Abstract
Purpose
To evaluate cMET and phospho-cMET (p-cMET) levels in breast cancer subtypes and its impact on survival outcomes.
Experimental Design
We measured protein levels of cMET and p-cMET in 257 breast cancers using reverse phase protein array. Regression tree method and Martingale residual plots were applied to find best cutoff point for high and low levels. Kaplan-Meier survival curves were used to estimate relapse-free (RFS) and overall (OS) survival. Cox proportional hazards models were fit to determine associations of cMET/p-cMET with outcomes after adjustment for other characteristics.
Results
Median age was 51years. There were 140 (54.5%) hormone receptor (HR)-positive, 53 (20.6%) HER2-positive and 64 (24.9%) triple-negative tumors. Using selected cutoffs, 181 (70.4%) and 123 (47.9%) cancers had high levels of cMET and p-cMET, respectively. There were no significant differences in mean expression of cMET (P<0.128) and p-cMET (P<0.088) by breast cancer subtype. Dichotomized cMET and p-cMET level was a significant prognostic factor for RFS (HR:2.44,95%CI:1.34-4.44,P=0.003 and HR:1.64,95%CI:1.04-2.60,P=0.033) and OS (HR:3.18,95%CI:1.43-7.11,P=0.003 and HR:1.92,95% CI:1.08-3.44,P=0.025). Within breast cancer subtypes, high cMET levels were associated with worse RFS (P=0.014) and OS (P=0.006) in HR-positive tumors, and high p-cMET levels were associated with worse RFS (P=0.019) and OS (P=0.014) in HER2-positive breast cancers. In multivariable analysis patients with high cMET had a significantly higher risk of recurrence (HR:2.06; 95%CI:1.08-3.94,P=0.028) and death (HR:2.81; 95%CI:1.19-6.64,P=0.019). High p-cMET level was associated with higher risk of recurrence (HR:1.79,95%CI 1.08-2.95.77,P=0.020).
Conclusions
High levels of cMET and p-cMET were seen in all breast cancer subtypes and correlated with poor prognosis.
Keywords: cMET, phospho-cMET, breast cancer prognosis, breast cancer subtype
Introduction
The proto-oncogene cMET (mesenchymal-epithelial transition factor gene) on chromosome 7q31 encodes a receptor tyrosine kinase (RTK) which acts as the receptor for hepatocyte growth factor (HGF) or scatter factor (SF) (1-3). This RTK is a single-pass type I transmembrane heterodimer protein made of a disulfide bridge that links an α-chain (50 kDa) and a β-chain (145 kDa) (4). The β-chain in cMET traverses the cell membrane and contains the cytoplasmic kinase domain and a docking site, comprised of tyrosine residues Y1349 and Y1356 (5). HGF binding to the extracellular domain activates the intrinsic kinase activity which phosphorylates the tyrosines at the carboxy-terminal docking site. Phosphorylated cMET (phospho-cMET or p-cMET) binds Grb2 (Growth factor receptor-bound protein 2) and Gab1 (GRB2-associated-binding protein 1) and activates downstream signaling molecules such as Phosphatidylinositol 3-kinase (PI3K/AKT) and Mitogen-activated protein kinase (ERK/MAPK) pathways (6, 7). Through these cellular signaling pathways HGF/cMET interaction plays a key role in cellular proliferation, survival, migration and invasion (8-10). The HGF/cMET axis contributes a critical physiological function in embryogenesis, angiogenesis and wound healing (11, 12).
Multiple germline and somatic mutations in tumors result in anomalous signaling via diverse mechanisms such as activating mutations of cMET gene and over-expression of HGF/SF or cMET (13-15). The HGF/cMET signaling cascade has been repeatedly shown to be dysregulated in a variety of tumors such as lung, kidney, head & neck and colorectal cancers (16-17). Increased HGF/cMET signaling in these tumors correlates with poor outcomes (18-20). Additionally, p-cMET has also been shown to be an important predictor of tumor aggressiveness, metastatic potential and poor survival (21, 22). In summary, aberrant HGF/cMET activation results in an aggressive phenotype and is associated with tumor progression and poor outcomes. The key role as a mitogenic, motogenic and angiogenic molecule makes HGF/cMET inhibition an attractive therapeutic strategy in cancer (23, 24).
Overexpression of cMET has been shown to contribute to the development of invasive phenotype during progression of breast cancer in vivo and in vitro (25). In addition, Lindemann et al. showed that differential expression of cMET between tumor and adjacent normal tissue was associated with aggressive ductal carcinoma in situ (DCIS) phenotype (26). Prior studies have shown that protein tyrosine phosphorylation profiling of basal-like breast carcinomas is characterized by elevated tyrosine phosphorylation of cMET (27, 28). Hence, the HGF/cMET axis seems to play a significant role in tumor progression in breast cancer (29).
We performed an analysis of primary breast cancer specimens to evaluate the protein levels of total cMET and p-cMET by breast cancer subtype using reverse phase protein arrays (RPPA), and their correlation with patient outcome.
Materials and Methods
Patients and tumor samples
Fine needle aspirates from 257 primary invasive breast cancers were obtained and snap frozen. All specimens were collected under Institutional Review Board (IRB)-approved protocols. The breast tumors were classified into three clinically relevant subtypes defined by immunohistochemistry (IHC) for estrogen receptor (ER) and progesterone receptor (PR) status and by IHC or fluorescent in situ hybridization (FISH) for HER2 status as per American Society of Clinical Oncology and College of American Pathologists (ASCO/CAP) guidelines (30). These subtypes were defined by the dominant traditional prognostic molecular marker (ER, PR and HER2). Hormone receptor-positive (HR-positive) tumors were ER-positive and/or PR-positive and HER2-negative. Similarly, HER2-positive group included all HER2 positive tumors irrespective of hormone receptor status. Triple negative subtype included all cases that were ER/PR and HER2 negative.
