Abstract
Brown adipose tissue (BAT) is classically activated by sympathetic nervous stimulation resulting from exposure to cold. Feeding a high-fat diet also induces development of brown fat, but is decreased by caloric restriction. Blood ketone bodies, which function as alternative energy substrates to glucose, are increased during caloric restriction. Here we discuss the unexpected observation that feeding an ester of ketone bodies to the mouse, which increases blood ketone body concentrations, results in an activation of brown fat. The mechanism of this activation of brown fat is similar to that occurring from cold exposure in that cyclic adenosine monophosphate (AMP) levels are increased as are levels of the transcription factor cyclic AMP–responsive element–binding protein, which is also increased by ketone ester feeding. Other effects of feeding ketone esters, in addition to their ability to induce brown fat, are discussed such as their ability to overcome certain aspects of insulin resistance and to ameliorate the accumulation of amyloid and phosphorylated tau protein in brain, and improve cognitive function, in a triple transgenic mouse model of Alzheimer’s disease.
Keywords: ketones, insulin resistance, mitochondria, Alzheimer’s disease
Introduction
Brown fat is hypertrophied by cold exposure and its development and metabolic activation are controlled by the sympathetic nervous system.1,2 Feeding a high-fat diet also increases the thermogenic activity of brown fat.3 The elevated blood lipids resulting from a high-fat diet increase the transcription factors, peroxisome proliferator–activated receptor α and γ,4 leading to the transcription of uncoupling protein 1 while also increasing brown fat adipocyte differentiation. In addition, it has been shown that simply overeating on the so-called cafeteria diet induces the development and activation of brown fat5 whereas caloric restriction decreases the development and activation of brown fat.6 The physiological response to fasting in the human is ketosis, where total blood ketone bodies rise to about 5–7 mM.7 Therefore the finding that ketosis induced by feeding ketone esters activated brown fat tissue in the mouse was surprising and is discussed further below.
Ketone ester feeding increases mitochondrial content of brown adipose tissue in the mouse
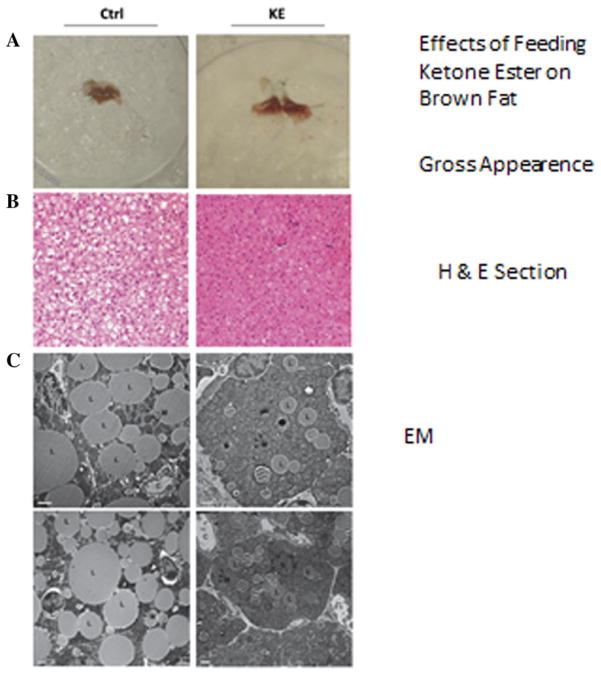
In a recent study investigating the effect of elevated ketone levels on brown adipose tissue (BAT) physiology,8 mice were pair-fed a diet containing 6% by weight of ketone esters and the control animals were pair-fed to the ketone ester–fed group, which consumed less food than the control group. The blood levels of D-β-hydroxybutyrate in ketone ester–fed mice were 7 mM. In addition, the ketone-fed mice had a twofold greater [18F]-fluordesoxyglucose (FDG) uptake than control animals. Feeding ketone ester was found to increase cyclic adenosine monophosphate (AMP) levels in BAT from 8 pmol to 13 pmol/mg protein, and doubled the transcription factor cyclic AMP–responsive element–binding protein, which is consistent with an increase in mitochondrial electron transport proteins. On the basis of this evidence, it would appear that feeding ketone esters increased the metabolic activity and presumably heat production in BAT in the mouse. The increase in FDG uptake in mice fed a ketone ester diet differed from mice fed a high-fat ketogenic diet.7 Figure 1 illustrates gross morphological, cellular, and subcellular comparisons of BAT from mice on either the control or ketone ester–based diets. Figure 1A depicts gross morphological changes in BAT, and Figure 2B and C illustrate lower amounts of lipid-containing vesicles in the BAT from ketone ester–fed mice compared to controls.
