Summary
The global spread of human immunodeficiency virus (HIV) is dependent on the ability of this virus to efficiently cross from one host to the next by traversing a mucosal membrane. Unraveling how mucosal exposure of HIV results in systemic infection is critical for the development of effective therapeutic strategies. This review focuses on understanding the immune events associated with the oral route of transmission (via breastfeeding or sexual oral intercourse), which occurs across the oral and/or gastrointestinal mucosa. Studies in both humans and simian immunodeficiency virus (SIV) monkey models have identified viral changes and immune events associated with oral HIV/SIV exposure. This review covers our current knowledge of HIV oral transmission in both infants and adults, the use of SIV models in understanding early immune events, oral immune factors that modulate HIV/SIV susceptibility (including mucosal inflammation), and interventions that may impact oral HIV transmission rates. Understanding the factors that influence oral HIV transmission will provide the foundation for developing immune therapeutic and vaccine strategies that can protect both infants and adults from oral HIV transmission.
Keywords: HIV, SIV, oral transmission, inflammation, SIV natural hosts, mucosal immunity
Introduction
The global spread of human immunodeficiency virus (HIV) is dependent on the ability of the virus to efficiently cross from one host to the next by traversing mucosal membranes. An effective vaccine or therapeutic agent that prevents HIV transmission most likely will need to function at or near the mucosal surface (1, 2). Therefore, it is critical that we thoroughly understand the multiple routes of virus exposure that can lead to HIV infection, including rectal, vaginal, penile, and oral HIV exposure. This review focuses on just one of these routes of exposure, oral transmission, when HIV infection occurs across the oral and/or gastrointestinal (GI) tract. Oral transmission of HIV primarily occurs in two distinct settings, breast milk consumption by infants born to HIV-infected women and oral-genital contact during adult sexual intercourse.
Access to antiretroviral treatment (ART) and bottle-feeding has dramatically reduced oral HIV transmission via breast milk in developed countries. However, in developing countries, over 300,000 new mother-to-child transmission (MTCT) events occur each year (3), and nearly 40% of these HIV infections are acquired through breastfeeding (4). Despite the risk of HIV acquisition via breastfeeding, exclusive breastfeeding of infants born to HIV-infected mothers is recommended by the World Health Organization (WHO), since, even in the presence of HIV, breastfeeding results in reduced infant mortality, partly due to immune benefits from passive transfer of antibodies and other bioactive products (5). Therefore, HIV transmission via breast milk continues to be a major route of infant HIV acquisition.
Oral transmission of HIV via oral-genital contact was initially difficult to quantify as it requires detailed and accurate knowledge of an individual’s sexual practices, which often include multiple high-risk behaviors. In fact, initial reports indicated that oral intercourse was a low risk sexual activity for transmitting HIV (6–15). However, more recent epidemiologic studies have provided clear evidence that HIV can be transmitted via receptive oral intercourse (ROI) (16–20), and studies in the simian immunodeficiency virus (SIV)/macaque animal model provide further proof that oral transmission of HIV can occur (21–25).
To investigate oral transmission of HIV, we discuss studies in both humans and SIV monkey models to provide a comprehensive understanding of this route of infection. This review evaluates the immune factors that influence HIV/SIV susceptibility as well as interventions that might be utilized to inhibit HIV transmission via oral HIV exposure.
Infant oral transmission
Infants born to HIV-infected mothers, who do not acquire HIV in utero or perinatally, continue to be at risk of acquiring HIV through breastfeeding. This risk was first observed by Ziegler et al. (26) in the mid-1980s and subsequently quantified in a Kenyan trial that randomized women to breast or formula feeding (27). In this large, randomized study, the risk of HIV transmission attributable to breastfeeding was 44.1% (27). Since then, other studies have confirmed that, without intervention, between 20 and 40% of MTCTs occur during breastfeeding (4, 28). There are also a handful of case reports where postnatal MTCT of HIV occurred in the absence of breastfeeding where premastication of food by the mother may have been the route of transmission (29). However, this route of transmission appears to be far less common than breast milk transmission of HIV (30).
Because of the risk of HIV transmission through breastfeeding, formula feeding is universally recommended to HIV-infected mothers in developed countries. However, postnatal transmission of HIV remains complex and difficult to prevent in settings where formula feeding is neither safe nor affordable. In developing countries, breastfeeding is recommended even for HIV-infected women, due to increased morbidity and mortality from malnutrition and gastroenteritis in formula-fed infants (5, 31–33). Therefore, in developing countries, postnatal transmission continues to account for up to 39% of MTCT (4, 28, 34).
It is unclear if any particular period of time during lactation presents a greater risk for HIV-1 transmission. Some studies suggest that colostrum, the breast milk produced shortly after delivery, has a higher viral load than non-colostrum breast milk, and therefore, transmission may be higher early in life (35), while others studies have not observed this phenomenon (36, 37). Regardless, the risk of MTCT transmission of HIV persists until the infant is fully weaned (4).
Factors associated with increased risk of breast milk HIV transmission include high maternal viral load, particularly breast milk viral load (38, 39), and duration of breastfeeding (4). Interestingly, low maternal CD4+ T-cell count is also a major determinant of transmission independent of viral load (36). Clinical and subclinical mastitis (which are associated with increased milk viral load in the involved breast), breast abscesses or other lesions, and infant oral thrush have also been shown to increase the risk of transmission (38–43). One particularly important risk factor for breast milk transmission is the consumption of non-breast milk foods. In four large prospective studies conducted in Africa, exclusive breastfeeding reduced the risk of MTCT by up to 11-fold (31, 32, 36, 44), compared to women who supplemented breastfeeding with other foods (known as mixed feeding). This increased risk was independent of maternal breast milk viral load and mastitis (38, 43).
Multiple trials have demonstrated that mother and infant antiretroviral therapy during breastfeeding can dramatically reduce HIV breast milk transmission (28, 45–47). Implementation of these interventions has made breastfeeding remarkably safer for HIV-exposed infants around the world. In communities that have made these interventions widely available, rates of transmission during breastfeeding have decreased to 1–4% (46). However, over 300,000 infants are still infected with HIV each year (3). In addition, the prophylactic use of antiretroviral drugs can result in drug resistance in infants that become infected despite prophylaxis (48, 49). Although much progress has been made in reducing HIV transmission through breastfeeding, additional interventions are still necessary to eliminate infant HIV acquisition.
Adult oral transmission
In contrast to MTCT of HIV, sexual transmission of HIV following oral exposure is still somewhat controversial. Since the beginning of the HIV epidemic, case reports have been published of HIV-infected individuals who likely acquired HIV through sexual oral exposure. These reports have implicated oral contact with female (50–54) and male genital fluids (55–69), oral-anal contact (50), and oral-oral contact (70). Accuracy of sexual history reporting has been called into question, and oral exposure rarely occurred in the absence of other exposures (61, 71–73). However, one transmission pair, where both individuals independently reported only oral-genital contact, was confirmed by viral sequencing (69). Interestingly, the incident case in several of the reports may have acquired HIV while their oral mucosa was compromised by dental procedures (55, 65, 74), allergies (56), pharyngitis (64), chemotherapy (59), or periodontal disease (63, 65, 66, 70). Although case reports alone are unable to evaluate the contribution of sexual oral transmission to the HIV epidemic, the number of reports over the last three decades would suggest that sexual oral transmission of HIV can occur, particularly when the oral mucosa is compromised.
