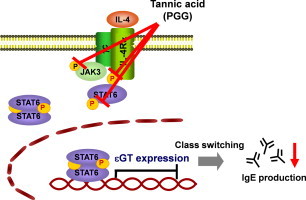
Abstract
Interleukin (IL)-4 is a critical stimulator that induces ɛ germline transcripts (ɛGT) for switch recombination to initiate immunoglobulin (Ig) E and is important in allergic disease pathogenesis. We found pentagalloylglucose (PGG) inhibited IL-4-induced ɛGT expression. PGG exerted its inhibitory function by suppressing IL-4-induced activation of IL-4Rα, JAK3 and STAT6. Furthermore, tannic acid, a higher galloylated PGG, attenuated ovalbumin-induced IgE production in vivo by inhibiting IL-4-induced ɛGT expression and the IL-4 signaling pathway. In conclusion, our results suggest that tannic acid may attenuate allergic diseases by suppressing IgE production by inhibiting IL-4-induced signaling.
Keywords: ɛ Germline transcript, IgE, Pentagalloylglucose, Signal transducers and activators of transcription6, Tannic acid
Abbreviations: ɛGT, ɛ germline transcript; IFN-γ, interferon-gamma; IgE, immunoglobulin E; IL, interleukin; JAK, Janus kinase; OVA, ovalbumin; PGG, 1,2,3,4,6-penta-O-galloyl-β-d-glucose; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor-beta
Graphical Abstract
Highlights
-
•
Tannic acid (TA) is highly galloylated pentagalloylglucose derived from oriental herbs.
-
•
Germline transcript (GT) expression is indispensable for immunoglobulin (Ig) E class switching.
-
•
PGG and TA inhibit ɛGT expression by attenuating IL-4 signaling.
-
•
TA attenuates ovalbumin-induced IgE production in vivo.
1. Introduction
The incidence of allergic disease has increased dramatically recently and in many countries, the prevalence has reached almost epidemic proportions [1]. Currently, a cure for these diseases does not exist although a wide range of treatments is used to control symptoms. Thus, allergies have become a major medical and social issue worldwide [2].
Interleukin (IL)-4 is thought to be a key cytokine in the early stages of allergic diseases because of its important role in regulating B-cell isotype switching to immunoglobulin (Ig) E production [3,4]. Its effects are mediated by a cell surface receptor unit, the IL-4 receptor α-chain (IL-4Rα) and the common γ-chain (γc). At the intracellular level, IL-4 activates the ɛ germline promoter that leads to the expression of ɛ germline transcript (ɛGT). This is essential for IgE production, through the tyrosine phosphorylation of signal transducers and activators of transcription (STAT) 6 molecule via the activation of Janus kinases (JAK) [5,6]. IgE is associated with immediate hypersensitivity reactions that underlie atopic conditions such as urticaria and eczema, seasonal allergy, food allergy, asthma, and anaphylaxis [7]. Therefore, inhibiting IgE production through blocking IL-4 signaling may be effective in attenuating allergic disease.
Although topical steroids, emollients and oral antihistamines are used as first-line therapy for allergy, many patients are still dissatisfied with present treatments and are worried about side effects. Therefore, novel, safe pharmaceutical agents that inhibit IgE production are required. If dietary intervention could inhibit the production of IgE resulting from allergic responses, it may be a useful strategy for therapy and prevention of allergy.
