Abstract
Antimycin A3 (AA) is used as an inhibitor of cyclic electron transport around photosystem I. However, the high concentrations of AA that are needed for inhibition have secondary effects, even in chloroplasts. Here, we screened for chemicals that inhibited ferredoxin-dependent plastoquinone reduction in ruptured chloroplasts at lower concentrations than those required for AA. We identified two AA-like compounds: AAL1 and AAL2. AAL1 likely shares an inhibitory site with AA, most probably in the PGR5–PGRL1 protein complex, and enhances O2 evolution in photosystem II, most likely via an uncoupler-like effect. AAL1 and AAL2 are unlikely to penetrate intact leaves. In ruptured chloroplasts, AALs are superior to AA as inhibitors of cyclic electron transport.
Keywords: Antimycin A, Chloroplast, Cyclic electron transport, PGR5, Photosynthesis
Abbreviations: AA, antimycin A3; AAL, antimycin A-like compound; Cyt, cytochrome; ETR, electron transport rate; Fd, ferredoxin; NDH, NADH dehydrogenase-like complex; NPQ, non-photochemical quenching of chlorophyll fluorescence; PQ, plastoquinone; PSI/II, photosystem I/II
Highlights
-
•
Antimycin A3 (AA) inhibits PSI cyclic electron transport.
-
•
AA-like compounds inhibit PSI cyclic electron transport at lower concentrations than AA.
-
•
AAL1 targets the same site as AA to inhibit PSI cyclic electron transport.
-
•
V3K alteration in PGR5 does not confer resistance to AAL2.
1. Introduction
The light reactions of photosynthesis convert light energy into chemical energy in the forms of ATP and NADPH. Linear electron transport is mediated by two photosystems and generates ATP and NADPH. In contrast, cyclic electron transport around photosystem I (PSI) is driven solely by PSI and preferentially generates ATP [1]. In angiosperms, PSI cyclic electron transport is mediated by two partially redundant pathways [2]. The main pathway depends on PROTON GRADIENT REGULATION 5 (PGR5) and PGR5-like PHOTOSYNTHETIC PHENOTYPE 1 (PGRL1) proteins [3–5], whereas the minor pathway is mediated by the chloroplast NADH dehydrogenase-like complex (NDH) [6–8].
Table 1.
Effects of inhibitors on linear electron transport.
| Control | AA | AAL1 | AAL2 | |
|---|---|---|---|---|
| O2 evolution rate (μmol ml−1 min−1) | 30.93 ± 1.01 | 27.47 ± 0.97 | 147.3 ± 4.59 | 31.7 ± 1.02 |
The O2 evolution rate was determined at 3000 μmol photons m−2 s−1 in the presence of 10 μM AA and 1 μM AALs. Data are averages ± standard deviations (n = 3).
On the basis of its sensitivity to antimycin A3 (AA), PGR5–PGRL1-dependent PSI cyclic electron transport is likely to be identical to the cyclic phosphorylation discovered by Arnon and coworkers [9,10]. Chloroplast NDH also accepts electrons from ferredoxin (Fd) but is resistant to AA [11]. AA was originally discovered to inhibit respiratory electron transport by binding to the Qi site of the cytochrome (Cyt) bc1 complex [12]. However, AA does not bind to the corresponding site of the Cyt b6f complex in chloroplasts. The site where AA inhibits PSI cyclic electron transport has long been unclear. Recently, AA was shown to inhibit electron transport from recombinant PGRL1 to the plastoquinone (PQ) analog 2,6-dimethyl-p-benzoquinone in vitro [5], and PGR5 may function in the Fd-dependent reduction of PGRL1 in vivo [4]. Consistent with these results, a single amino acid alteration in PGR5 confers resistance of PSI cyclic electron transport to AA [13]. AA most likely inhibits the function of the PGR5–PGRL1 protein complex, although the exact mode of inhibition is still unclear.
