SUMMARY
Dietary composition has major effects on physiology. Here we show that developmental rate, reproduction and lifespan are altered in C. elegans fed Comamonas DA1877 relative to those fed a standard E. coli OP50 diet. We identify a set of genes that change in expression in response to this diet, and use the promoter of one of these (acdh-1) as a dietary sensor. Remarkably, the effects on transcription and development occur even when Comamonas DA1877 is diluted with another diet, suggesting that Comamonas DA1877 generates a signal that is sensed by the nematode. Surprisingly, the developmental effect is independent from TOR and insulin signaling. Rather, Comamonas DA1877 affects cyclic gene expression during molting, likely through the nuclear hormone receptor NHR-23. Altogether, our findings indicate that different bacteria elicit various responses via distinct mechanisms, which has implications for diseases such as obesity and the interactions between the human microbiome and intestinal cells.
INTRODUCTION
The amount and nutritional content of food are important determinants of organismal health and influence life-history traits such as developmental rate and fecundity. Thus, cells and organisms must sense and interpret dietary state and alter their physiology accordingly. While the influence of caloric intake on organismal health and life-history traits is well appreciated, little is known about the physiological effects of different diets and the mechanisms involved in coordinating diet and physiology.
In response to differences in nutrient availability from different diets metabolic networks are modulated to meet cellular and organismal needs. Further, as it is not beneficial to turn on catabolic and anabolic fluxes simultaneously, metabolites generated by a specific catabolic pathway often act as inhibitors of the opposing anabolic pathway, and vice versa. Metabolic network modulation also occurs at the level of gene transcription: complex information processing mechanisms relay nutritional input to signal transduction pathways that impinge upon transcription factors to regulate metabolic gene expression.
The nematode C. elegans is a powerful genetic model to study the impact of diet on gene expression and life-history traits such as developmental rate, reproduction and lifespan. C. elegans is a soil-dwelling bacterivore with a simple anatomy of fewer than 1000 somatic cells, 20 of which form the intestine, a single organ that functions as both gut and liver with digestive as well as endocrine functions. In the laboratory, C. elegans can be fed a variety of bacterial species and strains (Avery and You, 2012; Coolon et al., 2009). The standard laboratory diet for C. elegans is Escherichia coli OP50. In the wild, however, C. elegans is not likely to encounter E. coli; rather its diet constitutes a variety of bacteria that grow on rotting vegetation. A number of bacteria have been isolated from soil samples containing C. elegans, including Comamonas (Avery and Shtonda, 2003). Altering the C. elegans diet can affect a number of traits, including roaming time, lifespan, and fecundity and pharyngeal pumping rate (Coolon et al., 2009; Shtonda and Avery, 2006; Soukas et al., 2009).
In response to starvation, animals can enter into L1 (larval stage 1) diapause, which is a short-term developmental delay. If food does not become available, C. elegans also employs a long-term survival strategy by entering into dauer, an alternate L3 phase (Sommer and Ogawa, 2011). Caloric restriction has been shown to decrease fecundity and developmental rate, and increase lifespan (Lakowski and Hekimi, 1998). Starvation and caloric restriction versus ample food availability exemplify extreme conditions in the dietary spectrum. However, challenges are not simply the presence or absence of food, but instead diverse food sources with different content and quality may be encountered.
Several nutrient-response systems have been studied in numerous model systems. The target of rapamycin (TOR) pathway, for instance, detects a variety of conditions, including amino acid availability, energy levels and stress, and affects numerous physiological processes including growth, metabolism and lifespan (Laplante and Sabatini, 2012). In C. elegans, the TOR ortholog let-363 is essential for development (Long et al., 2002). Other phenotypes associated with perturbation of the TOR pathway include decreased brood size and developmental rate, and increased lifespan (Honjoh et al., 2009; Korta et al., 2012; Pan et al., 2007; Soukas et al., 2009). The insulin/IGF signaling pathway is another major pathway that regulates lifespan, diapause and stress response, for instance in response to a lack of nutrients (Narasimhan et al., 2009). Insulin signaling occurs through the DAF-2 receptor and impinges on the FoxO transcription factor DAF-16. Finally, nuclear hormone receptors (NHRs) sense a variety of signals produced under specific metabolic and environmental conditions. NHRs are ligand-regulated transcription factors that affect numerous physiological processes including development, growth and metabolism (Pardee et al., 2011; Sonoda et al., 2008). Remarkably, the C. elegans genome encodes 271 NHRs (Reece-Hoyes et al., 2005), whereas the human genome encodes fewer than 50 (Reece-Hoyes et al., 2011; Sonoda et al., 2008). Most C. elegans NHRs are homologs of HNF4, and only a few have been characterized experimentally.
Here we use C. elegans to investigate the relationships between diet and life-history traits, and the mechanisms involved. We find that when fed the soil bacteria Comamonas, C. elegans develop faster, lay fewer eggs and live shorter than when fed E. coli OP50. By expression profiling, we identify a core set of C. elegans genes that differ in expression on the different diets. We establish a transgenic dietary sensor strain that harbors the promoter of one of these genes, acdh-1, to drive expression of the green fluorescent protein (GFP). On the standard laboratory diet of E. coli OP50, GFP expression levels are high. In contrast, GFP expression is barely detectable when the animals are fed Comamonas DA1877. This demonstrates that the dietary response occurs at the level of transcription. Remarkably, when Comamonas DA1877 is dramatically diluted with E. coli OP50 GFP expression is low and developmental rate is accelerated. This shows that the Comamonas DA1877 effect is ‘dominant’ over that of E. coli OP50 and that the response does not simply reflect differences in caloric intake, for instance by mimicking starvation. We show that the developmental acceleration caused by a Comamonas DA1877 diet is independent of TOR and insulin signaling. Instead, we find that Comamonas DA1877 affects cycling gene expression during larval molts, likely through NHR-23.