The samples used for the study were archived samples from previously collected tumor specimens from patient treated at M D Anderson Cancer Center between 1986 and 2007. The corresponding clinical data was obtained from the Breast Cancer Management System database at MD Anderson Cancer Center. Missing information from the database was collected by chart review.
Reverse phase protein lysate microarray (RPPA)
Protein was extracted from the human tumors and RPPA was performed in our laboratory as described previously (31). Briefly, lysis buffer was used to lyse frozen tumors by homogenization. Tumor lysates were normalized to 1 μg/μL concentration using bicinchoninic acid assay and boiled with 1% SDS, and the supernatants were manually diluted in six or eight 2-fold serial dilutions with lysis buffer. An Aushon Biosystems (Burlington, MA) 2470 arrayer created 1,056 sample arrays on nitrocellulose-coated FAST slides (Schleicher & Schuell BioScience, Inc.) from the serial dilutions. A slide was then probed with validated primary cMET and p-cMET antibodies (Cell Signaling Technology, Danvers, MA) and the signal was amplified using a DakoCytomation–catalyzed system. The antibodies for cMET (Mouse) and p-cMET (Rabbit, Y1235) were used at a dilution of 1:250 for RPPA. A secondary antibody was used as a starting point for amplification. The slides were scanned, analyzed, and quantitated using Microvigene software (VigeneTech Inc.) to generate serial dilution–signal intensity curves for each sample with the logistic fit model: ln(y) = a + (b − a) / (1 + exp {c*[d − ln(x)]}). A representative natural logarithmic value of each sample curve on the slide (curve average) was then used as a relative quantification of the amount of each protein in each sample. The level of cMET and p-cMET in each sample was expressed as a log mean centered value after correction for protein loading using the average expression levels of over 150 proteins as previously described (31).
Statistical Methods
Boxplots were generated for original and log2 transformed expressions of total cMET and p-cMET by breast cancer subtypes. The original expressions were right-skewed but the log2 transformation data was normally distributed. Hence, all following statistical analyses were based on the log2 transformation of the original expression values. P values less than 0.05 were considered statistically significant and all tests were two-sided. Statistical analyses were done with R statistical software version 2.12.0 (R Development Core Team, 2010, Vienna, Austria).
Mean and standard deviations were generated for total cMET and p-cMET by tumor subtypes. Linear regression models were used to determine if the mean total cMET and p-cMET expression was different by tumor subtypes. Martingale residual plots with lowess smooth for Cox’s model for total cMET and p-cMET separately as covariate by tumor subtypes suggested a non-linear effect of total and p-cMET. A regression tree method was applied to find the best cutoff point for total cMET and p-cMET expression. Combining the results from martingale residual plots and regression trees, total cMET expression was divided into high level (>0) expression and low level (≤0) expression. Similarly, p-cMET was divided as high level (>0.35) and low level (≤0.35). Patient and tumor characteristics including age, stage, grade and subtype were tabulated between high and low level expressions of total cMET and p-cMET individually. Groups were then compared with the Chi-square tests (32).
Overall survival (OS) and corresponding censoring were computed in months from diagnosis to death for each patient. Relapse-free survival (RFS) was regarded as the time to first relapse after diagnosis. Median RFS and OS were estimated nonparametrically with the use of Kaplan-Meier curves by patient characteristics and levels of total cMET and p-cMET expression and compared by the log-rank statistic. Log-rank tests were used to evaluate the hazard ratio by total cMET and p-cMET expression levels among all patients and patients within each subtype. Cox proportional hazards models were fit to determine the association of cMET and p-cMET levels with the risk of recurrence and death after adjustment for other patient and disease characteristics.
Results
Median patient age was 51 years (range 23-85 years). There were a total of 140 (54.5%) hormone receptor (HR)-positive tumors, 53 (20.6%) HER2-positive tumors and 64 (24.9%) triple receptor-negative (TN) tumors. Using the selected cutoffs, a total of 181 (70.4%) and 123 (47.9%) patients had high expression of cMET and p-cMET, respectively.
Patient and clinic characteristics by levels of total cMET and p-cMET are summarized in table 1. There was no statistically significant difference in clinical or pathologic parameters in patients with high or low level of total cMET. Patients with high p-cMET expression tended to be older (Age > 50: 60.2% vs. 41.0%, P = .003) and had fewer high grade tumors (Grade III: 60% vs. 72.2% P = .046).
Table 1. Patient and Clinical Characteristics by total cMET and phospho-cMET levels.