Early population studies of men who have sex with men (MSM) did not identify oral-genital contact as a risk factor for HIV seroconversion (6–15). However, these studies enrolled relatively small cohorts of very high-risk individuals, making them underpowered to detect transmission by lower risk sexual activity. One study in the 1980s evaluated oral-genital contact in serodiscordant, heterosexual couples and found a significantly higher amount of oral-genital contact among couples who transmitted HIV infection during the study (OR 7.29), especially in female partners of acquired immunodeficiency syndrome (AIDS) patients (75). Importantly, fewer sexual partners and less receptive anal intercourse (RAI), both well-established risk factors for sexual HIV transmission, were reported in this study compared to the MSM studies of the 1980s. The first report in MSM with no RAI showed a significantly higher proportion of seroconverters among individuals that reported oral-genital contact (OR 3.0), although this did not consistently retain significance in multivariate models (76). Subsequently, a large, more conclusive study found that, after adjusting for RAI, each additional receptive oral intercourse (ROI) partner significantly increased the risk of HIV seroconversion by 1% (compared to 5% for RAI) (18). While a handful of small studies in the early 1990s suggested that heterosexual HIV transmission through oral-genital contact may be a relatively rare event (77, 78), the contribution of oral-genital contact, specifically ROI, to heterosexual transmission of HIV was confirmed by a large study in New York City sex workers. In this study, the risk of HIV acquisition associated with oral sex was increased in individuals that smoked crack cocaine, which is known to cause oral lesions (20). In addition to confirming the potential of HIV sexual transmission, following oral exposure, this study also suggested that inflammation at the oral mucosa may increase susceptibility to HIV.
HIV acquisition via sexual oral exposure occurs at a lower rate than RAI transmission of HIV. A single study in MSM estimated that the per-contact risk of HIV infection through unprotected oral sex with an HIV-infected partner or a partner of unknown HIV status is 0.04%, in comparison to 0.27% for unprotected RAI (19). However, there are no other estimates of per-contact or per-act risk of oral-genital transmission of HIV. These key epidemiologic studies were rapidly followed by the first report of successful HIV infection of adult rhesus macaques by oral administration of SIV, confirming that HIV infection can occur through oral exposure to HIV (see Animal Models of Oral Transmission section). Although the exact frequency of oral sexual transmission of HIV is difficult to assess, by the late 1990s, the evidence for sexual transmission of HIV across the oral mucosa, particularly via ROI, had been well established.
Despite the lower rate of transmission compared to RAI, oral-genital contact significantly contributes to the HIV pandemic. Surveillance data between 2001 and 2003 from UK MSM that do not participate in unprotected anal intercourse showed that 2.6 to 5.2% of MSM acquired HIV through oral transmission (16). Oral sex is practiced frequently both by heterosexual and homosexual couples (78), and use of barrier protection during oral-genital contact, even in HIV serodiscordant couples, is rare (6, 68, 79). Many studies have reported a dramatic decrease in high-risk sexual activity among MSM between the 1980s and the 2000s (18, 79, 80), resulting in a marked reduction in HIV incidence (79). However, sexual oral contact is generally considered a lower risk activity, and rates of unprotected ROI remain high (79, 80). Reduction in higher risk activities paired with continuation of lower-risk activities is consistent with an increased role for lower risk sexual oral transmission in the MSM HIV pandemic.
The contribution of oral exposure to heterosexual HIV transmission is less clear, since no incidence studies have been performed to date. It is likely that transmission by receptive fellatio in heterosexuals is similar to those observed in MSM. However, the contribution of oral-genital contact via cunnilingus and oral-anal contact is still unknown. However, the HIV transmission rate per sexual act for vaginal intercourse is 5% of that observed for RAI (81, 82), making most heterosexual transmissions of HIV low-risk transmissions in comparison to RAI transmission of HIV. This would suggest that the relative contribution of sexual oral contact to the heterosexual HIV pandemic exceeds that observed in MSM. Importantly, the majority of the current HIV epidemic occurs in developing countries, predominantly in Sub-Saharan Africa (3), and HIV incidence per sexual act significantly varies by socio-economic setting (82), making it critical to study oral-genital transmission of HIV in developing countries. The variability in sexual HIV transmission, following vaginal and rectal sexual exposure, is largely associated with differences in mucosal immune activation at the site of HIV acquisition (83). This is also likely the case in oral HIV acquisition. However, studying oral HIV transmission is particularly challenging in human adults. Therefore, animal models are useful in determining the mechanisms underlying variation in oral mucosa susceptibility to HIV.
Animal models of oral transmission
To determine the immune events critical for protection against oral HIV exposure, we must understand the immune responses to HIV that occur prior to systemic virus dissemination and the establishment of latent reservoirs. In humans, identifying individuals within this time period is incredibly difficult due to the weeks to years before an individuals is diagnosed with HIV. Therefore, our understanding of early events, following oral transmission, comes largely from studies of simian immunodeficiency virus (SIV) infection in macaques (Table 1). The SIV strains utilized in these studies (generally SIVmac251) contain diverse quasispecies and can be used in macaques to mimic mucosal exposure to HIV (84–86). Initial studies of oral transmission in the SIV-macaque model by Baba et al. (22) determined that the infection was 6000 times more likely to occur during an oral exposure compared to a rectal exposure in adult macaques, although later studies have shown lower transmission rates with oral than rectal exposure, as predicted by human epidemiology studies (87). Subsequently, Van Rompay et al. (88) showed productive infection of infant macaques, following oral SIV exposure, and that transmission could be prevented by prophylactic treatment with ART. To evaluate the contribution of MALT to viral entry, Stahl-Hennig et al. (89) applied cell-free SIV directly to the tonsil. This study demonstrated that the tonsils are a potential site of transmission and an early site of explosive viral replication that plateaus 4–7 days post-inoculation. In a follow-up study, an attenuated SIV vaccine induced protection, following tonsillar administration of SIV in Rhesus macaques (90). Work in our laboratory has more comprehensively evaluated potential SIV entry sites, following non-traumatic application of high-dose SIV to the cheek pouch with subsequent swallowing, and has shown that in both infant and adult macaques SIV replication is concentrated in tissues proximal to the stomach, including the oral mucosa, esophagus, tonsils and draining lymph nodes, at days 1 and 2 post-exposure (24). Detailed evaluation of tissues from these macaques showed SIV-infected macrophages and CD4+ T cells at lymphoid tissues by 4 days post-exposure, suggesting that these cells play key roles in early viral replication and dissemination (24). These findings provide evidence that oral transmission of SIV, and by analogy HIV, occurs before the virus reaches the stomach and that the virus is able to disseminate rapidly to draining and then distant lymph nodes.
Table 1.
