Abstract
Iron-chelation therapy has its origins in the treatment of iron-overload syndromes. For many years, the standard for this purpose has been deferoxamine. Recently, considerable progress has been made in identifying synthetic chelators with improved pharmacologic properties relative to deferoxamine. Most notable are deferasirox (Exjade®) and deferiprone (Ferriprox®), which are now available clinically. In addition to treatment of iron overload, there is an emerging role for iron chelators in the treatment of diseases characterized by oxidative stress, including cardiovascular disease, atherosclerosis, neurodegenerative diseases and cancer. While iron is not regarded as the underlying cause of these diseases, it does play an important role in disease progression, either through promotion of cellular growth and proliferation or through participation in redox reactions that catalyze the formation of reactive oxygen species and increase oxidative stress. Thus, iron chelators may be of therapeutic benefit in many of these conditions. Phytochemicals, many of which bind iron, may also owe some of their beneficial properties to iron chelation. This review will focus on the advances in iron-chelation therapy for the treatment of iron-overload disease and cancer, as well as neurodegenerative and chronic inflammatory diseases. Established and novel iron chelators will be discussed, as well as the emerging role of dietary plant polyphenols that effectively modulate iron biochemistry.
Iron in mammalian systems
Iron is an essential element involved in many cellular processes that are necessary for life, including oxygen transport, oxygen sensing, electron transfer, energy metabolism and DNA synthesis [1–6]. The absolute requirement for iron in mammals has resulted in the evolution of complex mechanisms to efficiently acquire, transport and store this poorly soluble transition metal [7–9]. These mechanisms also shepherd iron safely, since unliganded or incompletely liganded iron ions can participate in ‘Fenton-type’ redox chemistry, reacting with hydrogen peroxide (H2O2) or lipid peroxides to generate the highly reactive hydroxyl radical (OH•) or lipid radicals such as LO• and LOO•. These reactive oxygen species (ROS) can damage lipid membranes, proteins and nucleic acids. Thus, excess iron can be toxic and patients with iron overload must be treated.
Medicinal interest in iron chelators originated with the treatment of such patients with acute or chronic iron overload [5,10–12]. However, as discussed in this review, this represents a limited subset of the potential medical benefits of iron chelators, which are currently being explored for their cardioprotective, neuroprotective, anti-cancer, anti-inflammatory and other properties. In this review, we focus on the potential medicinal benefits of iron chelators. We first provide a brief overview of systemic iron homeostasis (for more extensive reviews of this complex topic readers are referred elsewhere [5,13]). We then describe natural and synthetic iron chelators and discuss their current and potential therapeutic uses.
Systemic iron homeostasis
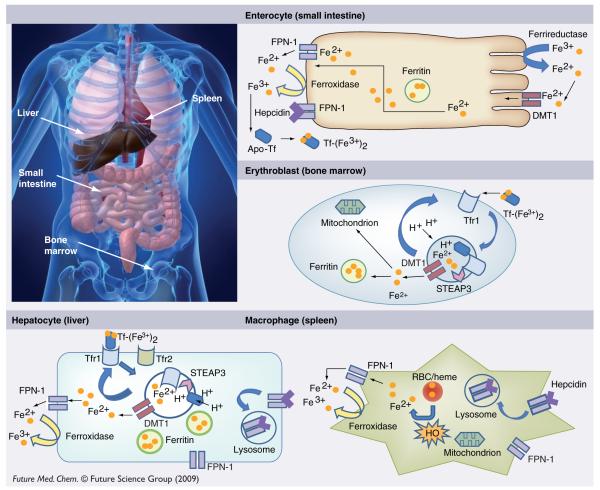
Absorption of dietary iron occurs in the proximal small intestine and is carefully regulated to maintain equilibrium between absorption and loss of body iron [14]. Figure 1 provides an overview of iron homeostasis. Most dietary nonheme iron is in the ferric (Fe3+) form, which must be reduced to ferrous (Fe2+) iron for efficient uptake. The activity of luminal ferrireductases such as duodenal cytochrome B (CYBRD1, also known as DCYTB) likely facilitates this reduction [13]. Absorptive enterocytes obtain Fe2+ through divalent metal transporter 1 (DMT1), the same iron transporter used for endosomal transfer in the transferrin cycle [13]. Upon entry into the intestinal epithelial cell, some iron remains in the cell for use or storage in ferritin, the cytosolic protein in which iron is stored. This iron will not be absorbed by the body; rather, it is lost as enterocytes are sloughed into the gut lumen [13].
Figure 1. Overview of iron homeostasis.
DMT1: Divalent metal transporter 1; FPN-1: Ferroportin-1; HO: Heme oxygenase; RBC: Red blood cell; STEAP3: Six-transmembrane epithelial antigen of prostate 3; Tf: Transferrin; Tfr1: Transferrin receptor 1; Tfr2: Transferrin receptor 2.
The majority of iron is delivered to the circulating plasma protein transferrin following efflux from the enterocyte through the basolateral iron transporter ferroportin (also known as FPN-1, IREG1, MTP1 and Slc40a1 [solute carrier family 4, member 1])[15–17]. Oxidation of iron by hephaestin or ceruloplasmin, both of which have ferroxidase activity (Fe2+→Fe3+) precedes its loading onto transferrin [18]. Extracellular iron circulates in the plasma in a nonreactive form bound to transferrin (Tf), which can be taken up by cells following binding to transferrin receptor 1 (Tfr1), present on cell surfaces [13]. Tf–Tfr1 complexes are taken into the cell by receptor-mediated endocytosis. Endosomes become acidified through proton influx, leading to conformational changes in both Tf and Tfr1 and promoting iron release [8,9,13]. Liberated inorganic iron is subsequently reduced by the ferrireductase six-transmembrane epithelial antigen of prostate (STEAP)3 to Fe2+ [19] and transported out of the endosome by DMT1 or (solute carrier family 11, member 2 (SLC11A2) [13,20–22]. Apo-Tf (iron-free) and Tfr1 are recycled to the cell surface to be used in further cycles of iron binding and uptake [5]. A second Tfr-like molecule, transferrin receptor 2 (Tfr2), can mediate cellular iron uptake and is expressed in liver cells, erythroid cell lines and duodenal crypt cells [23]. The affinity of Tfr2 for transferrin is substantially lower than that of Tfr1 and Tfr2 has been suggested to play an as yet poorly understood role as a systemic iron sensor [24]. Although virtually all circulating iron is bound to transferrin under physiological conditions, nontransferrin-bound iron (NTBI) can increase in iron overload and is proposed to contribute to the pathology of iron overload [25,26].
The major site of iron utilization is the bone marrow, where it is used in the synthesis of hemoglobin by red blood cell precursors. Due to the constant degradation of erythrocytes, conservation and recycling of iron is essential to replenish the iron contained within hemoglobin [27,28]. This recycling process occurs primarily within the macrophage, which is capable of phagocytozing erythrocytes, with subsequent liberation of iron in heme by heme oxygenase-1 (HO-1) [28–31].
Systemic iron homeostasis depends on the coordinate control of intestinal iron absorption, iron recycling and iron storage [13]. Hepcidin, a recently discovered peptide hormone, produced in the liver, has been shown to play a major role in this regulatory circuit. Hepcidin acts as a negative regulator of iron efflux by causing the degradation of ferroportin [32,33]. Ferroportin, the iron efflux pump involved in delivery of iron from enterocytes to the circulation, is also expressed at high levels in placenta, hepatocytes and macrophages and is an essential component of systemic iron homeostasis [16,34].
Synthesis of hepcidin is increased by iron loading and decreased by anemia and hypoxia [34]. The bone morphogenetic protein (BMP)/SMAD pathway is critical in the activation of hepcidin transcription: loss of hepatic SMAD4 results in severe iron overload due to failure of hepcidin-mediated degradation of ferroportin [35]. In addition to its role as an iron-regulatory hormone, hepcidin is induced by infection and inflammation. This results in hypoferremia, which contributes to defense against microbial infections [34]. A consensus signal transactivator of transcription 3 (STAT3)-binding site in the hepcidin gene has been shown to mediate hepcidin induction in inflammation through a signaling pathway triggered by the cytokine IL-6 [33,36,37]. ROS inhibit this effect [38], suggesting a link between ROS and iron metabolism [27].
Ferritin is an iron storage protein that is found both intracellularly and circulating in plasma. Cellular ferritin is composed of 24 subunits of heavy or heart (H-) and light or liver (L-) types, the ratios of which are tissue-specific and can be modified by disease and other stimuli [39]. The protein shell can hold up to 4500 iron ions in a redox-inactive ferrihydrite mineral form with phosphate and oxohydroxide anions. The primary function of ferritin is the sequestration of iron in a nontoxic form, which minimizes the ability of iron to catalyze the formation of ROS. Transcriptional and post-transcriptional controls increase cellular ferritin in response to environmental cues, as well as high iron levels [40]. While most ferritin is used to store iron within cells, a small amount is released into the serum and is measured clinically as a semiquantitative indicator of iron stores [28]. Serum ferritin has been reported to be rich in the L-subunit and glycosylated, although this characterization is based on ferritin isolated from non-normal (hemochromatitic) individuals [41]. The biological role of serum ferritin is unclear, although our laboratory has speculated that it may play a role in regulation of angiogenesis [42].
The expression of genes involved in angiogenesis, erythropoiesis and iron metabolism, including Tf and Tfr, is upregulated in response to hypoxia [43]. These pathways are tightly connected via regulation by hypoxia-inducible factor (HIF)-1, a transcription factor that binds to the hypoxia-response elements (HREs) of target genes [43]. The HIF-1 heterodimer consists of a regulatory subunit, HIF-1α, which is the oxygen-responsive component, and a constitutively expressed HIF-1β subunit, also known as the aryl hydrocarbon receptor nuclear trans-locator (ARNT) [43]. When oxygen is present, the regulatory subunit is hydroxylated on two prolines by iron-dependent prolyl-hydroxylases (PHDs). This recruits the von Hippel-Lindau (vHL) tumor-suppressor protein and triggers its degradation through the ubiquitin–proteosome pathway [43]. Under hypoxic conditions, prolyl hydroxylase activity is inhibited. This results in accumulation of HIF-1α and its translocation to the nucleus, where it binds ARNT/HIF-1β, and permits the transcription of HIF-dependent genes [43]. Iron chelators, such as deferoxamine (DFO) [44] and the flavonoid quercetin [45,46] inhibit the hydroxylation of HIF-1α, presumably by removing iron from PHD, which increases levels of HIF-1; thus, activation of HIF-1 may contribute to the biological effects of iron chelators [8,9].
Iron chelators & iron-chelation therapy: overview
While iron chelators are chemically quite diverse, they typically contain oxygen, nitrogen or sulfur-donor atoms that form coordinate bonds with bound iron. The donor atoms of the ligand affect the preference of the chelator for either the Fe(II) or Fe(III) oxidation states [7]. Chelators that prefer Fe(II) contain ‘soft’ donor atoms, such as nitrogen and sulfur, and, consequently, retain a relatively high affinity for other biologically important divalent metals such as Cu2+ and Zn2+ [47]. Iron can coordinate six ligands in an octahedral arrangement; thus, iron chelators with the highest affinity will normally be hexadentate, binding iron in a 1:1 ratio (chelator:iron), one example being DFO. By contrast, bidentate (3:1 ratio) or tridentate (2:1 ratio) chelators, which bind to only two or three of the available iron chelation sites, can potentially redox cycle and thereby promote free radical generation [26]. Effective iron chelators must efficiently compete with the biological ligands that normally bind iron; therefore, the affinity of chelators for iron and their stoichiometry of iron binding will greatly impact their activity as therapeutic agents [8,9]. Chemical structures of the most important iron chelators with medicinal applications are shown in Table 1.
Table 1.
Iron chelators and their therapeutic uses.