RESULTS
Dietary Modification of Life-History Traits
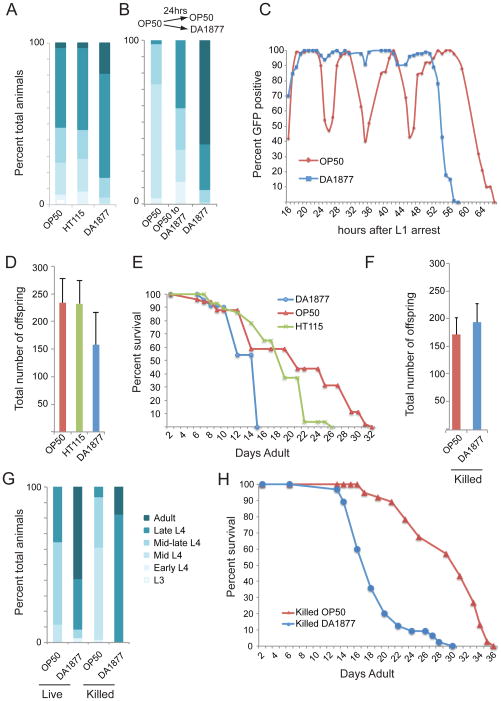
We measured developmental rate, fecundity and lifespan in C. elegans fed three different diets: E. coli OP50; E. coli HT115, a strain used in RNA interference (RNAi) by feeding experiments (Timmons et al., 2001); and Comamonas DA1877. The latter has been proposed to be a healthier diet because it alleviates developmental delays in Eat mutants that have defects in pharyngeal pumping and a compromised ability to eat (Avery and Shtonda, 2003; Shtonda and Avery, 2006). To assess developmental rate, we synchronized animals fed each of the diets in the L1 stage and selected one time point to monitor the developmental age of a population of animals (Figure S1). We found an increased number of older animals in the Comamonas DA1877-fed population than in that fed E. coli OP50 or HT115 suggesting that Comamonas DA1877-fed animals develop faster (Figure 1A). In order to separate the effects of diet on developmental rate from recovery from L1 starvation, we switched animals from an E. coli OP50 to a Comamonas DA1877 diet midway through development. Even following recovery from starvation on E. coli OP50, animals fed a Comamonas DA1877 diet displayed accelerated development (Figure 1B). This demonstrates that effects on development are not the result of differences in recovery from starvation and are not limited to early developmental time points.
Figure 1. A Comamonas DA1877 Diet Affects C. elegans Life-History Traits.
(A) Developmental progression on three different diets. Synchronized N2 wild type animals (L1 stage) were grown on three different diets as indicated on the x-axis, and scored after 43 hours. Larval stage was visually determined based on the stage of vulval development (see Figure S1).
(B) Developmental progression of animals at 48 hours post-L1 synchronization, OP50 to DA1877 indicates animals switched from E. coli OP50 food to Comamonas DA1877 at 24 hours. Development on Comamonas DA1877 is shown for comparison.
(C) Expression of GFP in a synchronized population of Pmlt-10::GFP-pest animals fed E. coli OP50 or Comamonas DA1877 throughout development.
(D) Brood size on three different diets. Wild type N2 animals were grown on three different diets indicated on the x-axis. Bars represent the average total number of progeny per animal, with the standard deviation indicated for all animals combined.
(E) Post-developmental lifespan of adult animals fed each of the indicated diets. OP50 – E. coli OP50; HT115 – E. coli HT115; DA1877 – Comamonas DA1877.
(F) Brood size of animals on killed E. coli OP50 or killed Comamonas DA1877. Bars represent the average total number of progeny per animal, with the standard deviation indicated for all animals combined.
(G) Developmental progression of animals grown on live or killed E. coli OP50 or Comamonas 1877 at 45 hours.
(H) Post-developmental survival of adult animals fed each of the indicated diets.
To investigate the timing of the changes in developmental rate, we used a transgenic strain harboring a molting-dependent reporter Pmlt-10::GFP-pest. This transgenic strain expresses a destabilized GFP protein under the control of the mlt-10 promoter, a gene whose expression oscillates with molting (Frand et al., 2005). We examined GFP expression during development in animals fed E. coli OP50 or Comamonas DA1877. As reported previously, animals fed E. coli OP50 displayed oscillatory GFP expression (Frand et al., 2005). Surprisingly, the amplitude of GFP oscillations was dramatically reduced in all four molting cycles in animals fed Comamonas DA1877 (Figure 1C). Thus, Comamonas DA1877 may alter developmental rate by modulating the molting program.
We measured two additional life-history traits in C. elegans fed the different diets and found that animals fed Comamonas DA1877 have a decreased average brood size of 158 eggs compared to 234 and 232 for animals fed E. coli OP50 or HT115, respectively (Figure 1D). Finally, animals fed Comamonas DA1877 exhibit a much-reduced lifespan (Figure 1E).
The Dietary Effect on Development and Lifespan is not Due to Pathogenic Infection
In addition to serving as food, some bacteria can also be pathogenic to C. elegans (Gravato-Nobre and Hodgkin, 2005; Tan et al., 1999). To test whether the life-history trait changes result from a pathogenic response, we used killed bacteria. Comamonas DA1877 proved difficult to kill using standard protocols (Sutphin and Kaeberlein, 2009)(data not shown). However, a combination of UV irradiation, peptone-free media and antibiotics effectively killed both E. coli OP50 and Comamonas DA1877. On killed bacteria both diets result in similar brood sizes (Figure 1F). In contrast, there was still a diet-induced difference in developmental rate and lifespan (Figures 1G and 1H). This demonstrates that the short lifespan and fast development are not the result of a pathogenic infection, but rather are more likely to be caused by a dietary effect. Thus, we focused on these phenotypes in the rest of our study.
Diet-Induced Changes in Gene Expression
Next, we examined the gene expression changes elicited by the three different bacterial diets in young adult animals using microarray expression profiling. In animals fed Comamonas DA1877 389 genes changed significantly (≥ 2 fold; p < 0.001) when compared to E. coli OP50 (Figure 2A and 2B).
Figure 2. Diet-Induced Changes in Gene Expression.
(A) Scatterplot indicating changes in gene expression in animals fed E. coli HT115 or Comamonas DA1877 relative to animals fed E. coli OP50 detected by microarray expression profiling. Each square indicates a gene. See also Table S1.
(B) Venn diagram indicating the total number of genes changing in response to diet on E. coli HT115 and Comamonas DA1877 diets and the overlap between these, relative to standard laboratory diet of E. coli OP50.
(C) Gene Ontology analysis of genes that decrease in expression on a Comamonas DA1877 diet. Numbers in parentheses indicate enrichment score. See also Table S1.
(D) Venn diagrams indicating a comparison of Comamonas DA1877-responsive genes at two different stages of development.
(E) Categorization of core dietary response genes. See also Table S2.