Pentagalloylglucose (PGG) is a polyphenolic and water soluble gallotannin isolated from various oriental herbs and plants such as gallnut of Rhus chinensis MILL. Botanically, PGG can exist in free form or as a core structure of tannic acid, a higher galloylated glucose [8]. PGG has various biomedical properties such as anti-diabetic [9,10] anti-oxidant [11], anti-cancer [12,13] and anti-inflammatory activities [14,15]. However, to our knowledge, no studies have reported that PGG has an anti-allergic effect by inhibiting the IL-4 signaling pathway, which is indispensable for class switching to IgE. Previously, we showed that strictinin, an ellagitannin isolated from tea leaves, could inhibit IgE production through the inhibition of IL-4-mediated signaling in B cells [16]. Because of a structural similarity between PGG and strictinin, the inhibitory effects of PGG on IL-4-induced allergic responses were analyzed. Furthermore, we also evaluated the effect of tannic acid, a higher galloylated PGG, on IgE production in mouse serum stimulated by ovalbumin. In this study, we found that PGG, a core structure of tannic acid, has a specific inhibitory effect on the IL-4 signaling pathway and that tannic acid has anti-IgE production activity.
2. Materials and methods
2.1. Chemicals
Tannic acid extracted from Rhus javanica L., Anacardiaceae, and PGG were donated from Dr. Toshio Miyase, University of Shizuoka [17].
2.2. Cells and cell culture
The human Burkitt's lymphoma cell line, DND39, in RPMI 1640 medium and mouse embryonic fibroblast cell line NIH3T3 cells in Dulbecco's modified Eagle's medium (DMEM) (Cosmo Bio, Tokyo, Japan) were maintained in media supplemented with 5% fetal bovine serum (FBS) (Intergen, Purchase, NY), 100 U/ml penicillin G, 100 μg/ml streptomycin, and 10 mM HEPES buffer at 37 °C with 5% CO2.
2.3. Analysis of ɛGT expression
DND39 cells (1 × 106 cells/ml) were stimulated with 250 U/ml human recombinant IL-4 (hrIL-4) (R&D Systems Inc., Minneapolis, USA) in RPMI 1640 medium containing 5% FBS. Total cellular RNA was prepared using TRIzol reagent (Invitrogen Corp Carlsbad, CA, USA) according to the manufacturer's recommended protocol. Ten micrograms RNA of total RNA was reverse transcribed to cDNA using 20 U of MMLV-reverse transcriptase (Amersham Pharmacia Biotech, Buckinghamshire, UK) and 0.5 μg of (dT)20 primer in a reaction volume of 20 μl. When synthesizing cDNA for detection of ɛGT, 1 μM of the ɛGT-R, an anti-sense direction primer for ɛGT, was used for reverse transcription. Reverse transcription-polymerase chain reaction (RT-PCR) was performed in a 10 μl reaction volume using 0.5 U of Amplitaq Gold DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT), 1 mM of each deoxynucleotide (Amersham Pharmacia Biotech), 1 μM of the appropriate primer for ɛGT or GAPDH, and 0.5 or 0.05 μg of total RNA-equivalent cDNA. Specific probes for ɛGT and GAPDH genes were made by labeling the following DNA: ɛGT, 5′-AGCTGTCCAGGAACCCCACAGGGAG-3′; GAPDH, PCR-amplified GAPDH genes. Sample were predenatured at 95 °C for 9 min followed by amplification at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s for 13 cycles (ɛGT) or 10 cycles (GAPDH), followed by a final 7 min extension step at 72 °C. The amplified PCR products were subjected to electrophoresis on a 1.5% agarose gel and transferred to a Hybond-N+ membrane (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Primer sequences used in this experiment were as follows: ɛGT-F, 5′-AGGCTCCACTGCCCGGCACAGAAAT-3′; ɛGT-R, 5′-ACGGAGGTGGCATTGGAGGGAATGT-3′; GAPDH-F, 5′-GCTCAGACACCATGGGGAAGGT-3′; GAPDH-R, 5′-GTGGTGCAGGAGGCATTGCTGA-3′.