Before the identification of mutants specifically defective in AA-sensitive PSI cyclic electron transport [3,4,14], AA was often used to inhibit PSI cyclic electron transport in physiological experiments using leaves [15,16]. However, to inhibit PSI cyclic electron transport, the concentrations of AA that were needed were 10- to 100-times higher than what was required to inhibit respiratory electron transport. This problem was serious in physiological experiments using leaves, in which respiratory electron transport was linked to photosynthetic electron transport [17]. Furthermore, high concentrations of AA caused a secondary effect on non-photochemical quenching (NPQ) of chlorophyll fluorescence induction in chloroplasts [18]. For these reasons, chemicals must be selected that inhibit PSI cyclic electron transport more specifically and at lower concentrations than what is required for AA. Furthermore, the isolation of such inhibitors may help to identify novel protein factors besides PGR5 and PGRL1 that are involved in Fd-dependent PQ reduction (i.e., through chemical genetics). In this study, we report two AA-like compounds (AALs) that inhibit PSI cyclic electron transport more efficiently than AA in ruptured chloroplasts.
2. Materials and methods
2.1. Plant materials and growth conditions
Arabidopsis thaliana (ecotype Columbia gl1) was grown in soil under growth-chamber conditions (50 μmol photons m−2 s−1, 16 h light/8 h dark cycle at 23 °C) for 3–4 weeks. The 35Sp::PtPGR5 lines were generated and characterized previously [19,20].
2.2. Chloroplast isolation
Chloroplasts were isolated from mature leaves as described previously [3].
2.3. In vitro assay of Fd-dependent PQ reduction
Fd-dependent PQ reduction was measured in ruptured chloroplasts (20 μg chlorophyll ml−1), as described previously [21], by using 5 mM spinach Fd (Sigma–Aldrich) and 0.25 mM NADPH (Oriental Yeast). The activity of PSI cyclic electron transport was monitored using a MINI-PAM photosynthesis yield analyzer (Walz) to detect the relative change in chlorophyll fluorescence. Fd-dependent PQ reduction activity was evaluated using the equation (F − Fo)/(Fm − Fo). Fo is the minimal chlorophyll fluorescence that is emitted from the open PSII reaction center in the dark, Fm is the maximal chlorophyll fluorescence that is emitted from PSII centers that are converted into the closed state (with reduced QA) by a saturating pulse (0.8 s, 8000 μmol photons m−2 s−1) in the light, and F is the fluorescence level 5 min after the addition of Fd. AA (Sigma–Aldrich) and the AALs were added to the medium before measurement.
2.4. Chlorophyll fluorescence analysis using leaves
Detached leaves were sandwiched by two layers of gauze that were soaked in medium (330 mM sorbitol, 20 mM HEPES/KOH [pH 7.6], 5 mM MgCl2, and 2.5 mM EDTA) containing 10 μM AA or AALs and were kept in the light for 10 min. After subsequent dark adaptation of the leaves, chlorophyll fluorescence parameters were determined using the MINI-PAM photosynthesis yield analyzer. The quantum yield of the conversion of photochemical energy in PSII was calculated using the equation (Fm′ − Fs)/Fm′, and the NPQ was calculated as (Fm − Fm′)/Fm′. Fs is the steady-state fluorescence level.
2.5. Oxygen evolution measurements
Thylakoid membranes (20 μg chlorophyll ml−1) were suspended in medium containing 0.3 M sorbitol, 50 mM HEPES/KOH (pH 7.6), 1 mM MgCl2, 0.5 mM KH2PO4, 2 mM EDTA, 5 mM Na pyrophosphate and 10 mM NaHCO3 in the presence of 1 mM methyl viologen and 1 mM KCN. Oxygen evolution was measured using a Clark-type oxygen electrode (Hanzatech) with an actinic light intensity of 3000 μmol photons m−2 s−1.
3. Results and discussion
To screen for chemicals that inhibited PSI cyclic electron transport at lower concentrations than what was required for AA, we tested 53 AA analogs. This chemical library was originally synthesized to study structure–activity relationships in the inhibition of the Cytbc1 complex [22]. To evaluate inhibitory effects, the Fd-dependent PQ reduction activity was monitored by using ruptured chloroplasts that were isolated from wild-type Arabidopsis leaves in medium containing 0.1, 1, 5, or 10 μM AA analogs (Fig. 1). In this assay, NADPH was required to reduce Fd via the reverse reaction of Fd-NADP+ reductase. Fd donates electrons to the PQ pool in both pathways of PSI cyclic electron transport [5,11], and the reduction in the PQ pool results in redox equilibrium with QA of PSII, which leads to the emission of chlorophyll fluorescence. Although adding NADPH to wild-type, ruptured chloroplasts did not reduce the PQ pool, subsequent addition of Fd did reduce the PQ pool (Fig. 1), which indicated that the electron flow depended on Fd. AA partially impaired the PQ reduction activity, and the remaining activity in the wild type depended on chloroplast NDH (Fig. 1) [2].
