Abstract
The peptide hormone apelin is translated as a 77-residue preproprotein, truncated to the 55-residue proapelin and, subsequently, to 13–36-residue bioactive isoforms named apelin-13 to -36. Proapelin is hypothesized to be cleaved to apelin-36 and then to the shorter isoforms. However, neither the mechanism of proapelin processing nor the endoproteases involved have been determined. We show direct cleavage of proapelin to apelin-13 by proprotein convertase subtilisin/kexin 3 (PCSK3, or furin) in vitro, with no production of longer isoforms. Conversely, neither PCSK1 nor PCSK7 has appreciable proapelin cleavage activity. Furthermore, we show that both proapelin and PCSK3 transcript expression levels are increased in adipose tissue with obesity and during adipogenesis, suggesting that PCSK3 is responsible for proapelin processing in adipose tissue.
Keywords: Proprotein convertase/subtisilin kexin 3, Furin, Prohormone processing, Adipocyte differentiation, Proapelin
Abbreviations: MALDI-MS, matrix assisted laser desorption ionization mass spectrometry; PCSK, proprotein convertase subtilisin/kexin; RP-HPLC, reverse phase high performance liquid chromatography; (q)PCR, (quantitative) polymerase chain reaction; RT-PCR, reverse transcription-PCR; TEV, tobacco etch virus
Graphical abstract
Highlights
-
•
No prohormone processing mechanisms have yet been shown for apelin.
-
•
Proapelin is cleaved into apelin-13 both specifically and preferentially by PCSK3.
-
•
Conversely, PCSK1 and PCSK7 do not cleave proapelin.
-
•
PCSK3 and apelin expression increase with adipocyte differentiation and obesity.
-
•
We show the first evidence of and propose a new theory for apelin bioactivation.
1. Introduction
Apelin is a peptide hormone present in many tissues and body fluids [1]. It is known to be a potent cardiac inotrope [2] and to modulate blood pressure [3]. Furthermore, apelin and its cognate G-protein coupled receptor (the apelin receptor) [4] are expressed in adipose tissue with significantly increased expression correlated to obesity [5]. Apelin was recently shown to inhibit adipocyte differentiation and breakdown of fat [6] and improve insulin sensitivity [7]. Thus, the apelin signalling system has been touted as having great therapeutic potential in treatment of obesity and cardiovascular diseases [3,7,8].
Apelin is expressed as a 77 residue pre-proprotein, which is cleaved to a 55 residue proprotein (proapelin) following removal of an N-terminal signal peptide [8]. In all bioactive forms of apelin, at least 12 residues at the C-terminus of proapelin are retained [9]. The current proposed mechanism for apelin processing suggests that proapelin is first cleaved to form apelin-36 (i.e., the 36 C-terminal residues of proapelin) in the cell and then further processed into shorter apelin isoforms retaining the C-terminus, most prevalently 13 and 17 amino acids long [3]. Spontaneous cyclization of the N-terminal Gln of apelin-13 produces the pyroglutamate-modified form (Pyr-apelin-13) [10]. All apelin isoforms bind to the apelin receptor to cause similar cellular effects; however, isoform potencies, efficacies, and receptor recycling kinetics differ [1,11]. Furthermore, studies have shown that there is tissue specificity in apelin isoforms production. For example, Pyr-apelin-13 is the dominant isoform present throughout the brain, hypothalamus, and heart, but the predominant isoform in the lungs, testis, and uterus is apelin-36 [12–14]. Despite this variability in isoform activity and localization, no studies have directly investigated proapelin processing or the endoproteases involved [1,3].
One of the endoprotease families known to activate prohormones is the proprotein convertase subtilisin/kexin (PCSK) family, comprising nine known subtypes of Ca2+ dependent endoproteases, known as PCSK1 through PCSK9 [15]. Substrates of many PCSKs contain dibasic residues immediately N-terminal to the cleavage site and usually have a β-turn at or near the cleavage site [16]. Since, proapelin has multiple dibasic motifs (Fig. 1A) and a β-turn at the cleavage site of apelin-13 [17], we hypothesized that PCSK enzymes are responsible for proapelin cleavage.
Fig. 1.