2.4. Immunoprecipitation and western blotting
hrIL-4-stimulated DND39 cells (2 × 106 cells/ml) were treated with PGG or tannic acid in RPMI 1640 medium for 10 or 30 min. Cells were lysed in lysis buffer containing 50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% (v/v) Triton X-100, 1 mM EDTA, 50 mM NaF, 30 mM Na4P2O7, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/ml aprotinin. The cell extracts were immunoprecipitated with the indicated antibodies followed by incubation with protein A-Sepharose beads (Amersham Bioscience). The washed immunoprecipitates were separated using an 8% acrylamide gel, and transferred onto nitrocellulose membranes (Schleicher & Schuell BioScience, Inc., NH, USA). Membranes were then probed with specific antibodies. The bound antibody was detected using ECL (Amersham Pharmacia Biotech). Anti-STAT6 (S-20), anti-phosphotyrosine (PY20), anti-STAT1 p84/p91 (E-23), anti-pSTAT1 (Tyr701), anti-human IL-4Rα (C-20) and anti-JAK3 (C-21) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phosphotyrosine antibody (4G10) was purchased from Upstate Biotechnology Inc. (Lake Placid, NY, USA).
2.5. Animals
Female 6-week-old C57BL/6J mice were purchased from Charles River Laboratories Japan Inc. (Yokohama, Japan). The mice were kept at the Biotron Institute of Kyushu University in a 12-h light/12-h dark cycle (lights on 8 a.m. to 8 p.m.) in an air-conditioned room (23–25 °C and 60% humidity under SPF conditions). This experiment was carried out according to the guidelines for animal experiments at the Faculty of Agriculture and the Graduate Course, Kyushu University, and the Law (No. 105) and Notification (No. 6) of the Japanese government. The approval number of the animal experiment was A24-052-1.
2.6. Immunization of mice with ovalbumin
C57BL/6J mice were divided into two groups of 5 mice each. Mice were administered water or water containing 4 mg/ml tannic acid for 17 days ad libitum. Mice were primed with ovalbumin (OVA) (Grade IV, Sigma-Aldrich Chemical, St. Louis, MO) to induce specific IgE responses as follows: 50 μg/ml OVA mixed with aluminum hydroxide (10 μg OVA/1 mg aluminum; LSL Inc., Japan) were intraperitoneally injected on day 3. At 1 month after administration, concentrations of OVA-specific IgE, IgM, and IgG were measured in serum by enzyme-linked immunosorbent assay Quantitation Kit (Bethyl Laboratories, Montgomery, TX, USA). Specific IgE, IgM, and IgG antibodies were quantified using horseradish peroxidase-conjugated antibody. The relative band intensities were calculated by densitometric analysis using KyPlot software, ver. 4.0.
2.7. Statistical analysis
Data were analyzed using the unpaired t-test using Graphpad Prism 4.0 software (Graphpad Software Inc., San Diego, CA, USA). Mean values were statistically significantly from the control group when p < 0.05.
3. Results
3.1. PGG inhibits IL-4-induced ɛGT expression
The expression of ɛGT is indispensable for IL-4-induced IgE class switching [18]. We first examined the effect of PGG on ɛGT expression in B cells induced by IL-4. Transforming growth factor (TGF)-β was used as a positive control for inhibiting IL-4-induced ɛGT expression [19]. PGG inhibited IL-4-induced ɛGT expression in a concentration-dependent manner (Fig. 1B).
Fig. 1.
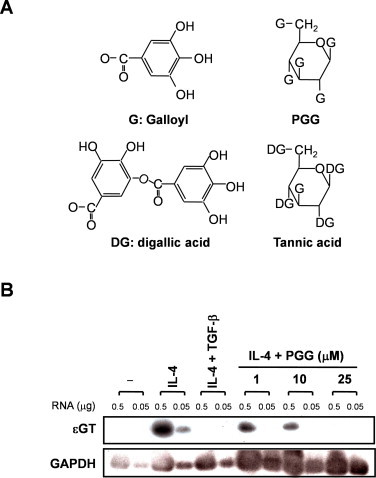
PGG inhibits IL-4-induced ɛGT expression. (A) The structure of galloyl group, PGG, digallic acid and tannic acid. (B) DND39 cells were treated with PGG (1, 10, 25 μM) or TGF-β (2 ng/ml, positive control) in the presence of IL-4 (250 U/ml) for 48 h, and then assessed for the expression of ɛGT by RT-PCR followed by Southern hybridization. Each experiment was repeated three times in triplicate.