| Name | Structure | Therapeutic use | ||
|---|---|---|---|---|
| Cell culture | Animal study | Human study | ||
| Siderophores | ||||
| Deferoxamine |
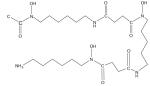
|
Type II diabetes [87–94], cancer chemotherapy [8,9,50, 95–99] |
Ischemia-reperfusion injuries [82], atherosclerosis [84–86], type II diabetes [87–94], cancer chemotherapy [8,9,50, 95–99] |
Current standard for iron chelation and treatment of iron overload diseases [60–62], Hematologic diseases characterized by iron overload including thalassemia major, sickle-cell disorders and myelodysplasia [25,67–77], type II diabetes [87–94], cancer chemotherapy [8,9,50, 95–99] |
| Desferrithiocin [2-(3-hydroxypyridin- 2-yl)-4-methyl-4,5- dihyrothiazole-4- carboxylic acid |
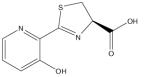
|
Potent antineoplastic activity [101,102] |
Treatment of iron overload disease [100] |
Treatment of iron overload disease [100] |
| Synthetic chelators | ||||
| Deferiprone (Ferriprox®) |
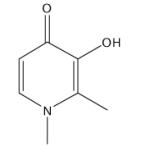
|
Analogues of deferiprone used to treat neuro- degeneration [106] |
Treatment of iron overload disease [60,63,70] and in combination with DFO [26,60,104], thalassemia major [68,93–95, 105], myelodysplastic syndromes (MDS) [74], sickle-cell disease[67], type II diabetes [90–94] |
|
| Clioquinol |
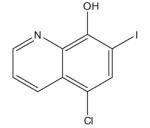
|
Alzheimer’s disease and Parkinson’s disease [110,111] |
Treatment of neuro-degeneration [54] | |
|
O-trensox (Tris-N-(2- aminoethyl-[8- hydroxyquinoline-5- sulfonato-7- carboxamido] amine) |
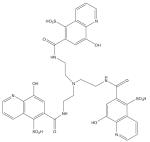
|
Cytotoxic to cancer cells [114,115] |
||
| Deferasirox (ICL670, Exjade®) |
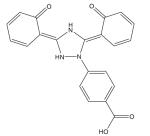
|
Treatment of chronic iron overload due to blood transfusions [67,68,118–121], myelodysplastic syndromes [76], Blackfan–Diamond syndrome and other rare anemias [122] |
||
| Tachpyr (N,N’,N’’- tris(2-pyridylmethyl)- cis,cis-1,3,5- triamino- cyclohexane) |
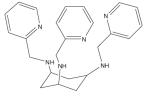
|
Cytotoxic to cancer cells [123–125,127], sensitized cancer cells to ionizing radiation [126] |
||
| Dexrazoxane (ICRF-197) |
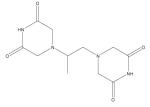
|
Antitumor agent [128], reduce or prevent the following: mitoxantrone toxicity, daunorubicin toxicity, bleomycin pulmonary toxicity, isoproterenol- induced cardiotoxicity, acetaminophen- induced hepatotoxicity, alloxan-induced diabetes, and hyperoxia-induced pulmonary damage [131–133] |
Cardioprotective agent to reduce doxorubicin- induced cardiotoxicity [128–130] and reduce or prevent the following: mitoxantrone toxicity, daunorubicin toxicity, bleomycin pulmonary toxicity, isoproterenol- induced cardiotoxicity, acetaminophen-induced hepatotoxicity, alloxan- induced diabetes, and hyperoxia-induced pulmonary damage [131–133] |
Cardioprotective agent to reduce doxorubicin-induced cardiotoxicity [128–130] |
| Triapine (3-aminopyridine-2- carboxaldehyde thiosemicarbazone) |
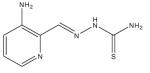
|
Anticancer therapy [50,134–138] |
Anticancer therapy [50,134–138] | |
| Pyridoxal isonicotinoyl hydrazone |
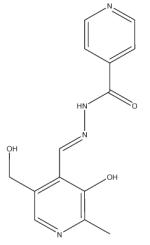
|
Cytotoxic to cancer cells [141,143–145], anti-retroviral therapy (anti-HIV) [142] |
||
| Di-2-pyridylketone thiosemicarbazone series |
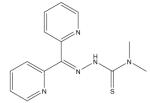
|
Cytotoxic to cancer cells [99,146–149] |
||
| Phytochemicals | ||||
| Flavan-3-ol |
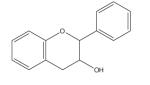
|
Chemo-preventive and cytotoxic to cancer cells [168] |
||
| Curcumin |
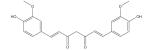
|
Cancer chemotherapy and iron chelation [170,172] |
Cancer chemotherapy and iron chelation [170,172], β-thallassemia [173], neuroprotection [176,180] |
Multiple myeloma, pancreatic cancer, myelodysplastic syndromes, colon cancer, psoriasis and Alzheimer’s disease [150] |
| Apocynin |
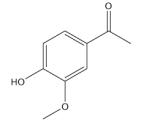
|
Neuroprotection [177–180] |
Neuroprotection [177–180] | |
| Kolaviron |
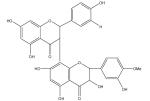
|
Atherosclerosis [181] | ||
| Floranol |
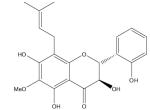
|
Atherosclerosis [182] | ||
| Baicalein |
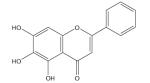
|
Cytotoxic to cancer cells [187,188] |
||
| Baicalin |
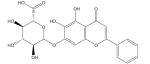
|
Iron-overload disease [189,190] |
||
|
Ligusticum wallichi Francha (ligustrazine) |
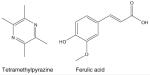
|
Neuroprotective [192] | Ischemia-reperfusion injuries [193,194] |
|
| Quercetin |
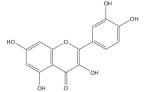
|
Inhibit tumor cell growth [197,201], vascular and neuroprotective [199,200] |
Vascular and neuroprotective [199,200]; iron overload [189,190] |
|
| Epigallocatechin gallate |
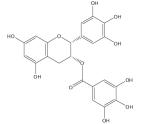
|
Cytotoxic to cancer cells [211], neuroprotective [223–228] |
Chemo-preventive [216–218], neuroprotective [223–228] |
|
| Theaflavin |
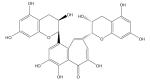
|
Cytotoxic to cancer cells [211–213] |
Chemo-preventive [216–220] | |
| Phytic acid |
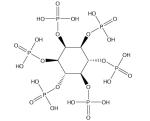
|
Cardiovascular disease [229–235] | ||
| Genistein (5,7,4′-tri- hydroxyisoflavone) |
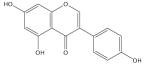
|
Insulin resistance and metabolic syndrome [238–241] |
Insulin resistance and metabolic syndrome [238–241], cancer prevention [237] |
Atherosclerosis and other inflammatory diseases [236] |
Iron-chelation therapy has largely focused on the treatment of iron overload in patients who receive multiple blood transfusions as supportive therapy for treatment of conditions such as β-thalassaemia, sickle cell disease and myelodysplasia [47,48]. As discussed below, however, the potential spectrum of activity of iron chelators is much broader than this. For example, due to their ability to sequester metals essential to tumor growth or to foster redox cycling of bound iron, chelators have been suggested to be useful as anticancer agents [8,9,49]. Indeed, numerous preclinical and clinical studies have suggested a role for iron chelators in the treatment of cancer [8,9,50]. In addition, iron chelators may play a role in diseases mediated by oxidative stress, including ischemia–reperfusion injury [51], liver [52], infectious [53] and neurologic [54] diseases, diabetes [55], inflammation [56] and atherosclerosis [57].
Chelators in current medicinal use include natural compounds derived from microorganisms (siderophores) and synthetic chelators. Additionally, there is a growing appreciation of the role of natural iron chelators that inhibit oxidative stress and are found in foods and plants. Below, we discuss recent advances in the medicinal applications of these three major classes of chelators: siderophores, synthetic chelators and food- and plant-derived chelators. Table 1 presents the structures of these chelators and some of the biological contexts in which they have been studied.
Siderophores
Iron-dependent microorganisms have evolved a strategy to solve the problems of bioavailability and toxicity of iron through the synthesis of siderophores (low-molecular mass Fe3+-specific chelators) employed for iron uptake and storage [58]. The iron-binding constant of siderophores can reach at least 1030 M, permitting microbial extraction of iron even from stainless steel [59].
Deferoxamine
Deferoxamine mesylate (Desferal®, desferrioxamine) is the best-known example of a medicinal siderophore and the most common one in medical use [8,9,27]. DFO has been used for decades to treat iron-overload diseases and remains the current standard for iron-chelation therapy [60–62]. Toxicity of iron overload is due largely to NTBI, as this pool of iron has both redox activity and the ability to access highly vascular tissues, especially hepatic, cardiac and endocrine tissue [25,26]. DFO has been shown to be a multifunctional therapeutic agent that can detoxify NTBI by chelation and heme proteins by ferryl reduction and radical scavenging [63]. DFO also acts as a reducing agent, preventing oxidation of membrane lipids by removing high-oxidation states of heme iron, such as ferryl myoglobin (Mb) or Hb [64–66].
Hematologic diseases for which iron-chelation therapy with DFO has shown clinical effectiveness include thalassemia major, sickle cell disorders and myelodysplasia [67]. β-thalassemia major is a rare recessively inherited disorder of hemoglobin synthesis resulting in transfusion-dependent anemia; consequently, chronic transfusion therapy results in a toxic accumulation of iron in the liver, spleen, endocrine organs and the myocardium [68]. Iron-chelation therapy benefits thalassemia major patients both by reducing the iron-loading burden and ameliorating iron-induced dysfunction within the heart [68–71]. Sickle cell disease (SCD) is an inherited disorder of hemoglobin synthesis characterized by lifelong hemolytic anemia [72]. Iron overload develops in 10–20% of SCD patients who receive repeated blood transfusions; these patients show similar rates of liver iron loading as patients with transfusion-dependent thalassemia, and are treated similarly [25,73]. Myelodysplastic syndromes (MDSs) are a common heterogeneous group of bone marrow disorders characterized by ineffective hematopoiesis and an increased risk of the development of acute myeloid leukemia [25,74]. Patients with MDSs may develop long-term dependence on blood transfusions, which generates higher levels of NTBI, requiring iron-chelation therapy [75,76]. The goal of chelation therapy in patients with MDS is primarily prevention of iron overload to preserve organ function, with the potential to improve patient survival [77]. Although there are few studies of efficacy for treatment of MDS, a retrospective study in patients with MDS indicated that iron chelation therapy using DFO was significantly associated with improved survival [74]. Few relevant clinical trials have been performed to evaluate the impact of iron chelation therapy in SCD and MDS or whether particular chelators could be more effective than others; however, documentation to date supports the use of DFO treatment in these diseases [67].
Iron chelation also may aid in reducing injury due to oxidant-related inflammation. Pro-oxidants play a significant role in the inflammation and tissue injury observed in many renal and hepatic diseases, including acute and chronic kidney disease, diabetic nephropathy, renal and hepatic ischemia–reperfusion injury (IRI) and nonalcoholic fatty liver disease (NAFLD) [78–81]. Since iron contributes to oxidative stress, siderophores such as DFO may play a role in the therapy of these diseases. While DFO was shown to ameliorate IRI in animal models, treatment using iron scavengers in ischemic syndromes is not in current clinical use [82].
Iron-chelation therapy with DFO has been studied in other diseases characterized by chronic inflammation, such as atherosclerosis and diabetes. Some epidemiological and many experimental studies suggest that development of atherosclerosis is associated with the amount of iron stored in the body [83]. Iron and oxidized lipids are both found in atherosclerotic lesions [84]. Iron depletion by DFO was shown to delay formation of atherosclerotic lesions [84–86], indicating that chelation therapy may aid in the treatment or prevention of atherosclerosis.
Deferoxamine also may have therapeutic value in the treatment of diabetes. Excess iron appears to be associated with insulin resistance and Type II diabetes, as well as increased risk of diabetic complications [55]. Use of DFO to lower elevated serum ferritin levels in Type II diabetic patients improved fasting glucose, triglyceride and HbA1c levels and correlated well with diabetes control [87]. Coronary metabolic vasodilation is impaired in diabetes, and administration of DFO to patients with Type II diabetes was shown to substantially improve coronary microvasculature function [88]. A recent study showed that treatment with DFO not only induced iron depletion in HepG2 hepatocytes and in rat liver, but also increased glucose uptake and upregulated insulin receptor activity and signaling [89]. The results of this study suggest that iron status may affect progression to hyperglycemia and may help to explain the pathogenesis of hepatic iron-overload insulin-resistance syndrome [89]. Thalassemic patients with iron overload have a high incidence of insulin resistance and diabetes; combination treatment of some of these patients with DFO and deferiprone (see ‘Synthetic chelators’ section) improved their diabetes and glucose tolerance [90,91] and decreased ferritin levels [92–94]. Further studies are required to confirm a beneficial role of iron chelators in other diabetic populations.
Iron chelators have also been explored as cancer chemopreventive and chemotherapeutic agents [8,9]. DFO was among the first to be explored in this context, largely due to its well-documented history of clinical safety and efficacy in the treatment of iron-overload disease [8,9,95]. DFO was observed to inhibit DNA synthesis and cell proliferation and induce apoptosis in multiple cancer cell lines. However, the ability of DFO to inhibit tumor growth in animal studies and clinical trials was limited [8,9,50,96–99]. The incomplete success of DFO as an anti-tumor agent has led to an emphasis on designing more effective iron chelators, with particular focus on chelator lipophilicity, membrane permeability and selective anti-tumor activity [50,99].
Synthetic alternatives to DFO have also been sought for the treatment of iron overload. While the long-term efficacy of DFO therapy in the treatment of iron overload has been demonstrated, its poor oral bioavailability requires an inconvenient regimen of parenteral administration. This has motivated the search for alternative, preferably oral, tridentate and bidentate chelators [26,60,62]. Efforts to substitute synthetic chelators for DFO in both the treatment of iron overload and cancer have met with substantial success, as discussed later (see ‘Synthetic chelators’ section).
Desferrithiocin
Desferrithiocin (DFT), a tridentate siderophore, is an orally effective iron chelator [100]. DFT was found to have potent antineoplastic activity against hepatocellular carcinoma cells in vitro with low levels of cytotoxicity for normal hepatocytes; studies with DFT and synthetic analogues that may be more selective for cancer cells are ongoing [101,102]. Although DFT showed much promise as a chelating agent, it exhibited severe nephrotoxicity [103]. The high oral activity of DFT made this chemical backbone a promising scaffold and structure–activity relationship studies have been performed to develop less-toxic derivatives of this pharmacophore [7].
Synthetic chelators
Although DFO is safe and effective in the treatment of iron overload, the requirement for prolonged subcutaneous infusions 5–7 days per week frequently results in patient non-compliance. This has led to the search for alternative chelators with superior pharmacological properties. The major synthetic focus has been on the design of Fe3+-selective chelators, which feature ‘hard’ oxygen donor atoms that are generally more selective for trivalent metal cations over divalent cations [7].
