Summary
Apoptosis and programmed necrosis balance each other as alternate first line host defense pathways against which viruses have evolved countermeasures. Intrinsic apoptosis, the critical programmed cell death pathway that removes excess cells during embryonic development and tissue homeostasis, follows a caspase cascade triggered at mitochondria and modulated by virus-encoded anti-apoptotic B cell leukemia (BCL)2-like suppressors. Extrinsic apoptosis controlled by caspase 8 arose during evolution to trigger executioner caspases directly, circumventing viral suppressors of intrinsic (mitochondrial) apoptosis and providing the selective pressure for viruses to acquire caspase 8 suppressors. Programmed necrosis likely evolved most recently as a “trap door” adaptation to extrinsic apoptosis. Receptor interacting protein (RIP)3 kinase (also called RIPK3) becomes active when either caspase 8 activity or polyubiquitylation of RIP1 is compromised. This evolutionary dialogue implicates caspase 8 as “supersensor” alternatively activating and suppressing cell death pathways.
Overview of intrinsic apoptosis, extrinsic apoptosis and programmed necrosis
Apoptosis is executed by two distinct signaling pathways that converge on the activation of executioner caspase (Casp)3 and Casp7, called intrinsic and extrinsic apoptosis. Either drives characteristic morphological changes (cell shrinkage, membrane blebbing, nuclear condensation and DNA fragmentation) dependent on caspase activity [1,2]. The intrinsic pathway relies on pro- and anti-apoptotic B cell lymphoma 2 (BCL2) family proteins [3] at mitochondria to sense stress, signal and execute dismantling of the cell. Two key pro-apoptotic players, BCL-2-associated X protein (BAX) and BCL-2 antagonist/killer (BAK), form a pore that releases pro-apoptotic factors (e.g. cytochrome c, SMAC/DIABLO) to form an Apaf1-dependent apoptosome responsible for Casp9-dependent activation of Casp3 and/or Casp7 [1,2]. Large DNA viruses such as adenoviruses, herpesviruses and poxviruses block apoptosis by elaborating anti-apoptotic functions that mimic BCL2 proteins [4–6]. These inhibitors have subtle effects in cultured cells but maintain cell viability and facilitate viral dissemination within monocyte-macrophage lineage cells during herpesvirus [7–10] and poxvirus [11] infections. For example, human cytomegalovirus (CMV) targets both BAX and BAK by expressing potent viral mitochondrial inhibitor of apoptosis (vMIA), encoded by the UL37x1 gene [12–16]. The biologically related murine CMV splits the job using two proteins: vMIA (m38.5 gene product) targeting BAX [17,18] and viral inhibitor of BAK oligomerization (vIBO), the m41.1 gene product [19]. Human CMV vMIA modulates apoptosis as well as a caspase-independent death pathway radiating from mitochondrial serine protease HtrA2 [16,20]. Murine CMV vMIA and vIBO facilitate dissemination by supporting viability of infected peripheral blood monocytemacrophages [7–10]. Virus-encoded cell death suppressors also influence respiration, metabolism, and morphology [16,21–25], working in tandem with interferon (IFN)-activated mediator, viperin [26] as well as non-coding RNA [24]. Virus-encoded suppressors of mitochondrial steps in apoptosis are numerous [6], but their natural roles are subtle.
This review focuses on recent advances in understanding the complex interplay of extrinsic cell death pathways where the suppressors encoded by large DNA viruses have striking roles in controlling cell fate, providing key insights to recently evolved host defense pathways. Understanding of extrinsic cell death comes largely from tumor necrosis factor (TNF) family death receptor studies. Extrinsic death is triggered via death receptors and pathogen sensors, which have evolved as components of the pathogen-host arms race. Extrinsic death pathways are a crucial first line of host defense, serving where intrinsic apoptosis has been compromised [27] (Fig. 1). A complex composed of Casp8, FAS-associated protein with death domain (FADD) and FLICE (e.g., Casp8) inhibitory protein (FLIP), controls extrinsic death pathways (Fig. 2). Casp8 inhibitors, either vFLIP-like proteins encoded by herpesviruses and poxviruses [28,29], or cellular FLIP [5,30,31], provide regulation of extrinsic death, particularly in macrophages and dendritic cells [16,27,32,33]. For example, the viral inhibitor of Casp8 activation (vICA) conserved in human and murine CMV [31] supports interaction with monocytemacrophages [34,35], aiding efficient dissemination of virus by maintaining viability of the infected monocyte-macrophage reservoir [36]. Although viral mimics override host regulation, cellular FLIP in the Casp8-FADD-FLIP complex dictates whether Casp8 homodimerization and self-activation occurs. Specifically, the long form of cellular FLIP (FLIPL) is catalytically inactive caspase paralog that heterodimerizes with unprocessed Casp8 to block self-activation (Fig. 2). Basal activity from this Casp8-FLIPL heterodimer suppresses RIP3 kinase-dependent pathways, preventing programmed necrosis [27]. When levels of FLIPL are too low, Casp8 homodimerization and self-activation triggers extrinsic apoptosis [27], following either of two pathways: (i) direct cleavage of Casp3 to trigger processing of the effector caspases or (ii) cleavage of pro-apoptotic BCL2 family member BH3-interacting domain (BID) to truncated (t)BID, which in turn triggers BAX-BAK activation at mitochondria, merging into intrinsic signaling [37,38].
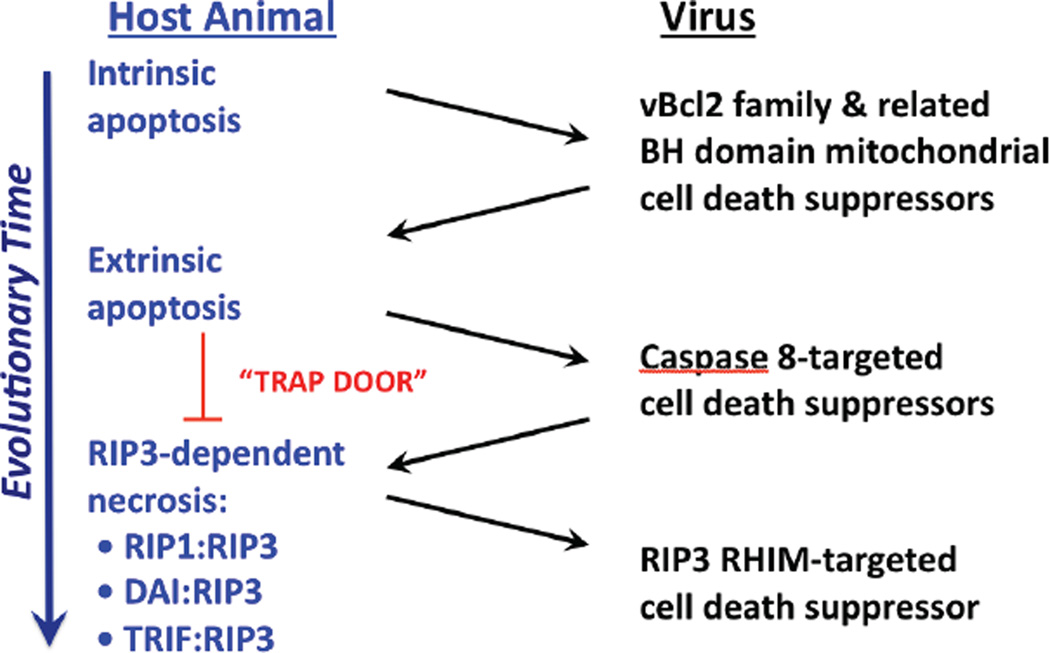
Figure 1. Evolution of cell death pathways in host defense.
