Abstract
Background:
Gmelina arborea Roxb (Verbenaceae), also known as “Gambhari”, is an important medicinal plant in the Ayurveda. There are no meticulous scientific reports on effect of the plant on inflammation and pain.
Objective:
To study the anti-inflammatory and anti-nociceptive properties of aqueous extracts (AE) and methanol extracts (ME) of G. arborea.
Materials and Methods:
The AE and ME of stembark of G. arborea was prepared by cold maceration and Soxhlet extraction technique respectively. Anti-inflammatory activity was determined in Wistar albino rats in a model of acute plantar inflammation induced by carrageenan. The anti-nociceptive activity was evaluated by using hot plate test and writhing test in Swiss albino mice. Significant differences between the experimental groups were assessed by analysis of variance.
Results:
AE and ME at dose of 500 mg/kg showed maximum inhibition in carrageenan induced inflammation up to 30.15 and 31.21% respectively. In hot plate test, the AE and ME showed the maximum response of 8.8 ± 0.97 (P < 0.01) and 8.2 ± 1.24 (P < 0.01) respectively at dose of 500 mg/kg when compared with control. AE showed maximum inhibition of writhing response (84.3%) as compared to ME (77.9%) in writhing test at a dose of 500 mg/kg.
Conclusion:
The findings suggested that G. arborea possess significant anti-inflammatory and anti-nociceptive activities.
Keywords: Analgesic, anti-nociceptive, Gmelina arborea, hot plate test, rat paw edema, writhing test
INTRODUCTION
Gmelina arborea Roxb (Verbenaceae) is a well-known medicinal plant in the Ayurveda, an ancient Indian system of medicine. The roots, leaves, flowers, fruits and bark are used for treating different ailments in traditional medicine. The literature suggests use of the plant in treatment of scorpion sting, snake-bites,[1] and diabetes.[2] The plant is anthelmintic and used for treating hallucinations, excess thirst, piles, abdominal pains, burning sensations, and fever.[3]
There are reports on phytoconstituents present in different parts of the plant. The isolation of luteolin[4] and indole alkaloids[5] from the leaves has been reported. Hentriacontanol[6] and lignans such as arboreol, isoarboreol, methyl arboreol, arborone, gmelanone, gummadiol, and 7-oxodihydrogmelinol[7,8,9] have been isolated from heartwood of the plant. Few coumarin glycosides[10] and iridoid glycosides[11] have been reported in roots and leaves, respectively. Three iridoid glycosides 6-O-(3“-O-benzoyl)-α-L-rhamnopyranosylcatalpol, 6-O-(3“-O-trans-cinnamoyl)-α-L-rhamnopyranosylcatalpol and 6-O-(3“-O-cis-cinnamoyl)-α-L-rhamnopyranosylcatalpol were isolated from aerial parts of G. arborea and structures were elucidated by spectral analysis.[12]
The bark of the plant indicated presence of tyrosol [2-(4-hydroxyphenyl) ethanol]; (+)-balanophonin, an 8-5′ neolignan, gmelinol, phenylethanoid glycoside {(−)-p-hydroxyphenylethyl [5“′-O-(3,4 dimethoxycinnamoyl)-b-D-apiofuranosyl (1“‘ → 6’)]-b-D-glucopyranoside}, 2,6-dimethoxy-p-benzoquinone and 3, 4, 5-trimethoxyphenol.[13]
Crude extracts of the plant are reported to possess wound-healing properties,[14] antidiarrheal activity,[15] antioxidant activity,[16] antidiabetic activity,[17,18] and antiulcer activity.[19] Toxicity studies of aqueous extract (AE) and methanol extracts (ME) have been reported by Kulkarni and Addepalli.[20,21]
However, little information is available on the effects of G. arborea on inflammation and nociception. Therefore, the present study was designed to examine the effects of AE and ME of G. arborea bark, as it is mainly used in Ayurveda.
MATERIALS AND METHODS
Plant material
The bark of the G. arborea was collected from Jawhar District-Thane), Maharashtra, India in the month of November. Identification and authentication of the bark was carried out by Dr. P. S. N. Rao, Botanical Survey of India, Pune, India. A voucher specimen (b-03) of the bark is deposited in department for future reference. The bark after drying at room temperature was grounded into a fine powder. The powdered bark was used to prepare the AE and ME.