Deferiprone
Deferiprone (Ferriprox®), one of hundreds of oral chelators that have been tested, is a bidentate ligand that has shown comparable efficacy to DFO and is more effective than DFO in the removal of excess iron from the heart [60,70]; however, major toxic side effects include agranulocytosis, musculoskeletal and joint pains, gastric intolerance and zinc deficiency [60,63]. An advantage of this compound is that the Fe(III) chelate of deferiprone carries no net charge and, therefore, can penetrate membranes easily, allowing removal of potentially toxic iron from tissues. In addition, the combination of DFO/deferiprone is increasingly used and no new toxic side effects have been reported for the combination [26,60,104]. Clinical data documenting the use of deferiprone in thalassemia major are limited; however, its use is associated with agranulocytosis and arthropathy [68]. A recent clinical study showed that deferiprone was well tolerated and effective in lowering serum ferritin levels in patients with thalassemia major, with few adverse effects [105]. The combination of deferiprone with DFO showed a greater efficacy in promoting iron excretion than either chelator alone, as well as rapid reduction of iron overload in the heart and liver and reversal of cardiac dysfunction in thalassemic patients [92–94]. Deferiprone was effective in only a small number of MDS patients [74]. Overall, the compliance with oral deferiprone therapy is greatly improved compared with that typical with DFO therapy [62].
Based on in vitro studies suggesting a neuroprotective effect of deferiprone, analogues of deferiprone are currently under investigation for treating neurodegeneration [106]. Neurodegeneration in Parkinson’s disease (PD) and Alzhemier’s diseases (AD) or other neurodegenerative diseases, such as Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS), are multifactorial. A complex set of toxic reactions, including induced expression of proapoptotic proteins and increase of iron and nitric oxide levels, lead to oxidative stress damage, inflammation, reduced expression of trophic factors and accumulation of protein aggregates, culminating in the death of neurons [107,108]. Metal ions such as iron and copper, have been cited as having key roles in the protein aggregation associated with many neurodegenerative diseases, including HD, AD, PD and Friedreich’s ataxia (FA) [54]. The accumulation of iron at sites of neuronal degeneration in HD, AD, PD and at significant levels within the mitochondria of FA patients supports a role for iron in the pathology of these diseases [109] and provides a rationale for the development of chelators as viable new therapeutic strategies [109].
8-hydroxyquinoline derivatives
To date, a range of 8-hydroxyquinoline analogues have demonstrated substantial potential for the treatment of neurodegeneration, and one compound, clioquinol (CQ), has entered clinical trials [54]. CQ is a small lipophilic bioavailable metal chelator that has shown great promise in both AD and PD animal models [110,111]. Although issues relating to the transport of iron chelators across the blood–brain barrier remain a significant problem, one recent study has shown a promising therapeutic role for nanoparticle-mediated delivery of iron chelators [112]. 8-hydroxyquinoline derivatives exhibited substantial cytotoxic activity against in vitro models of cancer and this could be related to blockade of voltage-activated K+ channels in the lipid membrane of the cancer cells [113]. Viability was significantly decreased in the rat hepatoma cell line FAO [114] and the human hepatoma cell line HepG2 [115] following treatment with the hexadentate hydroxyquinoline iron chelator O-trensox (tris-N-(2-aminoethyl-[8-hydroxyquinoline-5-sulfonato-7-carboxamido] amine). These studies offer insight into the protective efficiency of iron chelators against hepatocellular carcinoma, which is correlated with iron overload [116,117].
Deferasirox
Deferasirox (ICL670; Exjade®) is a tridentate oral chelator that has recently been approved by the US FDA for use in the treatment of chronic iron overload due to blood transfusions (transfusional iron overload). Deferasirox has proven effective in removing excess iron from the liver and shown acceptable tolerance, although special attention should be given to monitoring renal function [118–121]. Clinical studies using deferasirox in the treatment of some hematologic diseases suggest that it is as effective as DFO in maintaining iron balance. Adverse events with deferasirox included transient rash, gastrointestinal disturbances and mild nonprogressive increase in serum creatinine, as well as cases of renal failure and cytopenias [67,68]. Both deferiprone and deferasirox have shown efficacy in controlling body iron burden in SCD patients and no agranulocytosis was described [67]. A single study indicated that deferasirox may aid in controlling iron burden in MDS [76]. Combinations of DFO/deferasirox or the two orally active chelators deferiprone and deferasirox have been suggested as a treatment option for transfusional iron overload; however, the former would require slow subcutaneous or intravenous infusion of DFO and the latter may result in the formation of mixed (deferiprone–Fe–deferasirox) complexes that could raise new safety concerns [61].
Deferasirox also has been used in the treatment of rare anemias associated with iron overload. For example, serious complications of iron overload have been reported among patients with aplastic anemia and inherited bone marrow failure syndromes such as Diamond–Blackfan anemia (DBA) and Fanconi’s anemia. Recently, a 1-year prospective study showed that treatment with deferasirox was effective as a monotherapy in maintaining or decreasing iron loading in patients with DBA and other rare anemias, including aplastic anemia, α-thalassemia and sideroblastic anemia, myelofibrosis, pure red cell aplasia, pyruvate kinase deficiency, autoimmune hemolytic anemia, Fanconi’s anemia, hereditary sideroblastic anemia, erythropenia and β-thalassemia [122].
Tachpyridine
Tachpyridine (N,N’,N’’-tris(2-pyridylmethyl)-cis,cis-1,3,5-triaminocyclohexane) is a hexadentate metal chelator that chelates divalent metal ions and is toxic to cultured cancer cells, exhibiting an enhanced potency, relative to DFO [123–125]. Importantly, ferritin synthesis was depressed following tachpyridine treatment of cells, indicating that tachpyridine can effectively reduce levels of intracellular iron [123]. Additionally, tachpyridine sensitized cancer cells to ionizing radiation, suggesting that chelators may function in anticancer therapy, both as radio-enhancing agents and as cytotoxic agents [126]. Analysis of chelators based on the cis, cis-1,3,5-triaminocyclohexane (tach) backbone revealed that the most rapid cytotoxic response was achieved with chelators whose Fe(II) complexes underwent rapid oxidation, suggesting a path to further optimization of these chelators as anticancer agents [127].
Dexrazone
Dexrazone (ICRF-197; DZR), which belongs to a class of bis(2,6-dioxopiperazines), is a prodrug that is hydrolyzed to an iron-chelating EDTA-type structure (ADR-925) designed as an anti-tumor agent [128]. DZR is clinically approved as a cardioprotective agent to reduce doxorubicin-induced cardiotoxicity and it is supported by extensive animal studies [129] and clinical trials [130], showing it to be highly effective in reducing doxorubicin-induced cardiotoxicity, without affecting the anti-tumor activity of doxorubicin [128]. Additional studies have shown DZR can reduce or prevent the following: mitoxantrone toxicity, daunorubicin toxicity, bleomycin pulmonary toxicity, isoproterenol-induced cardiotoxicity, acetaminophen-induced hepatotoxicity, alloxan-induced diabetes and hyperoxia-induced pulmonary damage [131]. ADR-925 prevents formation of damaging ROS by removing Fe3+ from the Fe3+–anthracycline complex or by binding free iron in the cell [132,133].
Thiosemicarbazones
Thiosemicarbazones possess iron chelator activity and inhibit ribonucleotide reductase (RR), an iron-dependent enzyme that catalyzes the rate-limiting step in DNA synthesis [134]. Since RR is a validated drug target in cancer chemotherapy, effort has been devoted to the development of thiosemicarbazones in anticancer therapy [135]. Because RR participates in DNA repair, the effect of Triapine® (3-aminopyridine-2-carboxaldehyde thiosemicarbazone [3-AP]) on tumor cell radiosensitivity was investigated. Triapine significantly increased radiation-induced tumor growth delay in tumor-bearing mice [136]. Triapine is currently in clinical trials as an anticancer drug [50]. Considering the promising results observed from studies combining Triapine with gemcitabine [137] and cytarabine [138], Triapine may become the first iron chelator to be widely used as an anticancer drug [50].
Pyridoxal isonicotinoyl hydrazone analogs
Pyridoxal isonicotinoyl hydrazone (PIH) and its analogs are tridentate ligands, which bind iron via a carbonyl oxygen, an imine nitrogen and a phenolic oxygen and possess a high affinity and specificity for Fe3+ [139]. They are relatively lipophilic, compared with other chelators, resulting in rapid membrane permeability, and they exhibit excellent antioxidant properties [140].
Extensive structure–function studies on PIH analogs have been carried out. Interest in the anti-tumor activity of this group of compounds identified 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311) as among the most toxic to tumor cells [141]. This compound continues to be investigated; recent work has suggested that it may have potential anti-HIV activity [142]. Further studies on the chemistry of PIH-related compounds led to the development of the di-2-pyridylketone isonicotinoyl hydrazone (PKIH) analogs, which prevent iron uptake from Fe–Tf and are potent inhibitors of cell growth, in part due to free radical generation through the Fenton reaction [143,144]. For example, di-2-pyridylketone isonicotinoyl hydrazone (HPKIH) possesses significant antiproliferative activity against cancer cells that is influenced by the redox properties of the iron complexes [145].
Condensation of the di-2-pyridyl structure with thiosemicarbazides yielded a group of iron chelators, the DpT family, which shows selective toxicity for cancer cells [99,146–149]. Thiosemicarbazones are themselves chelators that inhibit RR activity (see earlier); thus, the strategic goal in the design of the DpT family was to incorporate this moiety into a ‘hybrid’ series. The most active compound in the DpT series is Dp44mT, with an average IC50 of 30 nM in 28 cancer cell types and good efficacy at inhibiting tumor growth in xenografts [149]. These novel iron chelators not only have potent and broad anti-tumor activity, but can overcome resistance to established chemotherapeutics and appear very promising as antineoplastic agents [99,148,149].
Phytochemicals
Evidence supporting the therapeutic efficacy of phytochemicals in conditions ranging from cancer to neurodegenerative disease has been steadily accumulating in recent years (Table 1). Although studies are, in many cases incomplete, data suggest that foods containing plant polyphenols and flavonoid compounds may have benefits not only as potent antioxidants, but also as iron chelators [27,150–152].
Proanthocyanidins, epicatechins, flavonols and anthocyanin contain an iron-binding motif similar to the catechol moiety that is a known iron-binding element of microbial siderophores [27,153–159]. Subclasses of flavonoids are based on chemical structure: flavanols (examples of rich sources: teas and red wine), flavanones (citrus foods), flavones (fruit skins, peppers and leafy vegetables), isoflavones (soy foods), flavonols (leeks, onions, leafy vegetables and tomatoes), anthocyanidins (berries) and proanthocyanidins (apples, chocolate and nuts) [160]. The activities of flavonoids are dependent on their chemical structures and the position and degree of hydroxylation appear particularly important for their biochemical and pharmacological actions [161]. The most well-absorbed flavonoids by humans are isoflavones, gallic acid, catechins, flavanones and quercetin glucosides, each with different kinetics [162,163]. The various terms applied to these polymeric flavon-3-ols have contributed to the confusing and often erroneous nomenclature used to describe these compounds [164]. The terms ‘proanthocyanidins’ and ‘condensed tannins’ are most frequently used in the literature (for a comprehensive description of proanthcyanidin nomenclature, see elsewhere [165]).
The most common group of flavonoids in the US diet, the flavan-3-ols and their polymeric condensation products, the proanthocyanidins, have been reported to exhibit antioxidant, anti-carcinogenic, cardioprotective, antimicrobial, antiviral and neuroprotective properties [165]. The general chemical structure (Table 1) includes a C15 (C6-C3-C6) skeleton joined to a chroman ring (benzopyran moiety) that in turn bears an aromatic ring at C2, C3 or C4. The ability of flavan-3-ol to chelate iron and other essential minerals limits growth of invasive microorganisms by causing severe mineral depletion and is vital to plant defense against pathogens [165]. Flavan-3-ols exhibit antioxidant activity via several mechanisms, including the scavenging of free radicals, chelation of transition metals and inhibition of enzymes (for a detailed review of antioxidant activity of flavon-3-ols, see elsewhere [166]). The flavan-3-ols and their microbial phenolic acid metabolites are particularly good antioxidants; they are considered better than vitamins C and E, due to the greater stability and less harmful nature of the derived radical compared with the initial radical species [165,167].
Flavonoids have been studied for their chemopreventive and anticancer effects, particularly through three mechanisms: prevention of carcinogenic metabolite formation, prevention of tumor cell proliferation and induction of tumor cell apoptosis [168]. Within the last decade, numerous studies have focused on the use of natural iron chelators as therapeutic agents; however, it is important to note that detrimental effects have also been noted for flavan-3-ol. Depending on the source, type, quantity and existence of other dietary factors, flavan-3-ol-related detrimental effects include activation of procarcinogens, ROS formation (pro-oxidant activity), hemorrhage formation, mutagenicity, initiation of hepatotoxicity, alteration of pharmacokinetics of therapeutic drugs, increased estrogenic plasma biochemistry, development of gastroenteritis, anti-nutritive activity and weight loss [165,169].
The following studies elucidate a role for phytochemicals as iron chelators and their therapeutic applications. They are summarized and presented according to flavon-3-ol-source: herbal remedy, grape or wine and other fruits and nuts, green tea, black tea and soy protein. Table 1 lists some of the phytochemicals with iron-binding properties disscussed in this review and their sources.
Although many results reported with phytochemicals are encouraging, caution is required when interpreting some of these studies. In particular, herbal remedies are occasionally used as extracts or powders of poorly defined composition. Limitations of such studies are therefore that, first, effects are not always attributable to a chemically defined component and, second, may be the result of a mixture.
Herbal remedies
Curcumin, the active ingredient in the traditional herbal remedy and dietary spice turmeric, is a free radical scavenger and hydrogen donor and exhibits both pro- and anti-oxidant activity [150]. It is remarkably nontoxic and exhibits limited bioavailability. Curcumin exhibits great promise as a therapeutic agent and is currently in human clinical trials for a variety of conditions, including multiple myeloma, pancreatic cancer, myelodysplastic syndromes, colon cancer, psoriasis and AD [150]. Cancer development and progression is inhibited by curcumin via its ability to target multiple steps in the pathway to malignancy (for a comprehensive review of curcumin, see elsewhere [150]).