Diagram representing evolutionary time (large arrow) together with host death pathways targeted by known virus-encoded cell death suppressors. Intrinsic apoptosis is a primordial property of multicellular organisms to eliminate excess cells during development that contributes to host defense. Herpesviruses, poxviruses and adenoviruses encode mitochondrial cell death suppressors that limit the impact of pro-apoptotic BCL2 family member signaling in host defense [4–6,13]. Extrinsic apoptosis represents an adaptation to avoid virus-encoded inhibitors of mitochondrial apoptosis, by employing Casp8 as a self-activating protease that can directly target executioner caspases, Casp3 and Casp7. Herpesviruses, poxviruses and adenoviruses acquired suppressors of Casp8 that limit the contribution of extrinsic apoptosis to elimination of virus-infected cells. Programmed necrosis represents an adaptation to take advantage of Casp8 inhibition, by opening a trap door via any of three signaling platforms, RIP1-RIP3, DAIRIP3 and TRIF-RIP3. Some viruses, such as vaccinia, remain susceptible to programmed necrosis; whereas, other viruses, such as murine CMV, encode suppressors that limit the impact of this pathway.
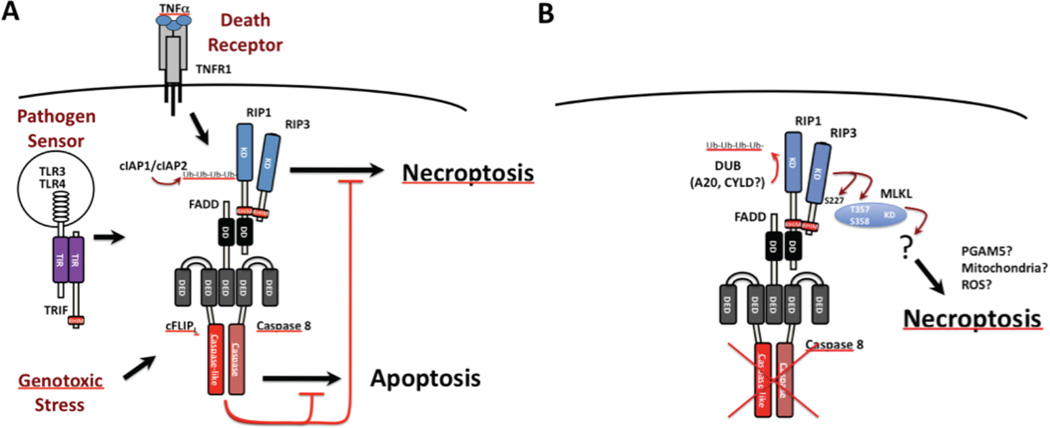
Figure 2. Caspase 8 regulation of extrinsic apoptosis and programmed necrosis.
A. Diagrammatic summary of the cytoprotective FADD-Casp8-cFLIPL-RIP1-RIP3 signaling complex (complex IIb or ripoptosome). The cytosolic complex forms downstream of cell surface death receptor (e.g. TNFR1 signaling) [87,117,118], endosomal pathogen sensor (e.g. TLR3/TLR4 signaling) [60,152] or intracellular genotoxic stress [61]. The cFLIPL-Casp8 heterodimer association with FADD prevents self-cleavage activation that is necessary to initiate apoptosis while maintaining sufficient basal activity to prevent unleashed RIP1-RIP3 necroptosis. E3 ubiquitin (Ub) ligases cIAP1 and cIAP2 also prevent RIP1-RIP3 necroptosis by adding K63 Ub chains to RIP1 and possibly other targets [61,62,69]. B. Diagrammatic summary of necroptosis unleashed by RIP3. In the presence of caspase compromise (red “X”) or when cIAP1 and cIAP2 are compromised and de-ubiquitinases (DUBs) such as A20 and CYLD predominate and eliminate poly Ub chains, RIP3 kinase becomes activated and is essential for activating MLKL kinase activity [76], autophosphorylating at S277 and also phosphorylating T357 and S358 on MLKL [78] to promote the direct interaction between these two kinases, an essential step in necroptosis.
Three potential outcomes emanate from death receptor activation [39,40]: (I) proinflammatory cytokine activation, (II) extrinsic apoptosis and (III) programmed necrosis. Importantly, a central Casp8-FADD-FLIP complex (Fig. 2) regulates outcome choices, but dictates Outcomes II and III. We have posited [27] that evolution of alternate death pathways have been driven by host adaptation to virus-encoded cell death suppressors (Fig. 1), such that the inflammatory consequences resulting from extrinsic cell death is the cost of effective innate host defense. Although pathogen sensors are recognized as contributing to innate NFκB (Outcome I) and IFN regulator factor (IRF)3 and IRF7 activation [41], sensors also activate extrinsic cell death (Outcomes II and III) via Casp8 in a manner paralleling death receptors [27,42]. In most vertebrates, Casp10 (not found in mice) also mediates extrinsic death. Human Casp8 and Casp10 bypass the mitochondria [43] and activate executioner caspases 3 and 7 directly, or merge with intrinsic apoptosis [1,43] by processing BID to tBID when x-linked inhibitor of apoptosis protein (XIAP) levels are sufficient [44]. In humans, Casp10 is downstream of FAS, such that autoimmune lymphoproliferative syndrome [45] is observed in Casp10 or FAS-deficient individuals [46], reminiscent of lymphoid hyperplasia in FAS-signaling deficient mice [47]. It remains unclearwhether Casp10 holds RIP3-dependent necrosis in check; however, phenotypes of humans with Casp8 or Casp10 deficiency suggests Casp10 sits in exclusive control of extrinsic apoptosis, whereas Casp8 regulates alternate activation of apoptosis and necrosis [27,48].
Nuts and bolts of extrinsic death pathways
TNF family receptors contribute to inflammation by activating NFκB and MAPK pathways, producing cytokines [27,49] independent of DD interactions [41,50,51]. Cytokine activation is subjected to modulation by virus-encoded suppressors [5,16,27,52,53]. Human death receptors (TNFR1, FAS, TRAIL-R1 and TRAIL-R2) signaling occurs via a death domain (DD) to FADD followed by self-cleavage activation of Casp8 or Casp10 [40,54–57]. Murine death receptors (TNFR1, FAS, TRAIL-R) all signal via Casp8 where death receptor signaling feeds into a Casp8-FADD-FLIPL-RIP1-RIP3 complex forms (Fig. 2). FADD-Casp8-FLIPL complex requires death effector domain (DED) interactions. Cellular FLIPL is anti-apoptotic, preventing homodimerization and self-activation of Casp8 [43,58]. It is the DD-containing subset of TNF family receptors that signal via the FADD-Casp8-FLIPL complex, and that recruit RIP1 to FADD via its DD. This leads to two alternatives: activation of NF-κB independent of RIP1 kinase activity [39] and triggering necrosis dependent on kinase activity [58,59]. An analogous cytosolic complex, called the ripoptosome, is triggered by toll-like receptor (TLR)3 signaling [60] as well as following genotoxic stress [61]. No matter how activation occurs, the ripoptosome controls cell fate (Outcomes II and III) [49,62], relying on DD-dependent association of RIP1 and FADD [39] and RIP homotypic interaction motif (RHIM)-dependent RIP1-RIP3 association. Death receptors and pathogen sensors rely on sustained basal Casp8 activity emanating from the FADD-Casp8-FLIPL complex to prevent RIP1-RIP3 kinase-dependent necrosis [27,63–66] (Fig. 2). Virus-encoded FLICE-inhibitory protein (vFLIP)-like proteins block this complex, preventing Casp8 homodimerization and self-activation [28–30]. Most vFLIPs act analogous to the short form cellular protein, FLIPS and completely shut down Casp8 homodimer activity [32,67]. This contrasts the more subtle role of FLIPL-Casp8 heterodimer, suppressing selfactivation while retaining basal caspase activity to suppress necrosis [68].