Figure 1.
Feeding the ketone monoester to the mouse resulted in (A) a darker color in the brown adipose tissue, (B) reduced lipid content in hematoxylin and eosin (H&E) sections, (C) reduced lipid droplet size and increased mitochondrial number in electron microscopy slides.8
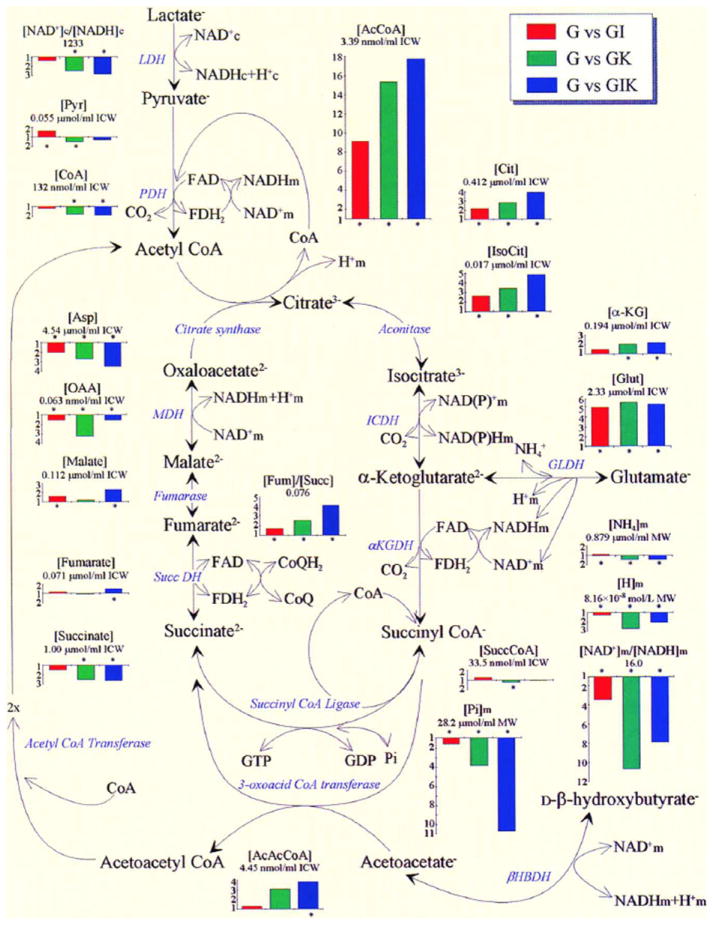
Figure 2.
A diagram depicting the Krebs cycle metabolite changes in the glucose-perfused working rat heart by addition of insulin, ketones, or a combination of the two.38
Ketogenic diet and ketone ester feeding compared
There are important differences between feeding ketone esters (as in the study described earlier) and feeding a ketogenic diet. Ketogenic diets have been fed to humans since they were proposed by Russell Wilder at the Mayo Clinic in 19219 following the finding by Hugh Conkin10 that fasting resulted in a cessation of seizures in a child with intractable epilepsy. Since that time, the ketogenic diet has been used to treat drug-resistant epilepsy.11 Because of their rapid absorption, blood ketone levels of any desired level can be achieved by feeding the ketone ester, whereas feeding a ketogenic diet may produce diverse levels of ketone bodies depending upon the amount of carbohydrate or protein of the diet. A high-fat diet can lead to significant elevation of blood ketones, but also to an elevation of blood free fatty acids which in humans leads to a deterioration in both physical and cognitive performance.12 In addition, feeding a ketogenic diet can lead to an elevation of blood cholesterol and triglycerides which is undesirable in anyone over 17 years of age because of it atherogenic potential.13
Ketone bodies and energy utilization
In addition to its effects on BAT physiology, feeding a ketone ester diet may be of benefit in the treatment of obesity since it decreased brain malonyl CoA, an important metabolic determinant of appetite.14 Although feeding ketone esters increased uncoupling protein and metabolic activity in BAT as estimated by FDG uptake, it is not proven that inducing ketosis would increase energy wasting in the whole animal. Indeed, based on the evidence from working perfused rat heart, the metabolism of ketones leads to an increase in the efficiency of the hydraulic work output of the heart, which reflects the inherent energy contained in the bond energy when comparing pyruvate versus D-β-hydroxybutyrate utilization.15 When comparing the hydraulic work (in Joules) per molecule of O2 consumed in the isolated working perfused heart (Table 1), the addition of either 4 mM ketone bodies or insulin increased the efficiency of work output by about 30%—similar to the heats of combustion of the substrate molecules (ketones versus glucose).