| Overall (N=257) |
cMET | p-cMET | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High cMET (>0) (N=181) |
Low cMET (≤0) (N=76) |
High p-cMET (>0.35) (N=123) |
Low p-cMET (≤0.35) (N=134) |
|||||||||
| N | % | N | % | N | % | P | N | % | N | % | P | |
| Age | ||||||||||||
| ≤ 50 | 128 | 49.8 | 83 | 45.9 | 45 | 59.2 | 0.069 | 49 | 39.8 | 79 | 59.0 | 0.003 |
| >50 | 129 | 50.2 | 98 | 54.1 | 31 | 40.8 | 74 | 60.2 | 55 | 41.0 | ||
| Stage | ||||||||||||
| I | 8 | 3.1 | 4 | 2.2 | 4 | 5.3 | 0.426 | 2 | 1.6 | 6 | 4.5 | 0.391 |
| II | 141 | 55.3 | 43 | 56.4 | 40 | 52.6 | 67 | 54.9 | 74 | 55.6 | ||
| III | 106 | 41.6 | 39 | 41.3 | 32 | 42.1 | 53 | 43.4 | 53 | 39.8 | ||
| Grade | ||||||||||||
| 1 | 12 | 4.7 | 9 | 5.1 | 3 | 4.0 | 0.473 | 9 | 7.5 | 3 | 2.3 | 0.046 |
| 2 | 73 | 28.9 | 55 | 30.9 | 18 | 24.0 | 39 | 32.5 | 34 | 25.6 | ||
| 3 | 168 | 66.4 | 114 | 64.0 | 54 | 72.0 | 72 | 60.0 | 96 | 72.2 | ||
| Chemotherapy | ||||||||||||
| Anthracycline based | 17 | 6.7 | 11 | 6.2 | 6 | 7.9 | 0.228 | 10 | 8.3 | 7 | 5.3 | 0.214 |
| Taxane based | 21 | 8.3 | 16 | 16.9 | 5 | 6.6 | 10 | 8.3 | 11 | 8.3 | ||
| Anthracycline & Taxane | 180 | 70.9 | 121 | 68.1 | 59 | 77.6 | 79 | 65.3 | 101 | 75.9 | ||
| No Chemotherapy | 36 | 14.1 | 30 | 16.8 | 6 | 7.9 | 22 | 18.2 | 14 | 10.5 | ||
| Hormone Therapy | ||||||||||||
| Yes | 107 | 40.5 | 70 | 38.7 | 37 | 48.7 | 0.178 | 59 | 48.0 | 48 | 35.8 | 0.065 |
| No | 150 | 59.5 | 111 | 61.3 | 39 | 51.3 | 64 | 52.0 | 86 | 64.2 | ||
| Radiation Therapy | ||||||||||||
| Yes | 164 | 63.8 | 108 | 59.7 | 56 | 73.7 | 0.046 | 79 | 64.2 | 85 | 63.4 | 0.998 |
| No | 93 | 36.2 | 73 | 40.3 | 20 | 26.3 | 44 | 35.8 | 49 | 36.6 | ||
| Subtype | ||||||||||||
| HER2-positive | 53 | 20.6 | 40 | 22.1 | 13 | 17.1 | 0.079 | 25 | 20.3 | 28 | 20.9 | 0.064 |
| HR-positive | 140 | 54.5 | 103 | 56.9 | 37 | 48.7 | 75 | 61.0 | 65 | 48.5 | ||
| Triple Negative | 64 | 24.9 | 38 | 31.0 | 26 | 34.2 | 23 | 18.7 | 41 | 30.6 | ||
cMET: Total cMET, p-cMET: Phospho-cMET, HR-positive: Hormone receptor positive
No significant differences in mean levels of total cMET expressions (P = 0.128) and p-cMET expressions (P = 0.088) were seen between different tumor subtypes, as seen in table 2. At a median follow up of 42.23 months (5.17-277.77 months), there were 76 (30%) relapses and 50 (20%) deaths.
Table 2. Total cMET and phospho-cMET expressions by tumor subtype.
| Total cMET | Phospho-cMET | ||||||
|---|---|---|---|---|---|---|---|
| Subtype | N | Mean | SD | F-test P Value |
Mean | SD | F-test P Value |
| HER2-positive | 53 | 0.355 | 0.0799 | 0.128 | 0.180 | 0.1027 | 0.088 |
| Hormone receptor-positive | 140 | 0.391 | 0.0541 | 0.257 | 0.0651 | ||
| Triple receptor-negative | 64 | 0.192 | 0.0904 | 0.001 | 0.0990 | ||
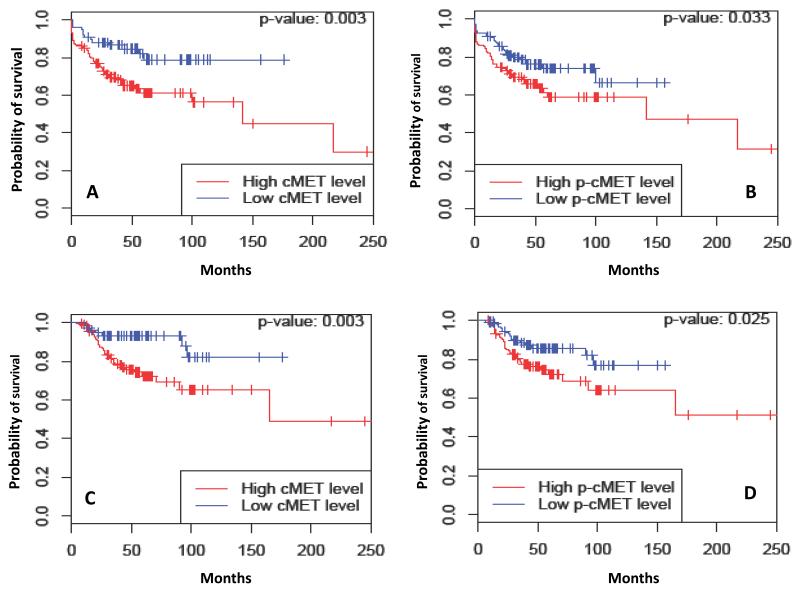
Table 3 summarizes the median RFS estimates by c-MET and p-cMET expression and by tumor subtypes. Dichotomized total cMET expression (cutoff point 0) was a significant prognostic factor for RFS (HR: 2.44, 95% CI: 1.34-4.44, P = 0.003). Estimated 5-year RFS rates were 61.3% (95% CI: 53.2%-70.7%) and 78.9% (95% CI: 68.6%-90.8%) for patients with high cMET and low cMET level, respectively (P = 0.003). Likewise, dichotomized p-cMET expression (cutoff point 0.35) was also a significant prognostic factor for RFS (HR: 1.64, 95% CI: 1.04-2.60, P = 0.033) and estimated 5-year RFS rates for patients with high p-cMET and low p-cMET levels were 58.9% (95% CI: 49.1%-70.7%) and 73.8% (95% CI: 65.6%-83.1%), respectively (P = 0.033). Total cMET was also a significant predictor of RFS within the HR-positive subtype (HR: 3.44, 95% CI: 1.21-9.81, P = 0.014). In contrast, p-cMET was a significant predictor of RFS within the HER2-positive subtype (HR: 3.02, 95% CI: 1.15-7.96, P = 0.019). The Kaplan-Meier survival curves for RFS for all patients and by breast tumor subtypes are as shown in figure 1. Although, there was a trend towards worse RFS with high cMET levels (HR 2.36; 95% CI: 0.86-6.51) in triple-negative subtype, this did not reach statistical significance (P = 0.086).