Gene Ontology (GO) analysis (Ashburner et al., 2000) of the genes that change on a Comamonas DA1877 diet revealed an enrichment of terms related to molting (Figure 2C). This is consistent with the ability of Comamonas DA1877 to affect the cycling expression of mlt-10 (Figure 1C). Thus, it is likely that Comamonas DA1877 affects the C. elegans molting program and hence the expression of associated genes. However, in contrast to what we observed with the mlt-10 reporter, the majority of the molting-associated genes that change in response to diet decrease in expression in response to Comamonas DA1877. Because animals fed E. coli OP50 and Comamonas DA1877 grow at different rates, animals were collected for RNA isolation based on visual examination of developmental age in the population. We therefore wondered whether some changes in the diet-induced expression profiles could result from differences in staging of the population. Therefore, we performed an additional microarray expression profiling experiment at a later time point that is well separated from the oscillations that occur during molting (gravid adults). At this stage, even more genes changed in expression in response to Comamonas DA1877 (Figure 2D). This is likely due, at least in part, to the onset of reproduction. A comparison between the two expression profiling experiments revealed a set of 87 ‘core’ genes that are affected by a Comamonas DA1877 diet at both stages (Figure 2E, Table S2). The core includes upregulated and downregulated genes that encode metabolic enzymes, as well as numerous worm-specific genes.
A Dietary Sensor in Living Animals
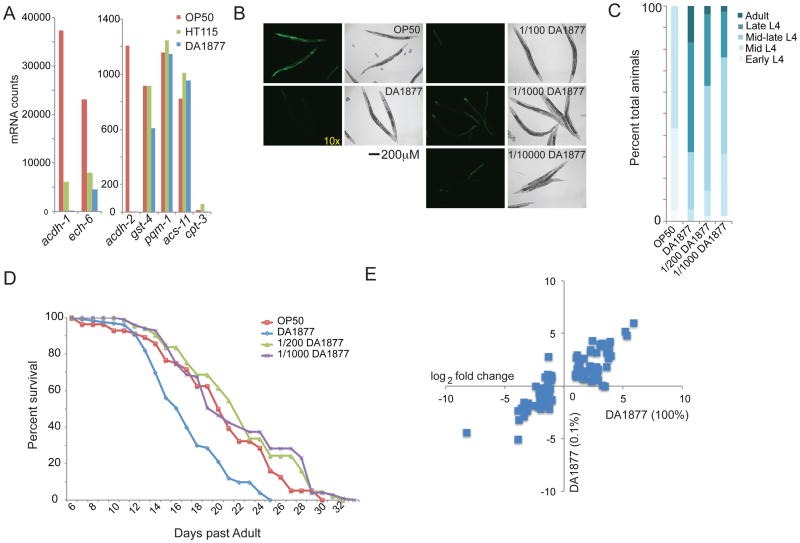
The expression profiles do not reveal whether these changes occur at the level of transcription or mRNA stability. Among the core genes, acdh-1 exhibits the most dramatic change in expression (Figure 2A, Table S1). We previously generated a transgenic strain that expresses GFP in the intestine and hypodermis under the control of the acdh-1 promoter, and wondered whether we could use this as a ‘dietary sensor’ in living animals (Arda et al., 2010). We integrated the Pacdh-1::GFP transgene into the C. elegans genome and fed the animals different diets. GFP expression was high on E. coli OP50, intermediate on E. coli HT115 and barely detectable on Comamonas DA1877, which recapitulates the microarray data (Figure 3A). The effect on GFP expression was transmitted to the progeny and maintained prior to the start of feeding (Figure 3B). To test whether the sensor can respond to individual nutrients, we exposed the Pacdh-1::GFP transgenic animals to 5 mM glucose on each of the three diets and found that this results in an increase in GFP expression, most notably on an E. coli HT115 diet (Figure 3C). Animals fed Comamonas DA1877 required higher glucose concentrations to induce acdh-1 promoter activity (Figure 3D). Previously, it has been shown that acdh-1 expression is reduced upon starvation (Van Gilst et al., 2005a). When deprived of food GFP expression was indeed reduced in Pacdh-1::GFP transgenic animals (Figure 3E). Altogether, these results demonstrate that acdh-1 expression is modulated in response to dietary conditions at least in part through the activity of its promoter, and thus at the level of transcription. We obtained similar results with a strain that expresses GFP under the control of acdh-2, which is homologous to acdh-1 and also changes in response to diet (Figure S2).
Figure 3. A Dietary Sensor in Living Animals.
(A) Pacdh-1::GFP transgenic animals respond transcriptionally to the different bacterial diets and can be used as a dietary sensor in living animals. The Comamonas DA1877 fluorescence image was acquired with a 25-fold longer exposure time (exp x25). See also Figure S2.
(B) Eggs display the maternal GFP expression.
(C) The addition of 5 mM glucose activates the dietary sensor.
(D) High levels of glucose can activate the dietary sensor on Comamonas DA1877.
(E) The dietary sensor is repressed upon starvation.
The Dietary Effect is Distinct from the Starvation Response
Both a diet of Comamonas DA1877 and starvation repress the dietary sensor. Previous studies have demonstrated that caloric restriction affects life-history traits (Lakowski and Hekimi, 1998), as does a Comamonas DA1877 diet. Although superficially this may suggest that caloric intake differs between the different bacterial diets, caloric restriction would be expected to reduce developmental rate and increase lifespan, which is opposite to the effects of Comamonas DA1877. We performed two experiments to test whether Comamonas DA1877 confers a (partial) starvation response. In the first experiment, we used nCounter technology (Geiss et al., 2008) to compare the expression changes of endogenous acdh-1 and acdh-2 to three starvation-induced genes, acs-11, gst-4 and cpt-3, as well pqm-1, a stress-responsive gene (Tawe et al., 1998; Van Gilst et al., 2005a). We did not observe significant changes in the expression of these genes on any diet (Figure 4A). In the second experiment, we reasoned that if Comamonas DA1877 lacks specific nutrients and thereby induces a starvation response, then combining it with E. coli OP50 would alleviate this and increase acdh-1 expression. Interestingly, however, a mixed diet of E. coli OP50 and Comamonas DA1877 resulted in barely detectable GFP expression, similar to Comamonas DA1877 alone (Figure 4B). Remarkably, even when diluted dramatically with E. coli OP50, Comamonas DA1877 still exerted a repressive effect (Figure 4B). Together, these observations demonstrate that Comamonas DA1877 is not simply nutrient-poor. Indeed, we did not observe major differences in bulk protein, carbohydrate or lipid between the different bacteria (Figure S3). A second implication of these observations is that Comamonas DA1877 generates a signal to which the animal responds.
Figure 4. Comamonas DA1877 Dietary Effect can be Diluted.
(A) nCounter analysis of gene expression of C. elegans fed different bacterial diets. gst-4 and pqm-1 are induced by oxidative stress. cpt-3 and acs-11 are starvation responsive genes.