Preparation of aqueous extract
Cold maceration technique was used to prepare AE of bark of G. arborea (AE). Powdered bark (100 g) was macerated with 1 L of distilled water for 7 days, with occasional shaking. After 7 days, the AE was filtered and the marc was again subjected to maceration with distilled water for complete extraction. After filtration the AEs were combined and concentrated with the help of rotary vacuum evaporator under reduced pressure. The AE was dried in vacuum dryer and stored at − 20°C until used. The yield of the AE was found to be 20% w/w, with respect to powdered bark. The extract was prepared freshly in double distilled water at the time of administration to experimental animals.
Preparation of methanol extract
Soxhlet extraction technique was used to prepare ME of bark of G. arborea (ME). One hundred gram of powdered bark was packed in soxhlet extractor and extracted with methanol. After complete extraction, the ME was filtered and concentrated under reduced pressure by using rotary vacuum evaporator. The ME was dried in vacuum dryer and stored at − 20°C until used. The yield of the ME was found to be 28% w/w with respect to powdered bark. The extract was prepared freshly in double distilled water at the time of administration to experimental animals.
Preliminary phytochemical screening
Preliminary phytochemical screening of the aqueous and ME was done to identify presence of diverse phytoconstituents by standard methods.[22]
Experimental animals
Wistar albino rats and Swiss albino mice were purchased from Haffkine Institute, Mumbai, India. The animals were maintained in polypropylene cages in the Animal Facility with 12 hours light: 12 hours dark cycle. All the animals were kept under laboratory conditions (temperature 25°C ± 2°C, relative humidity 75% ±5%) for an acclimatization period of 7 days before carrying out the experiments. Standard pellet diet (Nutrimix Laboratory Animal Feed, Maharashtra, India) and filtered water was provided ad libitum to all experimental animals. All the experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) constituted as per the norms of committee for the purpose of control and supervision of experiments on animals, India. All experimental procedures were performed according to the National Institutes of Health (NIH) guide for the care and use of laboratory animals.
Selection of doses
One tenth and 1/20th of the safe dose of the extracts were selected for the study.[20,21] The extracts were prepared in double distilled water at the time of administration. The volume of extract administered to the experimental animals was not greater than 2 ml/100 g.
Anti-inflammatory activity
Pedal inflammation in male Wistar rats (120-150 g) was produced according to the method described earlier.[23,24] An injection (s.c.) was made of 0.1 ml of 1% carrageenan into the right paw of each rat under the subplantar aponeurosis. The test groups were administered two doses of 250 and 500 mg/kg of both AE and ME, 1 hour before carrageenan injection. At the same time, the control group received 2 ml/kg distilled water and the reference group received 75 mg/kg phenylbutazone (p.o.). The paw volume was measured upto 6 huors after the injection of the edematogenic agent using a plethysmometer (IITC, USA).
Percentage reduction in edema volume was calculated by using the formula,

Where, V0 = Volume of the paw of control at time ‘t’, Vt = Volume of the paw of drug treated at time ‘t’.
Antinociceptive activity
To evaluate the anti-nociceptive activity of AE and ME, the effects of their treatments were assessed on two important experimental models: Hot plate test and writhing test.
Hot plate test
The hot-plate test was carried out as previously described.[25] Swiss albino mice (25-30 g) of either sex were fasted overnight with water given ad libitum. A hot-plate apparatus (IITC, USA) was used for determining the anti-nociceptive effect of the selected extracts. The animals were placed on the functioning hot plate (54°C ± 1°C) to determine baseline responsiveness. The time elapsed until the animal licked one of its hind paws or jumped was recorded (latency time, in seconds). Mice that presented baseline reaction of more than 15 seconds were eliminated from the test. After the baseline responsiveness determination the animals were divided into six groups. Group I (Control) received distilled water, Group II and III received AE at dose of 250 and 500 mg/kg, group IV and V received ME at dose of 250 and 500 mg/kg and group VI received pentazocine (17 mg/kg). After administration of doses the latency to lick one of the hind paws or to jump off plate was determined at 15, 30, 45 and 60 min. A cut off period of 15 seconds is observed to avoid the damage to paws. Maximum possible effect (MPE) was calculated by the formula:
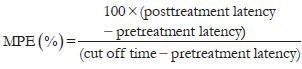
Writhing test
The writhing test in mice was carried out using the method of Koster.[26] The writhes were induced by 0.1 ml of intraperitoneal injection of 0.6% acetic acid (v/v). Group I (Control) received distilled water, Group II and III received AE at dose of 250 and 500 mg/kg respectively, the animals of group IV and V had ME at the same doses and group VI was administered standard drug (acetyl salicylic acid; 30 mg/kg), 45 min before an intraperitonial injection of acetic acid. The number of muscular contractions was counted over a period of 20 min after acetic acid injection. The data represent the total number of writhes observed during 20 min and is expressed as writhing numbers.