Table 1.
Effect of insulin and ketones on physiological parameters and efficiency of the working rat heart
| Control glucose n = 8 | Insulin n = 5 | Ketone n = 5 | Ketone + insulin n = 5 | |
|---|---|---|---|---|
| Cardiac output (mL/min/g wet wt) | 24.9 ± 0.6 | 26.7 ± 0.9 | 28.3 ± 0.9 | 25.8 ± 0.7 |
| Hydraulic work (J/min/g wet wt) | 0.30 ± 0.01 | 0.34 ± 0.01* | 0.37 ± 0.01* | 0.32 ± 0.01 |
| O2 consumption (μmol/min/g wet wt) | 6.5 ± 0.1 | 5.6 ± 0.1* | 6.0 ± 0.2* | 5.4 ± 0.4* |
| Cardiac efficiency (%) | 10.5 ± 0.3 | 13.4 ± 0.6 * | 13.0 ± 0.2* | 14.3 ± 1.3* |
P ≤ 0.05.
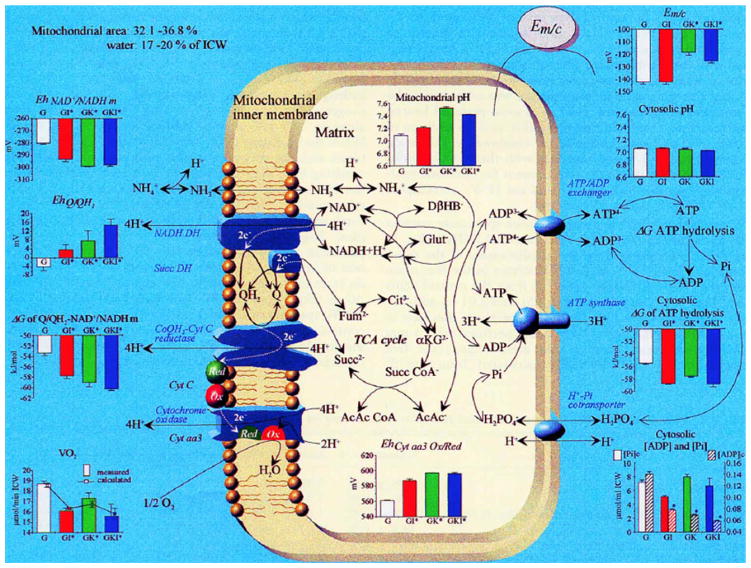
Insulin acts on energy production by activation of pyruvate dehydrogenase (PDH).16,17 This is shown by the ninefold increase in the product of PDH, acetyl CoA, by the addition of insulin to the glucose-perfused isolated working heart (Fig. 2). Adding ketone bodies to the perfusion increases acetyl CoA 15-fold, more than duplicating the metabolic effect of insulin (Fig. 2). The addition of both insulin and ketones leads to the reduction of free mitochondrial NAD+/NADH while at the same time increasing the fumarate/succinate ratio indicating an oxidation of the free coenzyme Q/reduced coenzyme Q ratio (Fig. 3). This increase in the redox span between sites I and II of the electron transport system results in an increase in ΔG′ of the mitochondrial NAD/Q couple and its resultant increase in the ΔG′ of mitochondria proton gradient and the ΔG′ of ATP hydrolysis. It should be emphasized that the values of the free nucleotide ratios are calculated from measured ratios of metabolites taking part in the near equilibrium reactions as described by Bucher and Klingenberg;18 Williamson et al.;19 Krebs and Veech;20 and Veech et al.21 Measurements of total NAD or NADH, as more recently reported in the cell biology literature,22–24 are insufficient energetic determinants because such values give no information about either the thermodynamics or kinetics of the intracellular reactions because of the near complete compartmentation of these nucleotides.
Figure 3.