(B) Mixing diets: numbers indicate proportion of Comamonas DA1877 to E. coli OP50.
(C) Animals grown on diluted Comamonas DA1877 develop faster relative to animals grown on OP50.
(D) Post-developmental survival of adult animals fed each of the indicated diets. OP50 – E. coli OP50; DA1877 – Comamonas DA1877; 1/200 and 1/1000 refers to the dilution of Comamonas DA1877 in E. coli OP50. All bacteria were seeded onto peptone-free plates to prevent bacterial growth.
(E) Changes in gene expression of animals fed a diet of Comamonas DA1877 diluted in E. coli OP50 compared to Comamonas DA1877 alone diet.
See also Figure S3.
Diluting Comamonas DA1877 with E. coli OP50 was sufficient to induce an increase in developmental rate (Figure 4C), but did not affect lifespan (Figures 4D). This suggests that developmental rate and lifespan are regulated by different Comamonas-derived signals. Alternatively, the effects may be caused by the same signal but lifespan may be more dose-dependent. Lifespan experiments require animals to be maintained on bacterial plates for longer periods of time during which the Comamonas DA1877 signal may diminish.
We performed microarray expression profiling on animals fed diluted Comamonas DA1877 (0.1%, Figure 4E). The expression profile of animals grown on diluted bacteria was very similar to that of animals grown on undiluted Comamonas DA1877. In fact, of 87 ‘core’ genes, 67 also changed on the diluted dietary condition, although in general the magnitude of these changes was lower (Figure 4E). These observations indicate that a small amount Comamonas DA1877 is sufficient to change gene expression as well as accelerate development.
Amphid Sensing is not Required for the Dietary Response
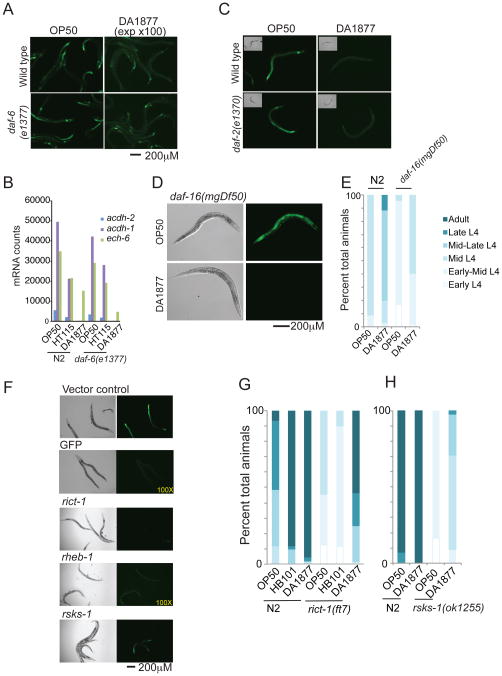
C. elegans senses many environmental cues neuronally and relays the information to the rest of the animal through the use of secreted ligands and neuropeptides (Eczurra et al., 2011). Therefore, we tested whether amphid neurons that are exposed to the environment and that sense external signals are involved in the response to Comamonas DA1877. We crossed the dietary sensor into daf-6(e1377) animals in which socket cells are abnormal, preventing the interaction of amphid neurons with the exterior environment (Albert et al., 1981). We found that daf-6(e1377) mutants still respond to Comamonas DA1877 (Figure 5A). The dietary response of endogenous core genes acdh-1, acdh-2 and ech-6 expression is also unaffected by a mutation in daf-6 (Figure 5B). Together, these observations demonstrate that the Comamonas DA1877 signal is not sensed by amphid neurons, but may rather be interpreted as the bacteria traverse the digestive tract upon ingestion.
Figure 5. Analysis of Known Genes and Pathways Indicates that the Comamonas DA1877 effect on Development is Independent of TOR and Insulin Signaling.
(A) The dietary sensor is not affected by a loss-of-function mutation in daf-6.
(B) nCounter analysis of diet-responsive genes in N2 wild type and daf-6(e1377) mutant animals.
(C) daf-2(e1370) mutant animals respond to Comamonas DA1877 like wild type animals.
(D) daf-16(mgDf50) mutant animals respond to Comamonas DA1877 like wild type animals.
(E) Developmental progression of animals at 43 hours post-L1 synchronization of daf-16(mgDf50) and wild type (N2) animals grown on E. coli OP50 or Comamonas DA1877.
(F) RNAi analysis of TOR pathway components. Animals harboring the dietary sensor were fed E. coli HT115 bacteria that express double stranded RNA for the indicated genes. See also Figure S4.
(G) Developmental progression of animals at 48 hours post-L1 synchronization of rict-1(ft7) mutant animals fed the indicated diets.
(H) Developmental progression of animals at 50 hours post-L1 synchronization of rsks-1(ok1255) mutant animals fed the indicated diets.
Effects of TOR and Insulin Pathways on the Dietary Response
Insulin and TOR signaling act as nutrient response pathways and affect life-history traits in many organisms (Laplante and Sabatini, 2012; Narasimhan et al., 2009). To test their possible roles in dietary response, we knocked down known pathway components and examined the effects on GFP expression in the dietary sensor using RNAi-by-feeding with E. coli HT115 bacteria on which the sensor displays intermediate levels of GFP expression (Figure 3A)(Timmons et al., 2001). Although acdh-1 was previously reported as a target of daf-16 (Murphy et al., 2003), knockdown of neither daf-2 nor daf-16 markedly affected GFP expression (Figure S4). We crossed the dietary sensor into daf-2(e1370) mutants and observed a slight increase in GFP on an E. coli OP50 diet (Figure 5C). When these animals were fed Comamonas DA1877, however, GFP expression decreased as in wild type sensor animals. Similarly, in the absence of daf-16, neither the dietary response nor the developmental acceleration by Comamonas DA1877 was affected (Figure 5D and 5E). Altogether, these findings demonstrate that the insulin signaling pathway is not required for the dietary sensor and developmental timing aspects of the response to Comamonas DA1877.