Iron chelation may contribute to the anti-cancer activity of curcumin [170]. The chemical properties of curcumin are consistent with iron-chelator activity [171], and our laboratory recently observed that liver cells treated with curcumin exhibited hallmarks of iron depletion, which included decreases in the iron-storage protein ferritin, increases in TfR1 and activation of iron-regulatory proteins [172]. Curcumin also acts as an iron chelator in vivo, particularly in the setting of mild iron deficiency [170,172]. Under these conditions, dietary curcumin exerted profound effects on systemic iron, inducing a decline in hematocrit, hemoglobin, serum iron and Tf saturation, the appearance of hypochromic red blood cells and decreases in spleen and liver iron content [170]. Curcumin also repressed synthesis of hepcidin, a peptide that plays a central role in regulation of systemic iron balance [170]. Consistent with these reports, curcumin reduced NTBI in a mouse model of β-thalassemia [173].
Some naturally occurring chelators cross the blood–brain barrier and can exert their antioxidant and iron-chelating properties in the brain [174,175]. Neuroprotective effects have been evaluated for curcumin [176], as well as apocynin, which is derived from the rhizome of Picrorhiza kurroa, a well-known herb in traditional Ayurvedic medicine [177–179]. While these studies provide experimental evidence in rodents that curcumin and apocynin act as neuroprotective agents for cerebrovascular diseases, further studies are necessary to determine the mechanism involved. Apocynin not only exhibited potent antioxidative properties by scavenging free radicals, but also acted on specific signaling pathways that regulate inflammatory responses in cultured human eosinophils and neutrophils [180]. Mucuna pruiens, another naturally occurring antioxidant used in traditional Ayurvedic Indian medicine, has also been shown to slow the progression of PD symptoms and to have none of the side effects of the current pharmaceutical l-dopa [174]. Mucuna pruiens inhibited the oxidation of lipids and deoxyribose sugars and exhibited divalent iron-chelating activity [174].
Kolaviron, a natural biflavonoid from Garcinia kola seeds that have been used in African herbal medicine, was shown to protect against oxidation of lipoproteins in rats through the chelating activity of kolaviron on Fe2+, suggesting kolaviron may offer protective effects against atherosclerosis [181]. The antioxidant activity of floranol (a new flavonoid isolated from the roots of Dioclea grandiflora, a vine that grows in Brazil) was shown to be related to its ability to efficiently bind Cu2+ and/or Fe3+. By preventing the reduction of these metals in heparanized blood collected from healthy, normolipidemic volunteers, floranol prevented the formation of free radicals that can oxidize low-density lipoprotein (LDL) [182]. This sequestering of metals makes floranol a beneficial candidate in reducing atherosclerosis [182], although the degree and duration of iron reduction required to normalize atherosclerotic plaque iron concentrations to that of healthy arterial tissue is not known and this will be a key question for future studies [57].
Pycnogenol™ (PYC), a standardized extract composed of a mixture of flavonoids, mainly procyanidins and phenolic acids obtained from a French maritime pine, may be shown to provide cardioprotective activity, which has been attributed to the strong free radical scavenging activity of its oligomeric procyanidin components [183]. Procyanidins extracted from Vitis vinifera, which have a composition similar to that of PYC, were observed to form a complex with ferric iron with a procyanidin–iron ratio of 1:2 [184]. Furthermore, the stability constant of the procyanidin–iron complex was comparable to another strong iron-chelating agent, nitrilotriacetate (NTA) [184]. Electron spin resonance observations indicate that PYC may act as an efficient metal chelator; thus, PYC may act as a preventive antioxidant via formation of redox-inert iron complexes and may inhibit the peroxidative process [183,185]. The mechanism of this complex interaction between radical species requires further investigation; furthermore, in vivo studies are necessary to evaluate the precise compounds that eventually exert biological activity.
Baicalein and its glycoside baicalin, the major bioactive compounds found in the Chinese herb Scutellaria baicalensis Georgi, have been found to strongly inhibit iron-promoted Fenton chemistry via a combination of chelation and radical scavenging mechanisms [186]. Baicalein also was shown to induce apoptosis in human breast cancer cells and human lung squamous carcinoma cells via alteration of the Bcl-2 family of mitochondrial proteins and activation of caspase-3 [187,188]. Baicalin and quercetin (see later) were shown to increase antioxidant status and decrease iron content and lipid peroxidation in the liver of mice with iron-overload-induced liver oxidative injury [189,190]. In addition, when added to rodent chow (1% w/w final) both flavonoids significantly reduced hydroxyproline content in liver homogenates, indicating that these flavonoids can protect liver from fibrosis, a complication of iron-overload disease that can lead to liver cirrhosis [189]. Although these flavonoids are generally regarded as poorly bioavailable, a study of an orally administered formulation that contained S. baicalensis reported an apparent 8-h elimination half-life for baicalein; thus, baicalein can remain in the body long enough to affect iron homeostasis and reduce oxidative stress [186]. These studies suggest that phenolic compounds with an ‘iron-binding motif’ are strong iron-chelating agents that may modulate the bioactivity and bioavailability of iron in the body [191].
Tetramethylpyrazine and ferulic acid, two active ingredients in the Chinese herbal medicine Ligusticum wallichi Francha (ligustrazine), can chelate ferrous iron and decrease the formation of hydroxyl radicals [192]. Ligustrazine may be useful in preventing neuronal diseases associated with oxidative stress as pretreatment of cultured neuronal cells with the active compounds found in this herb attenuated iron-induced oxidative neuronal damage [192]; however, pharmaceutical-grade tetramethylpyrazine and ferulic acid were used in this cell culture study and it is unclear if the levels of these active ingredients would be similar in an orally administered supplement of the herbal medicine ligustrazine. In additional studies, pretreatment with pharmaceutical-grade ligustrazine administered by intraperitoneal injection provided functional protection after renal IRI in mice by decreasing generation of ROS, inhibiting apoptosis, decreasing neutrophil infiltration and interfering with cell adhesion [193,194]. This study did not report whether any adverse systemic affects were observed following administration of ligustrazine.
Grape, wine, fruits & nuts
The iron-binding properties of the flavonol quercetin, the major phenolic phytochemical present in cranberries and other selected phenolic compounds (chrysin, 3-hydroxyflavone, 3′,4′-dihydroxy flavone, rutin and flavones), were investigated in aqueous media using UV/vis, NMR and electron paramagnetic resonance (EPR) spectropscopies and ESI–MS [191]. Quercetin was found to bind Fe2+ more strongly than the well-known Fe2+ chelator ferrozine. Quercetin can also bind Fe3+, Ga3+ and Zn2+. Interestingly, quercetin completely suppressed iron-promoted Fenton chemistry at micromolar levels, even in the presence of the major cellular iron chelators ATP or citrate. Data from this study indicate that the radical scavenging activity of quercetin provides only partial protection against Fenton chemistry-mediated damage. On the other hand, iron chelation by quercetin can completely inhibit the Fenton chemistry. Thus, quercetin’s antioxidant activity may be attributed to its iron-chelation activity [191]. Furthermore, superoxide radical-scavenging activity of flavonoid iron complexes was found to be more effective than the uncomplexed parent flavonoid [195].
Flavonols, including quercetin, are rapidly absorbed and reach maximum plasma concentration within a few hours, while the elimination of flavonols (e.g., quercetin metabolites) is quite slow, with reported elimination half-lives ranging from 11 to 28 h. Circulating flavonoids (and their metabolites) are further delivered into various organs, including liver, skin and brain [196]. Cranberry extracts rich in these compounds reportedly inhibit oxidative processes, including oxidation of LDL [197,198], oxidative damage to rat neurons during simulated ischemia [199] and oxidative and inflammatory damage to the vascular endothelium [200].
The potential of flavonols to act as anticancer agents was suggested by the ability of water-soluble cranberry phenolic extracts prepared from commercial cranberry powder to inhibit proliferation of several human tumor cell lines [201]. A total polyphenol extract containing a variety of flavonoids inhibited proliferation of two oral cancer cell lines (CAL27 and KB), four colon cancer cell lines (HT-29, HCT-116, SW480 and SW620) and three prostate cancer cell lines (RWPE-1, RWPE-2 and 22Rv1) [201]. Enhanced antiproliferative activity was obtained when cranberry extract was enriched in polyphenolic content [201]. The authors of these studies indicated that there were additive or synergistic antiproliferative effects, resulting from the combination of anthocyanidins, proanthocyanidins and flavonol glycosides compared with individual purified phytochemicals [201]. Further studies are needed to determine if the anti-tumor activity of these compounds is linked to their iron-chelation properties and to determine their bioavailability in cranberry juices and whole cranberry fruit.
Grape seed extract (GSE) contains various polyphenols, including gallic acid, catechin, epigallocatechin gallate (EGCG) and proanthocyanidins. GSE possesses antioxidant activity [202–205], which derives from the ability of these constituents to scavenge free radicals and to chelate metals such as iron [204]. Interestingly, GSE and EGCG inhibited nonheme iron absorption in human intestinal Caco-2 cells by reducing basolateral iron exit via FPN-1 rather than by decreasing apical iron import [204]. A study of brain cell iron chelation suggested that EGCG and GSE are membrane permeable and may enter cells as complex forms with Fe2+ [206]. The iron-chelating activity of EGCG was comparable with that of DFO [206]. In most of these studies, GSE or EGCG was commercially prepared and characterized for use in cell culture or animal diets. Comparisons between purified products and dietary sources of GSE or EGCG were not presented and will be an interesting direction for future research. The effect of these compounds on uptake of dietary iron as well as the uptake of iron–polyphenol complexes also needs to be assessed [204].
Green tea catechins & black tea theaflavins
Flavonoids extracted from tea leaves (catechins present in green teas and theaflavins present in black teas) act directly as radical scavengers and exert indirect antioxidant effects through activation of transcription factors and antioxidant enzymes [175,207]. The most abundant polyphenolic compound of green tea is EGCG [175]. In addition to their radical scavenging action, green tea catechins possess well-established metal-chelating properties, with the structurally important features defining their chelating potential being the 3′,4′-dihydroxyl group in the B ring as well as the gallate group [151,208–210]. Furthermore, tea polyphenols that were dried and purified from black and green teas have been shown to have a profound protective effect on red blood cells challenged with exogenous oxidants via formation of a redox-inactive complex with iron [210].
Green tea catechins and black tea theaflavins were evaluated for their ability to induce cell death in several human cancer cells including human breast (MCF-7), colon (HT-29), hepatoma (liver; HepG2) and prostate (PC-3) and compared with normal human liver and lung (HEL299) cells [211]. It was observed that water tea extracts generally exhibited lower effects than the ethanol extracts and the authors of the study suggest that aqueous ethanol should be used in the preparation of concentrated black and green tea extracts for sale as dietary supplements [211]. The tea extracts were also cytotoxic to normal liver but not to lung; therefore, it will be important to determine the ratio of effective preventive or therapeutic to toxic dose [211]. Further studies demonstrated that apoptotic effects of black tea theaflavins were mediated by p53 in a transcription-dependent manner through the mitochondrial death cascade [212]. Regulator of G-binding protein signaling 10 (RGS10), which was induced within 4 h of theaflavin treatment in human colon cancer cells, was identified as a target gene [213]. This finding may be significant, since dysregulation of G-protein signaling, including G proteins and RGS proteins, has been implicated in tumorigenesis [213–215].
In addition to their potential as chemotherapeutic agents, green and black tea extracts also demonstrated chemopreventive activity in rodent models of breast carcinogenesis [216–219]. Green tea catechins and black tea theaflavins administered in the drinking water to transgenic multiple mammary adenocarcinoma (TAg) mice were shown to delay mammary carcinogenesis accompanied by an antioxidant effect in the target organ. The delay of carcinogenesis was reflected by a slight prolongation of mouse survival and significant decrease in tumor volumes [216]. Further evidence of the chemopreventive efficacy of theaflavins and EGCG was provided by a study that showed these compounds could inhibit or delay benzo(a)pyrene-induced lung carcinogenesis in mice [220]. Mice exposed to theaflavins and EGCG exhibited reduced expression of the proliferation-associated gene cyclooxygenase 2 (COX-2) as well as upregulation and increased activation of the apoptosis-inducing genes caspase-3 and caspase-7 [220]. Additionally, black tea theaflavins were shown to be potent inducers of apoptosis in human mammary epithelial carcinoma cells.
Once considered primarily radical scavengers, green tea catechin polyphenols are now understood to be multimodal acting molecules that direct numerous cellular neuroprotection/neurorescue mechanisms involving iron chelation, scavenging of oxygen and nitrogen radical species and activation of protein kinase C signaling pathway and prosurvival genes. Their nontoxic, lipophilic (and thus, brain-permeable) nature is advocated for iron removal from those brain areas where it preferentially accumulates in neurodegenerative diseases [221]. Metal ions, and iron in particular, are critical to catalyzing redox cycling; thus, iron-chelation therapy should be considered as a valuable strategy for the treatment of neurodegenerative diseases [222].
EGCG has been shown to improve age-related cognitive decline and to protect against cerebral IRIs [223,224], brain inflammation and neuronal damage in experimental autoimmune encephalomyelitis [225]. Long-term administration of green tea catechins or EGCG improved spatial cognition and learning ability in rats and reduced cerebral amyloidosis in Alzheimer’s transgenic mice, respectively [226,227]. A recent study of the molecular mechanism of the neurorescue effect of EGCG demonstrated decreased protein levels and mRNA expression of the enzyme prolyl 4-hydroxylase, which belongs to the family of iron-oxygen sensors of HIF prolyl hydroxylases that regulate cell survival and differentiation in cultured human neuroblastoma cells [228]. While the source of EGCG was not identified in this study, proteins and genes related to various specific neuroprotective/neurorescue effects of EGCG were identified. These interactions should be investigated further in animal models of neurodegenerative diseases.