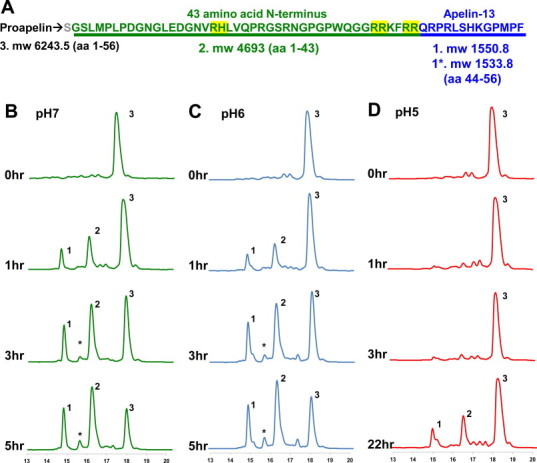
PCSK3 preferentially cleaves proapelin into apelin-13. Proapelin produced and purified from E. coli was incubated with recombinant PCSK3. (A) Proapelin and its fragments alongside predicted molecular weights and numbered 1–3 according to elution time. The gray Ser at the N-terminus is retained residue following TEV protease cleavage; the green residues represent the endogenous 42 amino acid N-terminal pro-domain; blue represents the 13 residue apelin-13, with 1* being Pyr-apelin-13. Proapelin incubation with PCSK3 resulted in production of 3 new products corresponding to apelin-13, Pyr-apelin-13, and the 43 amino acid N-terminal pro domain at (B) pH 7 (C) pH 6 and (D) pH 5. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In this report, we demonstrate for the first time proapelin cleavage into a bioactive isoform. Strikingly, PCSK3 directly and preferentially cleaves proapelin into apelin-13 in vitro with no evidence of longer isoforms. In contrast, neither PCSK1 nor PCSK7 shows appreciable proapelin cleavage activity. Furthermore, we show that both proapelin and PCSK3 transcript expression levels are increased in adipose tissue with obesity and during adipogenesis suggesting that PCSK3 is responsible for proapelin processing in adipose tissues.
2. Materials and methods
2.1. Proapelin expression and purification
To produce proapelin, proapelin (synthetic gene with Escherichia coli codon bias, BioBasic) with 6xHis tag and TEV protease cleavage site was cloned into the pEXP5-NT vector (Invitrogen) and expressed in E. coli C41(DE3) (Lucigen). The protein was purified by Ni-NTA agarose column (Qiagen). The 6xHis tag was cleaved by TEV protease (Addgene). Proapelin was purified by S Ceramic HyperD F cation exchange column (PALL Life Science).
2.2. In vitro enzymatic digestions
Active recombinant PCSKs (2 μg; RnD Systems) were mixed with proapelin (75 nmol) in appropriate buffer (PCSK1: 25 mM acetate or 2-(N-morpholino) ethanesulfonic acid, 5 mM CaCl2, 1% Brij-35, pH 6; PCSK3: 25 mM Tris or acetate, 1 mM CaCl2, 1% Brij-35, pH 5–7; PCSK7: 25 mM Tris, 0.4–1.5 mM CaCl2, 0.5% Brij-35, pH 7). In each case, the reaction was monitored at various time points by reverse phase high performance liquid chromatography (RP-HPLC) separation (Varian ProStar HPLC) using an analytical column C18-AR-II (4.6 × 250 mm, Cosmosil). The mobile phase components were Type II water (A) and acetonitrile (B), both containing 0.1% trifluoroacetic acid (v/v). Elution was carried out at a flow rate of 1 ml/min using a linear gradient from 2–100% B in A (2–20% in 5 min, 20–40% in 20 min, 40–100% in 15 min, and 100–2% in 2 min) or 2–40% (2–20% in 3 min, 20–40% in 20 min, and 40–2% in 2 min). UV chromatograms were recorded at 210 and 280 nm simultaneously and eluent masses determined by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS, C-CART Facility at Memorial University, St. John’s, Newfoundland).