Macaque Studies of Oral SIV Transmission
| Finding | Virus strain | Route of Virus Exposure |
Publication Date |
Reference |
|---|---|---|---|---|
| Adult macaques can be orally infected by cell-free virus alone |
SIVΔB67 | Drip with swallowing; variable dose |
1996 | (22) |
| Infants susceptible to oral SIV, and protected by prophylactic ART |
SIVmac251 | Drip with swallowing; high dose |
1998 | (88) |
| SIV can initiate infection via tonsils |
SIVmac251 | Direct application to tonsils; variable dose |
1999 | (89) |
| Attenuated SIV prevents SIV infection, implicating γδ T cells in protection |
SIVmac251 | Direct application to tonsils; high dose |
2004 | (90) |
| Oral SIV establishes infection in tissues proximal to the stomach |
SIVmac251 | Drip with swallowing; high dose |
2004 | (24) |
| Rapid induction of early innate genes in infants in tissues proximal to stomach |
SIVmac251 | Drip with swallowing; low dose |
2006 | (91) |
| Rapid induction of early innate genes in adults in tissues proximal to stomach |
SIVmac251 | Drip with swallowing; high dose |
2007 | (25) |
| Low dose challenge delays early innate gene activation |
SIVmac251 | Drip with swallowing; low dose |
2011 | (93) |
| Mucosal disruption (acetic acid) increases SHIV oral transmission |
SHIV-R5 clade |
Drip on site of acetic acid treatment; low dose |
2010 | (87) |
| Mucosal inflammation (gingivitis) does not increase oral SIV transmission, but does impact immune activation following SIV infection |
SIVmac251 | Drip on site of gingivitis; low dose |
2013 | (238) |
Oral infection of macaques with SIV elicits an innate immune response that can be measured within days post-infection (23, 25, 91). A study by Abel et al. (91) evaluated the innate immune changes at different tissue sites in infant macaques 7 days after oral exposure. Elevations of both anti-viral (MX, IFN-α) and proinflammatory (IL6, IL12, CXCL10) genes, particularly in upper gastrointestinal tissues, suggests that early, innate immune responses to SIV may both inhibit and potentiate the establishment of systemic infection (91). Studies in our laboratory have shown a correlation between increased innate immune activation at mucosal sites (OAS, CXCL9 and CXCL10) in orally inoculated SIV infected macaques and slower disease progression (25). In contrast, an increase in the same immune modulators in lymphoid tissues and in blood was associated with a more rapid progression to simian AIDS (23). Also, 1–4 days after oral SIV infection we observed a migration of the innate immune T cell subset gd T cells from mucosal sites to draining lymph nodes (92). In addition to the high-dose SIV administration used in the studies described above, macaques can be successfully infected with SIV through repeated, low dose challenges, which better replicate viral exposure via semen and breast milk. Macaques orally exposed to low-dose SIV have a slightly slower rate of innate immune response induction at both mucosa and lymph nodes (93). These findings indicate that following successful oral SIV infection, the innate immune system responds rapidly to the virus, which can be detected at both mucosal sites and lymph nodes within just a few days post-infection.
Comparison of oral SIV transmission with other mucosal transmission routes could provide clues for future HIV prevention strategies. Following high dose vaginal exposure to SIV, only a few clustered cells, predominantly CD4+ T cells, are SIV-infected up to 3–4 days post-infection (94, 95). During this initial, local infection, it is believed that the founder SIV population expands, triggering a localized innate immune response and recruitment of additional target cells that fuel viral replication and facilitate further dissemination of the virus into lymph nodes and distal tissues (96, 97). These findings contrast with oral SIV challenge studies, where high-dose virus rapidly spread through the mucosa and into the lymphoid tissues by 1 to 2 days post-infection (24). The differential rate of viral spread in these two transmission models might be due to inherent differences in the oral/GI tract and vaginal mucosa, or could be due to sensitivity of the principle assays utilized for each study (nested SIV DNA PCR for oral transmission and in situ hybridization for vaginal transmission). Either way, the SIV-macaque model has provided key insights into the earliest events, following mucosal transmission, which will be important for the design of effective, prophylactic HIV interventions.
Establishment of HIV infection
Documented cases of HIV acquisition after oral exposure have occurred predominantly through ROI, where individuals are exposed to virus in semen or pre-ejaculatory fluid, and breastfeeding, when virus is consumed in maternal breast milk. All three fluids are well populated by leukocytes, particularly macrophages (98–100), that can harbor infectious virus, and contain detectable titers of cell-free virus, although the viral load in these fluids is generally lower than that observed in the blood (99, 101–106). However, the relative contribution of cell-free and cell-associated virus to HIV transmission is still unclear.
Multiple lines of evidence have suggested that cell-associated virus may be important in oral transmission of HIV. Cell-associated virus can withstand low pH environments, such as the stomach, better than cell-free virus (100, 107); HIV-infected macrophages can penetrate infant oral epithelium, allowing direct viral access to the HIV target cells of the lamina propria (108); and epithelial transcytosis of virus (see below) is most efficient with cell-associated virus (109–112). However, treatment of HIV-infected mothers with antiretrovirals, which dramatically reduces the risk of HIV acquisition in breastfed infants, decreases cell-free viral load in breast milk without reducing either the DNA or RNA load in HIV-infected cells (113–116). This would suggest that cell-free virus is more important than cell-associated virus in infant oral transmission of HIV. However, it is possible that ART may also reduce the infectivity of cell-associated virus. Interestingly, epidemiologic studies have shown that cell-associated virus titers predict breast milk HIV transmission during the first 9 months of life, where cell-free viral titers better predict HIV transmission in older infants (41, 117). This suggests that both cell-associated and cell-free virus can mediate oral transmission of HIV.
Once HIV enters the oral cavity there are a number of distinct tissue sites along the GI tract that may permit viral entry in to the host’s tissue. These histologically distinct tissues include areas of stratified squamous epithelium with (e.g. gingiva) or without (e.g. esophagus and buccal mucosa) keratinization, mucosa-associated lymphoid tissue (MALT) (e.g. tonsils), and columnar epithelium (e.g. salivary glands, stomach and intestinal mucosa). Macaque models indicate that virus, following high-dose oral exposure, most likely initiates infection across the mucosa of the upper gastrointestinal tract, namely the mucosa of the oral cavity, the tonsils and the esophagus (24, 91). The tonsils have a relatively high proportion of HIV target cells (118), and ex vivo and macaque studies have suggested that the tonsils may play a particularly important role in HIV oral transmission (119, 120). However, ex vivo studies of HIV replication in tonsil tissue have consistently used tissue obtained from therapeutic tonsillectomies, which are most commonly performed to remove tonsils that are enlarged due to infection or excessive immune response. Therefore, unlike most other mucosal tissues, tonsil tissues studied ex vivo are collected under inflammatory conditions (118), which is known to increase HIV susceptibility (see Inflammation and HIV/SIV Susceptibility section). Evaluating acute HIV infection with physiologically relevant, low-dose viral exposure (better representing natural exposure), is very difficult, even in the macaque model, making it difficult to determine the exact site of HIV/SIV acquisition within the upper gastrointestinal tract.