Effects of the addition of insulin, ketone, or the combination on mitochondrial energy parameters in the glucose-perfused isolated working rat heart.38
The ability of ketones to mimic the metabolic and energetic effects of insulin demonstrates that ketones can overcome the effects of insulin resistance. Injury of any sort to the cell results in insulin resistance and is usually indicated by an increase in blood sugar levels characteristic of the injury. It then follows that resuscitation or fluids used in the treatment of, for example, a hemorrhage or burns would be more effective if they contained ketone bodies which could overcome the insulin resistance associated with injury.25,26 Use of fluids containing either D or L lactic acid provide no metabolic benefit in the presence of a metabolic block in PDH since the lactate cannot be further metabolized. Fluid replacements based on volumetric considerations alone without consideration of the metabolic and energetic properties of the cell are of little value and can, in fact, be harmful.27
The effects of ketone ester feeding on the triple transgenic mouse model of Alzheimer’s disease
Insulin resistance in the brains of patients with Alzheimer’s disease28 is associated with insulin-like growth factor 1 (IGF-1) resistance, impaired regulation of the insulin receptor substrate 1 (IRS-1), and cognitive decline. This insulin resistance is not corrected by direct addition of insulin to brain tissue28 where decreased cerebral glucose utilization observed by FDG-positron emission tomography (PET) precedes the cognitive impairment of Alzheimer’s disease.29,30 Direct addition of ketones to hippocampal neuronal cultures has been shown to decrease cell death that resulted from the addition of amyloid-β to the culture media.31 In the same study, the addition of ketones to cultures of mesencephalic neurons provided protection from cell death induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a free radical–inducing agent.
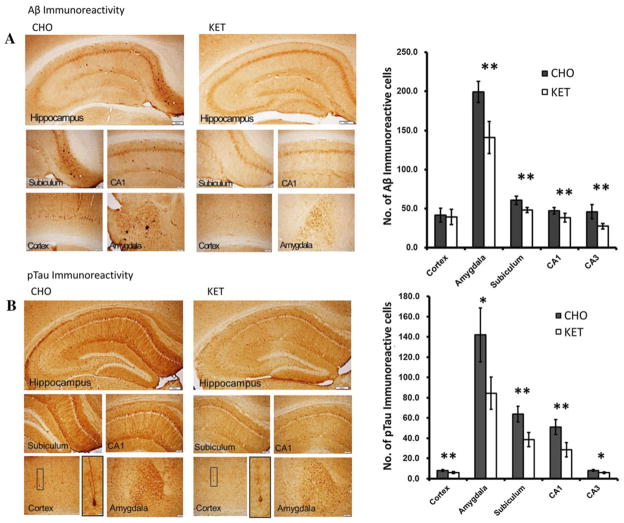
A recent study demonstrated that feeding ketone esters to triple transgenic mouse models of Alzheimer’s disease resulted in an improvement in cognitive function and a decrease in brain amyloid-β and phosphorylated tau, as observed by histological examination of brain sections stained for amyloid-β and phosphorylated tau proteins (Fig. 4).32 In a study recently reported from the Karolinska Institute, the addition of oral dietary energy substrates, including β-hydroxybutyrate, to standard animal diets reversed the energy deficits in a different transgenic mouse model of Alzheimer’s disease.33 These authors concluded that adding simple energy substrates to the diet could reverse the hyperexcitability observed in mouse models of Alzheimer’s disease. Demonstrations that mild ketosis can correct the pathological and behavioral changes in triple transgenic mouse models of Alzheimer’s disease suggests that ketosis could potentially be of therapeutic benefit in the treatment and prevention of Alzheimer’s disease in humans. Further, the ability of ketones to prevent free radical damage suggests the possibility that ketones could be an important protective agent in the treatment of both Parkinson and Alzheimer’s disease.
Figure 4.