Table 3. Relapse-Free Survival (RFS) and Overall Survival (OS) by total cMET and phospho-cMET levels and breast cancer subtype.
| Relapse-Free Survival (RFS) | Overall Survival (OS) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Subtypes | Protein Level | Patients | Events | 5-year RFS (%) |
95% CI | P | Events | 5-year OS (%) |
95% CI | P | |
| Overall | cMET | High | 181 | 63 | 61.3 | 53.2-70.7 | 0.003 | 43 | 72.4 | 64.7-81.0 | 0.003 |
| Low | 76 | 13 | 78.9 | 68.6-90.8 | 7 | 93.3 | 87.8-99.2 | ||||
| p-cMET | High | 123 | 45 | 58.9 | 49.1-70.7 | 0.033 | 32 | 72.4 | 63.7-82.3 | 0.025 | |
| Low | 134 | 31 | 73.8 | 65.6-83.1 | 18 | 85.8 | 79.4-92.7 | ||||
| Hormone receptor-positive |
cMET | High | 103 | 31 | 65.4 | 54.7-78.2 | 0.014 | 17 | 82.8 | 75.0-91.3 | 0.006 |
| Low | 37 | 4 | 85.3 | 78.2-100 | 1 | 100 | NA-NA | ||||
| p-cMET | High | 75 | 21 | 67.6 | 55.6-82.2 | 0.519 | 11 | 85.2 | 77.0-94.2 | 0.519 | |
| Low | 65 | 14 | 75.1 | 63.3-89.2 | 7 | 90.4 | 82.6-99.0 | ||||
| HER2-positive | cMET | High | 40 | 17 | 55.5 | 40.8-75.4 | 0.530 | 13 | 59.4 | 41.4-85.2 | 0.138 |
| Low | 13 | 4 | 0.00 | NA-NA | 1 | 100 | NA-NA | ||||
| p-cMET | High | 25 | 15 | 36.5 | 19.5-68.3 | 0.019 | 12 | 52.3 | 33.3-82.2 | 0.014 | |
| Low | 28 | 6 | 74.1 | 57.4-95.5 | 2 | 91.7 | 81.3-100 | ||||
| Triple receptor-negative |
cMET | High | 38 | 15 | 59.5 | 45.5-77.7 | 0.086 | 13 | 56.1 | 38.0-82.7 | 0.187 |
| Low | 26 | 5 | 80.4 | 66.4-97.4 | 5 | 66.2 | 66.2-97.4 | ||||
| p-cMET | High | 23 | 9 | 60.9 | 43.9-84.5 | 0.251 | 9 | 58.8 | 41.1-84.1 | 0.128 | |
| Low | 41 | 11 | 72.3 | 59.6-87.7 | 9 | 74.2 | 60.5-91.0 | ||||
CI: Confidence Interval, cMET: Total cMET, p-cMET: Phospho-cMET
Figure 1.
Kaplan-Meier estimates illustrating the Relapse free survival (RFS) of patients by (A) total cMET and (B) p-cMET expression levels and Overall survival (OS) of patients by (C) total cMET and (D) p-cMET expression levels. High: total cMET > 0, Low: total cMET ≤ 0. High: p-cMET > 0.35, Low: p-cMET ≤ 0.35.
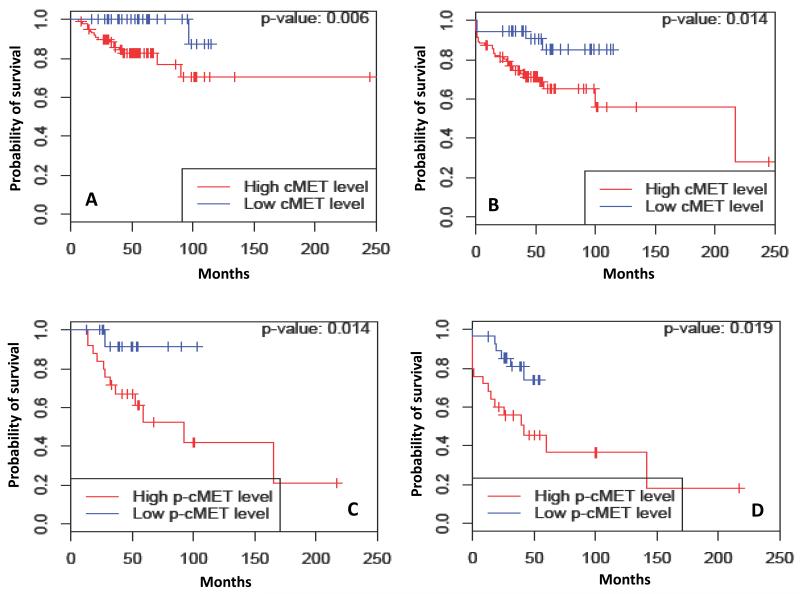
Table 3 summarizes the median OS estimates by c-MET and p-cMET expression and by tumor subtypes. At the time of analysis, 207 of the 257 patients (80.5%) were still alive. As was seen with RFS analysis, dichotomized cMET level was a significant prognostic factor of OS (HR: 3.18, 95% CI: 1.43-7.11, P = 0.003). Estimated 5-year OS rates were 72.4% (95% CI: 64.7%-81.0%) and 93.3% (95% CI: 87.8%-99.2%) for patients with high cMET and low cMET levels, respectively (P = 0.003). Dichotomized p-cMET level was a significant prognostic factor of OS (HR: 1.92, 95% CI: 1.08-3.44, P = 0.025). The estimated 5-year OS rates for patients with high p-cMET and low p-cMET levels were 72.4% (95% CI: 63.7%-82.3%) and 85.8% (95% CI: 79.4%-92.7%), respectively (P = 0.025). With regards to breast cancer subtypes, total cMET (HR: 8.28, 95% CI: 1.10-62.59, P = 0.006) and p-cMET (HR: 5.49, 95% CI: 1.20-25.10, P = 0.014) were significant predictor of OS within HR-positive tumors and HER2-positive tumors, respectively. The Kaplan-Meier survival curves for OS for all patients and by subtypes are as shown in figure 2. Although, there was a trend towards worse OS with high p-cMET levels (HR 2.02; 95% CI: 0.80-5.13) in triple-negative subtype, this did not reach statistical significance (P = 0.128).
