Abstract
Purpose
Selenium has been reported to have chemopreventive benefits in lung cancer. We conducted a double-blind, placebo-controlled trial to evaluate the incidence of second primary tumors (SPTs) in patients with resected non–small-cell lung cancer (NSCLC) receiving selenium supplementation.
Patients and Methods
Patients with completely resected stage I NSCLC were randomly assigned to take selenized yeast 200 μg versus placebo daily for 48 months. Participation was 6 to 36 months postoperatively and required a negative mediastinal node biopsy, no excessive vitamin intake, normal liver function, negative chest x-ray, and no other evidence of recurrence.
Results
The first interim analysis in October 2009, with 46% of the projected end points accumulated, showed a trend in favor of the placebo group with a low likelihood that the trial would become positive; thus, the study was stopped. One thousand seven hundred seventy-two participants were enrolled, with 1,561 patients randomly assigned. Analysis was updated in June 2011 with the maturation of 54% of the planned end points. Two hundred fifty-two SPTs (from 224 patients) developed, of which 98 (from 97 patients) were lung cancer (38.9%). Lung and overall SPT incidence were 1.62 and 3.54 per 100 person-years, respectively, for selenium versus 1.30 and 3.39 per 100 person-years, respectively, for placebo (P = .294). Five-year disease-free survival was 74.4% for selenium recipients versus 79.6% for placebo recipients. Grade 1 to 2 toxicity occurred in 31% of selenium recipients and 26% of placebo recipients, and grade ≥ 3 toxicity occurred in less than 2% of selenium recipients versus 3% of placebo recipients. Compliance was excellent. No increase in diabetes mellitus or skin cancer was detected.
Conclusion
Selenium was safe but conferred no benefit over placebo in the prevention of SPT in patients with resected NSCLC.
INTRODUCTION
Lung cancer is the leading cause of cancer-related death and an urgent target for prevention.1 However, the multiple factors that contribute to lung carcinogenesis and the high degree of heterogeneity of the disease complicate the development of effective preventive strategies.2,3 Cigarette smoking, the main risk factor, accounts for 70% to 90% of cases,1 and other environmental and occupational exposures, familial and dietary factors, and perhaps single-gene mutations3 are also associated with lung carcinogenesis. After smoking cessation, there is an increased risk of developing lung cancer for at least 30 to 40 years,4 and consequently, former smokers make up nearly 50% of all new lung cancer cases in developed countries.
Primary chemoprevention attempts to prevent cancer in healthy at-risk individuals. Secondary chemoprevention aims to retard or stop the development of cancer in precancerous lesions. Tertiary chemoprevention focuses on the prevention of a second primary tumor (SPT) in patients who have had presumably curative treatment. Previous attempts at primary and secondary chemoprevention with β-carotene,5 α-tocopherol,6 and several retinoids7,8 have largely shown no benefit of these agents.6,9 Positive outcomes for vitamin B12,10 folic acid,10 aspirin,11 and the prostaglandin pathway modulators celecoxib and iloprost12–14 have been achieved. Although smaller phase III trials attempting tertiary prevention of SPT development reported positive outcomes with isotretinoin15 and retinyl palmitate,16 larger phase III studies reported negative outcomes with lower doses of retinyl palmitate17 and 13-cis-retinoic acid.18,19
Supplementation with the trace element selenium, together with β-carotene and vitamin E, was associated with a significantly lower cancer mortality rate in a study conducted in the Chinese Linxian Province, a region with epidemic rates of squamous esophageal and adenomatous gastric cancers.20 A subsequent study in more than 2,000 participants showed a highly significant inverse association of serum selenium levels with the incidence of esophageal (relative risk, 0.47; 95% CI, 0.33 to 0.65) and gastric cancers (relative risk, 0.56; 95% CI, 0.44 to 0.71).21
The Nutritional Prevention of Cancer Trial randomly assigned 1,312 participants with a history of basal cell or squamous cell carcinoma of the skin to selenium supplements or placebo. A secondary analysis showed a statistically significant decrease in lung cancer incidence with selenium supplementation.22 After a longer follow-up of 7.9 years, this trend remained, but the difference was no longer significant.23 A subgroup analysis showed a nominally significant decrease among individuals with low baseline selenium concentrations (hazard ratio, 0.42; 95% CI, 0.18 to 0.96; P = .04). This finding has also been reported by others.24
On the basis of these results, we conducted a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of selenium supplementation in reducing the incidence of lung SPTs in patients who had been treated for stage I non–small-cell lung cancer (NSCLC) with complete surgical resection. Correlative studies for gene promoter methylation have been reported elsewhere.25
PATIENTS AND METHODS
The objectives of the study were to evaluate the efficacy of selenium supplementation in reducing the incidence of lung SPTs in patients who had been treated for stage I NSCLC; to evaluate the qualitative and quantitative toxicity of daily selenium supplementation; and to compare the incidence of specific cancers, mortality from cancer, and overall survival of patients treated with selenium supplementation versus placebo.
Patient Eligibility
Eligibility criteria included the following: age ≥ 18 years; 6 to 36 months from complete resection of histologically proven stage IA (pT1N0) or stage IB (pT2N0) NSCLC (carcinoid tumors were excluded); pathologic stage N0 confirmed by sampling at least one mediastinal lymph node at resection; chest x-ray or computed tomography scan ≤ 8 weeks before registration without sign of new or recurrent lung cancer; no concurrent cancers or any other prior cancer history within the past 5 years, except localized nonmelanoma skin cancer; no synchronous lesions (lung + nonlung) or metastasis, even if resectable; no history of greater than one lung cancer primary tumor at any time; normal hepatic function (total bilirubin and AST or ALT ≤ institutional upper limit of normal); laboratory values (including CBC) obtained within 8 weeks before registration; and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Patients with stage IA NSCLC should not have received any therapy other than surgery. Patients with stage IB NSCLC were allowed to have received other primary therapy (chemotherapy, radiotherapy, or biologic therapy) provided this was completed at least 6 months before study registration and all treatment-related symptoms had subsided before study registration.