3.2. PGG suppressed IL-4-induced STAT6 activation by inhibiting IL-4 signal pathway
STAT6 is a critical transcription factor that selectively induces ɛGT expression by IL-4 stimuli [20]. We evaluated the effect of PGG on the IL-4 induced signaling pathway. PGG inhibited IL-4-induced IL-4Rα phosphorylation and attenuated IL-4-induced JAK3 phosphorylation (Fig. 2). Furthermore, treatment of PGG for 30 min inhibited IL-4-induced STAT6 phosphorylation (Fig. 2). These results demonstrate that PGG inhibited IL-4-induced ɛGT expression and STAT6 phosphorylation by attenuating JAK3 and IL-4Rα activation.
Fig. 2.
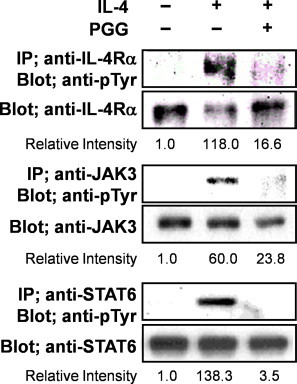
PGG suppresses IL-4-induced STAT6 activation by inhibiting the IL-4 signal pathway. DND39 cells were stimulated with IL-4 (250 U/ml) in the presence of 25 μM of PGG for 10 min or 30 min. Lysates were then immunoprecipitated with anti-JAK3, anti-IL-4Rα, or anti-STAT6 antibodies and analyzed by western blotting using anti-phosphotyrosine antibody (4G10) for phosphorylated JAK3 and IL-4Rα or anti-phosphotyrosine antibody (PY20) for phosphorylated STAT6. Each experiment was repeated three times in triplicate.
3.3. PGG specifically inhibits IL-4 and IL-13 signaling, but not IFN-γ signaling
To explore whether the inhibitory effect of PGG is specific for IL-4Rα signaling, we investigated whether PGG inhibited other cytokine signaling such as interferon (IFN)-γ, and IL-13. IFN-γ is a type II cytokine and IL-13 is a type I cytokine whose receptor shares IL-4Rα, to which IL-4 binds [21]. PGG did not inhibit phosphorylation of STAT1 (Fig. 3A). However, STAT6 phosphorylation induced by IL-13 was completely inhibited by PGG (Fig. 3B). These results suggest that PGG might specifically inhibit IL-4 and IL-13 induced signaling.
Fig. 3.
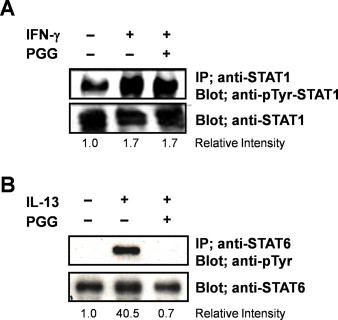
PGG specifically inhibits IL-4 and IL-13 signaling, but not IFN-γ signaling. (A) DND39 cells were treated with IFN-γ (400 ng/ml) and PGG for 30 min. Lysates were then immunoprecipitated with anti-STAT1 antibody and analyzed by western blotting using an anti-pTyr-STAT1 antibody. (B) NIH-3T3 cells were treated with IL-13 (10 ng/ml) and PGG (50 μM) for 30 min. Lysates were then immunoprecipitated with anti-STAT6 antibody and analyzed by western blotting using an anti-phosphotyrosine antibody (PY20). Each experiment was repeated three times in triplicate.