In addition, the FADD-Casp8-FLIPL-RIP1-RIP3 signaling platform (Fig. 2) [27] is tightly regulated by polyubiquitylation via cellular inhibitor of apoptosis protein (cIAP)1 and cIAP2 E3 ubiquitin (Ub) ligases [69] as well as by the linear Ub assembly complex (LUBAC) [70]. RIP3 is a potential substrate for cIAP1 or cIAP2 polyubiquitylation [71], although much remains to be clarified in this area. However, when Casp8 [72], cIAP1/cIAP2 E3 Ub ligase [69] or LUBAC [70] become compromised, de-ubiquitinases (DUBs), possibly A20 or cylindromatosis (CYLD), remove poly-Ub side chains decreasing the contribution of RIP1 activity in NFκB activation [72– 74] while also unleashing RIP1 and RIP3 kinase-dependent necrosis. These events influence TNF-induced extrinsic apoptosis, RIP1-RIP3 necrosis (called necroptosis) and inflammation [70]. The necroptosis ‘trap door’ involves RIP1-RIP3 oligomerization [75] via autophosphorylation of RIP3 at S227 and activation of MLKL kinase [76] by phosphorylation at T357 and S358. MLKL, recognized as a potent cell death mediator [77] prior to its implication in RIP3 necrosis, apparently acts in complex with RIP3 [78] (Fig. 2). The downstream execution of necrosis may proceed via PGAM5 and Drp1 recruitment to mitochondria [79], or via a reactive oxygen species (ROS) intermediates [80] (Fig. 2).
Consequences of dysregulating cell death pathways during development
Given that individual death receptors are dispensable for mammalian development, midgestational death of germ line Casp8 [81], FADD [82,83] or FLIP [84] deficient mice came as a surprise; however, the common embryonic day 10.5 (E10.5) lethality supported a coordinated FADD-Casp8-FLIPL contribution. Mice with endothelial cell-specific disruption of Casp8 exhibit the same E10.5 demise [85]. Normal development required enzymatic activity of Casp8, but not self-processing, eliminating the possibility of extrinsic apoptosis as the cause of death [86]. A caspase-independent function of Casp8 was suspected, and emerging experiments on RIP1–RIP3 necroptosis as well as demonstrating ability of viral suppressors to trigger [87,88] or block [89] this process opened understanding of FADD-Casp8-FLIPL complex-dependent host defense [27], harnessing machinery best understood in death receptor signaling Fig. 2.
Significant insight emerged when crossing Casp8- and RIP3-deficient mice produced viable Casp8−/−Rip3−/− mice [63,90] that became fertile adults able to mount immune control over virus infection [90]. Fadd−/−Rip3−/− and Fadd−/−Rip3−/−FLIP−/− mice [91] also develop into viable adults. Thus, the embryonic lethality of Casp8, FADD or FLIP deficiency results from dysregulated RIP3 function, confirming the contribution of the FADD-Casp8-FLIPL platform in restricting RIP3 pathways during development. Importantly, embryonic lethality due to germ line Casp8 deficiency in mice depends on RIP1 and RIP3 [63,90,92] and is not influenced by germ line elimination of the other RHIM-dependent interaction partners, DNA-dependent activator of IRFs (DAI) [93] or TIR-domain-containing adapter-inducing IFNβ (TRIF) [94]. Casp8−/−Rip3−/− mice also revealed that, once embryonic lethality is suppressed, the importance of Casp8 downstream of FAS becomes apparent. Abnormal B220+ T cells accumulate in aging mice similar to FAS signaling-deficiency [47]. Mice deficient in FADD and RIP1 (Fadd−/−Rip1−/−) survive to birth [92], further implicating FADD-Casp8-FLIPL signaling linked to RIP1- RIP3 in death [27,95]. The demonstration that germ line disruption of Casp8 unleashes RIP3 death provides clarification to disparate observations made in the fields of immunology, cell biology, development and signal transduction implicating the FADD-Casp8-FLIPL complex as a vital player in diverse cell cycle, NFκB activation, autophagy, cell adhesion and migration, and suppression of inflammation processes, particularly as relates to immunology [48,95,96]. Thus, Casp8 provides vital activity silencing RIP3-dependent pathways during development (Fig. 2), revealing gross dysregulation of normal processes as a cost of the evolutionary pressure to eliminate pathogen-infected cells [27] via RIP3 pathways (Fig. 3).
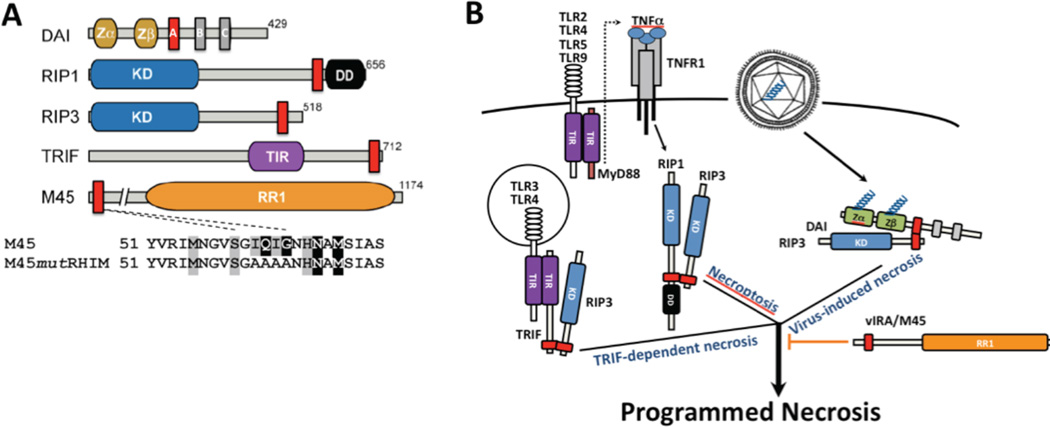
Figure 3. Signaling adaptors in control of RIP3 programmed necrosis.