Figure 2.
Kaplan-Meier estimates illustrating (A) Overall survival (OS) and (B) Relapse free survival (RFS) of patients by total cMET in Hormone receptor positive breast cancer. Kaplan-Meier estimates illustrating (C) Overall survival (OS) and (D) Relapse free survival (RFS) of patients by p-cMET in HER2 positive breast cancer. High: total cMET > 0, Low: total cMET ≤ 0. High: p-cMET > 0.35, Low: p-cMET ≤ 0.35.
Multivariable models for RFS and OS are summarized on table 4. After adjustment for patient factors, tumor characteristics and treatment, patients with tumors expressing high levels of cMET had a significant higher risk of recurrence (HR 2.06; 95% CI: 1.08-3.94; P = 0.028) and death (HR 2.81; 95% CI: 1.19-6.64; P = 0.019) compared to patients with low cMET levels. Also, patients with tumors expressing high levels of p-cMET had a significant higher risk of recurrence (HR 1.79; 95% C: 1.08-2.95; P = 0.020) compared to patients with high p-cMET levels.
Table 4. Multivariable Cox Proportional Hazards Model for Relapse Free Survival (RFS) and Overall Survival (OS) relative to total cMET and phospho-cMET expression.
| cMET |
p-cMET |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RFS | OS | RFS | OS | ||||||||||
|
| |||||||||||||
| Model | HR | 95% CI | P | HR | 95% CI | P | Model | HR | 95% CI | P | HR | 95% CI | P |
|
| |||||||||||||
| cMET: High vs. Low | 2.06 | 1.08-3.94 | 0.028 | 2.81 | 1.19-6.64 | 0.019 | p-cMET: High vs. Low | 1.79 | 1.08-2.95 | 0.020 | 1.84 | 0.97-3.48 | 0.062 |
| Subtype: HER2+ vs. HR+ | 1.03 | 0.54-1.98 | 0.925 | 1.24 | 0.53-2.90 | 0.621 | Subtype: HER2+ vs. HR+ | 1.00 | 0.52-1.92 | 0.999 | 1.11 | 0.48-2.60 | 0.803 |
| Subtype: TN vs. HR+ | 0.65 | 0.34-1.25 | 0.201 | 2.00 | 0.87-4.58 | 0.103 | Subtype: TN vs. HR+ | 0.59 | 0.31-1.14 | 0.115 | 1.62 | 0.71-3.73 | 0.249 |
| Age: >50 vs. ≤50 | 0.79 | 0.48-1.28 | 0.339 | 0.97 | 0.53-1.77 | 0.924 | Age: >50 vs. ≤50 | 0.83 | 0.51-1.34 | 0.441 | 1.06 | 0.58-1.93 | 0.848 |
| Chemotherapy: A vs. N | 0.06 | 0.01-0.25 | 0.0001 | 0.20 | 0.03-1.07 | 0.061 | Chemotherapy: A vs. N | 0.05 | 0.01-0.23 | 0.0001 | 0.15 | 0.03-0.78 | 0.024 |
| Chemotherapy: T vs. N | 0.13 | 0.05-0.33 | <0.0001 | 0.26 | 0.08-0.82 | 0.025 | Chemotherapy: T vs. N | 0.13 | 0.53-0.34 | <0.0001 | 0.28 | 0.08-0.89 | 0.032 |
| Chemotherapy: B vs. N | 0.10 | 0.05-0.18 | <0.0001 | 0.28 | 0.13-0.63 | 0.002 | Chemotherapy: B vs. N | 0.10 | 0.56-0.19 | <0.0001 | 0.26 | 0.13-0.54 | 0.0003 |
| Hormonal Therapy: Y vs. N | 0.21 | 0.10-0.43 | 0.0001 | 0.31 | 0.11-0.84 | 0.022 | Hormonal Therapy: Y vs. N | 0.16 | 0.08-0.33 | <0.0001 | 0.23 | 0.08-0.63 | 0.004 |
| Radiation Therapy: Y vs. N | 0.91 | 0.53-1.56 | 0.740 | 0.80 | 0.41-1.57 | 0.523 | Radiation Therapy: Y vs. N | 0.84 | 0.49-1.45 | 0.528 | 0.81 | 0.41-1.59 | 0.545 |
| Grade: I, II vs. III | 0.84 | 0.48-1.50 | 0.419 | 0.72 | 0.33-1.53 | 0.390 | Grade: I, II vs. III | 0.94 | 0.52-1.67 | 0.819 | 0.77 | 0.36-1.68 | 0.514 |
| Clinical Stage: I, II vs. III | 0.37 | 0.22-0.62 | 0.0002 | 0.67 | 0.33-1.34 | 0.243 | Clinical Stage: I, II vs. III | 0.40 | 0.24-0.69 | 0.0009 | 0.77 | 0.38-1.55 | 0.458 |
RFS: Relapse Free Survival, OS: Overall Survival, cMET: total cMET, p-cMET: phospho-cMET, HR: Hazard ratio, HR+: Hormone receptor positive, TN: Triple Negative, A: Anthracycline based, B: Both Anthracycline & Taxane, T: Taxane based
To evaluate whether cMET confers radioresistance, we performed an exploratory sub-group analysis among 164 patients who received radiation therapy. Dichotomized total cMET level was a significant prognostic factor for both RFS (HR 3.37; 95% CI: 1.50-7.57, P = 0.002) and OS (HR 4.03; 95% CI: 1.39-11.67, P = 0.006) for patients who received radiation therapy. Similarly, dichotomized p-cMET was a significant prognostic factor for RFS (HR 2.07; 95% CI: 1.12-3.84, P = 0.017) and OS (HR 2.25; 95% CI: 1.05-4.85, P = 0.033) in this group. In contrast, among 93 patients who did not receive radiation therapy, total cMET and p-cMET were not significant prognostic factors for either RFS or OS.