3.4. Tannic acid inhibits IgE production by suppressing IL-4-induced ɛGT expression and IL-4 signaling pathway
In various oriental herbs and plants, PGG exists as tannic acid [8]. Therefore, we examined the effect of tannic acid on IL-4-induced ɛGT expression, IL-4Rα, JAK3 and STAT6 activation in DND39 cells. Tannic acid (1 μg/ml) inhibited IL-4-induced ɛGT expression and suppressed IL-4-stimulated STAT6 phosphorylation by inhibiting IL-4Rα and JAK3 activation by IL-4 treatment (Fig. 4). To confirm whether tannic acid had an inhibitory effect on IgE production, we performed in vivo experiments for antigen-specific IgE production. Mice were administered water or water containing 4 mg/ml tannic acid ad libitum for 17 days. We primed mice by intraperitoneal injection of OVA/aluminum hydroxide on day 3. At 1 month after tannic acid administration, a blood sample was taken from their tails and the levels of total and OVA-specific IgE, IgM, and IgG were measured. The amounts of total (Fig. 5A) or OVA-specific IgE (Fig. 5B) were reduced in the tannic acid-administered mice compared with control mice. However, total and OVA-specific levels of other Ig isotypes (IgM and IgG) were not significantly affected (Fig. 5A and B). These results suggested that tannic acid selectively suppressed antigen-specific IgE production in vivo by inhibiting ɛGT expression and suppressing phosphorylation of STAT6 induced by IL-4.
Fig. 4.
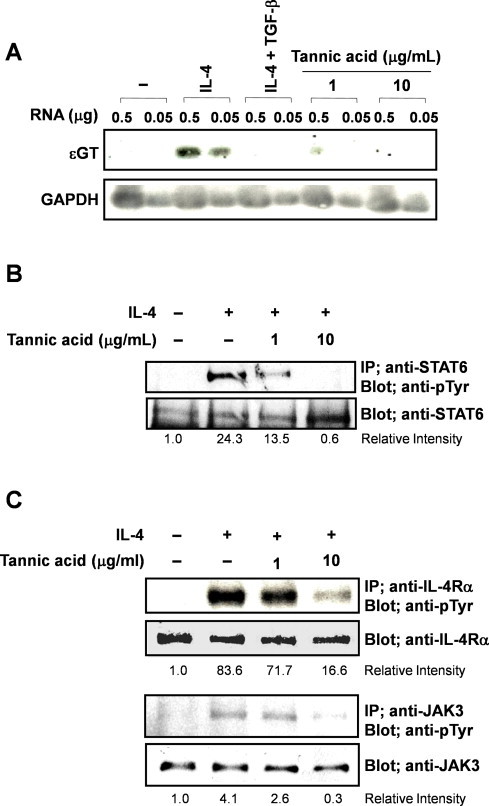
Tannic acid inhibits IL-4-induced ɛGT expression by suppressing the IL-4 signaling pathway. (A) DND39 cells were treated with tannic acid (1, 10 μg/ml) or TGF-β (2 ng/ml) in the presence of IL-4 (250 U/ml) for 48 h, and then assessed for the expression of ɛGT by RT-PCR followed by Southern hybridization. (B) Cells were treated with tannic acid (1, 10 μg/ml) in the presence of IL-4 (250 U/ml) for 30 min. (C) Cells were stimulated with IL-4 (250 U/ml) in the presence of tannic acid (1, 10 μg/ml) for 10 min. Lysates were then immunoprecipitated with an anti-STAT6, anti-JAK3 and anti-IL-4Rα antibodies and analyzed by western blotting using anti-phosphotyrosine antibody (4G10) for phosphorylated JAK3 and IL-4Rα or anti-phosphotyrosine antibody (PY20) for phosphorylated STAT6. Each experiment was replicated three times with triplicate.
Fig. 5.
