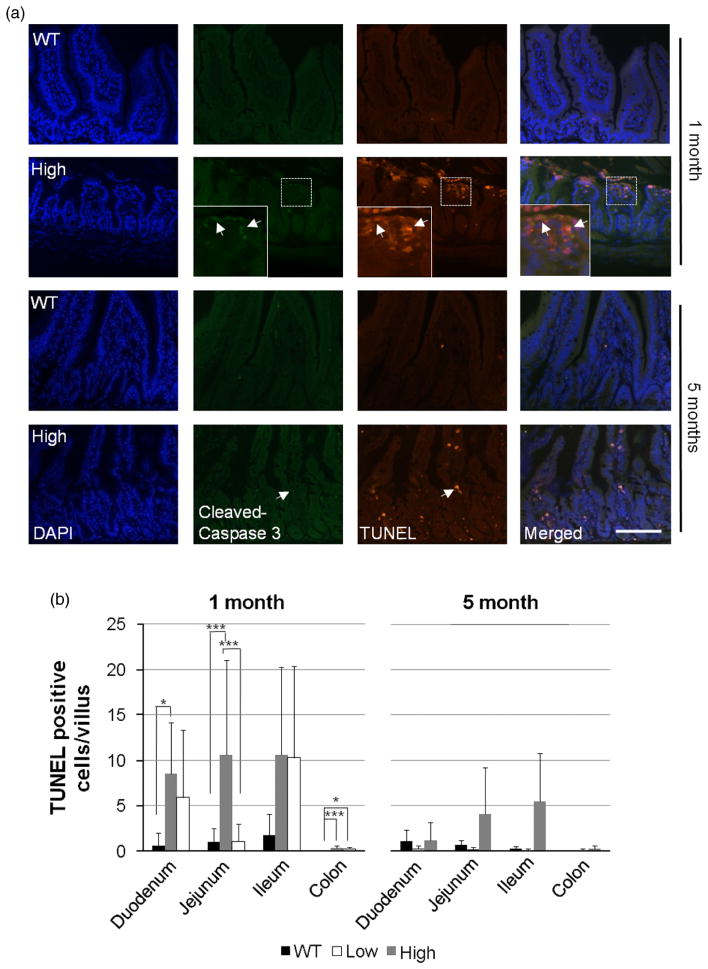
Cellular apoptosis in HB-EGF TG mice
Apoptotic cell death was examined in the epithelial cells of the villi using TUNEL staining and cleaved caspase 3 immunofluorescence staining (Figure 6A). Low expression TG mice had significantly increased apoptotic activity in the villi of the duodenum and jejunum compared to WT mice at 1 month of age (Figure 6B, p < 0.05 and p < 0.005, respectively). High expression TG mice did not have significantly increased numbers of apoptotic cells in the villi of the duodenum, jejunum, or ileum, compared to WT mice. In the colon, both TG mouse lines (low and high expression) showed increased numbers of TUNEL positive cells compared to WT mice (p < 0.005 and p < 0.05). At 5 months of age, there were no significant differences in apoptosis between HB-EGF TG and WT mice (Figure 6B).
Figure 6.
IEC apoptosis in HB-EGF TG and WT mice. (A) Apoptosis in ileal villi in high expression TG and WT mice at 1 and 5 months of age demonstrated by TUNEL staining (red) and anti-cleaved caspase 3 immunostaining (green), with DAPI nuclear counterstaining (blue). White arrows indicate examples of positively staining cells. The scale bar represents 50 μm. (B) Quantification of apoptotic activity in WT, low expression TG, and high expression TG villi at 1 and 5 months of age. Five to ten high power fields at 200 × magnification with the highest numbers of apoptotic cells by TUNEL staining were photographed at 200 × magnification using a Zeiss microscope and TUNEL positive cells/villus were counted. Values represent mean ± SD. Statistical significance was determined by one-way ANOVA (repeated measures). * p < 0.05; ** p < 0.01; *** p < 0.005; WT, wild type; low, low expression HB-EGF TG mice; high, high expression HB-EGF TG mice.