A. Diagrammatic depiction of host and viral RHIM-containing adaptors. Mouse DAI [148,150], RIP1 [153], RIP3 [154,155] and TRIF [145,146], together with murine CMV M45-encoded vIRA, an RR1 homolog [126]. The expanded regions depicts RHIM region from aa 51 and the tetra-alanine mutation employed to characterize M45 RHIM-specific inhibition [89,140]. Kinase domain (KD) of RIP1 and RIP3, death domain (DD) of RIP1, toll-IL-1 receptor (TIR) domain of TRIF and RIP homotypic interaction motif (RHIM; red boxes). DAI has three RHIM-like repeat (RLR) elements where RLR A is functional [148]. B. Summary diagram depicting three distinct triggers of RIP3 kinase-dependent programmed necrosis. TNFR1-induced RIP1-RIP3 necroptosis [87,117,118], DAI-RIP3 virus-induced necrosis [89,93] and TLR3 or TRL4 TRIF-dependent necrosis, as well as TLR2, TLR4, TLR5 and TLR9 MyD88-dependent activation of TNF and secondary induction of necroptosis [94]. These complexes depend on RHIM interactions and are blocked by murine CMV M45-encoded vIRA [89,93,94].
Programmed necrosis contributes to an array of serious defects and inflammatory consequences that arise from germ line [90] as well as cell-type-specific disruption of Casp8 or FADD in mice [49,55,85,86,97–109]. The phenotypes of mice carrying such disruptions have spawned debate as to whether necrosis contributes to inflammation or inflammation drives necrosis [42,49]. Pathologies associated with tissue-specific Casp8- or FADD-deficient mice are uniformly reversed when crossed into the Rip3−/− background, clearly showing that dysregulating RIP3 underlies tissue damage [42,91,105,110–114]. An array of different viral cell death suppressors [16,27,32,33] has clearly contributed to the evolution of the RIP3 ‘trap door’. This arms race (Fig. 1) has produced novel pro-death strategies to counterbalance virus-encoded anti-death functions, which in turn, contributes to inflammatory disease as the collateral damage of unleashed death intended to eliminate pathogens [27,115]. Acute and chronic diseases linked to viral infection are likely to radiate from such processes, particularly when the pathogen persists in the host.
Host defense value of programmed necrosis
In addition the interaction of RIP1 [116] and RIP3 [87,117,118], programmed necrosis follows RHIM-dependent association of RIP3 with DAI or TRIF, as depicted in Fig. 3. No matter whether RIP3 kinase is activated by RIP1, DAI or TRIF, an oligomeric complex forms and RIP3 kinase acts in collaboration with MLKL [94] (Fig. 2). Thus, studies that initially centered on RIP1 [69,72] paved the way to discovery of RIP3 [87,117,118] as well as to a conceptual framework where RIP3 in complex with MLKL is now recognized as the central mediator of programmed necrosis [27,89,93,94,119]. Vaccinia virus is highly susceptible to TNF-induced RIP1-RIP3 necroptosis [120], with cell death in infected organs dependent on TNFR1 and TNFR2 [87,88]. Rip3−/− mice are highly susceptible to vaccinia [87] and succumb to a dose of virus that WT C57BL/6 mice resist. Thus, necroptosis provides key antiviral control and tissue pathology [87,88]. Vaccinia-encoded B13R is a caspase inhibitor that blocks apoptosis [121,122]. Based on the increased susceptibility of Rip3−/− mice to WT vaccinia and the increased size of skin lesions formed by B13R mutant virus [123], vaccinia infection opens the necroptosis trap door by inhibiting caspases (Fig. 1). While highly susceptible to vaccinia, Rip3−/−mice are no more susceptible than WT mice to infection with murine CMV [89,93], lymphocytic choriomeningitis virus [96] or murine hepatitis virus [124]. Murine CMV encodes suppressors of both Casp8 and RIP3 [16,27,125]. RNA viruses are not known to actively suppress cell death pathways. Thus, the RIP3 pathway appears selective and specific regarding control of different viruses.
As large DNA viruses, both vaccinia and CMV encode a wide variety of immunomodulatory and cell death suppression functions that have helped to define host defense pathways crucial to the virus infection [13,16,27,125–127]. Whereas vaccinia encodes B13R caspase inhibitor that apparently opens the necroptosis ‘trap door’, murine CMV deploys viral inhibitor of RIP activation (vIRA) as a potent RHIM-dependent inhibitor of programmed necrosis to counteract the consequences of Casp8-inhibitor vICA [90] (Fig. 3). Infection with any large DNA virus is complicated by the overlapping nature of host defense as well as the myriad virus-encoded suppressor activities. Both vaccinia and murine CMV deflect many aspects of pathogen sensing, including activation of NF-κB [53,128,129], IFN [130,131] and PKR [131–133]. Murine CMV vIRA allows this virus to evade RIP1-RIP3 as well as DAI-RIP3 complex-dependent programmed necrosis (Fig. 3) [89,93]. In contrast, vaccinia is sensitive to necroptosis induced by TNF and causes histopathology consistent with widespread tissue necrosis in vivo [87,88]. Murine CMV is species specific and has evolved in dialogue with its host continuously for approximately 100 million years. Vaccinia infects a variety of mammalian hosts but its natural host is unknown [27,32]. The reasons underlying susceptibility of vaccinia to RIP1-RIP3 and murine CMV M45 mutant to DAI-RIP3 necrosis may stem from differences in modulation of mouse pathogen sensors. The Z-DNA-binding motifs of DAI [134] can replace the aminoterminal Z-DNA motif [135] of vaccinia E3L [136], and provide virulence characteristics [137], it remains uncertain whether this modulator interacts with or targets DAI. Thus, despite differences how necrosis is triggered, the programmed necrosis ‘trap door’ can open [27,32] (Figs. 1 and 3). In addition to these examples, the vFLIP proteins of Molluscum contagiosum virus, MC159, and equine herpesvirus 1, E8, inhibit TNF-induced necrosis when exogenously expressed [88], suggesting that further study of viral caspase inhibitors may add to the novel strategies for modulating necrosis.
Murine CMV-induced programmed necrosis occurs within 14 h in susceptible mouse or human cells following exposure to vIRA-deficient virus [89], acquiring morphological features of necrotic death, dying before viral DNA synthesis [89,138–140]. Moreover, this mutant virus is the most attenuated of any CMV cell death suppressor mutant [7–9,34,141], failing to disseminate from the inoculation site in immunocompetent or immunodeficient mice [89,142]. M45, which encodes vIRA, is located within in the cell death suppression locus (Fig. 4) along with Casp8 (vICA) and mitochondrial (vMIA and vIBO) inhibitors that are conserved in human CMV. The RHIM of vIRA is crucial (Fig. 3) and the phenotype of vIRA-deficient virus is normalized when either RIP3 or DAI is eliminated from the host [89,93]. The crucial role of the vIRA RHIM and the absence of a similar motif in human CMV raises questions of how suppression of programmed necrosis plays out in humans.
