Abstract
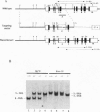
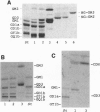
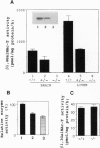
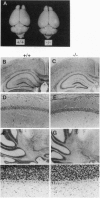
Gangliosides, sialic acid-containing glycosphingolipids, are abundant in the vertebrate (mammalian) nervous system. Their composition is spatially and developmentally regulated, and gangliosides have been widely believed to lay essential roles in establishment of the nervous system, especially in neuritogenesis and synaptogenesis. However, this has never been tested directly. Here we report the generation of mice with a disrupted beta 1,4-N-acetylgalactosaminyltransferase (GM2/GD2 synthase; EC 2.4.1.92) gene. The mice lacked all complex gangliosides. Nevertheless, they did not show any major histological defects in their nervous systems or in gross behavior. Just a slight reduction in the neural conduction velocity from the tibial nerve to the somatosensory cortex, but not to the lumbar spine, was detected. These findings suggest that complex gangliosides are required in neuronal functions but not in the morphogenesis and organogenesis of the brain. The higher levels of GM3 and GD3 expressed in the brains of these mutant mice may be able to compensate for the lack of complex gangliosides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975 Nov;23(4):896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- Cannella M. S., Oderfeld-Nowak B., Gradkowska M., Skup M., Garofalo L., Cuello A. C., Ledeen R. W. Derivatives of ganglioside GM1 as neuronotrophic agents: comparison of in vivo and in vitro effects. Brain Res. 1990 Apr 16;513(2):286–294. doi: 10.1016/0006-8993(90)90469-r. [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Garofalo L., Kenigsberg R. L., Maysinger D. Gangliosides potentiate in vivo and in vitro effects of nerve growth factor on central cholinergic neurons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2056–2060. doi: 10.1073/pnas.86.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaron M., Manev H., Alho H., Bertolino M., Ferret B., Guidotti A., Costa E. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7351–7355. doi: 10.1073/pnas.85.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G., Fabris M., Gorio A. Gangliosides enhance neurite outgrowth in PC12 cells. Brain Res. 1983 Jun;284(2-3):215–221. doi: 10.1016/0165-3806(83)90006-8. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Arita Y., Satomi N., Eisinger M., Lloyd K. O. Tumor necrosis factor enhances GD3 ganglioside expression in cultured human melanocytes. Arch Biochem Biophys. 1990 Aug 15;281(1):70–75. doi: 10.1016/0003-9861(90)90414-t. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Clausen H., Hakomori S., Sakamoto J., Look K., Lundblad A., Mattes M. J., Lloyd K. O. Analysis of the specificity of five murine anti-blood group A monoclonal antibodies, including one that identifies type 3 and type 4 A determinants. Biochemistry. 1985 Dec 17;24(26):7820–7826. doi: 10.1021/bi00347a047. [DOI] [PubMed] [Google Scholar]
- Ganser A. L., Kirschner D. A. Differential expression of gangliosides on the surfaces of myelinated nerve fibers. J Neurosci Res. 1984;12(2-3):245–255. doi: 10.1002/jnr.490120212. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Iuliano B. A., Schmelzer J. D., Thiemann R. L., Low P. A., Rodriguez M. Motor and somatosensory evoked potentials in mice infected with Theiler's murine encephalomyelitis virus. J Neurol Sci. 1994 May;123(1-2):186–194. doi: 10.1016/0022-510x(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Kuribara H., Tadokoro S. Circadian variation in methamphetamine- and apomorphine-induced increase in ambulatory activity in mice. Pharmacol Biochem Behav. 1982 Dec;17(6):1251–1256. doi: 10.1016/0091-3057(82)90129-0. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1992 Jun 15;267(17):12082–12089. [PubMed] [Google Scholar]