Supplements were defined as any nonfood compound taken by mouth or injection to provide dietary factors. Supplements containing ≥ 70 μg of selenium taken regularly (≥ three times per week for ≥ 4 consecutive weeks during the prior year) were required to be discontinued ≥ 1 month before registration. Supplements containing ≤ 70 μg of selenium were continued throughout study participation. Supplements not containing selenium were either discontinued ≥ 2 weeks before study entry or continued throughout study participation.
Random Assignment and Treatment
Compliance was tested over a 4-week run-in period, and patients who qualified as compliant (taking ≥ 75% of their daily placebo tablets) by patient diary review and pill count were randomly assigned 2:1 to receive either selenium in the form of selenized yeast or an identical-appearing placebo. Treatment assignments for patients at all institutions were obtained from the Central Randomization Desk at the ECOG Coordinating Center. Treatments were assigned using permuted blocks within strata with dynamic balancing within main institutions and their affiliate networks. Stratification factors were smoking status (current, former, or never), sex, and stage (IA v IB with other therapy v IB without other therapy). Patients took one tablet daily in the morning for eight cycles (one cycle = 6 months), for a total of 4 years. Compliance was reported every 3 months after random assignment using the E5597 Pill Count/Compliance Form.
Selenium was supplied for this study in tablets containing 200 μg in the form of selenized yeast or placebo yeast by Cypress Systems (Fresno, CA) and was distributed by Proclinical Pharmaceutical Services (Phoenixville, PA). Institutions obtained study drug or placebo from the National Cancer Institute by completing a drug request form.
Statistical Analysis
The original accrual goal for this phase III trial was 1,960 patients to enter the compliance run-in period. On the basis of prior clinical trials, ≥ 90% of participants were expected to prove compliant, resulting in a minimum of 1,764 patients to be randomly assigned.
The primary end point was the incidence of lung SPT, which the design assumed would occur at a constant rate of two per 100 person-years of follow-up.26 The study was designed to detect a reduction of 40% in this rate, to 1.2 per 100 person-years of follow-up. A two-sided, P = .05 level log-rank test was used to compare the groups, adjusted for sequential monitoring. The study had a power of 80% to detect the target alternative when a total of 180 events (occurrences of SPT) had been observed. Formal interim analyses were scheduled when 90 and 135 events had been observed (50% and 75% information, respectively). Early stopping for treatment differences was based on an O'Brien-Fleming boundary, with nominal two-sided P values of .003, .018, and .044 (the exact values changed slightly according to the number of observed events at the interim analyses).
The data as of June 2011 were analyzed based on the intent-to-treat principle, including all patients regardless of eligibility and treatment status. The distribution of time-to-event data (time to lung SPT, disease-free survival [DFS], and overall survival [OS]) was estimated using the Kaplan-Meier method. Differences in treatment effect were evaluated using the log-rank test. All reported P values are based on two-sided testing. Incidence rate was estimated by dividing the number of patients with lung SPT by total number of person-years followed.
Monitoring History
In 2003, the ECOG Data Monitoring Committee (DMC) reviewed the report of increased nonmelanoma skin cancer risk in patients with prior history of nonmelanoma skin cancer receiving selenium supplementation.27 The DMC recommended modifying the protocol to indicate that all basal and squamous cell skin cancers must be reported as adverse events. In 2007, the DMC discussed reports indicating a possible association of long-term selenium use with increased type 2 diabetes risk.28,29 Study E5597 was not designed to collect diabetes-related data, but adverse events reported were reviewed. The DMC recommended modifying the protocol to collect diabetes surveillance data, amending the consent form to indicate a potential slight increase in diabetes risk with long-term selenium use and providing letters to notify investigators and patients on study of the potential risk.
On October 21, 2009, the DMC reviewed the first planned interim analysis of the primary end point and determined that it was highly unlikely that this study could eventually show significant evidence of benefit from selenium. On the basis of the DMC recommendation, on November 5, 2009, the ECOG decided that accrual should be discontinued and all current patients should discontinue selenium/placebo tablets and enter the follow-up phase for recurrence and survival.