Effects of ketone feeding on brain amyloid and phosphorylated tau in a transgenic mouse model of Alzheimer’s disease.39
The ability of ketone metabolism to protect cells from free radical damage results from the ketone’s ability to reduce the free NADP nucleotide system.34 More recently it has been shown that D-β-hydroxybutyrate induces a transcription factor that increases the production of superoxide dismutase and metalothionine, both important in the destruction of free radicals. It has also recently been reported that a genetic variant in the triggering receptor expressed on myeloid cells 2 (TREM2), which increases free radical formation, is associated with late-onset Alzheimer’s disease.35,36 In addition, it has been shown that D-β-hydroxybutyrate is an endogenous inhibitor of histone deacetylase 1. This leads to increased acetylation at the FOXO3A and MT2 promoter sites, conferring protection against oxidative stress by induction of the FOX1 pathway and the enzymes superoxide dismutase and catalase, important in the detoxification of O2 free radicals.37
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev. 1969;49:330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 3.Mercer SW, Trayhurn P. Effect of high fat diets on the thermogenic activity of brown adipose tissue in cold-acclimated mice. J Nutr. 1984;114:1151–1158. doi: 10.1093/jn/114.6.1151. [DOI] [PubMed] [Google Scholar]
- 4.Barbera MJ, et al. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. doi: 10.1074/jbc.M006246200. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell NJ, Stock MJ. Effect of chronic food restriction on energy balance, thermogenic capacity, and brown-adipose-tissue activity in the rat. Biosci Rep. 1982;2:543–549. doi: 10.1007/BF01314214. [DOI] [PubMed] [Google Scholar]
- 7.Owen OE, et al. Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969;48:574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S, et al. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 2012;26:2351–2362. doi: 10.1096/fj.11-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilder RM. The effects of ketonemia on the course of epilepsy. Clin Bull. 1921;2:308. [Google Scholar]
- 10.Conklin HW. Cause and treatment of epilepsy. J Am Osteopath Assoc. 1922;26:11–14. [Google Scholar]
- 11.Freeman JM, Vining EPG. Intractable epilepsy. Epilepsia. 1992;33:1132–1136. doi: 10.1111/j.1528-1157.1992.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 12.Murray AJ, et al. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009;23:4353–4360. doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- 13.Kwiterovich PO, Jr, et al. Effect of a high-fat ke-togenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA. 2003;290:912–920. doi: 10.1001/jama.290.7.912. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwaya Y, et al. A ketone ester diet increased brain malonyl CoA and uncoupling protein 4 and 5 while decreasing food intake in the normal Wistar rat. J Biol Chem. 2010;285:25950–25956. doi: 10.1074/jbc.M110.138198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee C, Jungas RL. Activation of pyruvate dehydrogenase in adipose tissue by insulin. Evidence for an effect of insulin on pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1975;148:229–235. doi: 10.1042/bj1480229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denton RM, et al. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975;9:27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- 18.Bucher T, Klingenberg M. Wege des Wasserstoffs in der lebendigen Organisation. Angewandte Chemie. 1958;70:552–570. [Google Scholar]
- 19.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krebs HA, Veech RL. Pyridine nucleotide interrelations. In: Papa S, Tager JM, Quagliariello E, Slater EC, editors. The Energy Level and Metabolic Conntrol in Mitochondria. Bari: Adriatica Editrice; 1969. pp. 329–383. [Google Scholar]
- 21.Veech RL, et al. Cytosolic phosphorylation potential. J Biol Chem. 1979;254:6538–6547. [PubMed] [Google Scholar]
- 22.Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 23.Blander G, Guarente L. The sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard BP, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberthal W, et al. Comparison of the effects of a 50% exchange–transfusion with albumin, hetastarch, and modified hemoglobin solutions. Shock. 2002;17:61–69. doi: 10.1097/00024382-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Valeri CR, Ragno G, Veech RL. Severe adverse events associated with hemoglobin based oxygen carriers: role of resuscitative fluids and liquid preserved RBC. Transfusion Aphr Sci. 2008;39:205–211. doi: 10.1016/j.transci.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Veech RL. The toxic impact of parenteral solutions on the metabolism of cells: a hypothesis for physiological parenteral therapy. Am J Clin Nutr. 1986;44:519–551. doi: 10.1093/ajcn/44.4.519. [DOI] [PubMed] [Google Scholar]
- 28.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small GW, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashiwaya Y, et al. D-beta -Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashiwaya Y, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zilberter M, et al. Dietary energy substrates reverse early neuronal hyperactivity in a mouse model of Alzheimer’s disease. J Neurochem. 2013;125:157–171. doi: 10.1111/jnc.12127. [DOI] [PubMed] [Google Scholar]
- 34.Kashiwaya Y, King MT, Veech RL. Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Am J Cardiol. 1997;80:50A–64A. doi: 10.1016/s0002-9149(97)00458-x. [DOI] [PubMed] [Google Scholar]
- 35.Neumann H, Daly MJ. Variant TREM2 as risk factor for Alzheimer’s disease. N Engl J Med. 2013;368:182–184. doi: 10.1056/NEJMe1213157. [DOI] [PubMed] [Google Scholar]
- 36.Jonsson T, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimazu T, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, et al. Insulin, ketone bodies and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 39.Kashiwaya Y, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2012;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]