Morphology and cell proliferation in a second low expression HB-EGF TG mouse line
We analysed a second independent low expression HB-EGF TG mouse line and found that its phenotype resembled the first low expression HB-EGF TG mouse line. Its HB-EGF transgene expression in the jejunum (~120 fold higher than WT) was very close to that of the first low expression HB-EGF TG mouse line (~30 fold higher than WT) analysed, compared to the transgene expression in high expression HB-EGF TG mice (~1485 fold higher than WT) in the first month of life. Proliferative indices in the duodenum, jejunum, ileum, and colon (61.1 ± 0.8, 56.9 ± 3.7, 58.1 ± 2.3, and 11.7 ± 6.2, respectively) in this second low expression TG mouse line were similar to the first low expression HB-EGF TG mouse line in the first month of life. Duodenal villous length (626.9 ± 18.9) and width (140.1 ± 19.0) of 1 month old mice from the second low expression TG mouse line were significantly greater than that of WT and high expression HB-EGF TG mice, and were similar to the results for the first low expression HB-EGF TG mouse line.
Effects of HB-EGF on additional intestinal epithelial cell lineages
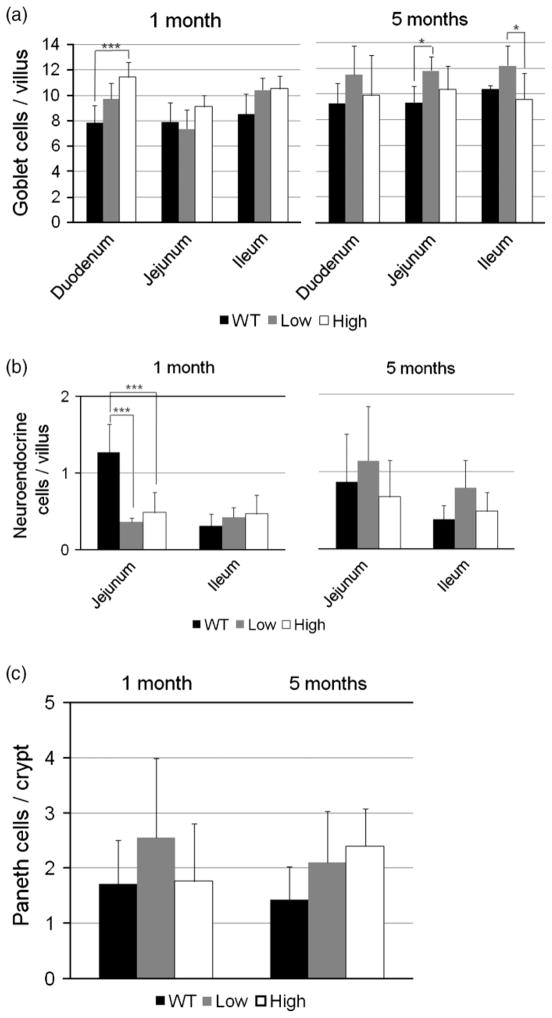
We next investigated the effects of overexpression of HB-EGF on goblet cells, granuled Paneth cells, and neuroendocrine cells (Figure 7). In 1-month-old mice, high expression TG mice had more goblet cells/villous in the duodenum compared to WT mice (11.4 ± 1.2 vs. 7.9 ± 1.4, p < 0.005) (Figure 7A). By 5 months of age, the low expression TG mice had more goblet cells/villous than WT mice in the jejunum and ileum. Neuroendocrine cells were generally not affected with the exception of decreased numbers in low expression and high expression TG mice in the jejunum at 1 month of age (Figure 7B). There were no significant differences in the numbers of granulated Paneth cells in the crypts of TG and WT mice (Figure 7C).