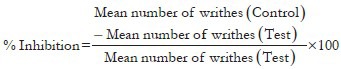
Statistical analysis
The differences among experimental and control groups were determined using the statistical software Sigmastat Ver. 2.03 for Windows. Significant differences between the experimental groups were assessed by analysis of variance. All data are expressed as mean ± standard error of mean (SEM); P value less than 0.05 was considered to be significant.
RESULTS
Preliminary phytochemical screening
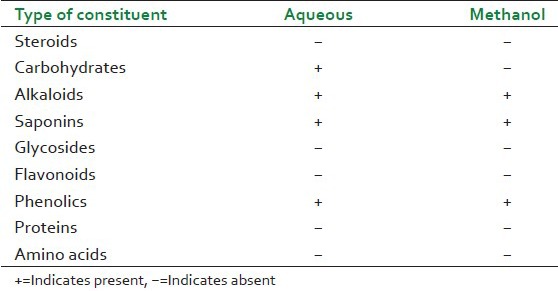
The results of the preliminary phytochemical screening are presented in Table 1.
Table 1.
Preliminary phytochemical screening of Gmelina arborea extracts
Anti-inflammatory activity
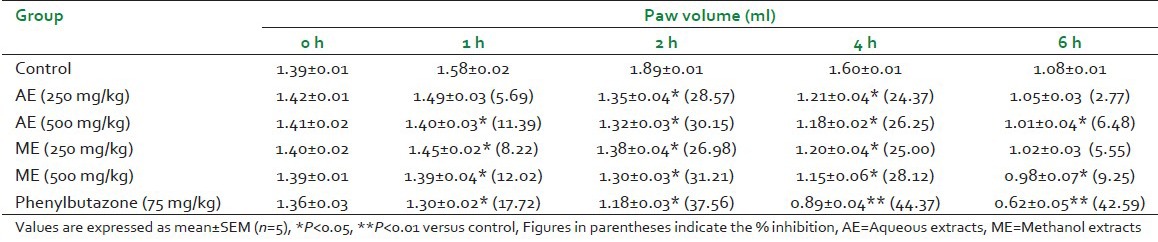
Subplantar injections of carrageenan provoked marked, time-related increase in the hind paw diameters of the ‘untreated’ control rats. Maximal swelling and/or edema occurred approximately 90 min following carrageenan administration. Table 2 shows effect of AE and ME in carrageenan induced rat paw edema. AE and ME showed significant reduction in paw volume when compared with control. AE at dose of 250 and 500 mg/kg showed maximum decrease of paw volume: 1.35 ± 0.04 (P < 0.05) and 1.32 ± 0.03 respectively when compared with control at 2 hours. ME at 500 mg/kg showed maximum decrease in paw volume 1.30 ± 0.03 (P < 0.05) as compared with control (1.89 ± 0.01) after 2 hours. The animals treated with AE and ME showed maximum reduction of 30.15% and 31.21% respectively, at 2 hours.
Table 2.
Effect of aqueous and methonolic extracts of Gmelina arborea on carrageenan induced rat paw edema
Anti-nociceptive activity
The effect of aqueous and ME of G. arborea was studied in hot plate test and acetic acid induced writhing method.