The predominant cell type in all mucosal tissues of the upper GI tract is epithelial cells, which do not express the classic HIV receptor CD4 and are not productively infected by HIV. However, cell-free and cell-associated HIV can be efficiently transcytosed across a wide range of epithelial tissues, allowing the virus to gain access to the HIV target cells of the lamina propria (108, 109, 112, 121–124). Virus transcytosed across the infant oral mucosa remains infectious (108). However, in adult oral mucosa, transcytosed HIV is not infectious, and successful penetration by cell-free virus only occurs when tight junctions are disrupted (112). Since epithelial cells are CD4 negative (118, 125), alternate receptors, namely galactosyl ceramide (GalCer) and heparin sulfate proteoglycans, are required for epithelial transcytosis (108, 109, 121–124, 126). The role of HIV coreceptors in transcytosis is still unclear. CXCR4 and CCR5 expression by mucosal epithelial cells varies, depending on the location of the mucosal tissue (118). For example, gingival epithelium expresses CXCR4 but little CCR5 (123, 127) whereas jejunal epithelium expresses substantial levels of CCR5 but no detectable CXCR4 (124). In tissues that express HIV co-receptors, blockade of the receptors by mAbs or inhibitors reduces transcytosis of the virus (123, 124), indicating that when present, HIV co-receptors can facilitate HIV transcytosis. However, both CXCR4- and CCR5-tropic viruses can be successfully transcytosed, regardless of the co-receptor expression of the mucosal tissue (108, 123). Interestingly, only CCR5-tropic virus has been observed to establish infection (see discussion below), suggesting that CCR5-dependent transcytosis of HIV is particularly important in HIV transmission or that selection of co-receptor tropism in transmitted HIV variants occurs after transcytosis. Although the transcytosis of HIV across mucosal epithelium has been well documented in ex vivo studies, its role in natural transmission is unknown.
HIV target cells, including CD4+ T cells, macrophages and Langerhans cells, are present in adult and infant upper GI tissues (108, 112, 128–130). However, in healthy upper GI tissue, Langerhans cells are the predominant immune cell type in the epithelium with CD4+ T cells and macrophages primarily observed in the lamina propria (108, 129, 131). Langerhans cells are tissue dendritic cells that express the HIV receptor CD4 (129) as well as the HIV co-receptor CCR5 (132). Although the cells are capable of internalizing HIV, they do not appear to produce many infectious virions, following viral integration (133, 134). However, like other dendritic cells, they can efficiently transfer virus to other HIV target cells of the lamina propria, including CD4+ T cells and macrophages, via a mechanism known as trans-infection (135, 136). Interestingly, the density of Langerhans cells in oral mucosa is significantly lower than that observed in the vaginal, cervical and foreskin mucosa, which may result in a lower risk of HIV acquisition across the oral mucosa (129). If HIV can survive the inhospitable environment of the stomach, the intestinal mucosa also has numerous CD4+ T cells present in the epithelium, making it possible for virus at this site to directly infect cells that have the capacity to spread HIV without the assistance of additional cell types.
Similar to HIV transmission through other mucosal routes, a narrow transmission bottleneck occurs during oral HIV transmission. One or two viral variants are responsible for establishing infection both in adult and infant oral transmission (137, 138), despite the diversity of HIV in the partner’s semen or mother’s breast milk. Higher SIV dose substantially increases the number of founder variants in macaque studies (93, 139–141), and a similar phenomenon has been observed in infants whose mothers seroconvert during breastfeeding (138, 142), likely due to high breast milk viral loads during acute infection. Also, similar to other routes of infection, only CCR5-tropic HIV viral variants establish infection in vivo (137, 142). Consistent with this observation, only macrophages infected with CCR5-tropic virus can migrate through infant oral mucosa to gain access to the HIV target cells of the lamina propria (108). Interestingly, HIV variants transmitted during breastfeeding have fewer glycosylation sites and shorter Env sequences compared to maternal viral variants (137), although this phenomenon was not recapitulated in the SIV/infant macaque model (138). There is some evidence that virus populations in both breast milk and semen can harbor unique viral variants relative to those observed in the blood (143). However, this compartmentalization is only observed in a subset of individuals, and the virus in breast milk and semen remains diverse and does not differ from blood viral variants in glycosylation sites or Env sequence length (40, 101, 105, 144–147). Inflammatory lesions at the mucosa can result in an increase in the number of variants that establish infection, suggesting that the viral bottleneck is greatest at the mucosa itself (148). However, it is also possible that an additional bottleneck may be present during dissemination of virus from mucosa to the systemic circulation. The narrow viral bottleneck in mucosal transmission of HIV despite the presence of cells that readily transcytose and replicate HIV suggests that anti-viral responses in the upper GI tract may serve a role in reducing, but not abolishing, HIV transmission following oral exposure.
Oral mucosa soluble, innate, and adaptive immunity to HIV
The ability of salivary immune components to inhibit HIV has been well studied. This has led to the discovery of a wide array of oral mucosa defenses against HIV, including both innate and adaptive immune responses (Table 2).
Table 2.
Anti-HIV Soluble Saliva Factors
| Anti-HIV Factor | Mechanism of Action | Concentration in Saliva | Reference |
|---|---|---|---|
| Alpha-Defensins | Unknown | 2715.2±1333.4 ug/mL | (183, 187, 192–194, 279) |
| Beta-Defensins | Inactivate transcytosed virus; Reduce CXCR4 expression |
hBD2: 1.2–21 ng/mL; hBD3: 50–931ng/mL |
(112, 190, 191,280) |
| CCL4 | CCR5 binding | 800ng/mL | (172, 281) |
| Cystatin | Unknown | 1–2ug/mL | (154) |
| Erythropoietin | Unknown | Present | (173, 282) |
| IL15 | Unknown | Present | (174, 283) |
| Lactoferrin | Bovine form blocks DC- SIGN binding; Human form unknown |
6–30ug/mL | (154, 168–170) |
| Long Chain Fatty Acids |
Unknown | Present | (175, 284) |
| Lysozyme | Unknown | 8–16ug/mL | (154) |
| Mucins | HIV aggregation; Strip gp120 from virus; DC- SIGN binding (Muc1) |
1.9mg/mL | (162–165, 285) |
| Salivary Agglutinin (gp340) |
Unknown | Present | (166, 167) |
| Secretory Leukocyte Protease Inhibitor (SLPI) |
Annexin II binding; Inactivates transcytosed virus |
4–24ug/mL | (112, 154, 169,176–179, 181) |
| sTLR2 | Unknown | Present | (171) |
| Theta-Defensins (retrocyclins) |
CD4, GalCer and HIV Env binding |
Unknown | (195–197) |
| Thrombospondin-a (TSP-1) |
HIV aggregation; Binds CD4 |
1.1–12.8ug/mL | (160, 161) |
Saliva can rapidly kill HIV-infected leukocytes: preincubation of HIV with saliva reduces HIV infectivity, and saliva inhibits HIV replication in infected cells (149–158). Filtration of HIV-incubated saliva prior to application to target cells dramatically reduced the infectivity of the inoculum, a phenomenon not seen with filtered HIV alone (153, 155, 159). This phenomenon may be due to the action of salivary components that can aggregate HIV, including thrombospondin-1 (TSP-1) (160, 161) and mucins, particularly 5B, 7A, and 7B (162, 163), some of which are also present in breast milk.