Figure 7.
Intestinal goblet cell, neuroendocrine cell, and Paneth cell quantification in HB-EGF TG and WT mice. (A) Goblet cell quantification. Tissue sections were stained with PAS to stain the cytoplasm of goblet cells pink. Quantification of goblet cells in the duodenum, jejunum, and ileum of WT, low expression TG mice, and high expression TG mice was performed by counting the numbers of PAS-positive goblet cells/villous. (B) Neuroedocrine cell quantification. Neuroendocrine cells were immunostained with rabbit anti-chromogranin A antibody, with nuclear counterstaining with DAPI. Quantification of neuroendocrine cells in the jejunum and ileum of WT, low expression TG mice, and high expression TG mice was performed by counting the numbers of positively stained cells/villous. (C) Paneth cell quantification. Paneth cells were identified as granule-containing cells at the base of the crypts of H&E-stained sections, with quantification performed by counting the granule-containing cells/crypt in the jejunum of WT, high expression TG, and low expression TG mice. All values shown represent mean ± SD. Statistical analyses were performed by one-way ANOVA (repeated measures). * p < 0.05; *** p < 0.005; WT, wild type; low, low expression HB-EGFTG mice; high, high expression HB-EGFTG mice.
Flow cytometric analysis of GALT in HB-EGF TG mice
Since the intestine harbors the largest collection of lymphoid tissue in the body, we next sought to determine whether overexpression of HB-EGF affects immune cell distribution in the intestine. We performed FACS analysis of cells isolated from Peyer’s patches of TG and WT mice. Under basal conditions, no differences were found in B cells, T helper cells, or T killer cells of high expression TG and WT mice (Figure 8). Additionally, immunohistochemical analysis of dendritic cells revealed no significant differences in cell numbers between high expression TG and WT mice (Figure 8).
Figure 8.
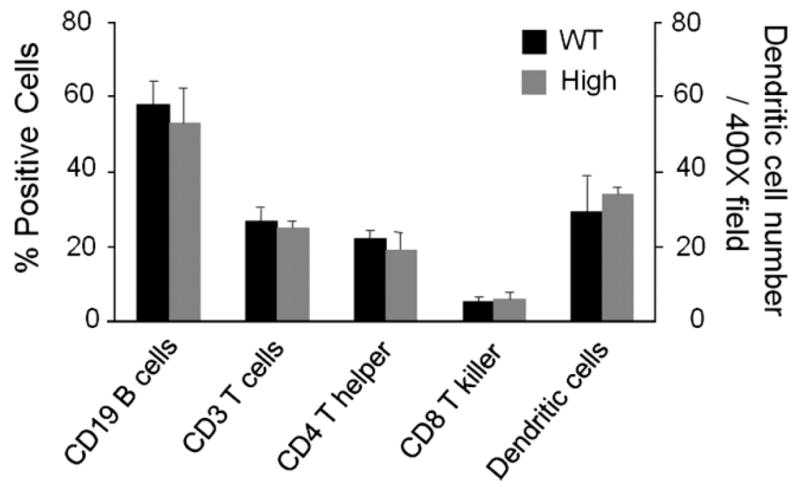
Analysis of gut-associated lymphoid tissue (GALT) and dendritic cells in HB-EGF TG and WT mice. Lymphocytes in HB-EGF TG and WT mice. Lymphocytes from Peyer’s patches were fluorescently labeled using antibodies specific for CD3, CD4, CD8, and immunoglobulin. GALT was analysed by flow cytometry. The intestinal dendritic cells were visualized by anti-dendritic cell IHC and quantified by counting the numbers of positively stained cells/400 × high power field. Statistical analyses were performed by one-way ANOVA (repeated measures). Values shown are mean ± SD. WT, wild type; high, high expression HB-EGF TG mice.