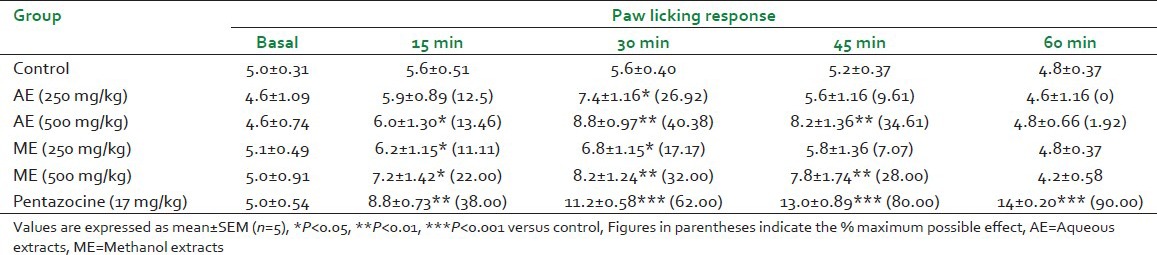
There was significant increase in pain threshold in mice towards the thermal stimuli by hot plate, after treatment with the two extracts [Table 3]. The time for paw licking response showed significant increase at 15, 30 and 45 min after treatment with AE and ME at doses of 250, 500 mg/kg. AE showed increase in paw licking response of 7.4 ± 1.16 (P < 0.05) and 8.8 ± 0.97 (P < 0.01) when compared with response before treatment at dose of 250 and 500 mg/kg respectively after 30 min of administration. ME at dose of 500 mg/kg exhibited significant increase (P < 0.01) in paw licking response after 30 min of administration. AE and ME showed maximum effect at 30 min at dose of 500 mg/kg.
Table 3.
Antinociceptive effect of Gmelina arborea extracts in hot plate test
The effects of AE, ME and acetyl salicylic acid on acetic acid induced writhing are presented in Table 4. All doses of the selected extracts (AE and ME) reduced acetic acid-induced writhing significantly. AE at dose of 250 and 500 mg/kg significantly (P < 0.001) reduced the number of writhings to 6.4 ± 0.93 and 4.0 ± 0.32 when compared with control (25.4 ± 0.81).
Table 4.
Effects of oral administration of Gmelina arborea extracts on acetic acid-induced writhing in mice
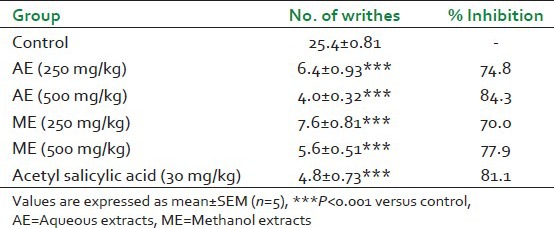
The AE at 500 mg/kg showed maximum inhibition of writhes (84.3%) which was more than the standard drug-acetyl salicylic acid (81.1%). ME showed significant reduction (P < 0.001) in writhings at doses of 250 and 500 mg/kg. The ME showed significant inhibition of 70% and 77.9% at dose of 250 and 500 mg/kg respectively.
DISCUSSION
Edema formation due to carrageenan in the rat paw is the biphasic event. The initial phase is attributed to the release of histamine and serotonin. The second phase of edema is due to the release of prostaglandins, protease and lysosome.[27,28] The second phase is sensitive to most clinically effective anti-inflammatory drugs.[27,29] The edema produced after injection of carrageenan in rodents is associated with the production of several inflammatory mediators, such as bradykinin, nitric oxide, prostaglandins and cytokines.[29,30,31,32,33] It has been found that the injection of carrageenan into the rat paw induces the liberation of bradykinin, which later induces the biosynthesis of prostaglandin and other autacoids, which are responsible for the formation of the inflammatory exudates.[34] Therefore, it is suggested that the mechanism of anti-inflammatory action of G. arborea extracts may be related to inhibition of prostaglandin and other autocoids.
In hot plate test pain induced by thermal stimulus is specific for centrally mediated nociception[35] and thought to involve opioids.[36] The hot plate test was selected to examine a central analgesic activity, because it had several advantages, particularly its sensitivity to strong analgesics and limited tissue damage. Maximum effect of the plant extracts at all doses was reached at 30 min. The higher dose (500 mg/kg) of the AE and ME prolonged the hot-plate latency with time, which was less potent as compared to pentazocine. The ability of the extracts to prolong the reaction latency suggests that the extracts have a central analgesic activity.