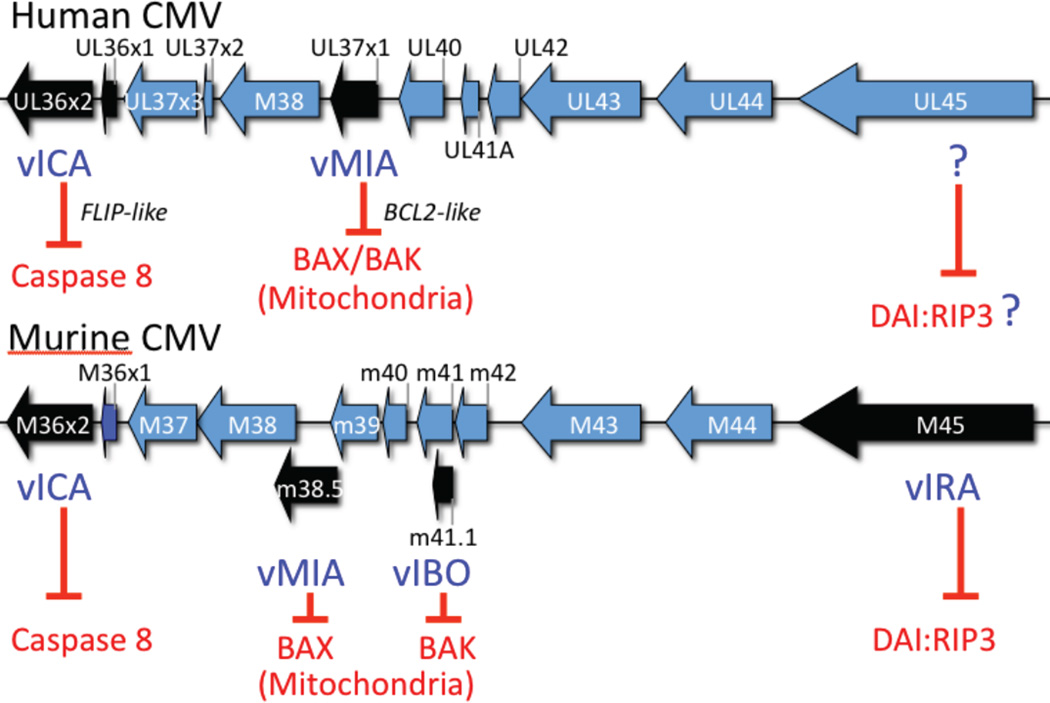
Figure 4. Cytomegalovirus cell death suppression locus.
Colinear regions of the human and murine CMV genomes showing the location of ORFs that encode cell death suppressors. vICA is a sequence homolog lacking a DED that nevertheless functions in a FLIP-like manner, interacting with Casp8 to prevent self-activation [30,31]. Human CMV encoded vMIA inhibits BAX [156: Poncet, 2004 #5807] and modulates BAK [157], whereas murine CMV encodes vMIA to inhibit BAX and vIBO to inhibit BAK at mitochondria. Murine CMV M45-encoded vIRA suppresses apoptosis and necrosis [89,138–140], acting as a RHIM inhibitor to suppress DAIRIP3 complex formation [93] (see Fig. 3).
Regardless of whether RIP1-RIP3, DAI-RIP3 or TRIF-RIP3 starts the process, once triggered, RHIM-containing proteins (Fig. 3) may be recruited into oligomers independent of their functional contribution to death [75,94]. In fibroblasts, TLR3-induced necrosis is triggered by TRIF-RIP3 independent of DAI or RIP1 [94], although RIP1 is nevertheless recruited into complexes. Necroptosis mediated by RIP1-RIP3 complexes independent of TRIF and DAI nevertheless include DAI [75].
In addition to cell death, RHIM-dependent signal transduction leads to activation NFκB and other transcription factors, via RIP1 [80]. The pathways controlled by RIP1 have been characterized [80,143,144], although TRIF [140,145,146] and DAI [147,148] also activate NFκB as well as IRF3/IRF7 and IFN [41]. Activation of NFκB (Outcome I), which increases FLIPL levels, prevents apoptosis (Outcome II), but not necrosis (Outcome III), whether induced by virus infection [93] or death receptor signaling [58,64]. This is likely due to the absence of any crosstalk between RIP3 and NF-κB [149]. Even though RIP3 partners, RIP1, TRIF and DAI have well-established impact on NF-κB, IRF3 and IFN, once RIP3 is activated, the value of increased FLIPL levels becomes moot. Indeed, initial observations on DAI signaling showed it to be a DNA-specific pathogen sensor activating TBK and IRF3 [150] as well as NFκB in a RHIMdependent manner [148]. Like the death pathway, DNA-induced DAI-dependent activation of NF-κB and IRF3 is blocked by vIRA [147]. Curiously, a large component of the human CMV virion-induced IFN-like response occurs via DAI [151]. Thus, the potency of M45-encoded vIRA to suppress RHIM signaling was obvious before the pathway or steps in virus-induced programmed necrosis had been defined. Like other viral immunomodulators, vIRA prevents proinflammatory signaling independent of RHIM-signaling late during infection via the RR1 domain of M45 (Fig. 3) interacting with the adaptor IKKγ/NEMO [53]. When necrosis is the outcome, it occurs within the first 14 h of infection and is independent of both NFκB activation and IFN signaling [93]. Thus, M45-encoded vIRA carries out additional RHIM-dependent and - independent functions during infection, however, it is the RHIM-dependent interference with RIP3-induced programmed necrosis that predominates in infection.
Conclusions and future directions
Evidence has accumulated that firmly establishes the evolutionary adaptation of cell death in host defense in animals and cell death suppressors that subvert host defense during infection by viruses. A new twist appears to be the way this evolution has created an opportunity for cell death pathways to become dysregulated and underlie developmental demise and inflammatory disease. As has repeatedly occurred in biology, model organisms, such as mice, and their natural pathogens have shown the path to discovery of pathways that are universal and will very likely continue to provide key insights into human disease.
Highlights.
Evolution of host cell death pathways was driven by virus-encoded cell death suppressors.
Caspase 8 regulates extrinsic apoptosis as well as programmed necrosis.
RIP3 (also called RIPK3) is the key mediator of programmed necrosis.
Programmed necrosis involves RIP3 RHIM-dependent interactions with RIP1, DAI or TRIF.
Programmed necrosis is suppressed by RHIM inhibitor vIRA encoded by MCMV.
Acknowledgements
Investigations were supported by NIH RO1 grants AI030363 and AI020211 (to ESM) and OD012198 (to WJK) as well as start-up funds from the University of Texas at Austin and the Cancer Prevention Research Institute of Texas (CPRIT) (to JWU).
References
- 1. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. The classic review of intrinsic cell death reliance on BCL2 family members.
- 2.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 3.Tait SW, Green DR. Mitochondria and cell signalling. J Cell Sci. 2012;125:807–815. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everett H, McFadden G. Apoptosis: an innate immune response to virus infection. Trends Microbiol. 1999;7:160–165. doi: 10.1016/s0966-842x(99)01487-0. [DOI] [PubMed] [Google Scholar]
- 5.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosby LN, McCormick AL, Mocarski ES. Gene products of the embedded m41/m41.1 locus of murine cytomegalovirus differentially influence replication and pathogenesis. Virology. 2013;346:274–283. doi: 10.1016/j.virol.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manzur M, Fleming P, Huang DC, Degli-Esposti MA, Andoniou CE. Virally mediated inhibition of Bax in leukocytes promotes dissemination of murine cytomegalovirus. Cell Death Differ. 2009;16:312–320. doi: 10.1038/cdd.2008.152. Physiological importance of murine CMV m38.5-encoded vMIA mitochondrial cell death suppressor.