RESULTS
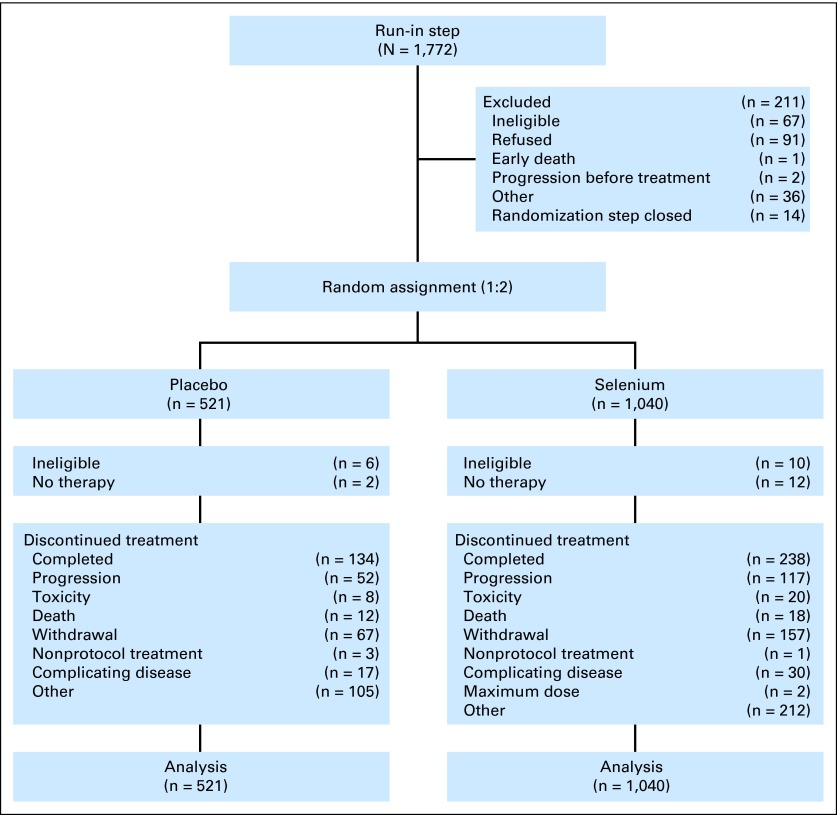
This study accrued 1,772 patients to step 1 and randomly assigned 1,561 patients to step 2 between October 6, 2000, and November 5, 2009 (Fig 1; Appendix Table A1, online only). Appendix Figure A1 (online only) displays the study schema, and Table 1 lists patients' baseline characteristics.
Fig 1.
CONSORT diagram.
Table 1.
Baseline Demographics and Clinical Characteristics of the Study Patient Population
| Characteristic | Treatment |
|||
|---|---|---|---|---|
| Placebo (n = 521) |
Selenium (n = 1,040) |
|||
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 66 | 66 | ||
| Range | 38-86 | 24-93 | ||
| Smoking history | ||||
| Active or stopped < 1 year ago | 150 | 28.8 | 298 | 28.7 |
| Stopped ≥ 1 year ago | 321 | 61.6 | 646 | 62.1 |
| Never smoked or ≤ 100 cigarettes | 50 | 9.6 | 96 | 9.2 |
| Sex | ||||
| Male | 250 | 48 | 509 | 49 |
| Female | 271 | 52 | 531 | 51 |
| Stage | ||||
| Ia | 339 | 65.1 | 677 | 65.1 |
| Ib with previous therapy* | 15 | 3.9 | 30 | 2.9 |
| Ib with no therapy | 159 | 30.5 | 312 | 30 |
| Missing | 8 | 1.5 | 21 | 2 |
Other primary therapy in addition to surgery (adjuvant chemotherapy, radiation therapy, or biologic agent).
At the first interim analysis, there were 83 cases of lung SPT, or 46% of the originally planned end points. The incidence rates of lung SPT were 1.91 per 100 person-years followed versus 1.36 per 100 person-years followed in the selenium and placebo treatment arms, respectively. Overall, the SPT incidence rate was higher in the selenium arm but not significantly. Five-year DFS was 72% for selenium versus 78% for placebo. The DMC recommended that this study be terminated based on the futility analysis.
As of June 2011, there were 252 reported SPTs among 224 participants (one patient developed four separate basal cell carcinomas, three patients reported three neoplasms, 19 patients developed SPT at two different sites, and the remainder developed one SPT; Table 2). Overall incidence rates of SPTs were 3.54 per 100 person-years followed versus 3.39 per 100 person-years followed in the selenium and placebo arms, respectively. Of these SPTs, 98 (from 97 patients) were lung cancers (69 patients from the selenium arm and 28 patients from the placebo arm), corresponding to 54% of the originally planned end points. The incidence rates of lung SPT were 1.62 per 100 person-years followed versus 1.30 per 100 person-years followed in the selenium and placebo arms, respectively. This difference was not significant by log-rank test (P = .294) after adjusting for the first interim analysis.
Table 2.
Incidence of SPTs by Treatment Arm (252 SPTs observed in 224 patients)
| Site of SPT | Placebo |
Selenium |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Breast | 2 | 2.4 | 8 | 4.7 |
| Colon | 3 | 3.6 | 3 | 1.8 |
| Rectum | 1 | 1.2 | 1 | 0.6 |
| Gastric | 0 | 0.0 | 1 | 0.6 |
| Pancreas | 3 | 3.6 | 5 | 3.0 |
| Esophagus | 0 | 0.0 | 1 | 0.6 |
| Liver, gallbladder, bile duct | 0 | 0.0 | 6 | 3.6 |
| Small Intestines | 0 | 0.0 | 1 | 0.6 |
| Head and neck | 2 | 2.4 | 4 | 2.4 |
| Thyroid | 2 | 2.4 | 2 | 1.2 |
| Myeloma | 0 | 0.0 | 2 | 1.2 |
| Chronic lymphocytic leukemia | 1 | 1.2 | 1 | 0.6 |
| Acute non–lymphocytic leukemia, AML | 1 | 1.2 | 0 | 0.0 |
| Chronic myelogenous leukemia | 0 | 0.0 | 1 | 0.6 |
| Myelodysplastic syndrome | 1 | 1.2 | 4 | 2.4 |
| Lung cancer | 23 | 28.1 | 58 | 34.1 |
| Small-cell lung | 3 | 3.6 | 3 | 1.8 |
| Non–small-cell lung | 2 | 2.4 | 9 | 5.4 |
| Melanoma | 2 | 2.4 | 5 | 3.0 |
| Basal cell carcinoma | 13 | 15.9 | 14 | 8.3 |
| Skin cancer not melanoma | 6 | 7.2 | 11 | 6.6 |
| Kaposi's sarcoma | 1 | 1.2 | 0 | 0.0 |
| Bladder, urinary tract | 6 | 7.3 | 9 | 5.2 |
| Prostate | 9 | 11.0 | 16 | 9.5 |
| Cervix | 0 | 0.0 | 1 | 0.6 |
| Chest wall | 0 | 0.0 | 1 | 0.6 |
| Vocal cord | 1 | 1.2 | 0 | 0.0 |
| Inguen (groin) | 0 | 0.0 | 1 | 0.6 |
| Left groin lymph node | 0 | 0.0 | 1 | 0.6 |
| Unknown | 1 | 1.2 | 0 | 0.0 |
| Total | 83 | 169 | ||
Abbreviations: AML, acute myeloid leukemia; SPT, second primary tumor.