Discussion
The cMET proto-oncogene encodes a RTK which binds with high affinity to its ligand HGF/SF (1). This receptor-ligand interaction leads to formation of phosphorylated cMET which activates downstream effectors such as PI3K (Phosphatidylinositol 3 kinase)-AKT, PLC γ (Phospholipase C γ), RAS-MAPK (mitogen-activated protein kinase), c-Src and STATs (signal transducer and activator of transcription) (4, 5). Together this cascade contributes to carcinogenesis and angiogenesis in a wide variety of human malignancies including cervical, endometrial, gastric, kidney, liver, sarcoma, lung, colorectal and breast (4).
In this analysis of 257 breast cancer samples we demonstrated that increased levels of total cMET and p-cMET using RPPA are seen in nearly 70% and 50% of breast cancers, respectively. We also show that there is no significant difference in the levels of total cMET and p-cMET among different breast cancer subtypes. Survival analysis reveals that total cMET and p-cMET levels are significant prognostic factors for both RFS and OS. Analysis of survival outcomes among various tumor subtypes show that high cMET levels is a poor prognostic factor for hormone receptor-positive breast cancer and high p-cMET levels is a poor prognostic factor for HER2-positive breast cancers.
A number of studies have shown correlation of cMET expression with progression, aggressive behavior and poor survival outcomes in breast cancers (33-36). However, no study, to the best of our knowledge, has yet investigated the significance of differential expression of cMET and p-cMET in the different breast cancer subtypes (HR-positive, HER2-positive and TNBC. Also, there are no studies reporting p-cMET levels as a prognostic factor in breast cancer. Previous work studying this receptor has been performed using enzyme-linked immunosorbent assay (ELISA), immunoperoxidase (IPO), immunohistochemistry (IHC) and Immunofluorescence (IF) techniques; however, cMET expression has not been evaluated to date using RPPA. Recent data from our laboratory has demonstrated significant correlations between RPPA and IHC in snap frozen primary breast tumors and has established reliability of RPPA in functional proteomic “fingerprinting” (31). Compared to IHC or ELISA, RPPA is more sensitive, reduces variability and avoids observer dependency (37). Applying RPPA analysis for molecular targets promotes practical development for clinical application since it allows for a cost-effective, quick, precise, reliable and reproducible quantification of protein expression and phosphorylation states in multiple samples simultaneously and requires only limited clinical material (38).
Both HGF expression and cMET expression have been shown to correlate with poor prognosis in breast cancer. These clinical outcomes are a consequence of multiple phenotypic properties that are imparted to tumor cells by HGF/cMET activation. HGF/cMET signaling enhances the transition from pre-invasive DCIS to invasive carcinoma (39). The pathway also promotes cell motility and angiogenesis (11, 40). HGF-dependent β-catenin stabilization leads to the establishment of bone metastasis in breast cancers (41). HGF/cMET receptor signals synergize with HER2 and promote breakdown of cell-cell junctions and enhance cell invasiveness (42). This cross talk is possibly responsible for poor prognosis seen in HER2-positive breast cancers with high levels of p-cMET (RFS: P = 0.019 and OS: P = 0.014).
Development of therapy resistance is a major cause of treatment failure in breast cancer. In vitro studies have shown that HGF/SF/cMET signaling pathway can confer resistance against induction of apoptosis by various DNA damaging-agents (radiation and cytotoxic agents such as anthracyclines and taxanes) (43). Moreover, the pathway is also involved in promoting cell survival by enhancing DNA repair (44). Studies also suggest overexpression of the HGF/SF and cMET contributes to resistance, both inherent and treatment-acquired, to endocrine therapy and to trastuzumab treatment (45, 46). Radiotherapy, anthracyclines, taxanes, endocrine therapy and trastuzumab form the backbone of breast cancer therapy. The anti-apoptotic prosurvival effect of the HGF/SF/cMET signaling pathway makes MET inhibition a potential therapeutic target for breast cancer that are resistant and refractory to conventional therapies. Since cMET participates in acquisition of resistance, a subset of breast cancers may benefit from combination of MET inhibitors as first line therapy with traditional treatments.
Recent literature has shown that MET expression confers radioresistance in cancer cells (47). De Bacco et al. demonstrated that human breast cancer cell lines (MDA-MB-231 and MDA-MB-435S) subjected to therapeutic doses of ionizing radiation (IR) showed increased MET expression, ligand-independent MET phosphorylation/signal transduction and promoted cell invasion and survival (47). Further, MET silencing by siRNA or treatment with kinase inhibitors counteracted these effects. Expression of cMET in oropharyngeal squamous cell carcinomas treated with radiotherapy has been shown to correlate with decreased rates of complete remission, shorter disease-free survival and overall survival (47). This data suggests that targeting MET may increase radiosensitivity of tumor cells and is an attractive target for radiosensitization.