Soy protein
Several studies have highlighted the beneficial effects of soy protein on CVD risk factors [229–231]. Age-related increase in iron stores is observed in both men and women and parallels the rise in CVD [232]. Consumption of commercially prepared powders of soy protein with native phytate after only 6 weeks was shown to reduce iron stores, as assessed by serum ferritin concentrations, in postmenopausal women [230]. Declines in serum iron and Tf saturation were also observed [230]. Phytic acid in soy protein isolates is a major inhibitor of nonheme iron absorption in humans [233,234]; thus, postmenopausal women who are at risk of excess iron may benefit by incorporating soy into their diets to reduce iron stores and lower CVD risk [230]. While consumption of soy protein in perimenopausal women did not reduce the rate of menopause-associated increases in iron stores, a beneficial effect on total antioxidant status was observed at 12 weeks in these women [235].
Lipoxygenase-mediated pathways play a major role in chronic inflammatory diseases. Results from a recent study reveal that soy isoflavones (purified from defatted soy flour and having an isoflavone content >95%) reduce the catalytically active ferric lipoxygenase to its resting ferrous form, indicating that soy isoflavones are natural redox inhibitors [236]. In a study of the signaling pathways underlying dietary tumor protection in rats, lifetime consumption of the soy isoflavone genistein and soy protein revealed that both dietary factors altered expression of mammary epithelial cell genes and identified a diverse subset of genes that may serve as potential biomarkers in other target tissues [237].
Additionally, soy proteins with isoflavones have been shown to impact the pathogenesis of insulin resistance and metabolic syndrome in obese male Zucker Diabetic Fatty (ZDF/Leprfa) rats [238]. In this study, both low and high isoflavone soy protein improved dyslipidemia and renal function in rats, compared with those on a casein-based diet; isoflavone-enriched soy protein had even greater antiadipogenic and hepatoprotective effects [238]. This study, along with previous investigations by this same group [238–242], indicates a potential therapeutic role for isoflavones in obesity and Type II diabetes and warrants further research to elucidate the mechanisms of soy phytochemicals on the development of insulin resistance and diabetes.
Future perspective
The spectrum of conditions that may be successfully treated with iron chelators has increased, as has the palette of chelators available to treat them. In particular, the development of deferiprone and deferasirox, new synthetic chelators with improved pharmacologic properties relative to deferoxamine, represent landmark accomplishments of the past few years. Although these chelators are not without their toxicities [120,243] and will continue to be evaluated, attention can now be more intensively focused on the application of iron chelators to a broad array of conditions related to oxidant stress, ranging from cardiovascular to neurodegenerative to inflammatory to malignant disease. Indeed, iron chelators appear very promising in many of these arenas, although investigations are largely still in preclinical and early clinical stages. The combined action of multiple chelators also offers fruitful ground for further investigation. Particularly encouraging has been the demonstration that smaller bidendate chelators can acquire iron from transferrin and shuttle it onto DFO [244] and the successful use of this combination in increasing cardiac function in patients with severe iron overload [245,246]. This strategy may find application not only in the treatment of iron overload, but in the treatment of neurodegenerative diseases and other conditions that represent known and potential targets for chelator therapy.
Perhaps most encouraging has been the recent recognition that a number of natural products contain endogenous substances that function as iron chelators. A caveat in interpretation of studies using natural products is that they are sometimes used as partially purified extracts, which can complicate the interpretation of results. Nevertheless, it is not unlikely that the chelating activity of naturally occurring dietary substances, such as the polyphenols, curcumin and others, may partially contribute to the widely acknowledged health benefit of dietary fruits and vegetables [247]. The recognition that dietary constituents long known to have beneficial health effects contain constituents with strong binding affinities for iron that can suppress Fenton chemistry [191] opens the prospect of extending the use of iron chelators beyond treatment for the prevention of disease. Optimization of the use of such agents in prevention will ultimately require studies of the potential of dietary iron and iron supplements to block their iron-chelation activity, as well as careful balancing of overall nutritional demands. Nevertheless, prospects for the use of iron chelators in the treatment and prevention of human disease appear greater than ever.
Executive summary.
Iron is the most abundant trace metal in the body
Iron is an essential element involved in oxygen transport and oxygen sensing, electron transfer, energy metabolism and DNA synthesis. Excess free iron that is not properly stored or chelated can catalyze the formation of tissue-damaging radicals and induce oxidative stress.
Iron chelators have been used to treat iron toxicity
Iron-chelation therapy originated in the treatment of iron-overload syndromes.
Preclinical and clinical studies suggest a role for iron chelators in the treatment of cancer and other diseases mediated by oxidative stress including ischemia–reperfusion injury, neurodegenerative and chronic inflammatory diseases.
Three major classes of iron chelators are chemically diverse
Siderophores are synthesized by microorganisms to manage iron uptake and storage. Deferoxamine mesylate is a medicinal siderophore and remains the current standard for iron-chelation therapy.
New orally effective synthetic iron chelators have shown promising clinical results in treating a broad spectrum of iron-dependent disorders.
A variety of phytochemicals with iron-binding properties are currently being investigated as potentially therapeutic iron chelators.
Conclusion
Iron-chelation therapy is an effective therapy for treating iron-overload disorders.
Iron chelators may have additional utility in the treatment of a broad array of conditions related to oxidant stress, ranging from cardiovascular to neurodegenerative to inflammatory to malignant disease.
Combinations of multiple chelators have shown promise and continue to be investigated.
The iron-chelating ability of many dietary substances likely contributes to the widely acknowledged health benefits of fruits and vegetables.
Acknowledgments
This work was supported in part by grants R01DK71892 (Suzy V Torti) and R37DK42412 (Frank M Torti) from the NIH. Heather C Hatcher and Ravi N Singh were supported in part by training grant T32CA079448. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Glossary
- Iron-overload disease
Characterized by excessive deposition with consequent injury or dysfunction of the heart, liver, anterior pituitary, pancreas or joints
- Neurodegenerative disease
Degenerative nerve diseases, including Alzheimer’s disease and Parkinson’s disease, that affect functions such as balance, movement, memory, talking, breathing and heart function
- Phytochemicals
Non-nutritive plant chemicals that have protective or disease-preventive properties
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NC. Disorders of iron metabolism. N. Engl. J. Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 3.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol. Aspects Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 4.Richardson DR. Therapeutic potential of iron chelators in cancer therapy. Adv. Exp. Med. Biol. 2002;509:231–249. doi: 10.1007/978-1-4615-0593-8_12. [DOI] [PubMed] [Google Scholar]
- 5.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 6.Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 7.Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol. Rev. 2005;57:547–583. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 8.Buss JL, Torti FM, Torti SV. The role of iron chelation in cancer therapy. Curr. Med. Chem. 2003;10:1021–1034. doi: 10.2174/0929867033457638. [DOI] [PubMed] [Google Scholar]
- 9.Buss JL, Greene BT, Turner J, Torti FM, Torti SV. Iron chelators in cancer chemotherapy. Curr. Top. Med. Chem. 2004;4:1623–1635. doi: 10.2174/1568026043387269. [DOI] [PubMed] [Google Scholar]
- 10.Galaris D, Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008;45:1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 13.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. ■ Comprehensive review of iron metabolism with a focus on several iron-disorder diseases.
- 14.Conrad ME, Umbreit JN. Pathways of iron absorption. Blood Cells Mol. Dis. 2002;29:336–355. doi: 10.1006/bcmd.2002.0564. [DOI] [PubMed] [Google Scholar]
- 15.Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 16.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T. Cellular iron: ferroportin is the only way out. Cell Metab. 2005;1:155–157. doi: 10.1016/j.cmet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Vulpe CD, Kuo YM, Murphy TL, et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the SLA mouse. Nat. Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- 19.Ohgami RS, Campagna DR, Greer EL, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl Acad. Sci. USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming MD, Trenor CC, 3rd, Su MA, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 22.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 23.Kawabata H, Nakamaki T, Ikonomi P, Smith RD, Germain RS, Koeffler HP. Expression of transferrin receptor 2 in normal and neoplastic hematopoietic cells. Blood. 2001;98:2714–2719. doi: 10.1182/blood.v98.9.2714. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata H, Tong X, Kawanami T, et al. Analyses for binding of the transferrin family of proteins to the transferrin receptor 2. Br. J. Haematol. 2004;127:464–473. doi: 10.1111/j.1365-2141.2004.05224.x. [DOI] [PubMed] [Google Scholar]
- 25.Barton JC. Optimal management strategies for chronic iron overload. Drugs. 2007;67:685–700. doi: 10.2165/00003495-200767050-00004. ■■ Recent clinical perspective of standard treatment for chronic iron overload.
- 26.Hider RC, Zhou T. The design of orally active iron chelators. Ann. NY Acad. Sci. 2005;1054:141–154. doi: 10.1196/annals.1345.017. [DOI] [PubMed] [Google Scholar]
- 27.Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23:95–104. doi: 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 30.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 31.Vitek L, Schwertner HA. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv. Clin. Chem. 2007;43:1–57. doi: 10.1016/s0065-2423(06)43001-8. [DOI] [PubMed] [Google Scholar]
- 32.De Domenico I, Ward DM, Langelier C, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol. Biol. Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 34.Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G199–G203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- 35.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 37.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi SO, Cho YS, Kim HL, Park JW. ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPα and STAT-3. Biochem. Biophys. Res. Commun. 2007;356:312–317. doi: 10.1016/j.bbrc.2007.02.137. [DOI] [PubMed] [Google Scholar]
- 39.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 40.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 41.Arosio P, Yokota M, Drysdale JW. Characterization of serum ferritin in iron overload: possible identity to natural apoferritin. Br. J. Haematol. 1977;36:199–207. doi: 10.1111/j.1365-2141.1977.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 42.Coffman LG, Parsonage D, D’Agostino R, Jr, Torti FM, Torti SV. Regulatory effects of ferritin on angiogenesis. Proc. Natl Acad. Sci. USA. 2009;106:570–575. doi: 10.1073/pnas.0812010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7:28–32. doi: 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- 44.O’Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ. Identification of hypoxically inducible mRNAs in HeLa cells using differential-display PCR. Role of hypoxia-inducible factor-1. Eur. J. Biochem. 1996;241:403–410. doi: 10.1111/j.1432-1033.1996.00403.x. [DOI] [PubMed] [Google Scholar]
- 45.Triantafyllou A, Liakos P, Tsakalof A, et al. The flavonoid quercetin induces hypoxia-inducible factor-1α (HIF-1α) and inhibits cell proliferation by depleting intracellular iron. Free Radic. Res. 2007;41:342–356. doi: 10.1080/10715760601055324. [DOI] [PubMed] [Google Scholar]
- 46.Jeon H, Kim H, Choi D, et al. Quercetin activates an angiogenic pathway, hypoxia inducible factor (HIF)-1-vascular endothelial growth factor, by inhibiting HIF-prolyl hydroxylase: a structural analysis of quercetin for inhibiting HIF-prolyl hydroxylase. Mol. Pharmacol. 2007;71:1676–1684. doi: 10.1124/mol.107.034041. [DOI] [PubMed] [Google Scholar]
- 47.Liu ZD, Hider RC. Design of clinically useful iron(III)-selective chelators. Med. Res. Rev. 2002;22:26–64. doi: 10.1002/med.1027. [DOI] [PubMed] [Google Scholar]
- 48.Porter JB. Evaluation of new iron chelators for clinical use. Acta. Haematol. 1996;95:13–25. doi: 10.1159/000203852. [DOI] [PubMed] [Google Scholar]
- 49.Le NT, Richardson DR. Competing pathways of iron chelation: angiogenesis or anti-tumor activity: targeting different molecules to induce specific effects. Int. J. Cancer. 2004;110:468–469. doi: 10.1002/ijc.20161. [DOI] [PubMed] [Google Scholar]
- 50.Yu Y, Wong J, Lovejoy DB, Kalinowski DS, Richardson DR. Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin. Cancer Res. 2006;12:6876–6883. doi: 10.1158/1078-0432.CCR-06-1954. [DOI] [PubMed] [Google Scholar]
- 51.Tang TY, Howarth SP, Miller SR, et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques in patients with asymptomatic carotid stenosis undergoing coronary artery bypass grafting: an ultrasmall superparamagnetic iron oxide enhanced magnetic resonance study. Eur. J. Vasc. Endovasc. Surg. 2008;35:392–398. doi: 10.1016/j.ejvs.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Deugnier Y, Brissot P, Loreal O. Iron and the liver: update 2008. J. Hepatol. 2008;48(Suppl. 1):S113–S123. doi: 10.1016/j.jhep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Ibrahim AS, Spellberg B, Edwards J., Jr Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr. Opin. Infect. Dis. 2008;21:620–625. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hider RC, Ma Y, Molina-Holgado F, Gaeta A, Roy S. Iron chelation as a potential therapy for neurodegenerative disease. Biochem. Soc. Trans. 2008;36:1304–1308. doi: 10.1042/BST0361304. [DOI] [PubMed] [Google Scholar]
- 55.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30:1926–1933. doi: 10.2337/dc06-2625. ■ Recent review of iron chelators and neurodegenerative diseases.