DFS and OS
DFS was defined as the time from random assignment to lung SPT or recurrence. A total of 337 patients experienced an event with respect to the DFS end point, including 97 patients with lung SPTs and 240 patients with recurrence, of whom 169 were from the selenium arm (54% local v 46% distant recurrence) and 71 were from the placebo arm (61% local v 39% distant recurrence). Among the selenium patients, active smokers had a 30% risk of recurrence or SPT versus a risk of 24% for former smokers and 20% for never-smokers. The 5-year DFS rate was 74.4% (SE, 1.0%) in the selenium arm and 79.6% (SE, 2.1%) in the placebo arm (P = .069, log-rank test; Fig 2A).
Fig 2.
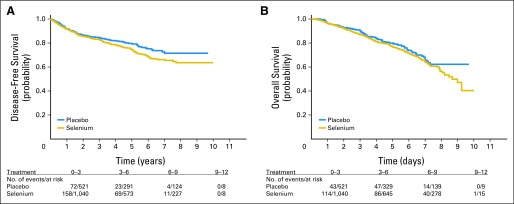
Kaplan-Meier plots of (A) disease-free survival and (B) overall survival by treatment.
OS was defined as the time from random assignment to death or last known follow-up date. The 5-year OS rate was 76.8% (SE, 1.6%) in the selenium arm and 79.9% (SE, 2.1%) in the placebo arm (P = .154, log-rank test; Fig 2B). The OS distribution was significantly different by smoking status (P = .027, log-rank test); in active smokers or those who had stopped smoking within 1 year, 3-year OS was 85.5% (SE, 1.7%) and 5-year OS was 74.9% (SE, 2.4%), whereas in never-smokers, 3-year OS was 90% (SE, 2.8%) and 5-year OS was 83.6% (SE, 3.6%). There were no significant differences in DFS distribution by smoking status (P = .245). When the initial date of surgery was used instead of the date of random assignment, the results of the DFS and OS analyses were unchanged (median time from surgery to random assignment was approximately 10 months in both arms).
Toxicity
Toxicity was assessed on 865 patients in the selenium arm and 477 patients in the placebo arm based on data processed as of June 2011. Rates were similar after adjusting for the 2:1 random assignment (Table 3). Of the patients with toxicity assessment, 31% in the selenium arm and 26% in the placebo arm reported grade 1 or 2 treatment-related toxicities as the worst degree. In addition, grade 3 or higher toxicity occurred in less than 2% of selenium patients versus 3% of placebo patients. One patient in the control arm had a constitutional lethal toxicity.
Table 3.
Incidence of Toxicity by Treatment Arm
| Toxicity Type | Treatment Arm (% of patients*) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 477) |
Selenium (n = 865) |
|||||||
| Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | |
| Allergic rhinitis | — | — | — | — | < 1 | — | — | — |
| Hearing, other | < 1 | — | — | — | < 1 | — | — | — |
| Hemoglobin | — | — | — | — | < 1 | — | — | — |
| Leukocytes | — | — | — | — | < 1 | — | — | — |
| Neutrophils | — | — | — | — | — | < 1 | — | — |
| Hematologic, other | — | — | — | — | < 1 | — | — | — |
| Palpitations | — | — | — | — | < 1 | — | — | — |
| Cardiac, ischemia | < 1 | — | — | — | — | — | — | — |
| Cardiac, left ventricular function | — | — | — | — | — | < 1 | — | — |
| Edema | < 1 | — | — | — | — | — | — | — |
| Hypertension | — | — | — | — | < 1 | — | — | — |
| Hypotension | — | — | — | — | — | < 1 | — | — |
| Thrombosis/embolism | — | — | — | — | — | — | < 1 | — |
| Fatigue | 7 | — | — | — | 7 | — | — | — |
| Sweating | 1 | — | — | — | 3 | — | — | — |
| Weight gain | 1 | — | — | — | 1 | — | — | — |
| Weight loss | 1 | — | — | — | 1 | — | — | — |
| Constitutional | < 1 | — | — | < 1 | 1 | — | — | — |
| Alopecia | 5 | — | — | — | 4 | — | — | — |
| Bruising | — | — | — | — | < 1 | — | — | — |
| Dry skin | 1 | — | — | — | 2 | — | — | — |
| Nail changes | 7 | — | — | — | 5 | — | — | — |
| Pigmentation | < 1 | — | — | — | — | — | — | — |
| Pruritus | 1 | — | — | — | 1 | — | — | — |
| Rash/desquamation | 2 | < 1 | — | — | 2 | < 1 | — | — |
| Urticaria | — | — | — | — | < 1 | — | — | — |
| Dermatitis | 2 | — | — | — | 3 | — | — | — |
| Skin, other | 1 | < 1 | — | — | 1 | — | — | — |
| Hot flashes | — | — | — | — | < 1 | — | — | — |
| Endocrine, other | < 1 | — | — | — | — | — | — | — |
| Anorexia | < 1 | — | — | — | 1 | < 1 | — | — |
| Colitis | — | < 1 | — | — | < 1 | — | — | — |
| Constipation | 5 | — | — | — | 3 | — | — | — |
| Dehydration | — | — | — | — | — | < 1 | — | — |
| Dyspepsia | 1 | — | — | — | 1 | — | — | — |
| Dysphagia | — | — | — | — | < 1 | — | — | — |