The writhing response by acetic acid consists of a contraction of the abdominal muscle together with a stretching of the hind limbs which perceives peripheral analgesia.[37] The test is unable to distinguish between central and peripheral analgesia.[38] Still this is a very sensitive method used to screen anti-nociceptive activity for compounds than other methods like tail flick test.[39] The effects were comparable to that of the standard drug acetyl salicylic acid. The acetic acid induced abdominal constrictions are associated with sensitization of nociceptive receptors to prostaglandins.[40] Thus we can predict that the anti-nociceptive effect of the selected extracts may therefore be due to its action on visceral receptors perceptive to acetic acid. The possible mechanism may also be linked to the inhibition of the production or action of prostaglandins.
Both extracts of G. arborea have shown presence of alkaloids and saponins which may be the possible chemical constituents responsible for its anti-inflammatory and anti-nociceptive activity.
CONCLUSION
The results obtained in the present study strongly suggest that G. arborea possesses significant anti-inflammatory and anti-nociceptive activities. The specific mechanisms through which AE and ME exerts their actions, as well as the specific bioactive compounds present in these extracts responsible for the activities, are currently under investigation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nadkarni KM. India: Popular Prakashan; 2000. Indian Materia Medica; pp. 584–5. [Google Scholar]
- 2.Khan IA, Khanum A. India: Ukaaz Publications; 2005. Herbal Therapy for Diabetes; pp. 34–5. [Google Scholar]
- 3.Kirtikar KR, Basu BD. India: International Book Distributors; 1999. Indian Medicinal Plants; pp. 1932–3. [Google Scholar]
- 4.Rao DV, Rao EV, Viswanathan N. Occurrence of luteolin in the leaves of Gmelina arborea Linn. Curr Sci. 1967;3:714. [Google Scholar]
- 5.Bhattacharjee AK, Das AK. Phytochemical survey of few mysore plants. Econ Bot. 1969;23:274–6. [Google Scholar]
- 6.Joshi KC, Prakash L, Singh LB. Extractives from heartwoods: Isolation of hentriacontanol from Gmelina arborea. J Ind Chem Soc. 1971;48:11756. [Google Scholar]
- 7.Govindachari TR, Parthasarathy PC, Dasai HK. Arboreol, a new lignan from Gmelina arborea. Ind J Chem. 1972;10:1120–2. [Google Scholar]
- 8.Anjaneyulu AS, Jaganmohan RK, Kameswara RV, Ramachandra RL, Subrahmanyam C, Pelter A, et al. The structures of lignans from Gmelina arborea. Tetrahedron. 1975;31 127785. [Google Scholar]
- 9.Satyanarayana P, Koteswara R, Ward RS, Pelter A. Arborone and 7-oxo-dihydrogmelinol: Two new keto-lignans from Gmelina arborea. J Nat Prod. 1986;49:10614. [Google Scholar]
- 10.Satyanarayana P, Subrahmanyam P, Kasai R, Tanka O. An apiose-containing coumarin glycoside from Gmelina arborea root. Phytochem. 1985;24:18623. [Google Scholar]
- 11.Hosny M, Rosazza JP. Gmelinosides A-L, twelve acylated iridoid glycosides from Gmelina arborea. J Nat Prod. 1998;61:734–42. doi: 10.1021/np970447u. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari N, Yadav AK, Srivastava P, Shanker K, Verma RK, Gupta MM. Iridoid glycosides from Gmelina arborea. Phytochemistry. 2008;69:2387–90. doi: 10.1016/j.phytochem.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Syamsul F, Takeshi K, Toshisada S. Chemical constituents from Gmelina arborea bark and their antioxidant activity. J Wood Sci. 2008;54:4839. [Google Scholar]
- 14.Shirwaikar A, Ghosh S, Rao PG. Effects of Gmelina arborea Roxb. leaves on wound healing in rats. J Nat Rem. 2003;3:45–8. [Google Scholar]
- 15.Agunu A, Yusuf S, Andrew GO, Zezi AU, Abdurahman EM. Evaluation of five medicinal plants used in diarrhoea treatment in Nigeria. J Ethnopharmacol. 2005;101:27–30. doi: 10.1016/j.jep.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Sinha S, Dixit P, Bhargava S, Devasagayam TP, Ghaskadbi S. Bark and fruit extracts of Gmelina arborea protect liver cells from oxidative stress. Pharm Biol. 2006;44:23743. [Google Scholar]