- 56.Li L, Frei B. Prolonged exposure to LPS increases iron, heme, and p22phox levels and NADPH oxidase activity in human aortic endothelial cells: inhibition by desferrioxamine. Arterioscler. Thromb. Vasc. Biol. 2009;29:732–738. doi: 10.1161/ATVBAHA.108.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan JL. Iron in arterial plaque: a modifiable risk factor for atherosclerosis. Biochim. Biophys. Acta. 2008;1790(7):718–723. doi: 10.1016/j.bbagen.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Haas H, Eisendle M, Turgeon BG. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008;46:149–187. doi: 10.1146/annurev.phyto.45.062806.094338. [DOI] [PubMed] [Google Scholar]
- 59.Askwith CC, de Silva D, Kaplan J. Molecular biology of iron acquisition in Saccharomyces cerevisiae. Mol. Microbiol. 1996;20:27–34. doi: 10.1111/j.1365-2958.1996.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 60.Vermylen C. What is new in iron overload? Eur. J. Pediatr. 2008;167:377–381. doi: 10.1007/s00431-007-0604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nick H. Iron chelation, quo vadis? Curr. Opin. Chem. Biol. 2007;11:419–423. doi: 10.1016/j.cbpa.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 62.Beutler E. Iron storage disease: facts, fiction and progress. Blood Cells Mol. Dis. 2007;39:140–147. doi: 10.1016/j.bcmd.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeder BJ, Hider RC, Wilson MT. Iron chelators can protect against oxidative stress through ferryl heme reduction. Free Radic. Biol. Med. 2008;44:264–273. doi: 10.1016/j.freeradbiomed.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Turner JJ, Rice-Evans CA, Davies MJ, Newman ES. Free radicals, myocytes and reperfusion injury. Biochem. Soc. Trans. 1990;18:1056–1059. doi: 10.1042/bst0181056. [DOI] [PubMed] [Google Scholar]
- 65.Miller YI, Felikman Y, Shaklai N. Hemoglobin induced apolipoprotein B crosslinking in low-density lipoprotein peroxidation. Arch. Biochem. Biophys. 1996;326:252–260. doi: 10.1006/abbi.1996.0073. [DOI] [PubMed] [Google Scholar]
- 66.Reeder BJ, Wilson MT. Desferrioxamine inhibits production of cytotoxic heme to protein cross-linked myoglobin: a mechanism to protect against oxidative stress without iron chelation. Chem. Res. Toxicol. 2005;18:1004–1011. doi: 10.1021/tx049660y. [DOI] [PubMed] [Google Scholar]
- 67.Maggio A. Light and shadows in the iron chelation treatment of haematological diseases. Br. J. Haematol. 2007;138:407–421. doi: 10.1111/j.1365-2141.2007.06666.x. ■ Clinical effectiveness of iron chelators in hematologic diseases including thalassaemia major, sickle-cell disorders and myelodysplasia.
- 68.Delea TE, Edelsberg J, Sofrygin O, et al. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. Transfusion. 2007;47:1919–1929. doi: 10.1111/j.1537-2995.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 69.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br. J. Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 70.Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006;107:3733–3737. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- 71.Davis BA, O’Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–269. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 72.Porter JB. Concepts and goals in the management of transfusional iron overload. Am. J. Hematol. 2007;82:1136–1139. doi: 10.1002/ajh.21100. [DOI] [PubMed] [Google Scholar]
- 73.Porter JB, Huehns ER. Transfusion and exchange transfusion in sickle cell anaemias, with particular reference to iron metabolism. Acta Haematol. 1987;78:198–205. doi: 10.1159/000205875. [DOI] [PubMed] [Google Scholar]
- 74.Leitch HA. Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk. Res. 2007;31(Suppl. 3):S7–S9. doi: 10.1016/S0145-2126(07)70460-5. [DOI] [PubMed] [Google Scholar]
- 75.Cazzola M, Malcovati L. Myelodysplastic syndromes – coping with ineffective hematopoiesis. N. Engl. J. Med. 2005;352:536–538. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 76.Balducci L. Transfusion independence in patients with myelodysplastic syndromes: impact on outcomes and quality of life. Cancer. 2006;106:2087–2094. doi: 10.1002/cncr.21860. [DOI] [PubMed] [Google Scholar]
- 77.Gattermann N. Guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Leuk. Res. 2007;31(Suppl. 3):S10–S15. doi: 10.1016/S0145-2126(07)70461-7. [DOI] [PubMed] [Google Scholar]
- 78.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J. Am. Soc. Nephrol. 2007;18:16–28. doi: 10.1681/ASN.2006050500. [DOI] [PubMed] [Google Scholar]
- 79.Aigner E, Theurl I, Theurl M, et al. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2008;87:1374–1383. doi: 10.1093/ajcn/87.5.1374. [DOI] [PubMed] [Google Scholar]
- 80.Ramm GA, Ruddell RG. Hepatotoxicity of iron overload: mechanisms of iron-induced hepatic fibrogenesis. Semin. Liver Dis. 2005;25:433–449. doi: 10.1055/s-2005-923315. [DOI] [PubMed] [Google Scholar]
- 81.Swaminathan S, High WA, Ranville J, et al. Cardiac and vascular metal deposition with high mortality in nephrogenic systemic fibrosis. Kidney Int. 2008;73:1413–1418. doi: 10.1038/ki.2008.76. [DOI] [PubMed] [Google Scholar]
- 82.de Vries B, Walter SJ, von Bonsdorff L, et al. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation. 2004;77:669–675. doi: 10.1097/01.tp.0000115002.28575.e7. [DOI] [PubMed] [Google Scholar]
- 83.Yuan XM, Li W. Iron involvement in multiple signaling pathways of atherosclerosis: a revisited hypothesis. Curr. Med. Chem. 2008;15:2157–2172. doi: 10.2174/092986708785747634. [DOI] [PubMed] [Google Scholar]
- 84.Ren M, Rajendran R, Ning P, et al. Zinc supplementation decreases the development of atherosclerosis in rabbits. Free Radic. Biol. Med. 2006;41:222–225. doi: 10.1016/j.freeradbiomed.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 85.Ferrara DE, Taylor WR. Iron chelation and vascular function: in search of the mechanisms. Arterioscler. Thromb. Vasc. Biol. 2005;25:2235–2237. doi: 10.1161/01.ATV.0000189303.45609.1f. [DOI] [PubMed] [Google Scholar]
- 86.Ishizaka N, Saito K, Mori I, Matsuzaki G, Ohno M, Nagai R. Iron chelation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin II-infused rats. Arterioscler. Thromb. Vasc. Biol. 2005;25:2282–2288. doi: 10.1161/01.ATV.0000181763.57495.2b. [DOI] [PubMed] [Google Scholar]
- 87.Cutler P. Deferoxamine therapy in high-ferritin diabetes. Diabetes. 1989;38:1207–1210. doi: 10.2337/diab.38.10.1207. [DOI] [PubMed] [Google Scholar]
- 88.Nitenberg A, Ledoux S, Valensi P, Sachs R, Antony I. Coronary microvascular adaptation to myocardial metabolic demand can be restored by inhibition of iron-catalyzed formation of oxygen free radicals in type 2 diabetic patients. Diabetes. 2002;51:813–818. doi: 10.2337/diabetes.51.3.813. [DOI] [PubMed] [Google Scholar]
- 89.Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am. J. Pathol. 2008;172:738–747. doi: 10.2353/ajpath.2008.070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Platis O, Anagnostopoulos G, Farmaki K, Posantzis M, Gotsis E, Tolis G. Glucose metabolism disorders improvement in patients with thalassaemia major after 24–36 months of intensive chelation therapy. Pediatr. Endocrinol. Rev. 2004;2(Suppl. 2):279–281. [PubMed] [Google Scholar]
- 91.Zheng Y, Li XK, Wang Y, Cai L. The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. Hemoglobin. 2008;32:135–145. doi: 10.1080/03630260701727077. [DOI] [PubMed] [Google Scholar]
- 92.Christoforidis A, Perifanis V, Athanassiou-Metaxa M. Combined chelation therapy improves glucose metabolism in patients with β-thalassaemia major. Br. J. Haematol. 2006;135:271–272. doi: 10.1111/j.1365-2141.2006.06296.x. [DOI] [PubMed] [Google Scholar]
- 93.Christoforidis A, Perifanis V, Tsatra I, Vlachaki E, Athanassiou-Metaxa M. Evolution of OGTT in patients with β-thalassaemia major in relation to chelation therapy. Diabetes Res. Clin. Pract. 2007;76:6–11. doi: 10.1016/j.diabres.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Farmaki K, Angelopoulos N, Anagnostopoulos G, Gotsis E, Rombopoulos G, Tolis G. Effect of enhanced iron chelation therapy on glucose metabolism in patients with β-thalassaemia major. Br. J. Haematol. 2006;134:438–444. doi: 10.1111/j.1365-2141.2006.06203.x. [DOI] [PubMed] [Google Scholar]
- 95.Pahl PM, Horwitz LD. Cell permeable iron chelators as potential cancer chemotherapeutic agents. Cancer Invest. 2005;23:683–691. doi: 10.1080/07357900500359976. [DOI] [PubMed] [Google Scholar]
- 96.Brard L, Granai CO, Swamy N. Iron chelators deferoxamine and diethylenetriamine pentaacetic acid induce apoptosis in ovarian carcinoma. Gynecol. Oncol. 2006;100:116–127. doi: 10.1016/j.ygyno.2005.07.129. [DOI] [PubMed] [Google Scholar]
- 97.Hoke EM, Maylock CA, Shacter E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic. Biol. Med. 2005;39:403–411. doi: 10.1016/j.freeradbiomed.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 98.Lee SK, Lee J, Min SK, et al. Iron chelator differentially activates macrophage inflammatory protein-3α/CCL20 in immortalized and malignant human oral keratinocytes. Arch. Oral Biol. 2008;53:801–809. doi: 10.1016/j.archoralbio.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 99.Richardson DR, Kalinowski DS, Lau S, Jansson PJ, Lovejoy DB. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim. Biophys. Acta. 2008;1790(7):702–717. doi: 10.1016/j.bbagen.2008.04.003. ■ Recent review examining novel synthetic iron chelators for cancer treatment.
- 100.Bergeron RJ, Wiegand J, Dionis JB, et al. Evaluation of desferrithiocin and its synthetic analogues as orally effective iron chelators. J. Med. Chem. 1991;34:2072–2078. doi: 10.1021/jm00111a023. [DOI] [PubMed] [Google Scholar]
- 101.Kicic A, Chua AC, Baker E. The desferrithiocin (DFT) class of iron chelators: potential as antineoplastic agents. Anticancer Drug Des. 2001;16:195–207. [PubMed] [Google Scholar]
- 102.Kicic A, Chua AC, Baker E. Desferrithiocin is a more potent antineoplastic agent than desferrioxamine. Br. J. Pharmacol. 2002;135:1393–1402. doi: 10.1038/sj.bjp.0704507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bergeron RJ, Streiff RR, Creary EA, et al. A comparative study of the iron-clearing properties of desferrithiocin analogues with desferrioxamine B in a Cebus monkey model. Blood. 1993;81:2166–2173. [PubMed] [Google Scholar]
- 104.Kattamis A, Ladis V, Berdousi H, et al. Iron chelation treatment with combined therapy with deferiprone and deferioxamine: a 12-month trial. Blood Cells Mol. Dis. 2006;36:21–25. doi: 10.1016/j.bcmd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 105.Sajid R, Ghani F, Adil S, Khurshid M. Oral iron chelation therapy with deferiprone in patients with Thalassemia Major. J. Pak. Med. Assoc. 2009;59:388–390. [PubMed] [Google Scholar]
- 106.Molina-Holgado F, Gaeta A, Francis PT, Williams RJ, Hider RC. Neuroprotective actions of deferiprone in cultured cortical neurones and SHSY-5Y cells. J. Neurochem. 2008;105(6):2466–2476. doi: 10.1111/j.1471-4159.2008.05332.x. [DOI] [PubMed] [Google Scholar]
- 107.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 108.Mandel S, Grunblatt E, Riederer P, Gerlach M, Levites Y, Youdim MB. Neuroprotective strategies in Parkinson’s disease: an update on progress. CNS Drugs. 2003;17:729–762. doi: 10.2165/00023210-200317100-00004. [DOI] [PubMed] [Google Scholar]
- 109.Whitnall M, Richardson DR. Iron: a new target for pharmacological intervention in neurodegenerative diseases. Semin. Pediatr. Neurol. 2006;13:186–197. doi: 10.1016/j.spen.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Cherny RA, Atwood CS, Xilinas ME, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 111.Kaur D, Yantiri F, Rajagopalan S, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 112.Liu G, Men P, Harris PL, Rolston RK, Perry G, Smith MA. Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci. Lett. 2006;406:189–193. doi: 10.1016/j.neulet.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 113.Shen AY, Wu SN, Chiu CT. Synthesis and cytotoxicity evaluation of some 8-hydroxyquinoline derivatives. J. Pharm. Pharmacol. 1999;51:543–548. doi: 10.1211/0022357991772826. [DOI] [PubMed] [Google Scholar]
- 114.Gaboriau F, Laupen-Chassay C, Pasdeloup N, Pierre JL, Brissot P, Lescoat G. Modulation of cell proliferation and polyamine metabolism in rat liver cell cultures by the iron chelator O-trensox. Biometals. 2006;19:623–632. doi: 10.1007/s10534-006-6888-y. [DOI] [PubMed] [Google Scholar]
- 115.Rakba N, Loyer P, Gilot D, et al. Antiproliferative and apoptotic effects of O-trensox, a new synthetic iron chelator, on differentiated human hepatoma cell lines. Carcinogenesis. 2000;21:943–951. doi: 10.1093/carcin/21.5.943. [DOI] [PubMed] [Google Scholar]
- 116.Ballardini G, Groff P, Zoli M, et al. Increased risk of hepatocellular carcinoma development in patients with cirrhosis and with high hepatocellular proliferation. J. Hepatol. 1994;20:218–222. doi: 10.1016/s0168-8278(05)80061-3. [DOI] [PubMed] [Google Scholar]
- 117.Tarao K, Shimizu A, Ohkawa S, et al. Development of hepatocellular carcinoma associated with increases in DNA synthesis in the surrounding cirrhosis. Gastroenterology. 1992;103:595–600. doi: 10.1016/0016-5085(92)90852-p. [DOI] [PubMed] [Google Scholar]
- 118.Cappellini MD, Cohen A, Piga A, et al. A Phase III study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 119.Piga A, Galanello R, Forni GL, et al. Randomized Phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873–880. [PubMed] [Google Scholar]
- 120.Vichinsky E. Clinical application of deferasirox: practical patient management. Am. J. Hematol. 2008;83:398–402. doi: 10.1002/ajh.21119. [DOI] [PubMed] [Google Scholar]
- 121.Yang LP, Keam SJ, Keating GM. Deferasirox: a review of its use in the management of transfusional chronic iron overload. Drugs. 2007;67:2211–2230. doi: 10.2165/00003495-200767150-00007. [DOI] [PubMed] [Google Scholar]