2.3. 3T3-L1 preadipocyte differentiation
3T3-L1 preadipocytes were obtained from ATCC (Manassas, Virginia) then subjected to differentiation [18]. Passages 4–10 were used for experiments, and tested negative for mycoplasma. Preadipocytes were seeded in 6-well plates and grown to confluence. At 2 days post-confluence (day 0), differentiation was initiated by changing the medium to induction media (DMEM containing 10% FBS, 1 μM dexamethasone (DEXA, D2915, Sigma Aldrich), 0.5 mM 3-isobutyl-1-methylxanthine (IBMX, I7018, Sigma Aldrich), and 1 μg/ml human insulin (HI-210, Eli Lilly) [18]. On day 2, the medium was replaced with insulin-containing medium (DMEM supplemented with 10% FBS, 1% P/S, and 10 μg/ml human insulin). Thereafter, the medium was replaced at 2-day intervals with FBS media (DMEM supplemented with 10% FBS). Differentiated cells were observed starting at day 4 and more than 90% of the cells were fully differentiated by day 8. During the differentiation protocol, mRNA was extracted from adipocytes and analyzed for proapelin and PCSKs expressions by RT-PCR. mRNA was extracted using Aurum Total RNA Fatty and Fibrous Tissue Kit (BioRad) following the manufacturer's guidelines.
2.4. Animals and tissue preparation
All animal studies were approved by the Dalhousie University Animal Care Committee and were performed in strict adherence to the policies of the Canadian Council for Animal Care. 6 week old male B6.V-Lep (ob)/j ob/ob mice and 12 week old male C57BL/6J mice that were fed a high fat diet (60% fat, 20% carbohydrate, 20% protein) from weeks 6 to 12, and their age matched controls who were fed a lower fat diet (10% fat, 70% carbohydrate, 20% protein) were purchased from Jackson Laboratory. Peri-gonadal white adipose tissues were removed and immediately snap frozen in liquid nitrogen, then stored at −80 °C until use. RNA extraction, cDNA synthesis and reverse transcription from tissues and cells were conducted as described previously [19]. Tissues extracted from control, DIO, and ob/ob mice were analyzed for the proapelin and PCSK3 mRNA by RT-PCR. The values are represented as mean ± standard deviation.
2.5. Real time PCR
All qPCR reactions were performed using 2 Å∼ Brilliant SYBR green master mix (Roche) with initial denaturation at 95 °C for 10 min followed by 40 cycles at 95 °C for 30 s, 60 °C for 1 min and 72 °C for 30 s. A list of all primers used is provided in the Supplementary Information (Table S1). mRNA concentrations were determined from a standard curve of known concentration amplicons. The amounts of mRNA were extrapolated from standard curves, and all data are expressed relative to 18S RNA (% 18S).
2.6. Statistical analysis
Data analysis was performed using Prism (v5.0, Graphpad). Data are reported as mean ± SEM. One way ANOVA was used for multiple comparisons between mouse strains, followed by Tukey's post hoc test. p < 0.05 was considered significant.
3. Results and discussion
3.1. PCSK3 preferentially activates proapelin into apelin-13
We initially studied the PCSK-mediated apelin processing with PCSK3 (also known as furin) because it is the most well studied PCSK subtype that cleaves C-terminally to dibasic sites. In addition, expression of PCSK3 is detected in tissues secreting apelin, such as the heart [20] and adipose tissue [21], suggesting the possibility for apelin processing by PCSK3.
Recombinant proapelin was produced in E. coli with an N-terminal His-tag, purified following proteolysis to remove the His-tag, and incubated with PCSK3 in vitro to test its suitability for cleavage by PSCK3. It should be noted that our recombinant proapelin retains an additional N-terminal Ser as a result of TEV protease cleavage. Proapelin assays were conducted at 37 °C and at pH 5, 6, and 7 to investigate the process across the pH gradient found along the secretory pathway [22]. Crude reaction mixture samples at given time points were subjected to RP-HPLC using a mobile phase gradient optimized for separation of apelin isoforms (details in the Supplementary Information). The constituents of all resulting peptide/protein containing fractions were identified by mass spectrometry.
Incubation of the 56 residue proapelin (6259 m/z) with PCSK3 resulted in production of two polypeptide species with distinct RP-HPLC elution properties at all tested pH conditions. These species were unambiguously identifiable as apelin-13 (1550.5 m/z, aa 44–56) and the N-terminal of proapelin (the “pro-domain”, 4709 m/z, aa 1–43) (Fig. 1B–D). Notably, these eluents were not observed when proapelin was incubated in the absence of PCSK3 (Fig. S1). Under all reaction conditions, both the pro-domain and uncleaved proapelin had a mass 16 m/z greater than expected, suggesting that oxidization had taken place. Following a 5-h incubation, the concentrations of apelin-13 and the pro-domain consistently exceeded that of proapelin at both pH 6 and 7. At the 5-h time point, another product became visible with a mass consistent with Pyr-apelin-13 (1533.8 m/z), presumably arising from spontaneous cyclization of the apelin-13 N-terminal Gln [10]. At pH 5, PCSK3 showed decreased enzymatic activity similar to previous studies [23] but the same preferential cleavage to apelin-13 and the pro-domain (Fig. 1D). In no instance were cleavage products observed corresponding to production of any of the longer or shorter apelin isoforms.