HB-EGF gene overexpression protects against intestinal barrier dysfunction in mice subjected to HS/R
We evaluated the effect of HB-EGF gene over-expression on the gut barrier function in mice subjected to HS/R by mucosal-to-serosal unidirectional clearance to FD4 using the everted gut sac method. All mice (HB-EGF TG and WT) subjected to HS/R had significantly increased intestinal permeability at 3 h of resuscitation compared to uninjured mice (Figure 9). High expression TG mice subjected to HS/R had significantly decreased mucosal permeability compared to WT mice subjected to HS/R at 3 h of resuscitation (FD4 clearance 13.06 ± 5.67 vs. 20.03 ± 7.81 nl/min/cm2, p = 0.02). This indicates that high expression TG mice have decreased intestinal permeability after HS/R, demonstrating that HB-EGF gene overexpression increases the preservation of gut barrier function during recovery of the intestine from injury.
Figure 9.
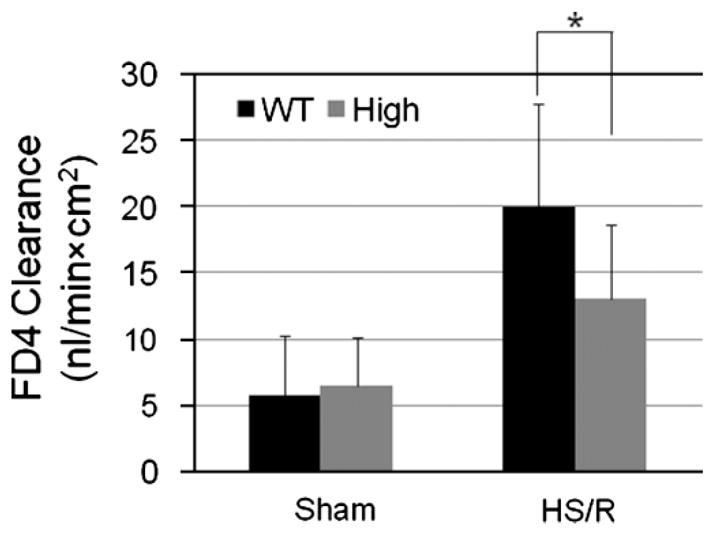
Overexpression of human HB-EGF in mice subjected to HS/R. HB-EGF TG and WT mice were subjected to hemorrhagic shock (30–35 mmHg) for 90 min, followed by resuscitation for 3 h. Intestinal permeability was determined using the everted gut sac method. *p < 0.05. Values shown are mean ± SD. WT, wild type; high, high expression HB-EGF TG mice.
Discussion
Multiple lines of evidence from our laboratory have demonstrated that HB-EGF is a potent intestinal cytoprotective agent. Administration of HB-EGF protects the intestine from injury in rat models of intestinal ischemia/reperfusion (El-Assal and Besner 2004), HS/R (El-Assal et al. 2007), and NEC (Feng et al. 2005). In the future, enteral administration of HB-EGF may be used clinically to protect the intestines from injury. In preparation for future clinical trials, we have established villin-human precursor-HB-EGF TG mice to investigate the effects of long-term overexpression of HB-EGF on the intestine in vivo. TG mice displayed human HB-EGF mRNA expression in the small and large intestine in a sustained, age-dependent, increasing manner at 1, 3, 5, and 7 months of age. Overproduction of HB-EGF protein was confirmed by IP-WB, which demonstrated that the majority of the HB-EGF produced was in the precursor forms, with less than 10% present as the cleaved mature form.