| Flatulence | 1 | — | — | — | < 1 | — | — | — |
| Gastritis | — | — | — | — | < 1 | — | — | — |
| Mouth dryness | < 1 | — | — | — | < 1 | — | — | — |
| Nausea | 2 | — | — | — | 4 | < 1 | — | — |
| Salivary | — | — | — | — | < 1 | — | — | — |
| Sense of smell | — | — | — | — | < 1 | — | — | — |
| Stomatitis | — | < 1 | — | — | < 1 | — | — | — |
| Taste disturbance | 3 | — | — | — | 4 | — | — | — |
| Vomiting | < 1 | — | — | — | 1 | < 1 | — | — |
| Diarrhea without prior colostomy | 4 | — | — | — | 3 | < 1 | — | — |
| GI, other | 1 | — | — | — | 1 | — | — | — |
| Epistaxis | < 1 | — | — | — | < 1 | — | — | — |
| Alkaline phosphatase | — | — | — | — | < 1 | — | — | — |
| Bilirubin | — | — | — | — | < 1 | — | — | — |
| AST | < 1 | — | — | — | < 1 | — | — | — |
| ALT | — | — | — | — | < 1 | — | — | — |
| Infection with unknown ANC | — | — | — | — | < 1 | — | — | — |
| Infection without neutropenia | < 1 | — | — | — | — | — | — | — |
| Infection, other | < 1 | < 1 | — | — | < 1 | — | — | — |
| CPK | — | — | — | — | < 1 | — | — | — |
| Hypercalcemia | — | — | — | — | < 1 | — | — | — |
| Hyperglycemia | 1 | < 1 | — | — | 1 | — | — | — |
| Hyperkalemia | < 1 | — | — | — | — | < 1 | < 1 | — |
| Hypernatremia | — | — | — | — | < 1 | — | — | — |
| Hypocalcemia | — | — | — | — | < 1 | — | — | — |
| Hyponatremia | < 1 | — | — | — | < 1 | < 1 | — | — |
| Hypophosphatemia | — | — | — | — | < 1 | — | — | — |
| Myositis | — | — | — | — | — | < 1 | — | — |
| Joint, muscle, bone, other | — | — | — | — | — | < 1 | — | — |
| Confusion | < 1 | — | — | — | < 1 | — | — | — |
| Dizziness/lightheadedness | < 1 | — | — | — | < 1 | — | — | — |
| Insomnia | < 1 | — | — | — | 1 | — | — | — |
| Memory loss | — | — | — | — | < 1 | — | — | — |
| Anxiety/agitation | < 1 | — | — | — | < 1 | — | — | — |
| Depression | — | — | — | — | < 1 | < 1 | — | — |
| Neuropathy, motor | < 1 | — | — | — | — | — | — | — |
| Neuropathy, sensory | 1 | — | — | — | 2 | — | — | — |
| Personality | 1 | — | — | — | 1 | — | — | — |
| Syncope | — | — | — | — | — | < 1 | — | — |
| Vertigo | — | — | — | — | < 1 | — | — | — |
| Tearing | — | — | — | — | < 1 | — | — | — |
| Ocular, other | < 1 | — | — | — | — | — | — | — |
| Abdominal pain | < 1 | — | — | — | < 1 | — | — | — |
| Arthralgia | < 1 | — | — | — | — | — | — | — |
| Bone pain | — | — | — | — | < 1 | — | — | — |
| Chest pain | < 1 | — | — | — | < 1 | < 1 | — | — |
| Headache | < 1 | — | — | — | < 1 | — | — | — |
| Myalgia | < 1 | — | — | — | < 1 | — | — | — |
| Neuropathic pain | — | — | — | — | < 1 | — | — | — |
| Pain, other | < 1 | — | — | — | < 1 | — | — | — |
| Cough | 1 | — | — | — | < 1 | — | — | — |
| Dyspnea | 1 | < 1 | — | — | 1 | < 1 | — | — |
| Hypoxia | < 1 | — | — | — | — | — | — | — |
| Pulmonary, other | — | — | — | — | < 1 | < 1 | — | — |
| Creatinine | — | — | — | — | — | < 1 | — | — |
| Urinary frequency/urgency | < 1 | — | — | — | — | — | — | — |
| Libido | — | — | — | — | < 1 | — | — | — |
| Worst degree | 26 | 2 | — | < 1 | 31 | 1 | < 1 | — |
Abbreviations: ANC, absolute neutrophil count; CPK, creatine phosphokinase.
Percentages were computed with a denominator corresponding to the number of patients with toxicity assessed in each of the two treatment arms.
Monitoring of Diabetic Incidences
Diabetes-related questions were added in the on-study, toxicity, and long-term follow-up forms per the DMC's 2007 recommendation. Since then, 26 patients in the selenium arm and 12 patients in the placebo arm reported a diagnosis of diabetes during the long-term follow-up period.
Monitoring for Skin Cancer Incidence
During the study, there were 44 reported nonmelanoma skin cancers (14 and 13 basal cell carcinomas and 11 and six squamous cell carcinomas in the selenium and placebo arms, respectively).
Treatment Compliance
Compliance data were available for 1,239 patients at month 3 after random assignment, and 96% of patients reported taking one pill per day almost always (Appendix Table A2, online only). Follow-up data for each subsequent 3-month period also indicated good compliance. There were no significant differences in the first year compliance data (proportion of patients taking one pill per day almost always and compliance ratio) between the two treatment arms at each 3-month interval.