Pre clinical data suggests that cMET inhibition in tumor cells impairs cell proliferation, survival, motility, invasion and angiogenesis (48, 49). Several small molecule cMET kinase inhibitors and antibodies against HGF and against cMET are in various stages of development as potential cancer therapies (24, 25, 50). Targeted therapy for breast cancers with pre-selection based on overexpression of cMET and p-cMET with MET inhibition needs further exploration after adequate optimization of predictive markers. Levels of total cMET and p-cMET are uniformly elevated in all breast cancers subtypes and were found to be significant prognostic factors for both RFS and OS. The predictive potential of cMET however cannot be reliably assessed from this retrospective analysis and needs evaluation in clinical trials of targeted therapy against cMET. cMET inhibition is a promising new strategy for treatment of breast cancers and deserves further assessment.
Statement of Translational Relevance.
The proto-oncogene cMET (mesenchymal-epithelial transition factor gene) encodes a tyrosine-kinase membrane receptor, which acts as a ligand for hepatocyte growth factor (HGF). Dysregulation of cMET and its phosphorylated form, phospho-cMET (p-cMET) have been implicated in tumor proliferation, survival, angiogenesis and migration. High expression of cMET has been shown to correlate with adverse survival outcomes in various tumor types and confers chemo-resistance and radio-resistance. The HGF/cMET axis also appears to play a key role in tumor progression in breast cancers. Clarifying the prognostic implication and differential impact of cMET expression on survival in breast cancer subtypes is a necessary first step to application of targeted therapy against cMET in breast cancers. We found that high levels of cMET and p-cMET were seen in all breast cancer subtypes and correlated with poor prognosis. Inhibition of the cMET axis is a promising new therapeutic strategy and needs further investigation.
Acknowledgments
Funding: This work was supported in part by the Kleberg Center for Molecular Markers at MD Anderson Cancer Center, NCI 1K23CA121994-01 (A.M.G.), The Susan G. Komen Foundation FAS0703849 (A. M. G., G. B. M.), and SAC100004 (A.M.G., G.R.B.) and The University of Texas MD Anderson’s Cancer Center Support Grant CCSG 2P30 CA016672.
References
- 1.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, Park M, Le Beau MM, Robins TS, Diaz MO, Rowley JD, et al. The human met oncogene is related to the tyrosine kinase oncogenes. Nature. 1985;318:385–8. doi: 10.1038/318385a0. [DOI] [PubMed] [Google Scholar]
- 3.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 4.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 5.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–71. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 6.Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–6. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 7.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001;98:247–52. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol. 1995;131:1573–86. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–42. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 10.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–41. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–5. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 13.Ma PC, Tretiakova MS, MacKinnon AC, Ramnath N, Johnson C, Dietrich S, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer. 2008;47:1025–37. doi: 10.1002/gcc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4:5–11. doi: 10.1097/JTO.0b013e3181913e0e. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt L, Duh FM, Chen F, Kishida T, Glenn G, Choyke P, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 16.Marshall DD, Kornberg LJ. Overexpression of scatter factor and its receptor (c-met) in oral squamous cell carcinoma. Laryngoscope. 1998;108:1413–7. doi: 10.1097/00005537-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Olivero M, Rizzo M, Madeddu R, Casadio C, Pennacchietti S, Nicotra MR, et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer. 1996;74:1862–8. doi: 10.1038/bjc.1996.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichimura E, Maeshima A, Nakajima T, Nakamura T. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn J Cancer Res. 1996;87:1063–9. doi: 10.1111/j.1349-7006.1996.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammula US, Kuntz EJ, Francone TD, Zeng Z, Shia J, Landmann RG, et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007;248:219–28. doi: 10.1016/j.canlet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Lo Muzio L, Farina A, Rubini C, Coccia E, Capogreco M, Colella G, et al. Effect of c-Met expression on survival in head and neck squamous cell carcinoma. Tumour Biol. 2006;27:115–21. doi: 10.1159/000092716. [DOI] [PubMed] [Google Scholar]
- 21.Miyata Y, Kanetake H, Kanda S. Presence of phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin Cancer Res. 2006;12:4876–81. doi: 10.1158/1078-0432.CCR-06-0362. [DOI] [PubMed] [Google Scholar]
- 22.Miyata Y, Sagara Y, Kanda S, Hayashi T, Kanetake H. Phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor growth and prognosis in patients with bladder cancer: correlation with matrix metalloproteinase-2 and -7 and E-cadherin. Hum Pathol. 2009;40:496–504. doi: 10.1016/j.humpath.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer. 46:1260–70. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Yao W, Newton RC, Scherle PA. Targeting the c-MET signaling pathway for cancer therapy. Expert Opin Investig Drugs. 2008;17:997–1011. doi: 10.1517/13543784.17.7.997. [DOI] [PubMed] [Google Scholar]