Morphometric analyses of our HB-EGF TG mice revealed modest but statistically significant increases in villous length, villous width, and muscle layer thickness in low expression TG mice compared to WT mice in the first month of life. These differences were temporary, and became insignificant in mice older than 1 month of age, despite documented increased HB-EGF expression at these later time points. We found thinner and shorter villi in the ileum of high expression TG mice compared to WT mice at 1 month of age. The etiology of the differences in morphology between high expression and low expression HB-EGF TG mice is not yet completely understood. Cell density data indicate that a larger enterocyte volume may contribute to the longer/wider villi seen in low expression TG mice at 1 month of age. Increased enterocyte volume, along with increased villous length and width, were not seen in high expression TG mice at 1 month of age. Thus, overexpression of human HB-EGF may play a biphasic role in which lower levels of overexpression promote an increase in enterocyte size, but higher levels of overexpression do not. Future studies will further explore this interesting phenomenon.
Our BrdU-labeling studies show that overexpression of HB-EGF moderately promotes cell proliferation in the crypts of TG mice. The proliferative effects of overexpressed HB-EGF are present in a dose-dependent manner, since the effects were more prominent in high expression TG mice compared to low expression TG mice. The mitogenic activity of overexpressed HB-EGF on IEC is consistent with a substantial body of previous studies, particularly with sHB-EGF, in the intestine and other cell types (Zushi et al. 1997; Xia et al. 2002; El-Assal and Besner 2005; Feng and Besner 2007; Wang et al. 2007; Zhuang et al. 2008). Intestinal renewal involves crypt epithelial cell proliferation and migration of crypt cells to the luminal surface (Freeman 2008). This process is dependent on SCs and multi-potent progenitor cells located in the crypts (May et al. 2008; Haegebarth and Clevers 2009). HB-EGF binds to ErbB1 and ErbB4 to induce a subset of mitotic genes including c-Myc through phosphoinositol-3-kinase (PI3K) and MAPK pathways, resulting in cell proliferation (Elenius et al. 1997; Mishra et al. 2002; Jin et al. 2005). It is likely that overexpressed sHB-EGF in high expression HB-EGF TG mice acts on either progenitor cells or SCs or both, resulting in increased cell proliferation, as has been described previously in liver and brain (Kiso et al. 2003; Jin et al. 2005; Sugiura et al. 2005). The increased BrdU positive cells seen in our high expression HB-EGF TG mice are probably multi-potent progenitor cells, since we did not observe any increase of total SC number or in SC proliferative activity in the crypts with prominen-1 staining.
Homeostasis of the intestinal mucosa is highly regulated by the fast-paced balance of cell proliferation and cell death (Gordon and Hermiston 1994). Based on this, we also examined the effect of overexpressed HB-EGF on IEC death. We found significantly increased numbers of apoptotic IEC in low expression TG mice. High expression TG mice also had higher apoptotic cell numbers compared to WT mice, although this did not reach statistical significance. The cytoplasm of some TUNEL positive cells also shows cleaved caspase 3, supporting typical apoptotic cell death. Previous studies have demonstrated that proHB-EGF enhances apoptosis in other cell types (Iwamoto et al. 1999; Lin et al. 2001; Pan et al. 2002; Khai et al. 2006; Rocks et al. 2008; Uetani et al. 2009). In cardioblasts, lack of shedding of proHB-EGF enhanced apoptosis through caspase-3 and C-JUN N-terminal kinase signaling (Uetani et al. 2009). The majority of targeted overexpressed HB-EGF in our HB-EGF TG mice is in the proHB-EGF form, which appears to promote apoptotic activity in the intestinal villi. In general, it appears that our HB-EGF TG mice have both increased IEC proliferation and increased IEC apoptosis. This seems to be well balanced, since no evidence of hyperplasia, polyps, or tumors appear in the intestinal mucosa after long-term targeted overexpression of HB-EGF for up to 1.5 years.
The development of the different IEC lineages appears to be only slightly or nonaffected by overexpression of HB-EGF. Similarly, overexpression of HB-EGF does not appear to affect GALT in terms of T cell, B cell, or dendritic cell numbers. However, our data showed that overexpression of HB-EGF in TG mice protects the intestines from injury. Additionally, preliminary studies from our laboratory show that HB-EGF TG mice have augmented healing of intestinal anastomoses compared to WT mice (unpublished observations).