In addition to HIV aggregation, there are also additional mechanisms by which salivary proteins protect against HIV. Mucin 1 from breast milk reduces DC-SIGN-dependent HIV infection by binding to specific carbohydrates on DC-SIGN (164). Mucin-containing components of human saliva can also strip gp120 from HIV viral particles, rendering them uninfectious (165). Salivary agglutinin, also known as gp340, inhibits CCR5- and CXCR4-tropic HIV infectivity by binding to gp120 (166, 167). Additionally, TSP-1 binds CD4 to block HIV entry into target cells (160). Lactoferrin, a protein present in high levels in both breast milk and saliva, is also an important component of salivary anti-HIV activity (168–170). Cystatin also shows anti-HIV activity at concentrations observed in saliva (154, 162). In addition, studies have also found a decreased risk of HIV transmission in mothers with elevated breast milk levels of sTLR2 (171), CCL4 (172), erythropoietin (173), IL15 (174) and specific long-chain fatty acids (175), indicating that there may be many additional host factors that can reduce HIV acquisition, following oral exposure.
Secretory leukocyte protease inhibitor (SLPI) has strong anti-HIV activity at concentrations found in human saliva (154) and is particularly effective at blocking HIV infection of monocytes (176, 177). Depletion of SLPI from whole saliva results in a substantial loss of salivary anti-HIV activity (154, 169, 178). In vivo, higher SLPI salivary levels in HIV-exposed infants reduce their susceptibility to HIV (179). However, breast milk levels of SLPI does not appear to correlate with reduced breastfeeding transmission of HIV (180). SLPI does not bind to HIV proteins or CD4, (154) but does bind Annexin II on macrophages (181). Blockade of Annexin II by SLPI or siRNA knockout of Annexin II reduces macrophage infection in vitro, suggesting a potential mechanism for SLPI’s inhibition of HIV (181). In addition to expression in salivary glands, SLPI is expressed both intracellularly and extracellularly in infant and adult tonsils, and inhibition of SLPI expression in adult human tonsil tissue results in decreased inactivation of transcytosed HIV (112).
Another class of potent anti-microbial proteins that are abundant throughout the gastrointestinal tract (182–189), human β-defensins (hBD), has a similar role as SLPI in inactivating transcytosed HIV (112). HBD-2 and 3, and possibly hBD1, reduce HIV replication in infected cells, and can reduce the expression of CXCR4, but not CCR5 (190, 191). Alpha-defensins 1, 2 and 3, similar small proteins with anti-HIV activity (192, 193), are also expressed throughout the GI tract (183, 187), and breast milk levels of alpha-defensins correlate with reduced HIV acquisition in breastfed infants (194). Theta-defensins, also known as retrocyclins, a new class of circular defensins first identified in non-human primates, have anti-HIV activity in humans by binding CD4, GalCer and glycosylated HIV Env (195–197).
Interestingly, the gastrointestinal microbiome may also play an important role in protecting against HIV acquisition. Bacteria isolated from breast milk, particularly lactobacillus and pediococcal bacteria, common gastrointestinal microbiota, can inhibit HIV infection of target cells (198). One potential mechanism for how specific microbes may modulate HIV susceptibility is the ability of bacteria, like lactobacilli, to produce hydrogen peroxide (199). Saliva contains high levels of peroxidases, which utilize hydrogen peroxide to produce reactive oxygen species that can rapidly inhibit HIV (200).
It is important to note that most of these innate, anti-viral factors have been documented at other mucosal sites, including the vaginal and rectal mucosa. Although elevated mucosal expression of many of these same factors is correlated with reduced vaginal transmission of HIV (201, 202), HIV transmission still occurs even in the presence of innate anti-HIV factors. Although these factors may certainly be responsible for the low rate of HIV transmission per exposure, they are unable to entirely prevent HIV acquisition.
Anti-HIV adaptive immune responses can also provide protection against HIV acquisition, following oral exposure to HIV. Genetic variations in HLA genes modify the antigens targeted by adaptive immune responses, and infants that share HLA genotypes with their mother have increased susceptibility to breast milk transmission of HIV (179). In addition, particular infant HLA genotypes are associated with a much higher risk of breast milk HIV acquisition (203), highlighting the importance of adaptive immunity in reducing oral HIV transmission.
Antibody responses, both mucosal and systemic, appear to be particularly important in protecting against oral transmission of HIV. Strikingly, high dose intravenous administration of cocktails of neutralizing, anti-HIV antibodies to infant macaques protects against SHIV challenge (204, 205). However, it is important to note that only a subset of neutralizing antibody cocktails show full protection in neonatal macaques (204, 205). Studies of anti-HIV responses in breast milk from HIV-infected mothers have shown that increased breast milk IgM responses (206) and antibody-dependent cellular cytotoxicity (ADCC) activity (207) decreases the risk of HIV infection in breastfed infants. Chronic exposure to HIV can induce anti-HIV antibody responses in the oral mucosa, namely IgA responses, as seen in HIV-uninfected partners of HIV-infected individuals (208). However, no studies have demonstrated protection by salivary anti-HIV antibodies in vitro, although these studies particularly focused on IgA antibodies (209), which are associated with increased risk of HIV transmission in breast milk (210). The ability of anti-HIV antibodies to block HIV transcytosis is somewhat unclear. Although some studies have observed effective inhibition of HIV transcytosis with breast milk-derived anti-HIV IgA and IgG (211, 212), other researchers have failed to observe this phenomenon (213). Interestingly, anti-HIV IgM and IgA applied to the basal side of epithelial cells can act within epithelial cells to block HIV transcytosis (214).
The role of T-cell responses in protecting against oral HIV transmission is less clear. Nearly 50% of HIV-exposed, uninfected infants in the absence of maternal art have detectable T cell responses (215, 216) and those responses are maintained for at least 6 months after birth (217). A recent analysis found that the breadth and magnitude of breast milk HIV-gag T cell responses correlate with reduced risk of breast milk MTCT (172). However, it is likely that both antibody and T-cell responses are important for full protection against HIV. Therefore, an HIV vaccine that could effectively block oral transmission of HIV will most likely elicit robust antibody and T cell responses at the site of virus exposure, the oral mucosa.
Inflammation and oral HIV/SIV susceptibility
Although infants are exposed to HIV-containing breast milk for many months, even in the absence of antiretroviral prophylaxis, only a relatively small number of infants born to HIV+ mothers will become infected (218). Indeed, if we assume a 40% MTCT infection rate, then each infant born to an HIV+ mother has a 16% chance of acquiring HIV during breastfeeding. This suggests that certain infants may be particularly susceptible to HIV. Understanding the factors that enhance oral HIV transmission will be critical for developing effective interventions to protect infants from postpartum transmission of HIV.
It is well documented that different types of mucosal inflammation at multiple sites impact sexual and vertical transmission of HIV (219–227). Oral mucosa inflammation is also a risk factor for oral HIV transmission. For example, infant oral candidiasis increases the risk of MTCT of HIV through breastfeeding (228). The risk of HIV acquisition associated with oral sex is increased in individuals that smoke crack cocaine, which is known to cause oral lesions (20), and oral sores are associated with HIV infection in crack cocaine users who performed ROI (OR 1.9) (229). In contrast, studies in HIV-uninfected, highly exposed individuals indicate that low levels of CD4+ T cell activation and quiescent CD4+ T-cell phenotypes are associated with reduced HIV susceptibility (230–233). These studies suggest that inflammation at the oral mucosa increases susceptibility to HIV.