Fig. 1.
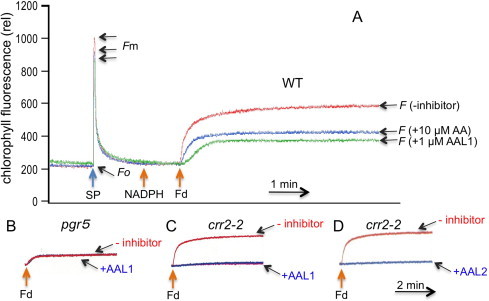
Screening of inhibitors using the Fd-dependent PQ reduction assay in ruptured chloroplasts. (A) An example of screening. PQ reduction was monitored as the chlorophyll fluorescence increased, and fluorescence levels were normalized against Fo levels. Fv/Fm values depended on the chloroplast preparation and were in the range of 0.7–0.8; this level of difference was unlikely to significantly affect the (F − Fo)/(Fm − Fo) values. This figure shows representative fluorescence patterns of wild-type, ruptured chloroplasts that were exposed to 10 μM AA, 1 μM AAL1, or no inhibitor, and the timings of addition of NADPH and Fd are indicated. “SP” indicates the saturation pulse for monitoring Fm, and the levels of Fo, Fm, and F are indicated. (B) The effect of 1 μM AAL1 on Fd-dependent PQ reduction in pgr5. (C and D) The effect of 1 μM AAL1 (C) and 1 μM AAL2 (D) on Fd-dependent PQ reduction in crr2–2.
Of the 53 AA analogs, two AA-like compounds (AAL1 and AAL2; Fig. 2) behaved similar to AA in this assay, but they did so at lower concentrations than what was required for AA. Fig. 1A shows an example of screening with AAL1. Adding 1 μM of AAL1 decreased the PQ reduction activity to the pgr5 level and did not affect PQ reduction further in pgr5 (Fig. 1B). The rate and level of PQ reduction were lower than those exposed to 10 μM AA, which was consistent with the previous observation that this level of AA did not completely inhibit PGR5-dependent PQ reduction [23], and addition of AAL1 did not affect NDH-dependent PQ reduction in pgr5 (Fig. 1B). In contrast, adding AAL1 to ruptured chloroplasts that were isolated from the crr2–2 mutant, which was defective in producing chloroplast NDH [7], nearly completely abolished the PQ reduction activity, and ALL2 also nearly completely inhibited PGR5-dependent PQ reduction in crr2–2 (Fig. 1D).
Fig. 2.
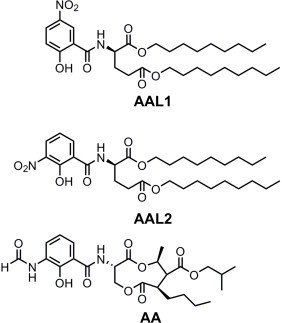
Structures of antimycin A3 (AA), AAL1, and AAL2.
To further characterize AAL1 and AAL2, we analyzed their concentration dependence in the inhibition of Fd-dependent PQ reduction, which was evaluated using the equation (F − Fo)/(Fm − Fo) (Figs. 1 and 3). AAL1 at 0.5 μM or AAL2 at 1 μM was sufficient to inhibit the PQ reduction activity to the level that was obtained after treatment with 5–10 μM AA (Fig. 3), and greater than 5 μM AAL1 increased the fluorescence level due to a decline in the Fm level, which was most likely a secondary effect of the high concentration of AAL1.
Fig. 3.
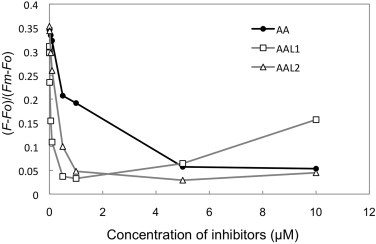
Concentration dependency of the inhibition of Fd-dependent PQ reduction in ruptured chloroplasts. Representative results that were obtained by using the same chloroplast preparation are shown.