- 9.Fleming P, Kvansakul M, Voigt V, Kile BT, Kluck RM, Huang DC, Degli-Esposti MA, Andoniou CE. MCMV-mediated Inhibition of the pro-apoptotic Bak protein Is required for optimal in vivo replication. PLoS Pathog. 2013;9:e1003192. doi: 10.1371/journal.ppat.1003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handke W, Luig C, Popovic B, Krmpotic A, Jonjic S, Brune W. Viral inhibition of BAK promotes murine cytomegalovirus dissemination to salivary glands. J Virol. 2013;87:3592–3596. doi: 10.1128/JVI.02657-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Everett H, Barry M, Lee SF, Sun X, Graham K, Stone J, Bleackley RC, McFadden G. M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J Exp Med. 2000;191:1487–1498. doi: 10.1084/jem.191.9.1487. Physiological importance of poxvirus M11L-encoded mitochondrial cell death suppressor.
- 12.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G. Viral proteins targeting mitochondria: controlling cell death. Biochim Biophys Acta. 2004;1659:178–189. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 14. Goldmacher VS. Cell death suppression by cytomegaloviruses. Apoptosis. 2005;10:251–265. doi: 10.1007/s10495-005-0800-z. Describes the identification and characterization of human CMV UL37x1-encoded vMIA and UL36-encoded vICA.
- 15. McCormick AL, Meiering CD, Smith GB, Mocarski ES. Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis. J Virol. 2005;79:12205–12217. doi: 10.1128/JVI.79.19.12205-12217.2005. Functional similarity of human and mouse CMV vMIA.
- 16.McCormick AL, Mocarski ES. Cell death pathways controlled by cytomegaloviruses. In: Reddehase MJ, editor. Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Caister Scientific Press; 2013. pp. 263–276. [Google Scholar]
- 17. Arnoult D, Skaletskaya A, Estaquier J, Dufour C, Goldmacher VS. The murine cytomegalovirus cell death suppressor m38.5 binds Bax and blocks Bax-mediated mitochondrial outer membrane permeabilization. Apoptosis. 2008;13:1100–1110. doi: 10.1007/s10495-008-0245-2. Molecular dissection of murine CMV vMIA function specific for BAX.
- 18. Jurak I, Schumacher U, Simic H, Voigt S, Brune W. Murine cytomegalovirus m38.5 protein inhibits Bax-mediated cell death. J Virol. 2008;82:4812–4822. doi: 10.1128/JVI.02570-07. Molecular dissection of murine CMV vMIA function specific for BAX.
- 19. Cam M, Handke W, Picard-Maureau M, Brune W. Cytomegaloviruses inhibit Bak- and Bax-mediated apoptosis with two separate viral proteins. Cell Death Differ. 2010;17:655–665. doi: 10.1038/cdd.2009.147. Description of murine CMV m41.1-encoded vIBO mitochondrial cell death suppressor specific for BAK.
- 20. McCormick AL, Roback L, Mocarski ES. HtrA2/Omi terminates cytomegalovirus infection and is controlled by the viral mitochondrial inhibitor of apoptosis (vMIA) PLoS Pathog. 2008;4:e1000063. doi: 10.1371/journal.ppat.1000063. Natura serine protease-dependent, caspase-independent cell death pathway suppressed by human CMV vMIA during infection.
- 21.Hardwick JM, Chen YB, Jonas EA. Multipolar functions of BCL-2 proteins link energetics to apoptosis. Trends Cell Biol. 2012;22:318–328. doi: 10.1016/j.tcb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick AL, Smith VL, Chow D, Mocarski ES. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J Virol. 2003;77:631–641. doi: 10.1128/JVI.77.1.631-641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharon-Friling R, Goodhouse J, Colberg-Poley AM, Shenk T. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc Natl Acad Sci U S A. 2006;103:19117–19122. doi: 10.1073/pnas.0609353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves MB, Davies AA, McSharry BP, Wilkinson GW, Sinclair JH. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science. 2007;316:1345–1348. doi: 10.1126/science.1142984. [DOI] [PubMed] [Google Scholar]
- 25.Kaarbo M, Ager-Wick E, Osenbroch PO, Kilander A, Skinnes R, Muller F, Eide L. Human cytomegalovirus infection increases mitochondrial biogenesis. Mitochondrion. 2011;11:935–945. doi: 10.1016/j.mito.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Seo JY, Yaneva R, Hinson ER, Cresswell P. Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science. 2011;332:1093–1097. doi: 10.1126/science.1202007. [DOI] [PubMed] [Google Scholar]
- 27. Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2011;12:79–88. doi: 10.1038/nri3131. Complete review of apoptosis and necrosis pathways and their modulation by viral cell death suppressors.
- 28. Bertin J, Armstrong RC, Ottilie S, Martin DA, Wang Y, Banks S, Wang GH, Senkevich TG, Alnemri ES, Moss B, et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. Initial characterization of viral FLIP-like cell death suppressors.
- 29. Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. Initial characterization of viral FLIP-like cell death suppressors.
- 30. Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci U S A. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. Identification of the human CMV UL36-encoded viral inhibitor of caspase 8 activation (vICA).
- 31.McCormick AL, Skaletskaya A, Barry PA, Mocarski ES, Goldmacher VS. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology. 2003;316:221–233. doi: 10.1016/j.virol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munoz-Pinedo C. Signaling pathways that regulate life and cell death: evolution of apoptosis in the context of self-defense. Adv Exp Med Biol. 2012;738:124–143. doi: 10.1007/978-1-4614-1680-7_8. [DOI] [PubMed] [Google Scholar]
- 34. Cicin-Sain L, Ruzsics Z, Podlech J, Bubic I, Menard C, Jonjic S, Reddehase MJ, Koszinowski UH. Dominant-negative FADD rescues the in vivo fitness of a cytomegalovirus lacking an antiapoptotic viral gene. J Virol. 2008;82:2056–2064. doi: 10.1128/JVI.01803-07. Physiological role of murine CMV M36-encoded vICA during infection.
- 35.McCormick AL, Roback L, Wynn G, Mocarski ES. Multiplicity-dependent activation of a serine protease-dependent cytomegalovirus-associated programmed cell death pathway. Virology. 2013;435:250–257. doi: 10.1016/j.virol.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daley-Bauer LP, Mocarski ES. Myeloid cell recruitment and function in cytomegalovirus immunity and pathogenesis. In. In: Reddehase MJ, editor. Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Caister Scientific Press; 2013. pp. 363–373. [Google Scholar]
- 37.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 38.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 39.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 40.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 42.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Salvesen GS. Caspases and apoptosis. Essays Biochem. 2002;38:9–19. doi: 10.1042/bse0380009. [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teachey DT. New advances in the diagnosis and treatment of autoimmune lymphoproliferative syndrome. Curr Opin Pediatr. 2012;24:1–8. doi: 10.1097/MOP.0b013e32834ea739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snow AL, Pandiyan P, Zheng L, Krummey SM, Lenardo MJ. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol Rev. 2010;236:68–82. doi: 10.1111/j.1600-065X.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. Summarizes FAS-signaling very effectively.
- 48. Hedrick SM, Ch'en IL, Alves BN. Intertwined pathways of programmed cell death in immunity. Immunol Rev. 2010;236:41–53. doi: 10.1111/j.1600-065X.2010.00918.x. Summarizes the situation in T cells very effectively.
- 49. Wallach D, Kovalenko A, Kang TB. 'Necrosome'-induced inflammation: must cells die for it? Trends Immunol. 2011 doi: 10.1016/j.it.2011.07.004. Presents the conundrum of "does necrosis lead to inflammation or does inflammation lead to necrosis" in tissues.