Reason for Treatment Termination
As of June 2011, 29 patients in the run-in step and 1,193 patients in the random assignment step reported reasons for treatment termination (Table 4), including 372 patients in the random assignment step who completed treatment. The most common reasons for treatment termination were patient withdrawal or refusal in both the run-in and random assignment steps, and progressive disease and patient withdrawal or refusal in the random assignment step. There was no significant difference in the distribution of reasons for treatment termination between the two arms.
Table 4.
Reasons for Treatment Termination by Treatment Arm
| Reason | Step |
|||||
|---|---|---|---|---|---|---|
| Run-In (n = 29) |
Random Assignment |
|||||
| No. of Patients | % | Placebo (n = 398) |
Selenium (n = 795) |
|||
| No. of Patients | % | No. of Patients | % | |||
| Completed treatment | — | — | 134 | 33.7 | 238 | 29.9 |
| Progressive disease | — | — | 52 | 13.1 | 117 | 14.7 |
| Excessive complication/toxicity | — | — | 8 | 2 | 20 | 2.5 |
| Death without progressive disease | — | — | 12 | 3 | 18 | 2.3 |
| Patient withdrawal or refusal | 20 | 69 | 67 | 16.8 | 157 | 19.8 |
| Patient started nonprotocol therapy | — | — | 3 | < 1 | 1 | < 1 |
| Other complicating disease | 1 | 3.4 | 17 | 4.3 | 30 | 3.8 |
| Maximum dose reached | — | — | 0 | 0 | 2 | < 1 |
| Other | 8 | 27.6 | 105 | 26.4 | 212 | 26.7 |
Selenium Levels
Sample collection to measure selenium blood levels was strongly encouraged but not mandatory. Samples were collected at baseline (n = 1,022), year 2 (n = 375), and year 4 (n = 194). Selenium testing was conducted initially by National Medical Services (Tucson, AZ) and, after July 2004, by Mayo Central Laboratory for Clinical Trials (Rochester, MN). Levels were scored as below normal, normal quartiles 1 to 4, and above normal. Baseline levels were normal in 647 patients (93.5%) in the selenium arm and 304 patients (92.1%) in the placebo arm (not significant), and higher than normal in 38 patients (5.5%) in the selenium arm and 24 patients (7.3%) in the placebo arm. There was no significant difference in the baseline selenium distribution by treatment (Appendix Table A3, online only). At years 2 and 4, there was a significantly increased selenium level in the group randomly assigned to selenium (Table 5; P < .001 using two-sample t test).
Table 5.
Changes in Selenium Levels by Time and Treatment Arm
| Change | Year 2–Baseline (n = 375) |
Year 4–Baseline (n = 194) |
Year 4–Year 2 (n = 164) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo |
Selenium |
Placebo |
Selenium |
Placebo |
Selenium |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Decrease | 44 | 32.4 | 5 | 2.1 | 27 | 36.5 | 1 | 0.8 | 21 | 36.2 | 11 | 10.4 |
| No change | 55 | 40.4 | 12 | 5.0 | 31 | 41.9 | 8 | 6.6 | 20 | 34.5 | 91 | 85.9 |
| Increase | 37 | 27.2 | 222 | 92.9 | 16 | 21.6 | 111 | 92.5 | 17 | 29.3 | 4 | 3.8 |
DFS and OS by Baseline Selenium Level
Overall DFS distribution of the 1,022 patients with baseline selenium levels was similar to the entire study cohort (data not shown). DFS was compared between patients according to their baseline selenium levels categorized as low (below normal and first quartile of normal), average (second and third quartiles of normal), and high (fourth quartile of normal and above normal). In the low, average, and high selenium groups, 5-year DFS rates in the selenium arm versus placebo arm were 75.5% (SE, 10.3%) versus 72.9% (SE, 12.7%), 75.6% (SE, 2.27%) versus 78.2% (SE, 3.3%), and 72.9% (SE, 4.5%) versus 80.9% (SE, 5.2%), respectively.
DISCUSSION
We report here the results of the largest lung cancer SPT prevention trial conducted by the US cancer cooperative groups. On the basis of promising data suggesting that selenium supplementation reduces lung cancer incidence, we conducted a randomized, double-blind, placebo-controlled trial in patients with resected stage I NSCLC. At the planned interim analysis, there was a trend toward a higher rate of lung SPTs in patients given selenium versus placebo (1.91 v 1.36 lung SPTs per 100 patient-years, respectively; P = .15), prompting the closure of the study for futility. At the final analysis, the incidence rates of lung SPTs were not significantly different (1.62 v 1.30 per 100 person-years in selenium and placebo arms, respectively; P = .294). The 5-year DFS rate was lower in the selenium arm but was not significantly different from placebo (74.4% [SE, 1.0%] v 79.6% [SE, 2.1%], respectively; P = .069, log-rank test). The DFS for both groups seemed virtually identical until the 28-month mark, at which time the curves diverged. This study was not powered to evaluate the interaction between selenium level and treatment; however, the descriptive data suggest that any beneficial effect of selenium is limited to patients with a low baseline selenium level. Compliance was excellent, and neither diabetes nor skin cancer risk was increased by selenium.
A recurring theme in this study and the retinoid SPT prevention trials in lung cancer18 and head and neck cancer19 is that the lowest rate of SPTs was seen in never-smokers, followed by former smokers. It is now clear that there is no demonstrable benefit in giving supplements such as selenium or retinoids to current smokers. However, the data suggest that a better approach might be to treat never-smokers with low serum selenium levels. Despite the earlier promising data, supplementation of patients with lung cancer with selenium in the presence of continued smoking trended toward a statistically inferior approach to placebo alone. Although the compelling data from Clark et al22 gave a strong rationale for pursuing selenium as a lung cancer chemopreventive approach, these data and those of the Selenium and Vitamin E Cancer Prevention Trial29 are consistent with the original observation by Lee et al7 suggesting that lung cancer chemopreventive approaches have the greatest likelihood of success in the absence of ongoing tobacco-driven carcinogenesis.