- 122.Porter J, Galanello R, Saglio G, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur. J. Haematol. 2008;80:168–176. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Torti SV, Torti FM, Whitman SP, Brechbiel MW, Park G, Planalp RP. Tumor cell cytotoxicity of a novel metal chelator. Blood. 1998;92:1384–1389. [PubMed] [Google Scholar]
- 124.Zhao R, Planalp RP, Ma R, et al. Role of zinc and iron chelation in apoptosis mediated by tachpyridine, an anti-cancer iron chelator. Biochem. Pharmacol. 2004;67:1677–1688. doi: 10.1016/j.bcp.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 125.Chong HS, Torti SV, Ma R, Torti FM, Brechbiel MW. Synthesis and potent antitumor activities of novel 1,3,5-cis,cis-triaminocyclohexane N-pyridyl derivatives. J. Med. Chem. 2004;47:5230–5234. doi: 10.1021/jm040076w. [DOI] [PubMed] [Google Scholar]
- 126.Turner J, Koumenis C, Kute TE, et al. Tachpyridine, a metal chelator, induces G2 cell-cycle arrest, activates checkpoint kinases, and sensitizes cells to ionizing radiation. Blood. 2005;106:3191–3199. doi: 10.1182/blood-2005-03-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Childers ML, Cho J, Regino CA, et al. Influence of ligand structure on Fe(II) spin-state and redox rate in cytotoxic tripodal chelators. J. Inorg. Biochem. 2008;102:150–156. doi: 10.1016/j.jinorgbio.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hasinoff BB, Herman EH. Dexrazoxane: how it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc. Toxicol. 2007;7:140–144. doi: 10.1007/s12012-007-0023-3. [DOI] [PubMed] [Google Scholar]
- 129.Herman EH, Ferrans VJ. Examination of the potential long-lasting protective effect of ICRF-187 against anthracycline-induced chronic cardiomyopathy. Cancer Treat. Rev. 1990;17:155–160. doi: 10.1016/0305-7372(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 130.Swain SM, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J. Cancer Res. Clin. Oncol. 2004;130:1–7. doi: 10.1007/s00432-003-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hasinoff BB, Hellmann K, Herman EH, Ferrans VJ. Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr. Med. Chem. 1998;5:1–28. [PubMed] [Google Scholar]
- 132.Hasinoff BB, Schroeder PE, Patel D. The metabolites of the cardioprotective drug dexrazoxane do not protect myocytes from doxorubicin-induced cytotoxicity. Mol. Pharmacol. 2003;64:670–678. doi: 10.1124/mol.64.3.670. [DOI] [PubMed] [Google Scholar]
- 133.Hasinoff BB. Dexrazoxane (ICRF-187) protects cardiac myocytes against hypoxiareoxygenation damage. Cardiovasc. Toxicol. 2002;2:111–118. doi: 10.1385/ct:2:2:111. [DOI] [PubMed] [Google Scholar]
- 134.Wadler S, Makower D, Clairmont C, Lambert P, Fehn K, Sznol M. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by 96-hour intravenous continuous infusion. J. Clin. Oncol. 2004;22:1553–1563. doi: 10.1200/JCO.2004.07.158. [DOI] [PubMed] [Google Scholar]
- 135.Shao J, Zhou B, Zhu L, et al. In vitro characterization of enzymatic properties and inhibition of the p53R2 subunit of human ribonucleotide reductase. Cancer Res. 2004;64:1–6. doi: 10.1158/0008-5472.can-03-3048. [DOI] [PubMed] [Google Scholar]
- 136.Barker CA, Burgan WE, Carter DJ, et al. In vitro and in vivo radiosensitization induced by the ribonucleotide reductase inhibitor Triapine (3-aminopyridine-2-carboxaldehydethiosemicarbazone) Clin. Cancer Res. 2006;12:2912–2918. doi: 10.1158/1078-0432.CCR-05-2860. [DOI] [PubMed] [Google Scholar]
- 137.Yen Y, Margolin K, Doroshow J, et al. A Phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother. Pharmacol. 2004;54:331–342. doi: 10.1007/s00280-004-0821-2. [DOI] [PubMed] [Google Scholar]
- 138.Yee KW, Cortes J, Ferrajoli A, et al. Triapine and cytarabine is an active combination in patients with acute leukemia or myelodysplastic syndrome. Leuk. Res. 2006;30:813–822. doi: 10.1016/j.leukres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 139.Lovejoy DB, Richardson DR. Iron chelators as anti-neoplastic agents: current developments and promise of the PIH class of chelators. Curr. Med. Chem. 2003;10:1035–1049. doi: 10.2174/0929867033457557. [DOI] [PubMed] [Google Scholar]
- 140.Simunek T, Klimtova I, Kaplanova J, et al. Study of daunorubicin cardiotoxicity prevention with pyridoxal isonicotinoyl hydrazone in rabbits. Pharmacol. Res. 2005;51:223–231. doi: 10.1016/j.phrs.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 141.Darnell G, Richardson DR. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents III. The effect of the ligands on molecular targets involved in proliferation. Blood. 1999;94:781–792. [PubMed] [Google Scholar]
- 142.Debebe Z, Ammosova T, Jerebtsova M, et al. Iron chelators ICL670 and 311 inhibit HIV-1 transcription. Virology. 2007;367:324–333. doi: 10.1016/j.virol.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Becker EM, Lovejoy DB, Greer JM, Watts R, Richardson DR. Identification of the di-pyridyl ketone isonicotinoyl hydrazone (PKIH) analogues as potent iron chelators and anti-tumour agents. Br. J. Pharmacol. 2003;138:819–830. doi: 10.1038/sj.bjp.0705089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chaston TB, Watts RN, Yuan J, Richardson DR. Potent antitumor activity of novel iron chelators derived from di-2-pyridylketone isonicotinoyl hydrazone involves fenton-derived free radical generation. Clin. Cancer Res. 2004;10:7365–7374. doi: 10.1158/1078-0432.CCR-04-0865. [DOI] [PubMed] [Google Scholar]
- 145.Bernhardt PV, Wilson GJ, Sharpe PC, Kalinowski DS, Richardson DR. Tuning the antiproliferative activity of biologically active iron chelators: characterization of the coordination chemistry and biological efficacy of 2-acetylpyridine and 2-benzoylpyridine hydrazone ligands. J. Biol. Inorg. Chem. 2008;13:107–119. doi: 10.1007/s00775-007-0300-4. [DOI] [PubMed] [Google Scholar]
- 146.Yuan J, Lovejoy DB, Richardson DR. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 147.Noulsri E, Richardson DR, Lerdwana S, et al. Antitumor activity and mechanism of action of the iron chelator, Dp44mT, against leukemic cells. Am. J. Hematol. 2009;84:170–176. doi: 10.1002/ajh.21350. [DOI] [PubMed] [Google Scholar]
- 148.Rao VA, Klein SR, Agama KK, et al. The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIα in breast cancer cells. Cancer Res. 2009;69:948–957. doi: 10.1158/0008-5472.CAN-08-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc. Natl Acad. Sci. USA. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. ■ Comprehensive overview of curcumin with insight into its chemical properties as an iron chelator.
- 151.Hider RC, Liu ZD, Khodr HH. Metal chelation of polyphenols. Methods Enzymol. 2001;335:190–203. doi: 10.1016/s0076-6879(01)35243-6. [DOI] [PubMed] [Google Scholar]
- 152.Morel I, Lescoat G, Cogrel P, et al. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993;45:13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- 153.Guo M, Harvey I, Yang W, et al. Synergistic anion and metal binding to the ferric ion-binding protein from Neisseria gonorrhoeae. J. Biol. Chem. 2003;278:2490–2502. doi: 10.1074/jbc.M208776200. [DOI] [PubMed] [Google Scholar]
- 154.Baker HM, Anderson BF, Baker EN. Dealing with iron: common structural principles in proteins that transport iron and heme. Proc. Natl Acad. Sci. USA. 2003;100:3579–3583. doi: 10.1073/pnas.0637295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alexeev D, Zhu H, Guo M, et al. A novel protein–mineral interface. Nat. Struct. Biol. 2003;10:297–302. doi: 10.1038/nsb903. [DOI] [PubMed] [Google Scholar]
- 156.Guo M, Harvey I, Campopiano DJ, Sadler PJ. Short oxo-titanium(IV) bond in bacterial transferrin: a protein target for metalloantibiotics. Angew. Chem. Int. Ed. Engl. 2006;45:2758–2761. doi: 10.1002/anie.200600260. [DOI] [PubMed] [Google Scholar]
- 157.Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc. Natl Acad. Sci. USA. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu H, Alexeev D, Hunter DJ, Campopiano DJ, Sadler PJ. Oxo-iron clusters in a bacterial iron-trafficking protein: new roles for a conserved motif. Biochem. J. 2003;376:35–41. doi: 10.1042/BJ20031283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 160.Rao YK, Geethangili M, Fang SH, Tzeng YM. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: a comparative study. Food Chem. Toxicol. 2007;45:1770–1776. doi: 10.1016/j.fct.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 161.Hu JP, Calomme M, Lasure A, et al. Structure-activity relationship of flavonoids with superoxide scavenging activity. Biol. Trace Elem. Res. 1995;47:327–331. doi: 10.1007/BF02790134. [DOI] [PubMed] [Google Scholar]
- 162.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 163.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 164.Khanbabaee K, van Ree T. Tannins: classification and definition. Nat. Prod. Rep. 2001;18:641–649. doi: 10.1039/b101061l. [DOI] [PubMed] [Google Scholar]
- 165.Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008;52:79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 166.Cos P, De Bruyne T, Hermans N, Apers S, Berghe DV, Vlietinck AJ. Proanthocyanidins in health care: current and new trends. Curr. Med. Chem. 2004;11:1345–1359. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 167.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 168.Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J. AOAC Int. 2006;89:1121–1134. [PubMed] [Google Scholar]
- 169.Shoji T, Akazome Y, Kanda T, Ikeda M. The toxicology and safety of apple polyphenol extract. Food Chem. Toxicol. 2004;42:959–967. doi: 10.1016/j.fct.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 170.Jiao Y, Wilkinson Jt, Di X, et al. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113:462–469. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bernabe-Pineda M, Ramirez-Silva MT, Romero-Romo MA, Gonzalez-Vergara E, Rojas-Hernandez A. Spectrophotometric and electrochemical determination of the formation constants of the complexes Curcumin–Fe(III)–water and Curcumin– Fe(II)–water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004;60:1105–1113. doi: 10.1016/S1386-1425(03)00344-5. [DOI] [PubMed] [Google Scholar]
- 172.Jiao Y, Wilkinson Jt, Christine Pietsch E, et al. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 173.Thephinlap C, Phisalaphong C, Fucharoen S, Porter JB, Srichairatanakool S. Efficacy of curcuminoids in alleviation of iron overload and lipid peroxidation in thalassemic mice. Med. Chem. 2009;5(5):474–482. doi: 10.2174/157340609789117912. [DOI] [PubMed] [Google Scholar]
- 174.Dhanasekaran M, Tharakan B, Manyam BV. Antiparkinson drug – mucuna pruriens shows antioxidant and metal chelating activity. Phytother. Res. 2008;22:6–11. doi: 10.1002/ptr.2109. [DOI] [PubMed] [Google Scholar]
- 175.Mandel SA, Avramovich-Tirosh Y, Reznichenko L, et al. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14:46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- 176.Rathore P, Dohare P, Varma S, et al. Curcuma oil: reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 2008;33:1672–1682. doi: 10.1007/s11064-007-9515-6. [DOI] [PubMed] [Google Scholar]
- 177.Kahles T, Luedike P, Endres M, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 178.Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J. Int. Med. Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- 179.Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia–reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 180.Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. Neuromolecular. Med. 2008;10:259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Farombi EO, Nwaokeafor IA. Anti-oxidant mechanisms of kolaviron: studies on serum lipoprotein oxidation, metal chelation and oxidative membrane damage in rats. Clin. Exp. Pharmacol. Physiol. 2005;32:667–674. doi: 10.1111/j.0305-1870.2005.04248.x. [DOI] [PubMed] [Google Scholar]
- 182.Botelho FV, Alvarez-Leite JI, Lemos VS, et al. Physicochemical study of floranol, its copper(II) and iron(III) complexes, and their inhibitory effect on LDL oxidation. J. Inorg. Biochem. 2007;101:935–943. doi: 10.1016/j.jinorgbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 183.Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 184.Maffei Facino R, Carini M, Aldini G, et al. Procyanidines from Vitis vinifera seeds protect rabbit heart from ischemia/reperfusion injury: antioxidant intervention and/or iron and copper sequestering ability. Planta. Med. 1996;62:495–502. doi: 10.1055/s-2006-957956. [DOI] [PubMed] [Google Scholar]
- 185.Yoshino M, Murakami K. Interaction of iron with polyphenolic compounds: application to antioxidant characterization. Anal. Biochem. 1998;257:40–44. doi: 10.1006/abio.1997.2522. [DOI] [PubMed] [Google Scholar]
- 186.Perez CA, Wei Y, Guo M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009;103:326–332. doi: 10.1016/j.jinorgbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee HZ, Leung HW, Lai MY, Wu CH. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005;25:959–964. [PubMed] [Google Scholar]
- 188.Lee JH, Li YC, Ip SW, et al. The role of Ca2+ in baicalein-induced apoptosis in human breast MDA-MB-231 cancer cells through mitochondria- and caspase-3-dependent pathway. Anticancer Res. 2008;28:1701–1711. [PubMed] [Google Scholar]
- 189.Zhang Y, Li H, Zhao Y, Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur. J. Pharmacol. 2006;535:263–269. doi: 10.1016/j.ejphar.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 190.Zhao Y, Li H, Gao Z, Xu H. Effects of dietary baicalin supplementation on iron overload-induced mouse liver oxidative injury. Eur. J. Pharmacol. 2005;509:195–200. doi: 10.1016/j.ejphar.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 191.Guo M, Perez C, Wei Y, et al. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007;21(43):4951–4961. doi: 10.1039/b705136k. ■■ Comprehensive study of the iron-binding properties of quercetin, the major phenolic phytochemical present in cranberries, and other selected phenolic compounds that provides insight into the mechanism of action of bioactive phenolics by confirming the identity of ‘iron-binding motifs’ in their structures.