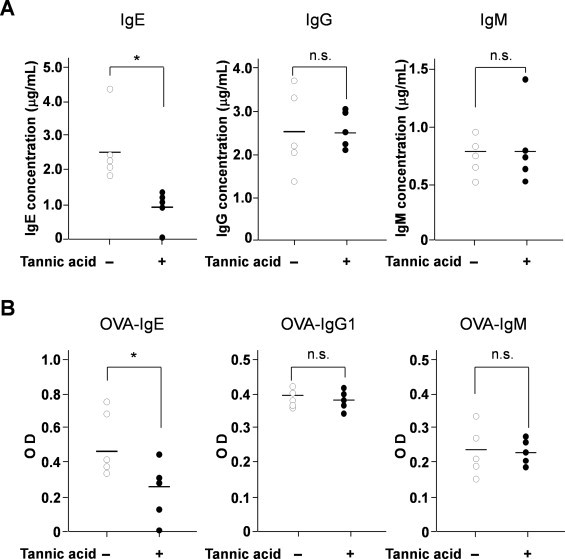
Tannic acid inhibits antigen-specific IgE production in ovalbumin-treated mice. Female, 6-week-old C57BL/6J mice were divided into two groups of 5 mice each. Mice were administered water or water containing 4 mg/ml tannic acid ad libitum for 17 days. Mice were intraperitoneally injected with 50 μg/ml OVA with aluminum hydroxide on day 3 to induce specific IgE responses. At 1 month after administration, the levels of total (A) and OVA-specific (B) IgE, IgM and IgG were measured in serum by ELISA. Statistical analysis was performed using unpaired t-test. Mean values were significantly different from the control group: *p < 0.05 (n = 5). n.s.: not significant.
4. Discussion
PGG is a representative gallotannin and a core structure of tannic acid derived from oriental herbs. A number of studies demonstrated that PGG has a wide range of biological effects such as anti-diabetic, anti-oxidant, anti-cancer and anti-inflammatory activities [9–15]. PGG especially exerts an anti-cancer effect by inhibiting the activation of JAK1 and STAT3 [12]. However, whether PGG possesses anti-allergic effects by inhibiting IL-4-induced signaling pathway is unclear. To our knowledge, this is the first report to demonstrate that PGG and tannic acid, higher galloylated PGGs, suppress IgE production by inhibiting IL-4 and IL-13-induced STAT6 phosphorylation and ɛGT expression.
IL-4-induced STAT6 activation and ɛGT expression are critical for the synthesis of IgE that is key in allergic disease [22,23]. In addition to IL-4, IL-13 binds to IL-4Rα, and therefore can induce similar responses generated by IL-4. The importance of IL-4 and IL-13 signaling was established in human asthma and animal models of asthma [24–26], suggesting that both cytokine signaling pathways are targets for therapeutic intervention of allergic diseases. Thus, the inhibition of IL-4 and IL-13-induced signaling activation may be effective in attenuating allergic disease. In the current study, PGG inhibited IL-4- and IL-13-induced STAT6 tyrosine phosphorylation, but not the IFN-γ signaling pathway. Because both IL-4 and IL-13 bind IL-4Rα to activate the same signaling pathway [21], IL-4Rα may be a target of PGG action.
An explanation why PGG has a specific inhibitory effect on IL-4-induced signaling pathways might be its structural similarity with strictinin, an ellagitannin isolated from tea leaves that inhibits IgE production by suppressing the IL-4 signaling pathway [16]. PGG and strictinin are hydrolysable tannins that are esters of gallic acid with a polyol (typically β-d-glucose). Substitution of 1-O-galloyl-β-d-glucosepyranose, the most simple member of this group, leads to the fully galloylated glucose derivative, PGG. This can be subjected to oxidation reactions that form linkages between suitably orientated galloyl residues to yield hexahydroxydiphenoyl (HHDP) moieties, thus giving rise to a second subclass of hydrolysable tannins known as ellagitannins [27]. Interestingly, the defining structural feature of hydrolysable tannins, including gallotannins (PGG) and ellagitannin (strictinin), is the inhibition of IgE production by inhibiting IL-4 and IL-13-induced signaling, although this needs to be explored further in future studies.