In summary, sustained overexpression of HB-EGF in the intestine appears to have relatively minor effects on the morphology of the intestines, or on the IEC lineage patterns present in the intestine, under basal conditions. However, overexpression of HB-EGF in TG mice results in protection of the intestines from stressful insults. Future studies will be designed to systematically examine the phenotype of HB-EGF TG compared with WT mice upon exposure to intestinal injury. Importantly, the long-term overexpression of HB-EGF in TG mice revealed no evidence of mucosal hyperplasia or tumor formation. These findings lend support to the possible future clinical administration of HB-EGF in studies designed to protect the intestines from injury.
Acknowledgments
We thank Dr Michael Robinson of the Transgenic and Embryonic Stem Cell Core at The Research Institute of Nationwide Children’s Hospital for assistance with generation of HB-EGF Transgenic mice, and Amy Stark Jingyuan Yang from The Ohio State University College of Medicine for assistance with the statistical analyses. This work was supported by NIH grants R01 GM61193 and R01 DK074611 (GEB).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahuja V, Dieckgraefe BK, Anant S. Molecular biology of the small intestine. Curr Opin Gastroenterol. 2006;22:90–94. doi: 10.1097/01.mog.0000203865.25384.65. [DOI] [PubMed] [Google Scholar]
- Besner GE, Klagsbrun M. Macrophages secrete a heparin-binding inhibitor of endothelial cell growth. Microvasc Res. 1991;42:187–197. doi: 10.1016/0026-2862(91)90086-q. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Loy A, Cen L, Chan C, Hsieh F-C, Cheng G, Wu B, Qualman SJ, Kunisada K, Yamauchi-Takihara K, et al. Signal transducer and activator of transcription 3 is involved in cell growth and survival of human rhabdomyosarcoma and osteosarcoma cells. BMC Cancer. 2007;7:111. doi: 10.1186/1471-2407-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KM, Brigstock DR, Johnson PR, Crissman-Combs MA, McCarthy DW, Downing MT, Besner GE. Production of glycosylated heparin-binding EGF-like growth factor in HeLa cells using vaccinia virus. Protein Expr Purif. 1996;8:57–67. doi: 10.1006/prep.1996.0074. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Xu DZ, Qi L. Different lymphocyte compartments respond differently to mitogenic stimulation after thermal injury. Ann Surg. 1990;211:72–77. doi: 10.1097/00000658-199001000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor and intestinal ischemia-reperfusion injury. Semin Pediatr Surg. 2004;13:2–10. doi: 10.1053/j.sempedsurg.2003.09.002. [DOI] [PubMed] [Google Scholar]
- El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2007;42:214–220. doi: 10.1016/j.jpedsurg.2006.09.055. [DOI] [PubMed] [Google Scholar]
- Feng J, El-Assal ON, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:167–174. doi: 10.1053/j.sempedsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006;41:742–747. doi: 10.1016/j.jpedsurg.2005.12.020. discussion 742–747. [DOI] [PubMed] [Google Scholar]
- Freeman H-J. Crypt region localization of intestinal stem cells in adults. World J Gastroenterol. 2008;14:7160–7162. doi: 10.3748/wjg.14.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JI, Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994;6:795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Mekada E. Heparin-binding EGF-like growth factor: A juxtacrine growth factor. Cytokine Growth Factor Rev. 2000;11:335–344. doi: 10.1016/s1359-6101(00)00013-7. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Handa K, Mekada E. Contact-dependent growth inhibition and apoptosis of epidermal growth factor (EGF) receptor-expressing cells by the membrane-anchored form of heparin-binding EGF-like growth factor. J Biol Chem. 1999;274:25906–25912. doi: 10.1074/jbc.274.36.25906. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Del Rio Guerra G, Jin L, Greenberg DA. Heparin-binding epidermal growth factor-like growth factor stimulates cell proliferation in cerebral cortical cultures through phosphatidylinositol 3′-kinase and mitogen-activated protein kinase. J Neurosci Res. 2005;81:497–505. doi: 10.1002/jnr.20510. [DOI] [PubMed] [Google Scholar]