Two hypotheses offer a mechanism for the observed increase in HIV transmission, following inflammatory events at the oral mucosa. The first is that inflammation leads to a break in the mucosal barrier, permitting viral access to target cells that reside beneath the difficult-to-penetrate stratified squamous epithelium of the oral cavity. Second, inflammatory events can recruit activated HIV target cells to mucosal tissues, while the mucosal barrier remains intact. Indeed, inflammatory cytokines can directly increase HIV replication (234–237), indicating that an inflammatory mucosal environment alone may promote productive HIV infection at mucosal sites.
Due to its unique ability to provide detailed mucosal information both before and during early infection, our understanding of inflammatory events that influence oral transmission relies on the SIV-macaque model (Table 1). In one study, Chenine et al. (87) utilized 10% acetic acid to induce an inflammatory sore on the inside cheek pouch with subsequent exposure to low dose oral SHIV challenge. These investigators identified an increased risk in SHIV infection in treated macaques, which likely resulted in both increased access to underlying target cells, recruitment of additional target cells to the site of inflammation as well as an activation of the target cells themselves (87). In contrast, a study from our laboratory experimentally induced gingival inflammation in adult rhesus macaques that mimicked the mild to moderate gingivitis common in humans (238). This was accomplished by tying silk ligatures around the base of the teeth and softening the macaque’s food with water. This treatment induced strong upregulation of multiple inflammatory markers in the oral tissue, including IL6, IL8, and IL18. Macaques were then orally exposed to repeated low dose challenge of SIVmac251 and, surprisingly, the rate of SIV transmission was similar in the gingivitis and control macaque groups, although an increase in the number of viral variants that establish the SIV infection was observed (238). One interesting distinction that may explain the different findings in these two studies is that while gingivitis does result in inflamed mucosa it does not necessarily compromised the mucosal barrier, which likely occurred with acetic acid treatment, potentially explaining the increased susceptibility in the acetic acid treated animals but not in the gingivitis animals.
In addition to inflammatory changes that are induced directly at the mucosa, activation of the systemic immune system may have an indirect impact on target cells at mucosal sites. Indeed, any infection, or even a vaccination, could potentially activate HIV target cells at both systemic and mucosal sites, potentially resulting in increased HIV transmission. This may have been the case in the STEP trial, where individuals who were previously exposed to the vaccine vector or were uncircumcised had an increased risk of acquiring HIV after vaccination (239). This suggests a complex interplay between vaccine-induced immune activation and mucosal tissues, which may have resulted in increased HIV susceptibility.
One perplexing observation worthy of additional study is the finding that mixed feeding, where infants receive breast milk and other foods, results in an increase in the rate of HIV breast milk transmission compared to exclusive breastfeeding. Indeed, in one study from KwaZulu-Natal, South Africa, infants who were mixed fed at any point after birth were almost 11 times more likely to acquire HIV than those who were received breast milk alone (36). In a second study, Kuhn and colleagues showed that the risk of postnatal HIV infection in the first four months of life was significantly lower among exclusively breastfed infants compared to mixed fed infants (31). To date, no difference in mastitis or maternal viral load, either in the systemic circulation or in breast milk, have been observed in mothers that mix feed, and mixed fed infants do not have an increase in GI permeability (38, 240, 241). However, mixed feeding is associated with an increased risk of a number of inflammatory conditions, including asthma, eczema, atopic dermatitis, food allergies, ear infections and diarrhea (242–244). We hypothesize that mucosal inflammation may be induced in mixed fed infants by exposure to novel antigens in food, exposure to food contaminants, including fungus-derived mycotoxins, physical disruption of the oral mucosa due to consumption of solid food or decreased exposure to the anti-inflammatory cytokines found in breast milk. The studies described here provide evidence that inflammation and activation of HIV target cells at the oral mucosa has the potential to increase the oral transmission of HIV.
Oral transmission in natural hosts
SIV natural hosts are African non-human primates who develop non-pathogenic SIV infection in the wild without progression to AIDS. These natural hosts include sooty mangabeys, African green monkey, mandrill, and many others. Key features of SIV infection of natural hosts include: high viremia (245, 246), normal peripheral CD4+ T-cell counts (246, 247), lack of microbial translocation despite significant loss of mucosal CD4+ T cells (248, 249), and lack of immune activation during chronic infection (247, 249–251). These studies have led to a working hypothesis that the lack of disease progression in natural hosts is due to a lack of chronic immune activation (252, 253).
In natural hosts, MTCT of SIV, including oral transmission, is a rare occurrence both in the wild and captivity (254–257). Early studies in Ethiopian wild grivet monkeys, SIV natural hosts, showed very low incidence of SIV seropositivity before the onset of sexual activity (257). In a subsequent study, captive infant African green monkeys show progressive decline in anti-SIV antibodies, with no detectable anti-SIV antibodies by one year of age, consistent with progressive loss of transferred maternal antibodies without MTCT of SIV (255). More recently, efficiency of breastfeeding transmission in natural hosts was directly tested in female mandrills infected with SIV one day after delivery. Interestingly, despite high peak and set-point plasma viral loads in the mothers, none of the offspring had serological or virological evidence of infection by the end of breastfeeding (6 months) (256). This finding is in direct contrast to the breast milk transmission studies performed in the pathogenic host, rhesus macaques, as mentioned above, where high rates of infant infection were seen in similar study designs (258, 259). It is important to note that observation of captive breeding colonies have revealed similar suckling frequency and total duration of breastfeeding in both natural and non-natural hosts (J. Else, personal communication).
The sooty mangabey has been extensively studied as a model of nonpathogenic SIV infection in a natural host species. Similar to other natural hosts, MTCT of HIV in sooty mangabeys is rare. An unprecedented study of viral sequences in fecal samples from wild sooty mangabeys in the Tai Forest in Cote d’Ivoire identified only two likely MTCT events (where both mother and infant had nearly identical virus) in the context of 59% adult prevalence of SIV infection (260). In a recent study, we investigated MTCT of SIV in sooty mangabeys in a large colony of naturally SIV-infected sooty mangabeys, using serological and virological methods. Of 161 sooty mangabey infants born to SIV-infected mothers only 11 infants (6.8%) were defined as ‘presumptively’ vertically infected based on repeated serologic sampling with confirmatory viral loads in the first year of life (254). In comparison, 25–45% of human infants born to HIV-infected mothers acquire HIV from their mother in the absence of intervention (218). Interestingly, SIV-infected sooty mangabey infants do not have increased morbidity or mortality, indicating that the infection is nonpathogenic even when acquired early in life (254). Interestingly, the viral load of SIV-infected sooty mangabey infants was ∼2-log lower than that observed in SIV-infected adult sooty mangabeys living in the same colony. These results confirm that vertical transmission, including breast milk transmission, is substantially less frequent in SIV-infected natural hosts than in HIV-infected humans or SIV-infected rhesus macaques.