Knockdown of several components of the TOR pathway reduced GFP expression in the dietary sensor, including rict-1 and ruvb-1 (Figure 5F, Figure S5). There are two TOR complexes, TORC1 and TORC2 that induce different signaling pathways, and inhibition of either pathway decelerates development (Pan et al., 2007; Soukas et al., 2009). Wild type animals fed E. coli HB101 develop faster than those grown on E. coli OP50 and this acceleration is suppressed by a mutation in rict-1, a TORC2 component (Soukas et al., 2009). To compare the effect of Comamonas DA1877 to that of E. coli HB101, we measured development in wild type and rict-1(ft7) mutant animals. As previously reported, E. coli HB101 accelerated growth in wild type but not rict-1(ft7) mutant animals. In contrast, Comamonas DA1877 accelerated growth in both wild type and rict-1(ft7) mutant animals (Figure 5G). We next tested whether rsks-1, a TORC1 pathway component, was required for accelerated growth on Comamonas DA1877. As previously reported (Pan et al., 2007), rsks-1 mutants develop more slowly than wild type animals, however they do develop faster on Comamomas DA1877 than on E. coli OP50 (Figure 5H). Together, these results demonstrate that, although the dietary sensor is affected by TOR pathway inhibition, the accelerated developmental rate of animals fed Comamonas DA1877 is independent of TOR.
Nuclear Hormone Receptors Affect the Dietary Sensor
NHRs are ligand-regulated transcription factors that act as sensors for hormones, vitamins and lipids and play broad roles in development and physiology (Sonoda et al., 2008). Therefore, they are excellent candidates to mediate the response to dietary signals. By RNAi, we found that nhr-49, a known nutrient-response mediator (Van Gilst et al., 2005b) does not affect the dietary sensor (Figure S4).
We previously identified NHR-10 as a direct regulator of acdh-1 (Arda et al., 2010). We crossed the dietary sensor into nhr-10(tm4695) mutant animals that carry a deletion in the nhr-10 gene. As expected, we observed a strong decrease in GFP expression in nhr-10 mutants on E. coli OP50 (Figure 6A)(Arda et al., 2010). However, GFP expression was still readily detectable, most notably in the posterior intestine. Interestingly, in nhr-10 mutants GFP expression was further reduced on Comamonas DA1877 (Figure 6A). In addition, while nhr-10 mutants developed more slowly than wild type animals, their development was still accelerated on a Comamonas DA1877 diet (Figure 6B). This demonstrates that NHR-10 is involved in acdh-1 regulation, but is not solely responsible for the dietary response.
Figure 6. Two Nuclear Hormone Receptors Affect the Dietary Sensor.
(A) nhr-10(tm4695) mutant animals have reduced acdh-1 promoter activity but still respond to Comamonas DA1877.
(B) Developmental progression of animals at 45 hours post-L1 synchronization of nhr-10(tm4695) mutant animals fed the indicated diets.
(C) Expression of GFP in a synchronized population of Pmlt-10::GFP-pest animals over time in animals fed E. coli OP50, E. coli HB101 or Comamonas DA1877.
(D) Developmental rate in N2 and Pnhr-23::NHR-23::GFP animals that overexpress NHR-23 (OE). Stages are as in (B).
(E) nhr-23 RNAi (diluted 1 in 20) greatly reduces acdh-1 promoter activity.
(F) nhr-23 RNAi (diluted 1 in 20) reduces acdh-1 promoter activity in nhr-10(tm4695) mutant animals.
The effect on the molting program by Comamonas DA1877 is either a cause or a consequence of accelerated development. In order to discriminate between these possibilities, we compared the oscillation of the mlt-10 molting reporter between animals fed Comamonas DA1877 and E. coli HB101. Remarkably, although animals fed E. coli HB101 develop more rapidly, the oscillatory pattern of GFP expression was not affected (Figure 6C). This demonstrates that the effect is specific to Comamonas DA1877 and not solely a result of accelerated growth.
NHR-23 is a regulator of molting and mlt-10 is a known transcriptional target of NHR-23. Therefore, we asked whether nhr-23 may be involved in the dietary response (Frand et al., 2005). In our microarray experiment of young adult animals, we found that the GO term ‘molting cycle’ is enriched in the genes that change in expression on a Comamonas DA1877 diet. Many of these genes also change in expression in a published study where nhr-23 knockdown was compared to control L1 larvae (Kouns et al., 2011). nhr-23 is an essential gene and its knockdown causes larval arrest due to molting defects (Kostrouchova et al., 1998). To circumvent this, we diluted bacteria producing nhr-23 dsRNA twenty-fold with bacteria containing vector alone. We examined larval development in both animals with decreased and increased nhr-23 expression (from transgene gaIs269 that expresses an NHR-23::GFP fusion protein). Surprisingly, both knockdown and overexpression of nhr-23 slowed development (Figure 6D). Knockdown of the exogenous copy of nhr-23 by GFP knockdown partially suppressed the latter effect, demonstrating that it is a result of an increase in nhr-23. Thus, both a reduction and an increase in nhr-23 expression decelerate development. The oscillations in mlt-10 expression are part of a tightly regulated system, controlled, at least in part by nhr-23. Changing nhr-23 expression levels may perturb these oscillations, and adjusting to these perturbations may result in a developmental delay.
Diluted nhr-23 RNAi results in a dramatic reduction in GFP expression in adults (Figure 6E). NHR-10 is a direct regulator of acdh-1 (Arda et al., 2010). NHR-23 could therefore act indirectly, for instance by activating NHR-10. Alternatively, NHR-23 may act in parallel to NHR-10 to affect acdh-1, either directly or indirectly. To test whether NHR-23 mediates its effect on acdh-1 expression through NHR-10, we performed nhr-23 RNAi in the nhr-10(tm4695) mutant animals carrying Pacdh-1::GFP. If NHR-23 affects acdh-1 expression by modulating NHR-10, we would expect that GFP levels following RNAi of nhr-23 in an nhr-10 null animal is the same as in the nhr-10 mutant alone. However, knocking down nhr-23 further reduced GFP expression (Figure 6F), demonstrating that the effect of nhr-23 on acdh-1 expression is not mediated through nhr-10.
DISCUSSION
Diet has major effects on the development and health of complex multicellular organisms. We, and others, have used the nematode C. elegans together with a variety of bacterial diets to unravel the phenotypic adjustments of the animal in response to diet, as well as the mechanisms involved (Coolon et al., 2009; Shtonda and Avery, 2006; Soukas et al., 2009; this study). Different bacteria likely provide different degrees and types of nutrition to the nematode. We focused on the effects of the soil bacterium Comamonas that was isolated together with C. elegans and therefore likely represents a natural food source (Avery and Shtonda, 2003). Relative to the standard laboratory diet of E. coli OP50, a diet of Comamonas DA1877 accelerates development, reduces fecundity and shortens lifespan. In contrast, unc-119(ed3) mutant animals produce more offspring on Comamonas DA1877 than on E. coli OP50 (data not shown). Thus, our findings indicate that bacterial diets are not simply healthy or unhealthy, but that specific qualities of these diets may be optimal under different conditions and for different life-history traits. In addition, the response to Comamonas DA1877 is likely not a trait that was acquired when C. elegans was established as a genetic model system in the laboratory because another nematode species, C. briggsae, exhibits similar changes in life-history traits when fed the different diets (data not shown).