Many PCSK3 substrates have a β-turn at their cleavage site and a consensus amino acid sequence N-terminal to the cleavage site of R-(X)-K/R-R, with X representing any amino acid other than Cys [15]. Exceptions to the consensus sequence are also observed, and it is the last two basic amino acids that are believed to be most critical for cleavage [24]. Strikingly, our previous structural investigations of apelin-17 demonstrated a β-turn over the residues R↓Q-R-P (with the apelin-13 cleavage site indicated by ↓) [17]. Furthermore, the sequence immediately N-terminal to the cleavage site is K-F-R-R, similar to the consensus sequence required by PCSK3 in terms of basic residues (Fig. 1A). Interestingly, apelin-17, which also has two Arg residues immediately N-terminal to its cleavage site, was not produced by PCSK3. This may simply be due to much lower affinity of PCSK3 to cleave following the G-G-R-R sequence N-terminal to apelin-17 vs. the K-F-R-R N-terminal to apelin-13. Alternatively, the absence of apelin-17 production may be due to the presence of ordered structures such as α-helices or β-strands upstream of apelin-17 cleavage site since structuring is associated with non-cleaved sites [24]. To date, no structural data is available for any apelin isoform longer than apelin-17 but circular dichroism studies of apelin-36 are certainly not indicative of extensive stable secondary structure [17,25]. In general, not all putative cleavage sites are processed by PCSKs, and the recognition of endoproteases is not correlated with the existence of a single consensus primary sequence around cleaved sites. Correspondingly, many factors involved in PCSK-mediated processing are still unknown. Therefore, it is difficult to attribute any single factor to the absence of apelin-17 production.
3.2. PCSK1 and PCSK7 do not cleave proapelin in vitro
We also investigated the capability of the neuroendocrine system-specific PCSK1 and ubiquitously expressed PCSK7 to process proapelin. During screening for cleavage by PCSK1 and 7, various Ca2+ concentrations and buffer compositions were employed, following previously reported optimized reaction conditions [23,26,27]. In the case of PCSK1, lower specific activity than PCSK3 was expected [28]. However, even after 3 days of incubation of proapelin with PCSK1, no processing to bioactive apelin isoforms was evident (Fig. S2). PCSK7, alternatively, has similar specific activity to PCSK3, and is known to process some of the same substrates [29]. Despite this, no evidence of proapelin cleavage by PCSK7 was observed (Fig. S2).
3.3. Proapelin and PCSK subtype expression levels correlate with adipocyte differentiation and obesity
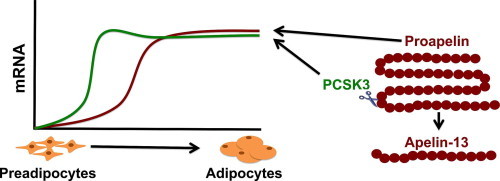
Apelin is expressed in adipose tissue and its expression increases with obesity [30,31]. To determine if proapelin and PCSK3 expression levels change during preadipocyte differentiation, mRNA levels were monitored over the course of the differentiation process of 3T3-L1 preadipocytes. Proapelin expression showed a sigmoidal increase in expression during early differentiation and peaked near day 5 (Fig. 2A). Notably, both PCSK3 and PCSK7 mRNA levels increased during differentiation, but differed in their expression profiles (Fig. 2B). PCSK1 mRNA, conversely, remains at its basal level throughout the process. PCSK3 expression levels exhibited bimodal behaviour, with peak expression at day 2 and 7, but remained relatively high after day 2 compared to other PCSK subtypes. PCSK7 expression remained low relative to PCSK3 until day 6 of differentiation, at which it started to increase. Therefore, high PCSK3 levels correlate strongly to increasing proapelin expression during adipocyte differentiation.
Fig. 2.