- Nussbaumer J. C., Van der Loos H. An electrophysiological and anatomical study of projections to the mouse cortical barrelfield and its surroundings. J Neurophysiol. 1985 Mar;53(3):686–698. doi: 10.1152/jn.1985.53.3.686. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pohlentz G., Klein D., Schwarzmann G., Schmitz D., Sandhoff K. Both GA2, GM2, and GD2 synthases and GM1b, GD1a, and GT1b synthases are single enzymes in Golgi vesicles from rat liver. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7044–7048. doi: 10.1073/pnas.85.19.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez O. A., Gomez R. A., Carrer H. F. Gangliosides improve synaptic transmission in dentate gyrus of hippocampal rat slices. Brain Res. 1990 Jan 8;506(2):291–293. doi: 10.1016/0006-8993(90)91264-h. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Schaeper R. J., Das K. K., Li Z., Basu S. In vitro biosynthesis of GbOse4Cer (globoside) and GM2 ganglioside by the (1-->3) and (1-->4)-N-acetyl beta-D-galactosaminyltransferases from embryonic chicken brain. Solubilization, purification, and characterization of the transferases. Carbohydr Res. 1992 Dec 15;236:227–244. doi: 10.1016/0008-6215(92)85018-u. [DOI] [PubMed] [Google Scholar]
- Schengrund C. L. The role(s) of gangliosides in neural differentiation and repair: a perspective. Brain Res Bull. 1990 Jan;24(1):131–141. doi: 10.1016/0361-9230(90)90297-d. [DOI] [PubMed] [Google Scholar]
- Schneider J. S., Pope A., Simpson K., Taggart J., Smith M. G., DiStefano L. Recovery from experimental parkinsonism in primates with GM1 ganglioside treatment. Science. 1992 May 8;256(5058):843–846. doi: 10.1126/science.1350379. [DOI] [PubMed] [Google Scholar]
- Steigerwald J. C., Basu S., Kaufman B., Roseman S. Sialic acids. Enzymatic synthesis of Tay-Sachs ganglioside. J Biol Chem. 1975 Sep 10;250(17):6727–6734. [PubMed] [Google Scholar]
- Suzaki K. The pattern of mammalian brain gangliosides. 3. Regional and developmental differences. J Neurochem. 1965 Dec;12(12):969–979. doi: 10.1111/j.1471-4159.1965.tb10256.x. [DOI] [PubMed] [Google Scholar]
- Takamiya K., Yamamoto A., Yamashiro S., Furukawa K., Haraguchi M., Okada M., Ikeda T., Shiku H., Furukawa K. T cell receptor-mediated stimulation of mouse thymocytes induces up-regulation of the GM2/GD2 synthase gene. FEBS Lett. 1995 Jan 16;358(1):79–83. doi: 10.1016/0014-5793(94)01395-h. [DOI] [PubMed] [Google Scholar]
- Thampoe I. J., Furukawa K., Vellvé E., Lloyd K. O. Sialyltransferase levels and ganglioside expression in melanoma and other cultured human cancer cells. Cancer Res. 1989 Nov 15;49(22):6258–6264. [PubMed] [Google Scholar]
- Yagi T., Nada S., Watanabe N., Tamemoto H., Kohmura N., Ikawa Y., Aizawa S. A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal Biochem. 1993 Oct;214(1):77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Haraguchi M., Yamashiro S., Fukumoto S., Furukawa K., Takamiya K., Atsuta M., Shiku H., Furukawa K. Heterogeneity in the expression pattern of two ganglioside synthase genes during mouse brain development. J Neurochem. 1996 Jan;66(1):26–34. doi: 10.1046/j.1471-4159.1996.66010026.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Yamashiro S., Takamiya K., Atsuta M., Shiku H., Furukawa K. Diverse expression of beta 1,4-N-acetylgalactosaminyltransferase gene in the adult mouse brain. J Neurochem. 1995 Dec;65(6):2417–2424. doi: 10.1046/j.1471-4159.1995.65062417.x. [DOI] [PubMed] [Google Scholar]
- Yamashiro S., Ruan S., Furukawa K., Tai T., Lloyd K. O., Shiku H., Furukawa K. Genetic and enzymatic basis for the differential expression of GM2 and GD2 gangliosides in human cancer cell lines. Cancer Res. 1993 Nov 15;53(22):5395–5400. [PubMed] [Google Scholar]
- Yu R. K., Iqbal K. Sialosylgalactosyl ceramide as a specific marker for human myelin and oligodendroglial perikarya: gangliosides of human myelin, oligodendroglia and neurons. J Neurochem. 1979 Feb;32(2):293–300. doi: 10.1111/j.1471-4159.1979.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Yu R. K., Macala L. J., Taki T., Weinfield H. M., Yu F. S. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988 Jun;50(6):1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- de Erausquin G. A., Manev H., Guidotti A., Costa E., Brooker G. Gangliosides normalize distorted single-cell intracellular free Ca2+ dynamics after toxic doses of glutamate in cerebellar granule cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8017–8021. doi: 10.1073/pnas.87.20.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]