A complication in understanding the influence of diet on C. elegans physiology is the fact that bacteria are not only a source of food; they can also be pathogenic (Tan and Shapira, 2011). The observation that Comamonas DA1877 can exert its effects on development and lifespan when killed demonstrates that these effects are not due to pathogenicity. However, we noted that several genes previously reported to change in response to specific pathogens were also changed in response to the diets we tested (data not shown). In studies of the C. elegans pathogen response, pathogenic bacteria also serve as food and, therefore, it will be important to disentangle dietary from pathogenic effects in the future.
In the wild, it is likely that C. elegans encounters complex mixtures of bacterial species. Our observation that the effect of Comamonas DA1877 on development and gene expression occurs even when mixed with E. coli OP50 illustrates that the animal is capable of responding to even low amounts of particular types of bacteria. Further, this indicates that it is not the caloric content that is responsible for the effects on physiology and gene expression, but rather that the bacteria generate a signal that is interpreted by the nematode. We demonstrate that this signal is not sensed by amphid neurons. Rather, it is likely that this response occurs after ingestion of the bacteria, potentially directly by cells in the intestine. Relative to E. coli OP50 diluted Comamonas DA1877 accelerated C. elegans development but did not affect lifespan. This may suggest that different effects of Comamonas DA1877 may be elicited by different signals. Future biochemical fractionation experiments with Comamonas DA1877 extracts may shed light on the nature of these signals, and thereby further illuminate the mechanism by which it is interpreted by the nematode.
TOR and Insulin represent major nutrient sensing pathways in a number of organisms (Laplante and Sabatini, 2012; Narasimhan et al., 2009). TOR signaling is regulated by amino acid availability and stress, and affects downstream processes including protein synthesis, energy metabolism and proliferation. Similarly, insulin signaling regulates the starvation response and lifespan. TOR and Insulin signaling pathways regulate developmental rate and likely adjust this rate in accordance with cellular and environmental states. Remarkably, the developmental acceleration of C. elegans in response to Comamonas DA1877 is independent of both of these pathways. We did, however, observe changes in the expression of GFP in the dietary sensor in response to perturbation of TOR. This is likely related to the response of the dietary sensor to food deprivation, as loss of TOR signaling may mimic starvation.
NHR-10 directly binds and activates the acdh-1 promoter (Arda et al., 2010)(this study). However, NHR-10 is not solely responsible for the dietary response. In the accompanying paper we find that NHR-10 is partly responsible for the response to endogenous metabolic network perturbations and identify additional NHRs that affect the dietary sensor (Watson et al., 2013). Here, we identify NHR-23 as a candidate mediator of the response to Comamonas DA1877. Together, these results indicate that a variety of NHRs function coordinately to ensure appropriate transcriptional and physiological responses to different exogenous and endogenous cues.
Comamonas DA1877 affects the C. elegans molting program, which is also regulated by nhr-23. Comamonas DA1877 dampens the oscillatory activity of the mlt-10 promoter. Further, in young adult animals many molting genes are repressed in response to this diet: of 266 genes downregulated by nhr-23 knockdown, 39 are also repressed by Comamonas DA1877 (Kouns et al., 2011) (this study). These changes could be either the cause or consequence of the effect that Comamonas DA1877 exerts on cyclic gene expression during molting. Our observation of dampened mlt-10 oscillations demonstrates that this diet does in fact affect molting. mlt-10 is a target of NHR-23, suggesting that this NHR may be activated by Comamonas DA1877. In adults fed this diet however, the expression of other NHR-23 targets is decreased, suggesting that NHR-23 may be repressed by this diet. NHRs can both activate and repress transcription (Pardee et al., 2011). nhr-23 mRNA expression does not change in response to diet (data not shown), suggesting a modulation of its activity at the protein level. The C. elegans genome encodes six NHR-23 variants that differ in their N-terminal domains. One possibility is that diet affects different NHR-23 variants that have distinct sets of target genes at different stages in the animal’s lifetime.
Oscillating gene expression is pivotal in numerous biological processes including the cell cycle, molting cycles and circadian rhythms. Our data show that diet can affect oscillatory gene expression, which likely affects developmental rate. Comamonas DA1877 both dampens mlt-10 oscillation and shortens its period. On the other hand, E. coli HB101 shortens the period without dampening the amplitude. Thus, both diets likely accelerate development by impinging on the molting program. While Comamonas DA1877 affects developmental rate in a TOR-independent manner, E. coli HB101 accelerates growth in a TOR-dependent manner. Nutrition also affects developmental rate in Drosophila (Layalle et al., 2008). In flies, the nutritional effect can occur via the TOR pathway which impinges on the molting hormone Ecdysone (Layalle et al., 2008). Ecdysone initiates a transcriptional cascade that activates the NHR-23 ortholog DHR3. This suggests an evolutionarily conserved mechanism that links nutrition, molting and developmental transitions. In humans, circadian oscillations in gene expression involve the NHR-23 ortholog, RORα. Like NHR-23, there are multiple isoforms of RORα. These vary in their N-termini and differ in their DNA binding specificity, suggesting that they may regulate different target genes (Giguere et al., 1994). Circadian rhythms are greatly affected by diet, and timing of food ingestion can have major effects on physiology (Froy, 2007). Further studies of dietary effects on oscillatory gene expression and the relationships to development, physiology and lifespan will shed more light on common as well as distinct mechanisms that have evolved in different species.
Our study has several implications for human health and nutrition. The first tantalizing implication relates to our observation that even small amounts of one diet can elicit a physiological response in the presence of another. This indicates that ‘unhealthy’ foods can illicit physiological responses in the presence of an otherwise ‘healthy’ diet or vice versa. This has major implications for treatments for diseases affected by diet such as diabetes, obesity and cancer. Another implication of our study for human health relates to the observation that bacteria can generate a signal that is interpreted by gene regulatory networks in nematode cells, most likely in the intestine. Numerous bacterial species, known as the microbiome, colonize the human intestine. These commensal bacteria provide numerous benefits to our health: they are important in immunity to ward off harmful bacteria, produce essential nutrients and vitamins, and regulate gut development. However, under adverse conditions, the gut flora can inflict infections or affect disease progression. It is likely that the microbiota generates a cacophony of signals that can affect the metabolic network of the surrounding intestinal cells. We propose that the nematode C. elegans can be used a model to provide further insights into the communication between microbes and mammalian cells.