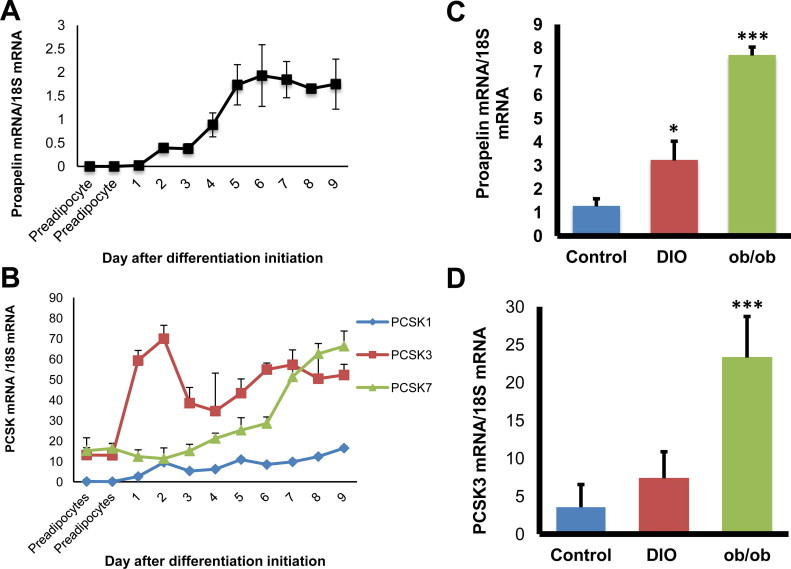
Proapelin, PCSK3 and PCSK7 mRNA expression are increased with adipocyte differentiation and obesity in mice. (A, B) During the differentiation protocol, mRNA was extracted from adipocytes and analyzed for proapelin and PCSKs expressions by RT-PCR. (C, D) Tissues were extracted from 12-week old control, DIO, and ob/ob mice and the proapelin and PCSK3 mRNA expressions were analyzed by RT-PCR. The values are represented as mean ± standard deviation.
To test this correlation in vivo, we investigated the expression of proapelin and PCSK3 expression in white adipose tissue from leptin deficient ob/ob mice and diet-induced obese mice. mRNA was extracted from peri-gonadal white adipose tissue from 12-week-old mice and analyzed expression using quantitative PCR. Proapelin and PCSK3 expression levels were significantly increased in mouse models of obesity compared to the lean controls (Fig. 2C and D). Given the high co-expression of PCSK3 and proapelin, apelin-13 may be the preferred isoform in adipose tissue. PCSK3 has also been shown to be highly expressed in the heart [20], suggesting that the prevalence of apelin-13 in this tissue may also be due to PCSK3.
3.4. A new proposed mechanism for apelin isoform production
Apelin is present in the body as many bioactive isoforms with potential tissue specific processing. We show for the first time that a specific endoprotease, PCSK3, is capable of producing apelin-13. The current proposed mechanism of apelin processing suggests that proapelin is initially cleaved to apelin-36 then further cleaved into shorter isoforms of increasing efficacies (Fig. 3A) [3]. However, we show that PCSK3 specifically and preferentially produces apelin-13 directly from proapelin without evidence of any apelin-36 or apelin-17 production. Furthermore, we showed that PCSK1 and 7 could not cleave proapelin, suggesting a specificity of PCSK3 for proapelin processing. We therefore propose a new mechanism of apelin processing in which proapelin is cleaved to various bioactive isoforms directly from its proprotein stage by various endoproteases, including PCSK3 (Fig. 3B). Although many regulators and other enzymes involved in proapelin processing still remain unknown, identifying PCSK3 as one of the proteases involved in processing is a key first step towards unraveling of apelin isoform level and activity differences as a function of both tissue type and of healthy or pathological conditions.
Fig. 3.
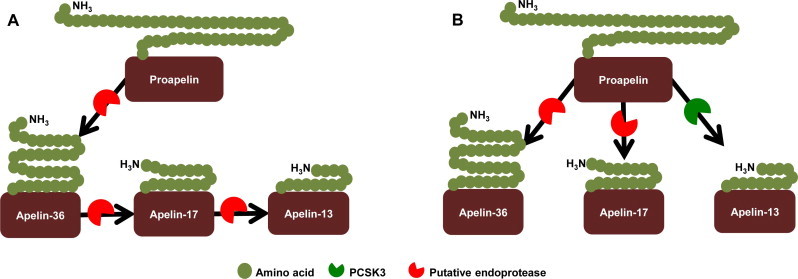
Overview of proposed apelin processing pathways. (A) The previously proposed mechanism for apelin processing [3] suggested that proapelin is cleaved to the longest isoform, apelin-36, and then further processed to shorter isoforms with increased efficacy. (B) Our new model of apelin processing suggests that PCSK3 directly produces apelin-13 from proapelin. The longer apelin-36 and -17 isoforms are putatively produced by other, as yet unidentified endoproteases.