- 192.Zhang Z, Wei T, Hou J, Li G, Yu S, Xin W. Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. Eur. J. Pharmacol. 2003;467:41–47. doi: 10.1016/s0014-2999(03)01597-8. ■ Overview of quercetin and other phenolic compounds and their efficacy in modulating cellular iron homeostasis.
- 193.Feng L, Ke N, Cheng F, et al. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J. Surg. Res. 2009 doi: 10.1016/j.jss.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 194.Feng L, Xiong Y, Cheng F, Zhang L, Li S, Li Y. Effect of ligustrazine on ischemiareperfusion injury in murine kidney. Transplant Proc. 2004;36:1949–1951. doi: 10.1016/j.transproceed.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 195.Moridani MY, Pourahmad J, Bui H, Siraki A, O’Brien PJ. Dietary flavonoid iron complexes as cytoprotective superoxide radical scavengers. Free Radic. Biol. Med. 2003;34:243–253. doi: 10.1016/s0891-5849(02)01241-8. [DOI] [PubMed] [Google Scholar]
- 196.Spencer JP, Abd-el-Mohsen MM, Rice-Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: implications for their bioactivity. Arch. Biochem. Biophys. 2004;423:148–161. doi: 10.1016/j.abb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 197.Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon) J. Agric. Food Chem. 2002;50:5844–5849. doi: 10.1021/jf0202234. [DOI] [PubMed] [Google Scholar]
- 198.Porter ML, Krueger CG, Wiebe DA, Cunningham DG, Reed JD. Cranberry proanthocyanidins associate with low-density lipoprotein and inhibit in vitro Cu2+-induced oxidation. J. Sci. Food Agric. 2001;81:1306–1313. [Google Scholar]
- 199.Neto CC, Sweeney-Nixon MI, Lamoureaux TL, Solomon F, Kondo M, MacKinnon SL. Cranberry Phenolics: Effects on Oxidative Processes, Neuron Cell Death and Tumor Cell Growth. ACS Books; Columbus, OH, USA: 2005. pp. 271–282. [Google Scholar]
- 200.Youdim KA, McDonald J, Kalt W, Joseph JA. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults (small star, filled) J. Nutr. Biochem. 2002;13:282–288. doi: 10.1016/s0955-2863(01)00221-2. [DOI] [PubMed] [Google Scholar]
- 201.Seeram NP, Adams LS, Hardy ML, Heber D. Total cranberry extract versus its phytochemical constituents: antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004;52:2512–2517. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- 202.Bagchi D, Sen CK, Ray SD, et al. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat. Res. 2003;523–524:87–97. doi: 10.1016/s0027-5107(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 203.Frederiksen H, Mortensen A, Schroder M, et al. Effects of red grape skin and seed extract supplementation on atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Mol. Nutr. Food Res. 2007;51:564–571. doi: 10.1002/mnfr.200700009. [DOI] [PubMed] [Google Scholar]
- 204.Kim EY, Ham SK, Shigenaga MK, Han O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J. Nutr. 2008;138:1647–1651. doi: 10.1093/jn/138.9.1647. [DOI] [PubMed] [Google Scholar]
- 205.Sato M, Maulik G, Ray PS, Bagchi D, Das DK. Cardioprotective effects of grape seed proanthocyanidin against ischemic reperfusion injury. J. Mol. Cell Cardiol. 1999;31:1289–1297. doi: 10.1006/jmcc.1999.0961. [DOI] [PubMed] [Google Scholar]
- 206.Reznichenko L, Amit T, Zheng H, et al. Reduction of iron-regulated amyloid precursor protein and β-amyloid peptide by (−)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer’s disease. J. Neurochem. 2006;97:527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 207.Cai F, Li CR, Wu JL, et al. Theaflavin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-inflammatory effect and modulation of STAT-1. Mediators Inflamm. 2006;2006:30490. doi: 10.1155/MI/2006/30490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta. 1996;1304:210–222. doi: 10.1016/s0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 209.Kumamoto M, Sonda T, Nagayama K, Tabata M. Effects of pH and metal ions on antioxidative activities of catechins. Biosci. Biotechnol. Biochem. 2001;65:126–132. doi: 10.1271/bbb.65.126. [DOI] [PubMed] [Google Scholar]
- 210.Grinberg LN, Newmark H, Kitrossky N, Rahamim E, Chevion M, Rachmilewitz EA. Protective effects of tea polyphenols against oxidative damage to red blood cells. Biochem. Pharmacol. 1997;54:973–978. doi: 10.1016/s0006-2952(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 211.Friedman M, Mackey BE, Kim HJ, et al. Structure–activity relationships of tea compounds against human cancer cells. J. Agric. Food Chem. 2007;55:243–253. doi: 10.1021/jf062276h. [DOI] [PubMed] [Google Scholar]
- 212.Lahiry L, Saha B, Chakraborty J, et al. Contribution of p53-mediated Bax transactivation in theaflavin-induced mammary epithelial carcinoma cell apoptosis. Apoptosis. 2008;13:771–781. doi: 10.1007/s10495-008-0213-x. [DOI] [PubMed] [Google Scholar]
- 213.Lu J, Gosslau A, Liu AY, Chen KY. PCR differential display-based identification of regulator of G protein signaling 10 as the target gene in human colon cancer cells induced by black tea polyphenol theaflavin monogallate. Eur. J. Pharmacol. 2008;601:66–72. doi: 10.1016/j.ejphar.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 214.Cao X, Qin J, Xie Y, et al. Regulator of G-protein signaling 2 (RGS2) inhibits androgen-independent activation of androgen receptor in prostate cancer cells. Oncogene. 2006;25:3719–3734. doi: 10.1038/sj.onc.1209408. [DOI] [PubMed] [Google Scholar]
- 215.Kelly P, Moeller BJ, Juneja J, et al. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc. Natl Acad. Sci. USA. 2006;103:8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kaur S, Greaves P, Cooke DN, et al. Breast cancer prevention by green tea catechins and black tea theaflavins in the C3(1) SV40 T,t antigen transgenic mouse model is accompanied by increased apoptosis and a decrease in oxidative DNA adducts. J. Agric. Food Chem. 2007;55:3378–3385. doi: 10.1021/jf0633342. [DOI] [PubMed] [Google Scholar]
- 217.Rogers AE, Hafer LJ, Iskander YS, Yang S. Black tea and mammary gland carcinogenesis by 7,12-dimethylbenz[a]anthracene in rats fed control or high fat diets. Carcinogenesis. 1998;19:1269–1273. doi: 10.1093/carcin/19.7.1269. [DOI] [PubMed] [Google Scholar]
- 218.Weisburger JH, Rivenson A, Garr K, Aliaga C. Tea, or tea and milk, inhibit mammary gland and colon carcinogenesis in rats. Cancer Lett. 1997;114:323–327. doi: 10.1016/s0304-3835(97)04693-4. [DOI] [PubMed] [Google Scholar]
- 219.Zhou JR, Yu L, Mai Z, Blackburn GL. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int. J. Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Banerjee S, Manna S, Mukherjee S, Pal D, Panda CK, Das S. Black tea polyphenols restrict benzopyrene-induced mouse lung cancer progression through inhibition of Cox-2 and induction of caspase-3 expression. Asian Pac. J. Cancer Prev. 2006;7:661–666. [PubMed] [Google Scholar]
- 221.Mandel S, Weinreb O, Reznichenko L, Kalfon L, Amit T. Green tea catechins as brain-permeable, non toxic iron chelators to “iron out iron” from the brain. J. Neural Transm. Suppl. 2006;71:249–257. doi: 10.1007/978-3-211-33328-0_26. [DOI] [PubMed] [Google Scholar]
- 222.Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MB. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J. Nutr. 2008;138:1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- 223.Lee S, Suh S, Kim S. Protective effects of the green tea polyphenol (−)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci. Lett. 2000;287:191–194. doi: 10.1016/s0304-3940(00)01159-9. [DOI] [PubMed] [Google Scholar]
- 224.Sutherland BA, Shaw OM, Clarkson AN, Jackson DN, Sammut IA, Appleton I. Neuroprotective effects of (−)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB. J. 2005;19:258–260. doi: 10.1096/fj.04-2806fje. [DOI] [PubMed] [Google Scholar]
- 225.Aktas O, Prozorovski T, Smorodchenko A, et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-κ B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 226.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J. Nutr. 2006;136:1043–1047. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 227.Rezai-Zadeh K, Shytle D, Sun N, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (−)-epigallocatechin-3-gallate. Free Radic. Biol. Med. 2007;43:546–556. doi: 10.1016/j.freeradbiomed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 229.Crouse JR, 3rd, Morgan T, Terry JG, Ellis J, Vitolins M, Burke GL. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch. Intern. Med. 1999;159:2070–2076. doi: 10.1001/archinte.159.17.2070. [DOI] [PubMed] [Google Scholar]
- 230.Hanson LN, Engelman HM, Alekel DL, Schalinske KL, Kohut ML, Reddy MB. Effects of soy isoflavones and phytate on homocysteine, C-reactive protein, and iron status in postmenopausal women. Am. J. Clin. Nutr. 2006;84:774–780. doi: 10.1093/ajcn/84.4.774. [DOI] [PubMed] [Google Scholar]
- 231.Vitolins MZ, Anthony M, Burke GL. Soy protein isoflavones, lipids and arterial disease. Curr. Opin. Lipidol. 2001;12:433–437. doi: 10.1097/00041433-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 232.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 233.Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am. J. Clin. Nutr. 1992;56:573–578. doi: 10.1093/ajcn/56.3.573. [DOI] [PubMed] [Google Scholar]
- 234.Reddy MB, Hurrell RF, Juillerat MA, Cook JD. The influence of different protein sources on phytate inhibition of nonheme-iron absorption in humans. Am. J. Clin. Nutr. 1996;63:203–207. doi: 10.1093/ajcn/63.2.203. [DOI] [PubMed] [Google Scholar]
- 235.Swain JH, Alekel DL, Dent SB, Peterson CT, Reddy MB. Iron indexes and total antioxidant status in response to soy protein intake in perimenopausal women. Am. J. Clin. Nutr. 2002;76:165–171. doi: 10.1093/ajcn/76.1.165. [DOI] [PubMed] [Google Scholar]
- 236.Mahesha HG, Singh SA, Rao AG. Inhibition of lipoxygenase by soy isoflavones: evidence of isoflavones as redox inhibitors. Arch. Biochem. Biophys. 2007;461:176–185. doi: 10.1016/j.abb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 237.Su Y, Simmen FA, Xiao R, Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol. Genomics. 2007;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 238.Davis J, Higginbotham A, O’Connor T, et al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metab. 2007;51:42–52. doi: 10.1159/000100820. [DOI] [PubMed] [Google Scholar]
- 239.Davis J, Iqbal MJ, Steinle J, et al. Soy protein influences the development of the metabolic syndrome in male obese ZDFxSHHF rats. Horm. Metab. Res. 2005;37:316–325. doi: 10.1055/s-2005-861487. [DOI] [PubMed] [Google Scholar]
- 240.Davis J, Steinle J, Higginbotham DA, Oitker J, Peterson RG, Banz WJ. Soy protein influences insulin sensitivity and cardiovascular risk in male lean SHHF rats. Horm. Metab. Res. 2005;37:309–315. doi: 10.1055/s-2005-861475. [DOI] [PubMed] [Google Scholar]
- 241.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 242.Peluso MR, Winters TA, Shanahan MF, Banz WJ. A cooperative interaction between soy protein and its isoflavone-enriched fraction lowers hepatic lipids in male obese Zucker rats and reduces blood platelet sensitivity in male Sprague-Dawley rats. J. Nutr. 2000;130:2333–2342. doi: 10.1093/jn/130.9.2333. [DOI] [PubMed] [Google Scholar]
- 243.Kwiatkowski JL. Oral iron chelators. Pediatr. Clin. North Am. 2008;55:461–482. doi: 10.1016/j.pcl.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 244.Devanur LD, Evans RW, Evans PJ, Hider RC. Chelator-facilitated removal of iron from transferrin: relevance to combined chelation therapy. Biochem. J. 2008;409:439–447. doi: 10.1042/BJ20070823. [DOI] [PubMed] [Google Scholar]
- 245.Fabio G, Minonzio F, Delbini P, Bianchi A, Cappellini MD. Reversal of cardiac complications by deferiprone and deferoxamine combination therapy in a patient affected by a severe type of juvenile hemochromatosis (JH) Blood. 2007;109:362–364. doi: 10.1182/blood-2006-04-016949. [DOI] [PubMed] [Google Scholar]
- 246.Peng CT, Chow KC, Chen JH, Chiang YP, Lin TY, Tsai CH. Safety monitoring of cardiac and hepatic systems in β-thalassemia patients with chelating treatment in Taiwan. Eur. J. Haematol. 2003;70:392–397. doi: 10.1034/j.1600-0609.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 247.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc. Res. 2007;73:341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]