To test whether AAL1 and AAL2 targeted the same inhibitory site as what was targeted by AA, we analyzed the sensitivity of ruptured chloroplasts that were isolated from a 35Sp::PtPGR5 line that was resistant to AA [13]. The PtPGR5 gene was isolated from loblolly pine (Pinus sativa). It was discovered that the third residue of the mature PtPGR5 protein was lysine; however, this residue was valine in AtPGR5 in Arabidopsis, and this V3K substitution was necessary and sufficient to confer resistance to AA [13]. AAL1 inhibited PQ reduction at lower concentrations than did AA in the wild type, but the 35Sp::PtPGR5 line was resistant to AAL1 and AA (Fig. 4A and B). The V3K substitution conferred resistance to AA and AAL1, which suggested that AA and AAL1 targeted the same inhibitory site. In contrast, two lines that exhibited concentration-dependency for the inhibition to AAL2 overlapped, indicating that the 35Sp::PtPGR5 and wild-type lines were sensitive to AAL2 (Fig. 4C). This result suggests that AAL2 inhibits Fd-dependent PQ reduction by binding to a site that is different from the site that is bound by AA and AAL1. More likely, AAL2 may bind to the same site as AA and AAL1, but the V3K substitution may not influence the accessibility of AAL2 to this site.
Fig. 4.
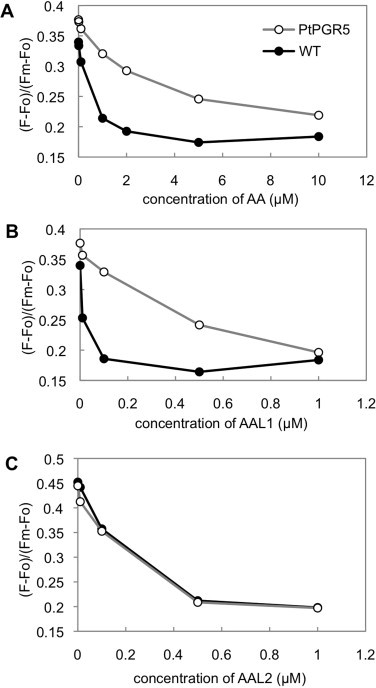
Concentration dependency of the inhibition of Fd-dependent PQ reduction in ruptured chloroplasts from wild-type Arabidopsis (AtPGR5) and a line that over-accumulates PtPGR5 (PtPGR5 + AtPGR5). Ruptured chloroplasts were treated with AA (A), AAL1 (B), or AAL2 (C). Representative results that were obtained by using the same chloroplast preparation are shown in A and B, but an independent preparation was used in C. The ranges of inhibitor concentrations were different between AA (A) and AALs (B and C).
We also evaluated the inhibitory effect of AALs on PSI cyclic electron transport in intact leaves. We infiltrated 10 μM of AA or AALs into wild-type leaves and then analyzed the light intensity dependence of the chlorophyll fluorescence parameters (Fig. 5). As previously reported, infiltration of AA decreased the electron transport rate (ETR) and NPQ in wild-type leaves [13], although the effect on NPQ was milder than what was reported previously, possibly because we used a different infiltration procedure (see Section 2). Infiltration of AAL1 or AAL2 did not affect electron transport, although the ETR was slightly reduced by AAL2 (Fig. 5). Because AAL1 and AAL2 inhibited PSI cyclic electron transport at lower concentrations than did AA in ruptured chloroplasts, the AALs most likely did not penetrate into the cells or chloroplasts. Because root growth depends on respiration, AA inhibited the growth rate in the dark (Supplemental Fig. 1), and consistent with our observations of leaves, root growth was not affected by 10 μM AAL1 or AAL2 (Supplemental Fig. 1). We cannot eliminate the possibility that the affinity of AAL1 and AAL2 for the Cytbc1 complex is reduced, but both results suggest that the AALs do not penetrate into the cells. Although AAL1 and AAL2 can be used as efficient inhibitors of PSI cyclic electron transport in ruptured chloroplasts, they cannot be used in assays in intact leaves.
Fig. 5.
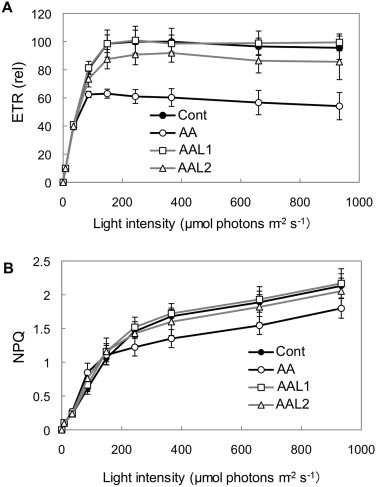
The light-intensity dependence of the relative ETR (A) and NPQ (B) that was measured in detached leaves of wild-type Arabidopsis. The concentration of inhibitors was 10 μM, and the control leaves were treated with medium that lacked inhibitors.