Acknowledgements
This work was supported by a Canadian Institutes of Health Research (CIHR) Operating Grant (MOP-111138 to JKR); a Nova Scotia Health Research Foundation (NSHRF) Scotia Support Grant (to J.K.R.); a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant (to X.Q.L.); and, the IWK Research Foundation (to Y.A.). Key equipment was provided by an NSERC Research Tools & Instruments Grant (J.K.R.), by Canadian Foundation for Innovation Leaders Opportunity Fund grants (to J.K.R. and Y.A.) and by a Dalhousie Medical Research Foundation grant (to J.K.R., X.Q.L. and Y.A.). J.K.R. is supported by a CIHR New Investigator Award and K.S. by an NSERC Canada Graduate Scholarship. We thank Bruce Stewart for technical support and Drs. David Langelaan and Jeffrey Gagnon for initial preproapelin vector design and helpful discussions.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Younes Anini, Email: younes.anini@dal.ca.
Jan K. Rainey, Email: jan.rainey@dal.ca.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fob.2013.08.001.
Appendix. Supplementary materials
This document contains a Section on "in vitro assay optimization", Supplementary Figs. S1 and S2, and Tables S1 and S2.
References
- 1.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C., Kurokawa T., Onda H., Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 2.Japp A.G., Newby D.E. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem. Pharmacol. 2008;75:1882–1892. doi: 10.1016/j.bcp.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Kleinz M.J., Davenport A.P. Emerging roles of apelin in biology and medicine. Pharmacol. Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.O’Dowd B.F., Heiber M., Chan A., Heng H.H., Tsui L.C., Kennedy J.L., Shi X., Petronis A., George S.R., Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 5.Soriguer F., Garrido-Sanchez L., Garcia-Serrano S., Garcia-Almeida J.M., Garcia-Arnes J., Tinahones F.J., Garcia-Fuentes E. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes. Surg. 2009;19:1574–1580. doi: 10.1007/s11695-009-9955-y. [DOI] [PubMed] [Google Scholar]
- 6.Than A., Cheng Y., Foh L.C., Leow M.K., Lim S.C., Chuah Y.J., Kang Y., Chen P. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell. Endocrinol. 2012;362:227–241. doi: 10.1016/j.mce.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Castan-Laurell I., Dray C., Knauf C., Kunduzova O., Valet P. Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol. Metab. 2012;23:234–241. doi: 10.1016/j.tem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Masri B., Knibiehler B., Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell. Signal. 2005;17:415–426. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Tatemoto K., Takayama K., Zou M.X., Kumaki I., Zhang W., Kumano K., Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 10.Pitkin S.L., Maguire J.J., Bonner T.I., Davenport A.P. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010;62:331–342. doi: 10.1124/pr.110.002949. [DOI] [PubMed] [Google Scholar]
- 11.Lee D.K., Ferguson S.S., George S.R., O’Dowd B.F. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem. Biophys. Res. Commun. 2010;395:185–189. doi: 10.1016/j.bbrc.2010.03.151. [DOI] [PubMed] [Google Scholar]
- 12.Kawamata Y., Habata Y., Fukusumi S., Hosoya M., Fujii R., Hinuma S., Nishizawa N., Kitada C., Onda H., Nishimura O., Fujino M. Molecular properties of apelin: tissue distribution and receptor binding. Biochim. Biophys. Acta. 2001;1538:162–171. doi: 10.1016/s0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 13.De Mota N., Lenkei Z., Llorens-Cortes C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology. 2000;72:400–407. doi: 10.1159/000054609. [DOI] [PubMed] [Google Scholar]
- 14.Maguire J.J., Kleinz M.J., Pitkin S.L., Davenport A.P. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54:598–604. doi: 10.1161/HYPERTENSIONAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 15.Seidah N.G. The proprotein convertases, 20years later. Methods Mol. Biol. 2011;768:23–57. doi: 10.1007/978-1-61779-204-5_3. [DOI] [PubMed] [Google Scholar]