- Khai NC, Takahashi T, Ushikoshi H, Nagano S, Yuge K, Esaki M, Kawai T, Goto K, Murofushi Y, Fujiwara T, et al. In vivo hepatic HB-EGF gene transduction inhibits Fas-induced liver injury and induces liver regeneration in mice: A comparative study to HGF. J Hepatol. 2006;44:1046–1054. doi: 10.1016/j.jhep.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Kiso S, Kawata S, Tamura S, Higashiyama S, Ito N, Tsushima H, Taniguchi N, Matsuzawa Y. Role of heparin-binding epidermal growth factor-like growth factor as a hepatotrophic factor in rat liver regeneration after partial hepatectomy. Hepatology. 1995;22:1584–1590. [PubMed] [Google Scholar]
- Kiso S, Kawata S, Tamura S, Inui Y, Yoshida Y, Sawai Y, Umeki S, Ito N, Yamada A, Miyagawa J-I, et al. Liver regeneration in heparin-binding EGF-like growth factor transgenic mice after partial hepatectomy. Gastroenterology. 2003;124:701–707. doi: 10.1053/gast.2003.50097. [DOI] [PubMed] [Google Scholar]
- Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: Inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- Liaudet L, Soriano F, Szabó E, Virág L, Mabley J, Salzman A, Szabo C. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose) polymerase. Proc Natl Acad Sci USA. 2000;97:10203–10208. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Hutchinson L, Gaston SM, Raab G, Freeman MR. BAG-1 is a novel cytoplasmic binding partner of the membrane form of heparin-binding EGF-like growth factor: A unique role for proHB-EGF in cell survival regulation. J Biol Chem. 2001;276:30127–30132. doi: 10.1074/jbc.M010237200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, double cortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- Mishima K, Higashiyama S, Nagashima Y, Miyagi Y, Tamura A, Kawahara N, Taniguchi N, Asai A, Kuchino Y, Kirino T. Regional distribution of heparin-binding epidermal growth factor-like growth factor mRNA and protein in adult rat forebrain. Neurosci Lett. 1996;213:153–156. doi: 10.1016/0304-3940(96)12850-0. [DOI] [PubMed] [Google Scholar]
- Mishra R, Leahy P, Simonson MS. Gene expression profiling reveals role for EGF-family ligands in mesangial cell proliferation. Am J Physiol Renal Physiol. 2002;283:F1151–F1159. doi: 10.1152/ajprenal.00103.2002. [DOI] [PubMed] [Google Scholar]
- Mori S, Tanaka M, Nanba D, Nishiwaki E, Ishiguro H, Higashiyama S, Matsuura N. PACSIN3 binds ADAM12/meltrin alpha and up-regulates ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J Biol Chem. 2003;278:46029–46034. doi: 10.1074/jbc.M306393200. [DOI] [PubMed] [Google Scholar]
- Nanba D, Mammoto A, Hashimoto K, Higashiyama S. Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. J Cell Biol. 2003;163:489–502. doi: 10.1083/jcb.200303017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37:S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- Nishi E, Prat A, Hospital V, Elenius K, Klagsbrun M. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J. 2001;20:3342–3350. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Sengoku K, Goishi K, Takuma N, Yamashita T, Wada K, Ishikawa M. The soluble and membrane-anchored forms of heparin-binding epidermal growth factor-like growth factor appear to play opposing roles in the survival and apoptosis of human luteinized granulosa cells. Mol Hum Reprod. 2002;8:734–741. doi: 10.1093/molehr/8.8.734. [DOI] [PubMed] [Google Scholar]