EXPERIMENTAL PROCEDURES
Strains
C. elegans strains were cultured and maintained by standard protocols (Brenner, 1974). Construction Pacdh-1::GFP was previously described (Arda et al., 2010). The extrachromosomal array was integrated by UV irradiation using standard methods (Evans, 2006) to generate VL749 wwIs24 [Pacdh-1::GFP + unc-119(+)]. Integrated lines were outcrossed three times to N2 wild type animals. The nhr-10(tm4695) mutant was kindly provided by the National Bioresource Project, Japan. Additional strains were obtained from the C. elegans Genetics Center (CGC). The daf-6(e1377) mutant was crossed into VL749 to generate VL840. Animals were genotyped and dye filling was performed to verify the identity of the daf-6(e1377) mutant, which are dye-filling defective. Bacterial strains E. coli OP50, E. coli HT115(DE3) and Comamonas DA1877 were obtained from the CGC. GR1395 mgIs49 [mlt-10::GFP-pest; ttx-1::GFP] was used to monitor molting (Frand et al., 2005). The RW10429 strain [gaIs269 [nhr-23::TY1::EGFP::3xFLAG + unc-119(+)]. (TY1::EGFP::3xFLAG tag inserted in fosmid P000006_G11) was obtained from the CGC.
Phenotypic Analysis of Life-History Traits
Animals were grown at 20°C. For all diet-specific assays, animals were grown on the appropriate diet for at least one generation prior to the assay. We measured development by first synchronizing animals by L1 arrest. Briefly, animals were grown on the relevant diet, eggs were collected by bleaching, washed three times in M9 buffer and allowed to hatch in M9 buffer for 18 hours. Following synchronization, animals were transferred to NGM plates and incubated at 20°C. At the indicated times in the Figures, animals were washed off the plates, mounted on agarose pads and examined on a compound microscope. Animals were visually categorized into age groups based on the development of the vulva (Figure S1). At least 40 animals were scored for each diet.
Brood sizes were determined by picking individual L4 animals onto plates containing different diets. Animals were transferred daily and number of offspring on the plates was counted.
For lifespan analysis L4 animals were transferred onto NGM plates seeded with either of the three diets. The following day, the animals were transferred to NGM plates seeded with the appropriate bacteria. Every two days, animals were checked for pharyngeal pumping. If pumping was not observed, animals were lightly prodded with a platinum wire, if animals did not respond, they were considered dead, scored and removed. For lifespan analysis using diluted bacteria, animals were cultured on Peptone-free NGM. Killed bacterial lawns were prepared using an overnight culture of bacteria (E. coli OP50 or Comamonas DA1877). Bacterial cultures were concentrated 2-fold and seeded on peptone-free NGM containing tetracycline and spectinomycin. When lawns were dry, plates were UV-irradiated using a Strategene cross-linker as described (Sutphin and Kaeberlein, 2009).
Expression Profiling Analysis
N2 wild type animals were grown on each diet for one generation prior to egg collection. Eggs were collected and synchronized in L1. All animals were grown on standard NGM plates. Animals fed Comamonas DA1877 developed faster, and thus all samples could not be collected simultaneously, but were instead collected when most animals on the plates reached the young adult stage as judged by the presence of a fully developed vulva or as gravid adults as judged by the presence of eggs in the gonad. Animals were washed twice in M9 buffer, pelleted by centrifugation and frozen at −80°C in Trizol. RNA was collected using Trizol extraction followed by DNAse I treatment and cleanup using RNeasy prep kit (Qiagen). Three biological replicates were prepared for each condition. Microarray expression profiling was performed by the Genomics Core facility at University of Massachusetts Medical School using C. elegans genome arrays that contain probes for predicted coding sequences (Affymetrix). The RMA method in the Affy package from Bioconductor was used in R to summarize the probe level data and normalize the dataset to remove across-array-variation. Log transformed data were used in subsequent analyses. Moderated T statistics in Limma (Smyth et al., 2004) was used to calculate significance. Significance was determined using an adjusted p value (Benjamini and Hochberg, 1995). Changes in gene expression that were 2 fold (p < 0.001) or greater were considered significant. Microarray data has been submitted to GEO (GSE43959).
nCounter mRNA Quantification
nCounter assays were performed as per manufacturer’s instructions (nanostring technologies). Probes were hybridized with 300 ng of total RNA. Resulting counts were normalized to ama-1 mRNA levels. Probe sequences are provided in Table S3.
Mixing Bacterial Diets
Liquid cultures were grown overnight at 37°C in LB broth. E. coli OP50 and Comamonas DA1877 bacterial cultures were diluted to the same OD600 and mixed in ratios indicated in Figures 3F and 3G. Bacterial suspensions were spread onto peptone-free NGM to minimize bacterial growth. Eggs were immediately added to plates and animals were allowed to develop to adults. For growth assays, eggs were prepared by hypochlorite bleaching of animals grown on E. coli OP50. Eggs were allowed to hatch in M9 to generate a synchronized L1 population.
Starvation Assay
To assay the response to starvation, animals were first grown on E. coli OP50 bacteria. L4 animals were washed five times in M9, and then transferred to unseeded peptone-free NGM plates. After 24 hours, animals were collected and examined for GFP expression.
Gene Ontology Analysis
Gorilla software (Eden et al., 2009) was used to identify GO term enrichment. Up- or down regulated genes were compared to all genes present on the array. GO terms with a p-value < 10−4 are provided in Figure 2C.
RNAi Gene Knockdown
Plates were prepared by adding IPTG to a final concentration of 5 mM to NGM agar. RNAi clones were obtained either from the ORFeome RNAi library (Rual et al., 2004), the Ahringer RNAi library (Kamath et al., 2003), or cloned into the RNAi Gateway Destination vector from ORFeome clones or using genomic DNA. E. coli HT115 RNAi cultures were grown in LB broth containing 100 μg/mL ampicillin to log phase and were concentrated by centrifugation, resuspended in 1/10 volume of M9 and added to RNAi feeding plates. VL749 or N2 animals were synchronized by hypochlorite bleaching and washed in M9 buffer, and added to the E. coli HT115 seeded plates. For nhr-23 RNAi, cultures were grown as described above. Following growth of the cultures, HT115 bacteria containing the nhr-23 RNAi construct were diluted 1/20 with HT115 bacteria containing the empty RNAi vector alone.