- 16.Brakch N., Rholam M., Boussetta H., Cohen P. Role of beta-turn in proteolytic processing of peptide hormone precursors at dibasic sites. Biochemistry. 1993;32:4925–4930. doi: 10.1021/bi00069a029. [DOI] [PubMed] [Google Scholar]
- 17.Langelaan D.N., Bebbington E.M., Reddy T., Rainey J.K. Structural insight into G-protein coupled receptor binding by apelin. Biochemistry. 2009;48:537–548. doi: 10.1021/bi801864b. [DOI] [PubMed] [Google Scholar]
- 18.Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 19.Morash M.G., Gagnon J., Nelson S., Anini Y. Tissue distribution and effects of fasting and obesity on the ghrelin axis in mice. Regul. Pept. 2010;163:62–73. doi: 10.1016/j.regpep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Beaubien G., Schafer M.K., Weihe E., Dong W., Chretien M., Seidah N.G., Day R. The distinct gene expression of the pro-hormone convertases in the rat heart suggests potential substrates. Cell Tissue Res. 1995;279:539–549. doi: 10.1007/BF00318166. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto T., Shibata R., Ohashi K., Kambara T., Kataoka Y., Uemura Y., Yuasa D., Murohara T., Ouchi N. Regulation of adipolin/CTRP12 cleavage by obesity. Biochem. Biophys. Res. Commun. 2012;428:155–159. doi: 10.1016/j.bbrc.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 22.Paroutis P., Touret N., Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology. 2004;19:207–215. doi: 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- 23.Molloy S.S., Bresnahan P.A., Leppla S.H., Klimpel K.R., Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- 24.Rholam M., Fahy C. Processing of peptide and hormone precursors at the dibasic cleavage sites. Cell. Mol. Life. Sci. 2009;66:2075–2091. doi: 10.1007/s00018-009-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan X., Zhou N., Zhang X., Mukhtar M., Lu Z., Fang J., DuBois G.C., Pomerantz R.J. Structural and functional study of the apelin-13 peptide, an endogenous ligand of the HIV-1 coreceptor, APJ. Biochemistry. 2003;42:10163–10168. doi: 10.1021/bi030049s. [DOI] [PubMed] [Google Scholar]
- 26.Scamuffa N., Basak A., Lalou C., Wargnier A., Marcinkiewicz J., Siegfried G., Chretien M., Calvo F., Seidah N.G., Khatib A.M. Regulation of prohepcidin processing and activity by the subtilisin-like proprotein convertases Furin, PC5, PACE4 and PC7. Gut. 2008;57:1573–1582. doi: 10.1136/gut.2007.141812. [DOI] [PubMed] [Google Scholar]
- 27.Jean F., Basak A., Rondeau N., Benjannet S., Hendy G.N., Seidah N.G., Chretien M., Lazure C. Enzymic characterization of murine and human prohormone convertase-1 (mPC1 and hPC1) expressed in mammalian GH4C1 cells. Biochem. J. 1993;292(Pt. 3):891–900. doi: 10.1042/bj2920891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remacle A.G., Shiryaev S.A., Oh E.S., Cieplak P., Srinivasan A., Wei G., Liddington R.C., Ratnikov B.I., Parent A., Desjardins R., Day R., Smith J.W., Lebl M., Strongin A.Y. Substrate cleavage analysis of furin and related proprotein convertases. A comparative study. J. Biol. Chem. 2008;283:20897–20906. doi: 10.1074/jbc.M803762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegfried G., Basak A., Cromlish J.A., Benjannet S., Marcinkiewicz J., Chretien M., Seidah N.G., Khatib A.M. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J. Clin. Invest. 2003;111:1723–1732. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boucher J., Masri B., Daviaud D., Gesta S., Guigne C., Mazzucotelli A., Castan-Laurell I., Tack I., Knibiehler B., Carpene C., Audigier Y., Saulnier-Blache J.S., Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 31.Castan-Laurell I., Vitkova M., Daviaud D., Dray C., Kovacikova M., Kovacova Z., Hejnova J., Stich V., Valet P. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur. J. Endocrinol. 2008;158:905–910. doi: 10.1530/EJE-08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document contains a Section on "in vitro assay optimization", Supplementary Figs. S1 and S2, and Tables S1 and S2.