- 50.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verma S, Benedict CA. Sources and signals regulating type I interferon production: lessons learned from cytomegalovirus. J Interferon Cytokine Res. 2011;31:211–218. doi: 10.1089/jir.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedict CA, Banks TA, Ware CF. Death and survival: viral regulation of TNF signaling pathways. Curr Opin Immunol. 2003;15:59–65. doi: 10.1016/s0952-7915(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 53. Fliss PM, Jowers TP, Brinkmann MM, Holstermann B, Mack C, Dickinson P, Hohenberg H, Ghazal P, Brune W. Viral mediated redirection of NEMO/IKKgamma to autophagosomes curtails the inflammatory cascade. PLoS Pathog. 2012;8:e1002517. doi: 10.1371/journal.ppat.1002517. Second function of murine CMV vIRA, independent of RHIM interactions.
- 54.Valmiki MG, Ramos JW. Death effector domain-containing proteins. Cell Mol Life Sci. 2009;66:814–830. doi: 10.1007/s00018-008-8489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tourneur L, Chiocchia G. FADD: a regulator of life and death. Trends Immunol. 2010;31:260–269. doi: 10.1016/j.it.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Kersse K, Verspurten J, Vanden Berghe T, Vandenabeele P. The death-fold superfamily of homotypic interaction motifs. Trends Biochem Sci. 2011;36:541–552. doi: 10.1016/j.tibs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 58. Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. Review of extrinsic apoptosis and programmed necrosis.
- 59.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 60. Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, Macfarlane M, Hacker G, Leverkus M. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. Description of the ripoptosome forming downstream of pathogen sensor TLR3.
- 61. Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, Macfarlane M, Cain K, et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. Description of the ripoptosome forming downstream of genotoxic stress.
- 62. Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. Important contribution of E3 Ub ligases mediating K63 linkages to regulation of RIP3 activity.
- 63. Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. Describes the regulation of caspase 8 by FLIP to restrict programmed necrosis.
- 64.Weinlich R, Dillon CP, Green DR. Ripped to death. Trends Cell Biol. 2011;21:630–637. doi: 10.1016/j.tcb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Raam BJ, Salvesen GS. Proliferative versus apoptotic functions of caspase-8 Hetero or homo: the caspase-8 dimer controls cell fate. Biochim Biophys Acta. 2012;1824:113–122. doi: 10.1016/j.bbapap.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feoktistova M, Geserick P, Panayotova-Dimitrova D, Leverkus M. Pick your poison: The ripoptosome, a cell death platform regulating apoptosis and necroptosis. Cell Cycle. 2012;11:460–467. doi: 10.4161/cc.11.3.19060. [DOI] [PubMed] [Google Scholar]
- 67.Li FY, Jeffrey PD, Yu JW, Shi Y. Crystal structure of a viral FLIP: insights into FLIP-mediated inhibition of death receptor signaling. J Biol Chem. 2006;281:2960–2968. doi: 10.1074/jbc.M511074200. [DOI] [PubMed] [Google Scholar]
- 68. Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grutter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. Shows that the caspase 8-FLIPL heterodimer retains basal protease activity.
- 69. Moulin M, Anderton H, Voss AK, Thomas T, Wong WW, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, et al. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. Shows that E3 Ub ligases cIAP1 and cIAP2 provide overlapping roles protecting from a TNF-induced and RIP3-dependent midgestational lethality.
- 70. Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. Demonstrates that LUBAC is involved in suppression of RIP1-dependent signaling.
- 71.Bertrand MJ, Lippens S, Staes A, Gilbert B, Roelandt R, De Medts J, Gevaert K, Declercq W, Vandenabeele P. cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor interacting proteins kinases 1 to 4 (RIP1-4) PLoS One. 2011;6:e22356. doi: 10.1371/journal.pone.0022356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. Shows that diverse apoptotic mechanisms emanate from caspase 8.
- 73.Ikner A, Ashkenazi A. TWEAK Induces Apoptosis through a Death-signaling Complex Comprising Receptor-interacting Protein 1 (RIP1), Fas-associated Death Domain (FADD), and Caspase-8. J Biol Chem. 2011;286:21546–21554. doi: 10.1074/jbc.M110.203745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K, Vucic D, Fulda S, Vandenabeele P, Bertrand MJ. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18:656–665. doi: 10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. Shows the role of MLKL as an interaction partner and protein kinase activated downstream of RIP3 to execute programmed necrosis.
- 77.Mollenhauer J, Hudler M, Blaich S, Wittig R. Use of MLKL in cancer therapy. US Patent. 2010
- 78. Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. Shows the role of MLKL as a phosphorylation target and interaction partner of RIP3 in programmed necrosis.
- 79.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 80. Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. Comprehensive review of RIP1 describing roles in NF-κB activation, receptor signaling and cell death.
- 81. Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. Demonstration that germ line disruption of caspase 8 leads to midgestational death.
- 82. Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. Demonstration that germ line disruption of FADD leads to midgestational death.
- 83.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 84. Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. Demonstration that germ line disruption of FLIP leads to midgestational death.
- 85. Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. Evaluation of tissue specific disruption of caspase 8 revealing diverse inflammatory outcomes.
- 86. Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, Wallach D. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181:2522–2532. doi: 10.4049/jimmunol.181.4.2522. Demonstration of the requirements of caspase 8 enzymatic activity but not self-processing in midgestational death.
- 87. Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. Description of RIP1-RIP3 programmed necrosis and importance in vaccinia virus host defense.
- 88.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 89. Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. Identification of murine M45-encoded vIRA as a RHIM inhibitor blocking programmed necrosis.
- 90. Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. Demonstration that midgestational demise of caspase 8-deficient mice is due to dysregulated RIP3 kinase.
- 91.Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-Caspase-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Upton JW, Kaiser WJ, Mocarski ES. DAI (ZBP1/DLM-1) complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. Description of DAI-RIP3 pathway leading to programmed necrosis and importance in host defense.
- 94. Kaiser WJ, Sridharan H, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via a TRIF-RIP3 complex completely independent of RIP1 kinase. J Biol Chem. 2013 doi: 10.1074/jbc.M113.462341. (submitted) Description of TRIF-RIP3 pathway leading to necrosis.
- 95.Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS. RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell. 2011;44:9–16. doi: 10.1016/j.molcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ch'en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208:633–641. doi: 10.1084/jem.20110251. Comprehensive evaluation of the impact of programmed necrosis in antigen-stimulated caspase 8-deficient T cells and rescue with cross to RIP3-deficient mice.
- 97.Ben Moshe T, Barash H, Kang TB, Kim JC, Kovalenko A, Gross E, Schuchmann M, Abramovitch R, Galun E, Wallach D. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–1024. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]
- 98.Maelfait J, Beyaert R. Non-apoptotic functions of caspase-8. Biochem Pharmacol. 2008;76:1365–1373. doi: 10.1016/j.bcp.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 99.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee P, Lee DJ, Chan C, Chen SW, Ch'en I, Jamora C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122:4094–4104. doi: 10.1172/JCI64115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 105.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 Blocks Kinase RIPK3-Mediated Activation of the NLRP3 Inflammasome. Immunity. 2012;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 106. Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, Du J, Wallach D. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. Description of the inflammatory response consequences of caspase 8 deficiency.