- Perrella MA, Maki T, Prasad S, Pimental D, Singh K, Takahashi N, Yoshizumi M, Alali A, Higashiyama S, Kelly RA. Regulation of heparin-binding epidermal growth factor-like growth factor mRNA levels by hypertrophic stimuli in neonatal and adult rat cardiac myocytes. J Biol Chem. 1994;269:27045–27050. [PubMed] [Google Scholar]
- Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–F199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- Rocks N, Estrella C, Paulissen G, Quesada-Calvo F, Gilles C, Gueders MM, Crahay C, Foidart JM, Gosset P, Noel A, et al. The metalloproteinase ADAM-12 regulates bronchial epithelial cell proliferation and apoptosis. Cell Prolif. 2008;41:988–1001. doi: 10.1111/j.1365-2184.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocourt DV, Mehta VB, Besner GE. Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. J Surg Res. 2007;139:269–273. doi: 10.1016/j.jss.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol. 2008;22:391–409. doi: 10.1016/j.bpg.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: Intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Singh AB, Sugimoto K, Harris RC. Juxtacrine activation of epidermal growth factor (EGF) receptor by membrane-anchored heparin-binding EGF-like growth factor protects epithelial cells from anoikis while maintaining an epithelial phenotype. J Biol Chem. 2007;282:32890–32901. doi: 10.1074/jbc.M702677200. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Kitagawa K, Tanaka S, Todo K, Omura-Matsuoka E, Sasaki T, Mabuchi T, Matsushita K, Yagita Y, Hori M. Adenovirus-mediated gene transfer of heparin-binding epidermal growth factor-like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke. 2005;36:859–864. doi: 10.1161/01.STR.0000158905.22871.95. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Higashiyama S, Wood K, Pollitt NS, Damm D, McEnroe G, Garrick B, Ashton N, Lau K, Hancock N. Characterization of sequences within heparin-binding EGF-like growth factor that mediate interaction with heparin. J Biol Chem. 1994;269:2541–2549. [PubMed] [Google Scholar]
- Uetani T, Nakayama H, Okayama H, Okura T, Higaki J, Inoue H, Higashiyama S. Insufficiency of pro-heparin-binding epidermal growth factor-like growth factor shedding enhances hypoxic cell death in H9c2 cardiomyoblasts via the activation of caspase-3 and c-Jun N-terminal kinase. J Biol Chem. 2009;284:12399–12409. doi: 10.1074/jbc.M900463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li J, Ying Wang C, Yan Kwok AH, Leung FC. Epidermal growth factor (EGF) receptor ligands in the chicken ovary: I. Evidence for heparin-binding EGF-like growth factor (HB-EGF) as a potential oocyte-derived signal to control granulosa cell proliferation and HB-EGF and kit ligand expression. Endocrinology. 2007;148:3426–3440. doi: 10.1210/en.2006-1383. [DOI] [PubMed] [Google Scholar]
- Xia G, Martin AE, Michalsky MP, Besner GE. Heparin-binding EGF-like growth factor preserves crypt cell proliferation and decreases bacterial translocation after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2002;37:1081–1087. doi: 10.1053/jpsu.2002.33881. [DOI] [PubMed] [Google Scholar]
- Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434–439. doi: 10.1053/jpsu.2003.50075. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Kinsey GR, Rasbach K, Schnellmann RG. Heparin-binding epidermal growth factor and Src family kinases in proliferation of renal epithelial cells. Am J Physiol Renal Physiol. 2008;294:F459–F468. doi: 10.1152/ajprenal.00473.2007. [DOI] [PubMed] [Google Scholar]
- Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Tsutsui S, Sugimachi M, Higashimoto Y, Kanayama S, Matsuzawa Y. Role of heparin-binding EGF-related peptides in proliferation and apoptosis of activated ras-stimulated intestinal epithelial cells. Int J Cancer. 1997;73:917–923. doi: 10.1002/(sici)1097-0215(19971210)73:6<917::aid-ijc26>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]