- 25.Beviglia L, Matsumoto K, Lin CS, Ziober BL, Kramer RH. Expression of the c-Met/HGF receptor in human breast carcinoma: correlation with tumor progression. Int J Cancer. 1997;74:301–9. doi: 10.1002/(sici)1097-0215(19970620)74:3<301::aid-ijc12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Lindemann K, Resau J, Nahrig J, Kort E, Leeser B, Annecke K, et al. Differential expression of c-Met, its ligand HGF/SF and HER2/neu in DCIS and adjacent normal breast tissue. Histopathology. 2007;51:54–62. doi: 10.1111/j.1365-2559.2007.02732.x. [DOI] [PubMed] [Google Scholar]
- 26.Hochgrafe F, Zhang L, O’Toole SA, Browne BC, Pinese M, Porta Cubas A, et al. Tyrosine phosphorylation profiling reveals the signaling network characteristics of Basal breast cancer cells. Cancer Res. 70:9391–401. doi: 10.1158/0008-5472.CAN-10-0911. [DOI] [PubMed] [Google Scholar]
- 28.Gastaldi S, Comoglio PM, Trusolino L. The Met oncogene and basal-like breast cancer: another culprit to watch out for? Breast Cancer Res. 12:208. doi: 10.1186/bcr2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L, Fuchs A, Schnitt SJ, Yao Y, Joseph A, Lamszus K, et al. Expression of scatter factor and c-met receptor in benign and malignant breast tissue. Cancer. 1997;79:749–60. doi: 10.1002/(sici)1097-0142(19970215)79:4<749::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Hammond ME, Hayes DF, Wolff AC. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. doi: 10.1200/JCO.2011.35.2245. [DOI] [PubMed] [Google Scholar]
- 31.Hennessy BT, Lu Y, Gonzalez-Angulo AM, Carey MS, Myhre S, Ju Z, et al. A Technical Assessment of the Utility of Reverse Phase Protein Arrays for the Study of the Functional Proteome in Non-microdissected Human Breast Cancers. Clin Proteomics. 6:129–51. doi: 10.1007/s12014-010-9055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leo B. Classification & Regression Trees Wadsworth Co. 1984. [Google Scholar]
- 33.Camp RL, Rimm EB, Rimm DL. Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer. 1999;86:2259–65. doi: 10.1002/(sici)1097-0142(19991201)86:11<2259::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Lengyel E, Prechtel D, Resau JH, Gauger K, Welk A, Lindemann K, et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113:678–82. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 35.Tolgay Ocal I, Dolled-Filhart M, D’Aquila TG, Camp RL, Rimm DL. Tissue microarray-based studies of patients with lymph node negative breast carcinoma show that met expression is associated with worse outcome but is not correlated with epidermal growth factor family receptors. Cancer. 2003;97:1841–8. doi: 10.1002/cncr.11335. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita J, Ogawa M, Yamashita S, Nomura K, Kuramoto M, Saishoji T, et al. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994;54:1630–3. [PubMed] [Google Scholar]
- 37.Berg D, Hipp S, Malinowsky K, Bollner C, Becker KF. Molecular profiling of signalling pathways in formalin-fixed and paraffin-embedded cancer tissues. Eur J Cancer. 46:47–55. doi: 10.1016/j.ejca.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Grote T, Siwak DR, Fritsche HA, Joy C, Mills GB, Simeone D, et al. Validation of reverse phase protein array for practical screening of potential biomarkers in serum and plasma: accurate detection of CA19-9 levels in pancreatic cancer. Proteomics. 2008;8:3051–60. doi: 10.1002/pmic.200700951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edakuni G, Sasatomi E, Satoh T, Tokunaga O, Miyazaki K. Expression of the hepatocyte growth factor/c-Met pathway is increased at the cancer front in breast carcinoma. Pathol Int. 2001;51:172–8. doi: 10.1046/j.1440-1827.2001.01182.x. [DOI] [PubMed] [Google Scholar]
- 40.Kermorgant S, Aparicio T, Dessirier V, Lewin MJ, Lehy T. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis. 2001;22:1035–42. doi: 10.1093/carcin/22.7.1035. [DOI] [PubMed] [Google Scholar]
- 41.Previdi S, Maroni P, Matteucci E, Broggini M, Bendinelli P, Desiderio MA. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and beta-catenin/Wnt pathways. Eur J Cancer. 46:1679–91. doi: 10.1016/j.ejca.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 42.Khoury H, Naujokas MA, Zuo D, Sangwan V, Frigault MM, Petkiewicz S, et al. HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol Biol Cell. 2005;16:550–61. doi: 10.1091/mbc.E04-07-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan S, Wang JA, Yuan RQ, Rockwell S, Andres J, Zlatapolskiy A, et al. Scatter factor protects epithelial and carcinoma cells against apoptosis induced by DNA-damaging agents. Oncogene. 1998;17:131–41. doi: 10.1038/sj.onc.1201943. [DOI] [PubMed] [Google Scholar]
- 44.Fan S, Ma YX, Wang JA, Yuan RQ, Meng Q, Cao Y, et al. The cytokine hepatocyte growth factor/scatter factor inhibits apoptosis and enhances DNA repair by a common mechanism involving signaling through phosphatidyl inositol 3′ kinase. Oncogene. 2000;19:2212–23. doi: 10.1038/sj.onc.1203566. [DOI] [PubMed] [Google Scholar]
- 45.Hiscox S, Jordan NJ, Jiang W, Harper M, McClelland R, Smith C, et al. hronic exposure to fulvestrant promotes overexpression of the c-Met receptor in breast cancer cells: implications for tumour-stroma interactions. Endocr Relat Cancer. 2006;13:1085–99. doi: 10.1677/erc.1.01270. [DOI] [PubMed] [Google Scholar]
- 46.Shattuck DL, Miller JK, Carraway KL, 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471–7. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 47.De Bacco F, Luraghi P, Medico E, Reato G, Girolami F, Perera T, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 103:645–61. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 48.Davies G, Watkins G, Mason MD, Jiang WG. Targeting the HGF/SF receptor c-met using a hammerhead ribozyme transgene reduces in vitro invasion and migration in prostate cancer cells. Prostate. 2004;60:317–24. doi: 10.1002/pros.20068. [DOI] [PubMed] [Google Scholar]
- 49.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–17. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 50.Stella GM, Benvenuti S, Comoglio PM. Targeting the MET oncogene in cancer and metastases. Expert Opin Investig Drugs. 19:1381–94. doi: 10.1517/13543784.2010.522988. [DOI] [PubMed] [Google Scholar]