We propose three non-mutually exclusive hypotheses to explain the restriction of breast milk SIV transmission in natural hosts: (i) lower levels of SIV in natural host breast milk than those observed in pathogenic infections, (ii) a relatively non-permissive breast milk and/or gastrointestinal microenvironment, with lower immune activation and the presence of innate and adaptive inhibitory factors, and (iii) insufficient target cells for establishment of infection in the natural host infant. To date, no definitive studies have yet been performed that test one hypothesis while controlling for all other potential confounding factors (and may be impossible to perform), but a preponderance of data suggests target cell restriction in the infant GI tract is a defining feature of natural hosts that limits MTCT.
In humans, breast milk viral load and breast milk cell-associated HIV correlates with the risk of MTCT (38, 39), but similar levels of cell-free SIV are seen in both SIV-infected African green monkeys and rhesus macaques (261). SIV RNA was measured in breast milk from mandrills infected with SIV postpartum, and high levels of breast milk RNA were found on days 7–14 post infection, but interestingly, the virus became undetectable in milk by day 21 (256). However, this phenomenon has not been observed in lactating African green monkeys and sooty mangabeys. Cell-associated SIV in chronically-infected African green monkeys is 1-log lower than in chronically SIV-infected rhesus macaques (261). This is likely due to the lower level of CD4+ T cells found in breast milk of SIV-infected African green monkeys, but it is unclear whether these lower levels of cell-associated SIV influence transmission rates. It should be noted that there was no difference in the level of cell-free and cell-associated SIV between blood and breast milk in African green monkeys.
Second, the maternal breast milk or infant oral microenvironment, including innate and adaptive immune responses, may be less favorable for transmission in natural hosts. Analysis of human milk and saliva has revealed numerous factors that inhibit HIV replication in vitro, including lactoferrin, SLPI, and mucins (see ‘Innate and adaptive oral mucosa immunity to HIV’ section). Other milk constituents, such as sTLR2 (171), CCL4 (172), and IL15 (174), correlate with reduced MTCT in epidemiologic studies. An exhaustive characterization of potential inhibitory factors in breast milk of natural hosts has not been performed, and, to date, there have been no studies of salivary inhibitory factors in natural hosts. However, we have analyzed the ability of breast milk collected from both sooty mangabeys and macaques to neutralize the infectivity of SIV and found a significant inhibition of infection with breast milk from both species. However, there was no difference between milk from the natural vs. non-natural hosts (unpublished). It has been proposed that African green monkeys have higher breast milk levels of neutralizing antibodies than rhesus macaques (261). This potential protective mechanism has not been tested in other natural hosts. However, given that cell-free virus levels are similar in the milk of natural and non-natural hosts, it seems unlikely that the milk of natural hosts contains potent inhibitors of SIV replication.
Successful HIV/SIV transmission via breast milk requires adequate target cells at infant GI mucosal tissues. Pandrea et al. (262) have demonstrated that the level of CD4+CCR5+ T cells in adult GI mucosa is extremely low in five different species of natural hosts, and that expression of CCR5 in adult sooty mangabeys is restricted to the effector memory populations (263). In infant mandrills, the percent of circulating CD4+CCR5+ T cells was less than 0.5% (256). We have extensively analyzed infant sooty mangabey target cells from multiple sites along the GI tract, including oral mucosa, esophagus and tonsils, and found a paucity of CD4+CCR5+ T cells (<10%) (authors’ unpublished data). In recent work, both esophageal and jejunal CD4+CCR5+ T cells were remarkably few in young African green monkeys, but increased with age (264). In the same work, intrarectal SIV infection of African green monkeys was found to be dependent on the level of CD4+CCR5+ T cells at the site of exposure, with a significant positive correlation between the percentage of CD4+CCR5+ T cells and the number of transmitted founder viruses. These data suggest that a critical threshold for establishment of infection may not be reached in natural host infants exposed to SIV via the oral route.
In the rare sooty mangabeys who become infected via vertical transmission as well as experimentally infected neonatal African green monkeys, viral loads are 1–2 logs lower than those seen in animals who become infected later in life by the horizontal route (254, 265). This is particularly striking given that neonates and infants have a significantly higher percentage and absolute number of CD4+ T cells compared to adults and further underlines the important role of target cell restriction in determining the outcome of neonatal exposure to SIV.
By looking at both inflammatory conditions and natural hosts, it becomes clear that the mucosal immune environment, especially mucosal target cell levels, predicts HIV/SIV susceptibility. Increased mucosal inflammation in infant and adult oral mucosa facilitates the establishment of HIV and SIV infection. Evidence suggests that increased HIV target cells in inflamed oral mucosa paired with an increase expression of the HIV/SIV co-receptor, CCR5, results in increased infection of HIV target cells in the oral mucosa (Fig. 1, left). On the other hand, SIV natural hosts, whose infants have a dramatically lower risk of SIV acquisition, have lower levels of target cells in GI mucosa. Of particular importance is the paucity of CD4+CCR5+ T cells, suggesting that these cells are particularly important in facilitating HIV/SIV infection (Fig. 1, right).
Fig. 1. Oral mucosa immune activation and HIV susceptibility.
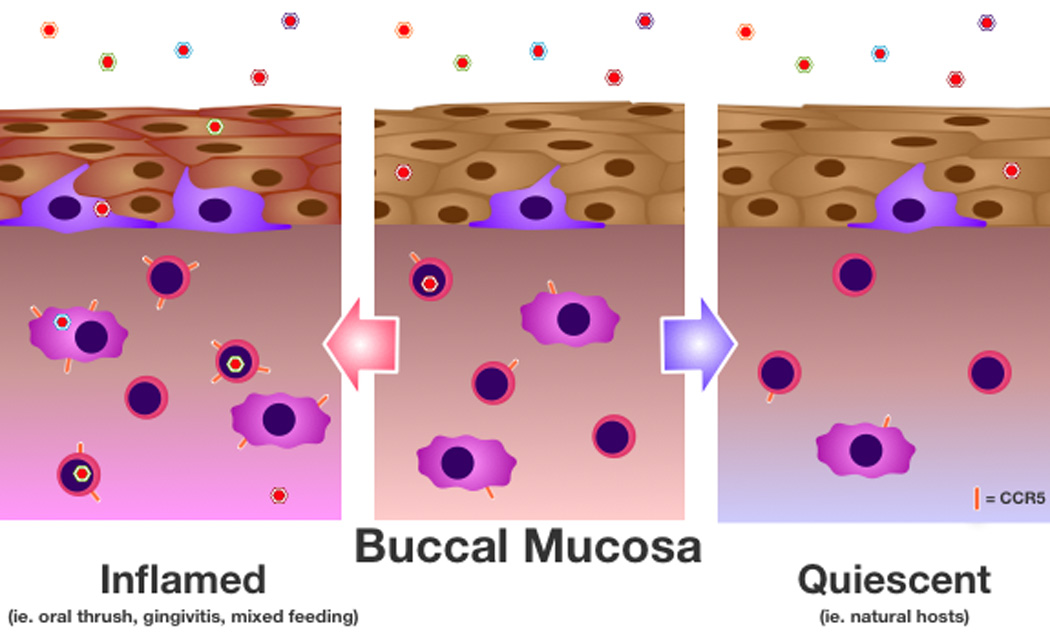
The buccal mucosa, non-keratinized stratified squamous epithelium that lines the inner cheek, is shown during exposure to an HIV/SIV quasispecies (variation in virus color reflects the variability in viral genetic sequence) under inflammatory (left side) and immune quiescent (right side) conditions. HIV/SIV can cross through the epithelium either through transcytosis or by migrating between disrupted cell-cell junctions, which can occur in response to inflammatory stimuli. This disruption of tight junctions results in an increase in the number of viral variants that successfully enter the lamina propria. Langerhans cells, which facilitate virus transfer to productively infected cells, reside in the basal layer of the epithelium and may increase in response to inflammatory stimuli. CD4+ HIV/SIV target cells of the lamina propria, primarily macrophages and T cells, can express variable levels of the HIV/SIV co-receptor CCR5. Notably, CCR5 expression is enhanced, particularly on T cells, under inflammatory conditions, while CCR5-expressing cells are rare in quiescent mucosa. CD4+ cells with high CCR5 expression are most readily infected with HIV, resulting in more productively-infected cells in inflamed conditions and a paucity of infected cells in quiescent conditions. These infected cells then produce additional virus and facilitate dissemination of the virus throughout the systemic circulation, leading to the establishment of productive HIV/SIV infection.