In summary, our data show that PGG causes the strong suppression of IL-4-induced ɛGT expression, which is indispensable for IgE class switching and that the inhibition of ɛGT expression may occur by attenuating activation of the IL-4 signaling pathway. Tannic acid, a higher galloyl glucose, also inhibits IgE production by suppressing IL-4-induced ɛGT expression. In addition, the suppressive effect exerted by PGG is specific to IL-4 and IL-13 cytokines that influence the development of allergic disease. These findings also highlight the therapeutic potential of tannic acid and PGG as potential pharmaceutical agents that may be useful to inhibit the progression of IgE-mediated disease such as food allergy, seasonal allergy, and asthma.
Acknowledgments
This work was supported by Grants for project research (Development of fundamental technology for analysis and evaluation of functional agricultural products and functional foods), in part by JSPS KAKENHI Grants Number 22228002, and Grant-in-Aid for JSPS Fellows (25.2691).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Ppulsen L.K., Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med. 2007;39:440–456. doi: 10.1080/07853890701449354. [DOI] [PubMed] [Google Scholar]
- 2.Mancini A.J., Kaulback K., Chamlin S.L. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol. 2008;25(2):1–6. doi: 10.1111/j.1525-1470.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 3.Ricci M. IL-4: a key cytokine in atopy. Clin Exp Allergy. 1994;24:801–812. doi: 10.1111/j.1365-2222.1994.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 4.Pauwels R.A., Brusselle G.G., Tournoy K.G., Lambrecht B.N., Kips J.C. Cytokines and their receptors as therapeutic targets in asthma. Clin Exp Allergy. 1998;28:1–5. [PubMed] [Google Scholar]
- 5.Quelle F.W., Shimoda K., Thierfelder W. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995;15:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schindler U., Wu P., Rothe M., McKnight S.L. Components of a Stat recognition code: evidence for two layers of molecular selectivity. Immunity. 1995;2:689–697. doi: 10.1016/1074-7613(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 7.Corry D.B., Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402:B18–B23. doi: 10.1038/35037014. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Shaik A.A., Zhang J., Nhkata K., Wang L., Zhang Y., Xing C., Kim S.H., Lü J. Preparation of penta-O-galloyl-β-d-glucose from tannic acid and plasma pharmacokinetic analyses by liquid-liquid extraction and reverse-phase HPLC. J Pharm Biomed Anal. 2011;54:545–550. doi: 10.1016/j.jpba.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X., Kim J.K., Li Y., Li J., Liu F., Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J Nutr. 2005;135:165–171. doi: 10.1093/jn/135.2.165. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Kim J., Li J., Liu F., Liu X., Himmeldirk K., Ren Y., Wagner T.E., Chen X. Natural anti-diabetic compound penta-O-galloyl-d-glucopyranose binds to insulin receptor and activates insulin mediated glucose transport signaling pathway. Biochem Biophys Res Commun. 2005;336:430–437. doi: 10.1016/j.bbrc.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.J., Jeong S.J., Lee H.J., Lee E.O., Bae H., Lieske J.C., Kim S.H. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose reduces renal crystallization and oxidative stress in a hyperoxaluric rat model. Kidney Int. 2011;79:538–545. doi: 10.1038/ki.