In the current era of molecularly targeted therapies for lung cancer, it seems that persisting with broad approaches in genomically unselected patient populations who continue to smoke is highly unlikely to be successful.30–33 This contention is supported by recent observations of robust efficacy noted with targeted agents in selected subpopulation of lung cancer. More aggressive smoking cessation approaches, combined with agents inhibiting critical metabolic pathways during lung carcinogenesis, are currently being explored.34 Although promising recent data suggest that iloprost,14 a prostacyclin agonist, and celecoxib,12,13 a cyclo-oxygenase-2 inhibitor, seem to be effective at reversing premalignant lesions of the lung in former smokers and never-smokers, the overwhelming weight of the data suggest that there are few compelling reasons to continue with large-scale lung cancer prevention trials of natural or synthetic compounds in genomically unselected patients who continue to smoke. Defining the optimal patient population, appropriate clinical setting, risk categorization, and best tolerated and most active chemopreventive agents for lung cancer remains a challenge despite major strides in early detection of lung cancer.35
Supplementary Material
Acknowledgment
We thank the participants in this clinical trial, the study nurses and research coordinators, and the Eastern Cooperative Oncology Group clinical trials personnel. We also thank Anthea Hammond, PhD, in the Department of Hematology and Medical Oncology at Emory University for editing the manuscript.
Appendix
Table A1.
Total Accrual to Steps 1 and 2 by Institutions
| Institution | No. of Patients |
|
|---|---|---|
| Step 1 | Step 2 | |
| Eastern Cooperative Oncology Group | 604 | 537 |
| Radiation Therapy Oncology Group | 135 | 122 |
| North Central Cancer Treatment Group | 105 | 87 |
| Cancer and Leukemia Group B | 174 | 156 |
| National Cancer Institute of Canada | 462 | 404 |
| Southwest Oncology Group | 297 | 255 |
| Total | 1,772 | 1,561 |
NOTE. This report was generated using the data as of June 2011. The main analysis presented in this report is the intent-to-treat analysis on 1,561 patients randomly assigned to step 2. All patients with data, regardless of eligibility or treatment status, were included in the analysis.
Table A2.
Summary of Compliance for the First 2 Years of Study
| Compliance | Follow-Up Time |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0-3 Months | 3-6 Months | 6-9 Months | 9-12 Months | 12-15 Months | 15-18 Months | 18-21 Months | 21-24 Months | |
| No. of patients | 1,237 | 1,245 | 932 | 1,000 | 786 | 864 | 671 | 732 |
| Count estimate | ||||||||
| Pill count | ||||||||
| No. of patients | 129 | 1,143 | 79 | 930 | 82 | 815 | 45 | 691 |
| % | 10 | 92 | 8 | 93 | 6 | 94 | 7 | 94 |
| Telephone | ||||||||
| No. of patients | 1,180 | 103 | 853 | 70 | 738 | 49 | 626 | 41 |
| % | 90 | 8 | 92 | 7 | 94 | 6 | 93 | 6 |
| Taking 1 pill a day almost always | ||||||||
| No./Total No. | 1,182/1,234 | 1,159/1,236 | 901/927 | 950/992 | 757/780 | 826/857 | 650/666 | 701/727 |
| % | 96 | 94 | 97 | 96 | 97 | 96 | 98 | 96 |
| Compliance ratio | ||||||||
| By pill count | ||||||||
| Median | 1.00 | 0.99 | 1.00 | 0.99 | 1.00 | 0.99 | 1.00 | 0.99 |
| Interquartile range | 0.95-1.00 | 0.97-1.00 | 0.92-1.00 | 0.95-1.00 | 0.91-1.00 | 0.96-1.00 | 0.95-1.00 | 0.95-1.00 |
| By telephone | ||||||||
| Median | 1.10 | 1.00 | 1.09 | 0.96 | 1.08 | 0.90 | 1.09 | 0.82 |
| Interquartile range | 1.00-1.20 | 0.84-1.07 | 1.00-1.20 | 0.80-1.01 | 0.99-1.19 | 0.81-1.05 | 0.99-1.20 | 0.74-0.95 |
Table A3.
Baseline Selenium Levels (n = 1,022)
| Treatment | Selenium Level |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (below normal) |
1 (normal, first quartile) |
2 (normal, second quartile) |
3 (normal, third quartile) |
4 (normal, fourth quartile) |
5 (above normal) |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Placebo (n = 330) | 2 | < 1 | 26 | 7.9 | 95 | 28.8 | 127 | 38.5 | 56 | 17.0 | 24 | 7.3 |
| Selenium (n = 692) | 7 | 1 | 43 | 6.2 | 192 | 27.7 | 310 | 44.8 | 102 | 14.7 | 38 | 5.5 |
Fig A1.
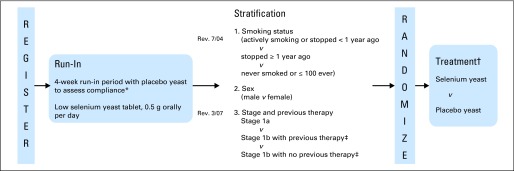
Study schema. (*) At least 75% of tablets must be consumed to be randomly assigned. (†) Double-blind design. The selenium yeast or placebo yeast will be administered for 4 years, with continued follow-up anticipated for the duration of the 10-year study. (‡) (Revised March 2007) Previous therapy: other primary therapy in addition to surgery (adjuvant chemotherapy, radiation therapy, or biologic agent).