- 107.Scharner D, Rossig L, Carmona G, Chavakis E, Urbich C, Fischer A, Kang TB, Wallach D, Chiang YJ, Deribe YL, et al. Caspase-8 is involved in neovascularization-promoting progenitor cell functions. Arterioscler Thromb Vasc Biol. 2009;29:571–578. doi: 10.1161/ATVBAHA.108.182006. [DOI] [PubMed] [Google Scholar]
- 108.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ben Moshe T, Kang TB, Kovalenko A, Barash H, Abramovitch R, Galun E, Wallach D. Cell-autonomous and non-cell-autonomous functions of caspase-8. Cytokine Growth Factor Rev. 2008;19:209–217. doi: 10.1016/j.cytogfr.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 111.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Donnell MA, Ting AT. NFkappaB and ubiquitination: partners in disarming RIPK1-mediated cell death. Immunol Res. 2012 doi: 10.1007/s12026-012-8321-7. [DOI] [PubMed] [Google Scholar]
- 113.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silke J, Strasser A. The FLIP Side of Life. Sci Signal. 2013;6:pe2. doi: 10.1126/scisignal.2003845. [DOI] [PubMed] [Google Scholar]
- 115.Galluzzi L, Vanden Berghe T, Vanlangenakker N, Buettner S, Eisenberg T, Vandenabeele P, Madeo F, Kroemer G. Programmed necrosis from molecules to health and disease. Int Rev Cell Mol Biol. 2011;289:1–35. doi: 10.1016/B978-0-12-386039-2.00001-8. [DOI] [PubMed] [Google Scholar]
- 116.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 117. He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. Demonstration that RIP1-RIP3 complex controls necrosis.
- 118. Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. Demonstration that RIP1-RIP3 complex controls necrosis.
- 119.Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis. 2013;4:e465. doi: 10.1038/cddis.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sambhi SK, Kohonen-Corish MR, Ramshaw IA. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci U S A. 1991;88:4025–4029. doi: 10.1073/pnas.88.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith GL. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol. 1997;78(Pt 3):677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 122. Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. Description of RIP1 in programmed necrosis.
- 123.Tscharke DC, Reading PC, Smith GL. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. J Gen Virol. 2002;83:1977–1986. doi: 10.1099/0022-1317-83-8-1977. [DOI] [PubMed] [Google Scholar]
- 124. Lu JV, Weist BM, van Raam BJ, Marro BS, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS, Walsh CM. Complementary roles of FADD and RIPK3 in T cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A. 2011;108:15312–15317. doi: 10.1073/pnas.1102779108. Comprehensive evaluation of the impact of programmed necrosis in antigen-stimulated FADD-deficient T cells and rescue with cross to RIP3-deficient mice.
- 125.Brune W. Inhibition of programmed cell death by cytomegaloviruses. Virus Res. 2011;157:144–150. doi: 10.1016/j.virusres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 126.Lembo D, Brune W. Tinkering with a viral ribonucleotide reductase. Trends Biochem Sci. 2009;34:25–32. doi: 10.1016/j.tibs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 127.McCormick AL. Control of apoptosis by human cytomegalovirus. Curr Top Microbiol Immunol. 2008;325:281–295. doi: 10.1007/978-3-540-77349-8_16. [DOI] [PubMed] [Google Scholar]
- 128.Bahar MW, Graham SC, Chen RA, Cooray S, Smith GL, Stuart DI, Grimes JM. How vaccinia virus has evolved to subvert the host immune response. J Struct Biol. 2011;175:127–134. doi: 10.1016/j.jsb.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bowie AG. Insights from vaccinia virus into Toll-like receptor signalling proteins and their regulation by ubiquitin: role of IRAK-2. Biochem Soc Trans. 2008;36:449–452. doi: 10.1042/BST0360449. [DOI] [PubMed] [Google Scholar]
- 130.Le VT, Trilling M, Zimmermann A, Hengel H. Mouse cytomegalovirus inhibits beta interferon (IFN-beta) gene expression and controls activation pathways of the IFN-beta enhanceosome. J Gen Virol. 2008;89:1131–1141. doi: 10.1099/vir.0.83538-0. [DOI] [PubMed] [Google Scholar]
- 131.Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 132.Valchanova RS, Picard-Maureau M, Budt M, Brune W. Murine cytomegalovirus m142 and m143 are both required to block protein kinase R-mediated shutdown of protein synthesis. J Virol. 2006;80:10181–10190. doi: 10.1128/JVI.00908-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Child SJ, Hanson LK, Brown CE, Janzen DM, Geballe AP. Double-stranded RNA binding by a heterodimeric complex of murine cytomegalovirus m142 and m143 proteins. J Virol. 2006;80:10173–10180. doi: 10.1128/JVI.00905-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim K, Khayrutdinov BI, Lee CK, Cheong HK, Kang SW, Park H, Lee S, Kim YG, Jee J, Rich A, et al. Solution structure of the Zbeta domain of human DNA-dependent activator of IFN-regulatory factors and its binding modes to B- and Z-DNAs. Proc Natl Acad Sci U S A. 2011;108:6921–6926. doi: 10.1073/pnas.1014898107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beattie E, Paoletti E, Tartaglia J. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L- and E3L-mutant viruses. Virology. 1995;210:254–263. doi: 10.1006/viro.1995.1342. [DOI] [PubMed] [Google Scholar]
- 136.Valentine R, Smith GL. Inhibition of the RNA polymerase III-mediated dsDNA-sensing pathway of innate immunity by vaccinia virus protein E3. J Gen Virol. 2010;91:2221–2229. doi: 10.1099/vir.0.021998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim YG, Muralinath M, Brandt T, Pearcy M, Hauns K, Lowenhaupt K, Jacobs BL, Rich A. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci U S A. 2003;100:6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brune W, Menard C, Heesemann J, Koszinowski UH. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science. 2001;291:303–305. doi: 10.1126/science.291.5502.303. [DOI] [PubMed] [Google Scholar]
- 139. Mack C, Sickmann A, Lembo D, Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc Natl Acad Sci U S A. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. Identifies RIP1 as an interacting partner of murine CMV M45-encoded vIRA.
- 140. Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. Identifies RIP1 as an interacting partner of murine CMV M45-encoded vIRA and shows that cell death suppression requires an intact RHIM.
- 141.Handke W, Krause E, Brune W. Live or let die: manipulation of cellular suicide programs by murine cytomegalovirus. Med Microbiol Immunol. 2012;201:475–486. doi: 10.1007/s00430-012-0264-z. [DOI] [PubMed] [Google Scholar]
- 142.Lembo D, Donalisio M, Hofer A, Cornaglia M, Brune W, Koszinowski U, Thelander L, Landolfo S. The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis. J Virol. 2004;78:4278–4288. doi: 10.1128/JVI.78.8.4278-4288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 144. Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. Description of RIP1-deficient mice.
- 145.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 146.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 147.Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. Initial description of DAI as a RHIM-containing protein that stimulates NF-κB activation in a RHIM-dependent fashion.
- 149. Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. Description of RIP3-deficient mice.
- 150.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 151.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol. 2010;84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. Description of TLR-dependent activation fo RIP1-RIP3 necrosis.
- 153.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 154.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 155.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 156. Arnoult D, Bartle LM, Skaletskaya A, Poncet D, Zamzami N, Park PU, Sharpe J, Youle RJ, Goldmacher VS. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc Natl Acad Sci U S A. 2004;101:7988–7993. doi: 10.1073/pnas.0401897101. Description of human CMV vMIA inhibitor of BAX.
- 157.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]