Generation of Molting Curves
In order to generate molting curves, embryos were collected from E. coli OP50 grown adults by hypochlorite bleaching. Animals were allowed to hatch and synchronized in L1 by incubation in M9 for 18 hours. Animals were then added to the specified foods and incubated at 20°C. For the longer time course (Figure 1), two experiments were started 12 hours apart and animals were scored every hour for 12 hours. Animals were scored on a fluorescence-equipped dissecting microscope. Animals with appreciable GFP expression were scored positive. At least 50 animals were scored for each time point.
Supplementary Material
Figure 7.
Model for Dietary Regulation of Developmental Rate
HIGHLIGHTS.
Developmental acceleration in C. elegans by Comamonas, even under dilute conditions
Comamonas bacteria affect oscillating gene expression during development
Comamonas-induced developmental acceleration is TOR and insulin-independent
NHR-23 is a candidate mediator of the dietary response
Acknowledgments
We thank members of the Walhout laboratory and Amy Walker for discussion and critical reading of the manuscript. We thank Heidi Tissenbaum for RNAi clones and discussions. We thank Victor Ambros for discussions. Some nematode strains used in this work were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by NIH grant DK068429 to A.J.M.W., a CIHR post-doctoral fellowship to L.T.M. and an American Heart Association fellowship to E.W.
References
- Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- Arda HE, Taubert S, Conine C, Tsuda B, Van Gilst MR, Sequerra R, Doucette-Stam L, Yamamoto KR, Walhout AJM. Functional modularity of nuclear hormone receptors in a C. elegans gene regulatory network. Molecular Systems Biology. 2010;6:367. doi: 10.1038/msb.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Shtonda BB. Food transport in the C. elegans pharynx. J Exp Biol. 2003;206:2441–2457. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, You YJ. C. elegans feeding. Wormbook; 2012. pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolon JD, Jones KL, Todd TC, Carr BC, Herman MA. Caenorhabditis elegans genomic response to soil bacteria predicts environment-specific genetic effects on life history traits. PLoS genetics. 2009;5:e1000503. doi: 10.1371/journal.pgen.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eczurra M, Tanizawa Y, Swoboda P, Schafer WR. Food sensitizes C. elegans avoidance behaviours through acute dopamine signaling. Embo J. 2011;30:1110–1122. doi: 10.1038/emboj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TC. Transformation and microinjection. Wormbook; 2006. [Google Scholar]
- Frand AR, Russel S, Ruvkun G. Functional genomic analysis of C. elegans molting. PLoS biology. 2005;3:e312. doi: 10.1371/journal.pbio.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O. The relationship between nutrition and circadian rhythms in mammals. Frontiers in Neuroendocrinology. 2007;28:61–71. doi: 10.1016/j.yfrne.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Geiss GK, Bumgarnder RE, Birditt B, Dahl T, Dowidar DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes & development. 1994;8:538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Gravato-Nobre MJ, Hodgkin J. Caenorhabditis elegans as a model for innate immunity to pathogens. Cell Microbiol. 2005;7:741–751. doi: 10.1111/j.1462-5822.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457:726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Korta DZ, Tuck S, Hubbard EJ. S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. Development. 2012;139:859–870. doi: 10.1242/dev.074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrouchova M, Krause M, Kostrouch Z, Rall JE. CHR3: a Caenorhabditis elegans orphan nuclear hormone receptor required for proper development and molting. Development. 1998;125:1617–1626. doi: 10.1242/dev.125.9.1617. [DOI] [PubMed] [Google Scholar]
- Kouns NA, Nakielna J, Behensky F, Krause MW, Kostrouch Z, Kostrouchova M. NHR-23 dependent collagen and hedgehog-related genes required for molting. Biochem Biophys Res Commun. 2011;413:515–520. doi: 10.1016/j.bbrc.2011.08.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layalle S, Arquier N, Leopold P. The TOR pathway couples nutrition and developmental timing in Drosophila. Developmental cell. 2008;15:568–577. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Long X, Spycher C, Han ZS, Rose AM, Muller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Current biology : CB. 2002;12:1448–1461. doi: 10.1016/s0960-9822(02)01091-6. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Current biology : CB. 2009;19:R657–666. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee K, Necakov AS, Krause H. Nuclear Receptors: Small Molecule Sensors that Coordinate Growth, Metabolism and Reproduction. Subcell Biochem. 2011;52:123–153. doi: 10.1007/978-90-481-9069-0_6. [DOI] [PubMed] [Google Scholar]
- Reece-Hoyes JS, Barutcu AR, Patton McCord R, Jeong J, Jian L, MacWilliams A, Yang X, Salehi-Ashtiani K, Hill DE, Blackshaw S, et al. Yeast one-hybrid assays for high-throughput human gene regulatory network mapping. Nature Methods. 2011;8:1050–1052. doi: 10.1038/nmeth.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece-Hoyes JS, Deplancke B, Shingles J, Grove CA, Hope IA, Walhout AJM. A compendium of C. elegans regulatory transcription factors: a resource for mapping transcription regulatory networks. Genome Biol. 2005;6:R110. doi: 10.1186/gb-2005-6-13-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer RJ, Ogawa A. Hormone signaling and phenotypic plasticity in nematode development and evolution. Curr Biol. 2011;21:R758–R766. doi: 10.1016/j.cub.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Pei L, Evans RM. Nuclear receptors: decoding metabolic disease. FEBS Letters. 2008;582:2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphin GL, Kaeberlein M. Measuring Caenorhabditis elegans life span on solid media. Journal of visualized experiments : JoVE. 2009 doi: 10.3791/1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MW, Shapira M. Genetic and molecular analysis of nematode-microbe interactions. Cell Microbiol. 2011;13:497–507. doi: 10.1111/j.1462-5822.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- Tawe WN, Eschbach ML, Walter RD, Henkle-Duhresen K. Identification of stress-responsive genes in Caenorhabditis elegans using RT-PCR differential display. Nucleic Acids Res. 1998;26:1621–1627. doi: 10.1093/nar/26.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Yamamoto KR. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A. 2005a;102:13496–13501. doi: 10.1073/pnas.0506234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gilst MR, Hajivassiliou H, Jolly A, Yamamoto KR. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005b;3:e53. doi: 10.1371/journal.pbio.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E, MacNeil LT, Arda HE, Zhu LJ, Walhout AJM. Integration of metabolic and gene regulatory networks modulates the C. elegans dietary response. 2013 doi: 10.1016/j.cell.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.