Footnotes
See accompanying editorial on page 4169
Written on behalf of the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair), together with the Cancer and Acute Leukemia Group B, National Cancer Institute of Canada Clinical Trials Group, North Central Cancer Treatment Group, Radiation Therapy Oncology Group, and Southwest Oncology Group.
Supported in part by Public Health Service Grants No. CA037403, CA14958, CA80775, CA73590, CA107868, CA49957, CA31946, CA33601, CA32102, CA20319, CA25224, CA21661, and CA37422 and grants from the National Cancer Institute, National Institutes of Health, and Department of Health and Human Services.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: David H. Johnson, Peloton Therapeutics (C), Mirna Therapeutics (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Daniel D. Karp, Sandra J. Lee, Steven M. Keller, Gail Shaw Wright, David H. Johnson, Gerald Clamon, Randolph Marks, Worta McCaskill-Stevens, Scott M. Lippman, John Ruckdeschel, Fadlo R. Khuri
Provision of study materials or patients: Daniel D. Karp, Steven M. Keller, David H. Johnson, Michael R. Johnston, Gary Goodman, Gerald Clamon, Gordon Okawara, Randolph Marks, Eric Frechette, Worta McCaskill-Stevens, Scott M. Lippman, John Ruckdeschel, Fadlo R. Khuri
Collection and assembly of data: Daniel D. Karp, Seena Aisner, David H. Johnson, Michael R. Johnston, Gary Goodman, Gordon Okawara, Randolph Marks, Eric Frechette, Worta McCaskill-Stevens, Scott M. Lippman, John Ruckdeschel, Fadlo R. Khuri
Data analysis and interpretation: Daniel D. Karp, Sandra J. Lee, Steven M. Keller, Seena Aisner, Steven Alan Belinsky, Fadlo R. Khuri
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stewart SL, Cardinez CJ, Richardson LC, et al. Surveillance for cancers associated with tobacco use: United States, 1999-2004. MMWR Surveill Summ. 2008;57:1–33. [PubMed] [Google Scholar]
- 2.Mao L, Lee JS, Kurie JM, et al. Clonal genetic alterations in the lungs of current and former smokers. J Natl Cancer Inst. 1997;89:857–862. doi: 10.1093/jnci/89.12.857. [DOI] [PubMed] [Google Scholar]
- 3.Suda K, Tomizawa K, Yatabe Y, et al. Lung cancers unrelated to smoking: Characterized by single oncogene addiction? Int J Clin Oncol. 2011;16:294–305. doi: 10.1007/s10147-011-0262-y. [DOI] [PubMed] [Google Scholar]
- 4.Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103:1058–1068. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 6.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers: The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Lippman SM, Benner SE, et al. Randomized placebo-controlled trial of isotretinoin in chemoprevention of bronchial squamous metaplasia. J Clin Oncol. 1994;12:937–945. doi: 10.1200/JCO.1994.12.5.937. [DOI] [PubMed] [Google Scholar]
- 8.Kurie JM, Lee JS, Khuri FR, et al. N-(4-hydroxyphenyl)retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium. Clin Cancer Res. 2000;6:2973–2979. [PubMed] [Google Scholar]
- 9.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 10.Heimburger DC, Alexander CB, Birch R, et al. Improvement in bronchial squamous metaplasia in smokers treated with folate and vitamin B12: Report of a preliminary randomized, double-blind intervention trial. JAMA. 1988;259:1525–1530. [PubMed] [Google Scholar]
- 11.Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 12.Mao JT, Roth MD, Fishbein MC, et al. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev Res (Phila) 2011;4:984–993. doi: 10.1158/1940-6207.CAPR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim ES, Hong WK, Lee JJ, et al. Biological activity of celecoxib in the bronchial epithelium of current and former smokers. Cancer Prev Res (Phila) 2010;3:148–159. doi: 10.1158/1940-6207.CAPR-09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keith RL, Blatchford PJ, Kittelson J, et al. Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev Res (Phila) 2011;4:793–802. doi: 10.1158/1940-6207.CAPR-11-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 16.Pastorino U, Infante M, Maioli M, et al. Adjuvant treatment of stage I lung cancer with high-dose vitamin A. J Clin Oncol. 1993;11:1216–1222. doi: 10.1200/JCO.1993.11.7.1216. [DOI] [PubMed] [Google Scholar]
- 17.van Zandwijk N, Dalesio O, Pastorino U, et al. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer: For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 18.Lippman SM, Lee JJ, Karp DD, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–618. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 19.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 20.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 21.Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753–1763. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- 22.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: A randomized controlled trial—Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 23.Reid ME, Duffield-Lillico AJ, Garland L, et al. Selenium supplementation and lung cancer incidence: An update of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:1285–1291. [PubMed] [Google Scholar]
- 24.Fleet JC. Dietary selenium repletion may reduce cancer incidence in people at high risk who live in areas with low soil selenium. Nutr Rev. 1997;55:277–279. doi: 10.1111/j.1753-4887.1997.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 25.Belinsky SA, Klinge DM, Dekker JD, et al. Gene promoter methylation in plasma and sputum increases with lung cancer risk. Clin Cancer Res. 2005;11:6505–6511. doi: 10.1158/1078-0432.CCR-05-0625. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 27.Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 28.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 29.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Lippman SM. Molecular signatures of lung cancer: Toward personalized therapy. N Engl J Med. 2007;356:76–78. doi: 10.1056/NEJMe068218. [DOI] [PubMed] [Google Scholar]
- 31.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61:91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 32.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen–induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.