To analyze the effects of AALs on linear electron transport, we used 1 mM methyl viologen as an electron acceptor to analyze the rate of linear electron transport-dependent O2 evolution in the presence of AA or AALs. Whereas 10 μM AA or 1 μM AAL2 did not affect linear electron transport, unexpectedly, adding 1 μM of AAL1 drastically increased O2 evolution. It is well known that uncouplers activate linear electron transport by relaxing ΔpH [24]. Therefore, AAL1 may act as an uncoupler in the thylakoid membrane, as suggested by its structure (Fig. 2), and AAL1 may inhibit Fd-dependent PQ reduction via this uncoupler-like behavior. However, adding 5 mM NH4Cl did not affect PQ reduction [21], whereas adding AAL1 impaired PQ reduction in the presence of NH4Cl (data not shown), which suggests that AAL1 inhibits PSI cyclic electron transport independent of its uncoupler-like function.
AAL2 is superior to AAL1 because it lacks uncoupler-like behavior. Furthermore, AAL2 may inhibit PSI cyclic electron transport by binding to a site that is different from that bound by AA or AAL1. However, AAL1 inhibits PSI cyclic electron transport at a lower concentration (0.5 μM) than does AAL2 (Figs. 3 and 4). Despite the differences in their effects, AAL1 and AAL2 differ only in the position of their substitution of the nitro group (Fig. 2), and it may be possible to use AAL1 to biochemically identify the site at which AA binds to the machinery of PGR5–PGRL1-dependent, AA-sensitive PSI cyclic electron transport. Although AALs are not ideal inhibitors of PSI cyclic electron transport when used in leaves or intact chloroplasts, the assay utilizing ruptured chloroplasts or isolated thylakoids is a powerful tool for fully understanding the route of electrons in AA-sensitive, PSI cyclic electron transport.
Acknowledgements
The authors thank Tsuyoshi Endo and Kentaro Ifuku for their support in oxygen evolution analysis. T.S. was supported by Grants 22114509 and 22247005 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fob.2013.09.007.
Appendix. Supplementary materials
Supplementary materials for Antimycin A-like molecules inhibit cyclic electron transport around photosystem I in ruptured chloroplasts.
References
- 1.Shikanai T. Cyclic electron transport around photosystem I; genetic approaches. Annu. Rev. Plant Biol. 2007;58:199–217. doi: 10.1146/annurev.arplant.58.091406.110525. [DOI] [PubMed] [Google Scholar]
- 2.Munekage Y., Hashimoto M., Miyake C., Tomizawa K., Endo T., Tasaka M., Shikanai T. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature. 2004;429:579–582. doi: 10.1038/nature02598. [DOI] [PubMed] [Google Scholar]
- 3.Munekage Y., Hojo M., Meurer J., Endo T., Tasaka M., Shikanai T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002;110:361–371. doi: 10.1016/s0092-8674(02)00867-x. [DOI] [PubMed] [Google Scholar]
- 4.DalCorso G., Pesaresi P., Masiero S., Aseeva E., Schünemann D., Finazzi G., Joliot P., Barbato R., Leister D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell. 2008;132:273–285. doi: 10.1016/j.cell.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 5.Hertle A.P., Blunder T., Wunder T., Pesaresi P., Pribil M., Armbruster U., Leister D. PGRL1 is the elusive ferredoxin–plastoquinone reductase in photosynthetic cyclic electron flow. Mol. Cell. 2013;49:511–523. doi: 10.1016/j.molcel.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Shikanai T., Endo T., Hashimoto T., Yamada Y., Asada K., Yokota A. Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc. Natl. Acad. Sci. USA. 1998;95:9705–9709. doi: 10.1073/pnas.95.16.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto M., Endo T., Peltier G., Tasaka M., Shikanai T. A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 2003;36:541–549. doi: 10.1046/j.1365-313x.2003.01900.x. [DOI] [PubMed] [Google Scholar]
- 8.Ifuku K., Endo T., Shikanai T., Aro E.-M. Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits. Plant Cell Physiol. 2011;52:1560–1568. doi: 10.1093/pcp/pcr098. [DOI] [PubMed] [Google Scholar]