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H.J., Seo N.J., Jeong S.J., Park Y., Jung D.B., Koh W., Lee H.J., Lee E.O., Ahn K.S., Ahn K.S., Lü J., Kim S.H. Oral administration of penta-O-galloyl-b-d-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis. 2011;32:804–811. doi: 10.1093/carcin/bgr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W.S., Jeong S.J., Kim J.H., Lee H.J., Song H.S., Kim M.S., Ko E., Lee H.J., Khil J.H., Jang H.J., Kim Y.C., Bae H., Chen C.Y., Kim S.H. The genome-wide expression profile of 1,2,3,4,6-penta-O-galloyl-b-d-glucose-treated MDA-MB-231 breast cancer cells: molecular target on cancer metabolism. Mol cells. 2011;32:123–132. doi: 10.1007/s10059-011-2254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu M., Gu Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. Evid Based Complement Altern Med. 2009;6:57–63. doi: 10.1093/ecam/nem066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.H., Park H.H., Kim J.E., Kim J.A., Kim Y.H., Jun C.D., Kim S.H. Allose gallates suppress expression of pro-inflammatory cytokines through attenuation of NF-kappaB in human mast cells. Planta Med. 2007;73:769–773. doi: 10.1055/s-2007-981553. [DOI] [PubMed] [Google Scholar]
- 16.Tachibana H., Kuboa T., Miyase T., Taninoa S., Yoshimoto M., Sano M., Yamamoto-Maeda M., Yamada K. Identification of an inhibitor for interleukin 4-induced germline transcription and antigen-specific IgE production in vivo. Biochem Biophys Res Commun. 2001;280:53–60. doi: 10.1006/bbrc.2000.4069. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi W., Miyase T., Sano M., Umehara K., Warashina T., Noguchi H. Prolyl endopeptidase inhibitors from the roots of Lindera strychnifolia F. VILL Biol Pharm Bull. 2002;25:1049–1052. doi: 10.1248/bpb.25.1049. [DOI] [PubMed] [Google Scholar]
- 18.Gauchat J.F., Lebman D.A., Coffman R.L., Gascan H., de Vries J.E. Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to IgE production. J Exp Med. 1990;172:463–473. doi: 10.1084/jem.172.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichiki T., Takahashi W., Watanabe T. The effect of cytokines and mitogens on the induction of C epsilon germline transcripts in a human Burkitt lymphoma B cell line. Int Immunol. 1992;4:747–754. doi: 10.1093/intimm/4.7.747. [DOI] [PubMed] [Google Scholar]
- 20.Yanagihara Y., Ikizawa K., Kajiwara K., Koshio T., Basaki Y., Akiyama K. Functional significance of IL-4 receptor on B cells in IL-4-induced human IgE production. J Allergy Clin Immunol. 1995;96:1145–1151. doi: 10.1016/s0091-6749(95)70199-0. [DOI] [PubMed] [Google Scholar]
- 21.Leonard W.J., O’Shea J.J. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 22.Holgate S.T. The epidemic of allergy and asthma. Nature. 1999;402:B2–B4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 23.Nelms K., Keegan A.D., Zamorano J., Ryan J.J., Paul W.E. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 24.Mueller T.D., Zhang J.L., Sebald W., Duschl A. Structure, binding, and antagonistst in the IL-4/IL-13 receptor system. Biochim Biophys Acta. 2002;1592:237–250. doi: 10.1016/s0167-4889(02)00318-x. [DOI] [PubMed] [Google Scholar]
- 25.Izuhara K. The role of interleukin-4 and interleukin-13 in the non-immunologic aspects of asthma pathogenesis. Clin Chem Lab Med. 2003;41:860–864. doi: 10.1515/CCLM.2003.130. [DOI] [PubMed] [Google Scholar]
- 26.Hahn C., Teufel M., Herz U., Renz H., Erb K.J., Wohlleben G., Bröcker E.B., Duschl A., Sebald W., Grunewald S.M. Inhibition of the IL-4/IL-13 receptor system prevents allergic sensitization without affecting established allergy in a mouse model for allergic asthma. J Allergy Clin Immunol. 2003;111:1361–1369. doi: 10.1067/mai.2003.1527. [DOI] [PubMed] [Google Scholar]
- 27.Niemetz R., Gross G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry. 2005;66:2001–2011. doi: 10.1016/j.phytochem.2005.01.009. [DOI] [PubMed] [Google Scholar]