- 17.Kulkarni YA, Addepalli V. Antidiabetic activity of aqueous extract of Gmelina arborea bark in rats. Altern Ther Health Med. 2009;15:183–4. [Google Scholar]
- 18.Kulkarni YA, Addepalli V. Effect of Gmelina arborea extract in STZ induced type I diabetic rats. FASEB J. 2011;25:805–9. [Google Scholar]
- 19.Giri M, Divakar K, Goli D, Dighe SB. Anti ulcer activity of leaves of Gmelina arborea plant in experimentally induced ulcer in Wistar rats. Pharmacol. 2009;1:10210. [Google Scholar]
- 20.Kulkarni Y, Addepalli V. Toxicologcal studies on aqueous extract of Gmelina arborea in rodents. Pharm Biol. 2010;48 doi: 10.3109/13880209.2010.489228. 141320. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni YA, Veeranjaneyulu A. Toxicological Evaluation of the Methanol Extract of Gmelina arborea Roxb. Bark in Mice and Rats. Toxicol Int. 2012;19:125–31. doi: 10.4103/0971-6580.97203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harborne JB. London: Chapman and Hall; 1998. Phytochemical Methods. [Google Scholar]
- 23.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni YA, Gokhale SB, Veeranjaneyulu A, Surana SJ, Tatiya AU. Effect of Persea macrantha against acute inflammation and adjuvant-induced arthritis in rats. Pharm Biol. 2009;47:304–8. [Google Scholar]
- 25.Vogel GH. Drug discovery and evaluation: Pharmacological assays. Berlin, Heidelberg, New York: Springer; 2002. Analgesic, anti-inflammatory, and anti-pyretic activity; p. 696. [Google Scholar]
- 26.Koster R, Anderson M, DeBeer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412. [Google Scholar]
- 27.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 28.Crunkhorn P, Meacock SC. Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol. 1971;42:392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 30.Henriques MG, Silva PM, Martins MA, Flores CA, Cunha FQ, Assreuy-Filho J, et al. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20:243–9. [PubMed] [Google Scholar]
- 31.Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc. 1987;46:118–26. [PubMed] [Google Scholar]
- 32.Posadas I, Bucci M, Roviezzo F, Rossi A, Parente L, Sautebin L, et al. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol. 2004;142:331–8. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha AC, Fernandes ES, Quintão NL, Campos MM, Calixto JB. Relevance of tumour necrosis factor-alpha for the inflammatory and nociceptive responses evoked by carrageenan in the mouse paw. Br J Pharmacol. 2006;148:688–95. doi: 10.1038/sj.bjp.0706775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueno A, Naraba H, Ikeda Y, Ushikubi F, Murata T, Narumiya S, et al. Intrinsic prostacyclin contributes to exudation induced by bradykinin or carrageenin: A study on the paw edema induced in IP-receptor-deficient mice. Life Sci. 2000;66:155–60. doi: 10.1016/s0024-3205(00)00420-3. [DOI] [PubMed] [Google Scholar]
- 35.Magaji M, Anuka J, Abdu-Aguye I, Yaro A, Hussaini I. Preliminary studies on anti-inflammatory and analgesic activities of Securinega virosa (Euphorbiaceae) in experimental animal models. J Med Plants Res. 2008;2:3944. [Google Scholar]
- 36.Besra SE, Sharma RM, Gomes A. Antiinflammatory effect of petroleum ether extract of leaves of Litchi chinensis Gaertn. (Sapindaceae) J Ethnopharmacol. 1996;54:1–6. doi: 10.1016/0378-8741(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 37.Derardt R, Jongney S, Delvalcee F, Falhout M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;51:1724. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 38.Chen YF, Tsai HY, Wu TS. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995;61:2–8. doi: 10.1055/s-2006-957987. [DOI] [PubMed] [Google Scholar]
- 39.Bentley GA, Newton SH, Starr J. Evidence for an action of morphine and the enkephalins on sensory nerve endings in the mouse peritoneum. Br J Pharmacol. 1981;73:325–32. doi: 10.1111/j.1476-5381.1981.tb10425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mekonnen T, Urga K, Engidawork E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J Ethnopharmacol. 2010;127:433–9. doi: 10.1016/j.jep.2009.10.020. [DOI] [PubMed] [Google Scholar]