- 9.Arnon D.I., Allen M.B., Whatley F.R. Photosynthesis by isolated chloroplasts. Nature. 1954;174:394–396. doi: 10.1038/174394a0. [DOI] [PubMed] [Google Scholar]
- 10.Tagawa K., Tsujimoto H.Y., Arnon D.I. Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc. Natl. Acad. Sci. USA. 1963;49:567–572. doi: 10.1073/pnas.49.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H., Peng L., Fukao Y., Shikanai T. An Src homology 3 domain-like fold protein forms a ferredoxin-binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell. 2011;23:1480–1493. doi: 10.1105/tpc.110.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia D., Yu C.A., Kim H., Xia J.Z., Kachurin A.M., Zhang L., Yu L., Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto K., Okegawa Y., Tohri A., Long T.A., Covert S.F., Hisabori T., Shikanai T. A single amino acid alteration in PGR5 confers resistance to antimycin A in cyclic electron transport around PSI. Plant Cell Physiol. 2013 doi: 10.1093/pcp/pct098. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa Y., Yamamoto H., Okegawa Y., Wada S., Sato N., Taira Y., Sugimoto K., Makino A., Shikanai T. PGR5-dependent cyclic electron transport around PSI contributes to the redox homeostasis in chloroplasts rather than CO2 fixation and biomass production in rice. Plant Cell Physiol. 2012;53:2117–2126. doi: 10.1093/pcp/pcs153. [DOI] [PubMed] [Google Scholar]
- 15.Miyake C., Schreiber U., Asada K. Ferredoxin-dependent and antimycin A-sensitive reduction of cytochrome b-559 by far-red light in maize thylakoids; participation of a menadiol-reducible cytochrome b-559 in cyclic electron flow. Plant Cell Physiol. 1995;36:743–748. [Google Scholar]
- 16.Joët T, Cournac L, Horvath E.M., Medgyesy P., Peltier G. Increased sensitivity of photosynthesis to antimycin A induced by inactivation of the chloroplast ndhB gene. Evidence for a participation of the NADH-dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol. 2001;125:1919–1929. doi: 10.1104/pp.125.4.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida K., Terashima I., Noguchi K. Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant Cell Physiol. 2006;47:22–31. doi: 10.1093/pcp/pci219. [DOI] [PubMed] [Google Scholar]
- 18.Horton P., Ruban A.V., Walters R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 19.Long T.A., Okegawa Y., Shikanai T., Schmidt G.W., Covert S.F. Conserved role of PROTON GRADIENT REGULATOR 5 (PGR5) in the regulation of PSI cyclic electron transport. Planta. 2008;228:907–918. doi: 10.1007/s00425-008-0789-y. [DOI] [PubMed] [Google Scholar]
- 20.Okegawa Y., Long T.A., Iwano M., Takayama S., Kobayashi Y., Covert S.F., Shikanai T. Balanced PGR5 level is required for chloroplast development and optimum operation of cyclic electron transport around photosystem I. Plant Cell Physiol. 2007;48:1462–1471. doi: 10.1093/pcp/pcm116. [DOI] [PubMed] [Google Scholar]
- 21.Okegawa Y., Kagawa Y., Kobayashi Y., Shikanai T. Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol. 2008;49:825–834. doi: 10.1093/pcp/pcn055. [DOI] [PubMed] [Google Scholar]
- 22.Tokutake N., Miyoshi H., Nakazato H., Iwamura H. Inhibition of electron transport of rat-liver mitochondria by synthesized antimycin A analogs. Biochim. Biophys. Acta. 1993;1142:262–268. doi: 10.1016/0005-2728(93)90154-8. [DOI] [PubMed] [Google Scholar]
- 23.Peng L., Fukao Y., Fujiwara M., Takami T., Shikanai T. Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell. 2009;21:3623–3640. doi: 10.1105/tpc.109.068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evron Y., McCarty R.E. Simultaneous measurement of ΔpH and electron transport in chloroplast thylakoids by 9-aminoacridine fluorescence. Plant Physiol. 2000;124:407–414. doi: 10.1104/pp.124.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials for Antimycin A-like molecules inhibit cyclic electron transport around photosystem I in ruptured chloroplasts.