Interventions
Antiretroviral therapy has significantly reduced the morbidity and mortality associated with HIV infection, but there is still neither a cure nor a vaccine for this disease. In this context, MTCT of HIV represents an enormous health care problem, with nearly 300,000 new pediatric HIV infections per year (3). Of these cases of HIV MTCT, almost half can be attributed to breastfeeding (27). In developing countries that are most affected by the AIDS epidemic, formula feeding, which can effectively eliminate HIV breast milk transmission, is neither safe nor affordable. Maternal and infant antiretroviral prophylaxis reduces the rate of transmission via breast milk to as low as 1–2% risk of transmission, but these therapies are associated with high cost, infant toxicity, viral resistance in both mothers and infants and unknown long-term consequences. Therefore, it is crucial that alternative interventions be developed to protect infants from HIV acquisition via breast milk (Table 3).
Table 3.
Current and Future HIV Interventions to Reduce Oral HIV Transmission
| Intervention | Benefits | Challenges |
|---|---|---|
| Antiretroviral Prophylaxis |
Proven efficacy Currently available for clinical use |
Long duration of treatment Suboptimal adherence Drug resistance |
| Replacement Feeding |
Proven efficacy Eliminates breast milk transmission |
Increases infant mortality in developing settings Expensive Social acceptability |
| Exclusive Breastfeeding |
Proven efficacy |
Substantial HIV transmission Necessity to introduce solid food |
| Maternal Breast Milk Treatment |
Effective at inactivating HIV | No clinical trials to date Expensive May remove beneficial components of breast milk |
| Infant Immune- Modulating Therapeutics |
Permit safe use of multiple feeding methods |
Target not yet identified Potential to modulate susceptibility to other pathogens |
| Vaccine | Block HIV transmission via multiple routes, including oral transmission |
Only one trial in adults showed any efficacy Efficacy of current vaccine is very low |
There have been a number of studies of extended infant postnatal prophylaxis to prevent breastfeeding transmission of HIV, testing the efficacy of a variety of different drug regimens (266). These studies have shown comparable efficacy to maternal antiretroviral prophylaxis during the breastfeeding period. Current WHO guidelines recommend daily nevirapine to breastfeeding infants until 1 week after breastfeeding has finished for infants born to mothers who are not on a triple antiretroviral regimen (Option A) or daily nevirapine or zidovudine for 4–6 weeks for infants born to mothers who are on a triple antiretroviral regimen (Option B) (5). An interesting question has been raised recently with the description of an infant ‘cured’ of HIV, following initiation of highly active antiretroviral therapy at birth (267). While this particular infant was not breastfed, one wonders whether the use of highly active combination antiretrovirals during breastfeeding may reduce reservoir establishment and viral latency in infants infected in the late postpartum or intrapartum periods.
Interventions to reduce mucosal immune activation in breastfed infants would be a welcome addition to the prophylactic antiretrovirals currently in use. WHO guidelines that encourage HIV-infected women to exclusively breastfeed their infants for 6 months, if replacement feeding is neither safe (due to contamination of water or food) nor readily available, likely result in a reduction in infant mucosal inflammation by delaying the introduction of solid foods (5). However, mixed feeding is a biological necessity, since all infants must at some point be weaned. Therefore, if we can identify specific exposures in mixed fed infants that result in increased mucosal immune activation, we may be able to reduce the risk of HIV acquisition associated with mixed feeding. Alternatively, by understanding the biological mechanisms underlying mucosal immune activation in the oral mucosa, immunomodulating therapeutics can be developed to counteract the mucosal immune activation induced by mixed feeding.
Another approach that may be able to supplement current antiretroviral therapy is treatment of maternal breast milk to reduce its infectivity. Many sterilization and filtration processes have been devised that can effectively remove or reduce HIV viral infectivity (268–273). Unfortunately, most of these technologies require tools not commonly available in households in developing countries or are prohibitively expensive. In addition, some of the immunologic benefits of breast milk may be lost with aggressive treatment (274), potentially reducing the benefit of breastfeeding compared to replacement feeding.
An effective HIV vaccine for newborns ideally would be given at birth with extremely high safety and proven efficacy to reduce HIV acquisition and/or modulate disease progression should HIV infection occur. Indeed, passive immunization of neonatal rhesus macaques can provide protection against oral SIV infection (88, 204), highlighting the potential of vaccines to induce potent antibody responses for prevention of oral HIV infection in newborns (88, 205, 275). DNA vaccines that express specific viral antigens may be useful in the setting of neonatal immunization due to their generally lower safety concerns and ability to induce both humoral and cellular immunity. A study from Van Rompay et al. (276) demonstrated that systemic administration of a canarypox virus vector-based SIV vaccine (ALVAC-SIV) or modified vaccinia virus Ankara (MVA-SIV) expressing Gag, Pol, and Env did not show protection from infection but did result in reduced viremia, following oral SIV challenge in infant rhesus macaques. Other work has tested oral vaccination with a vesicular stomatitis virus based SIV vaccine (VSV-SIV) expressing Gag, Pol, and Env followed by a boost with intramuscular immunization of MVA-SIV, but this also failed to provide protection against oral SIV challenge despite robust antibody and cell-mediated immune responses at both systemic and mucosal sites (277, 278). Thus far, none of the potential vaccine candidates have shown high efficacy in preventing SIV infection of infant macaques.
The development of HIV vaccines to prevent oral transmission through breastfeeding in infants may be more difficult compared to other routes of transmission, due to the potential for frequent, high dose HIV exposure over months to years as well as the infant’s immature immune system. More studies are needed to better understand infant immunity, the interaction between HIV and maternal immune modulators in newborns, and perhaps how to utilize our knowledge of the protection from oral transmission in natural hosts to impact the lives of children born to HIV-infected mothers.
Conclusions
The mucosal immune environment is a key determinant for a host’s susceptibility to HIV. The studies presented here evaluate our current knowledge of HIV oral transmission in both infants and adults, the use of SIV models in understanding early immune events, oral immune factors that modulate HIV/SIV susceptibility (including mucosal inflammation), and interventions that may impact oral HIV transmission rates. One take-home message from these numerous studies is that understanding the mechanisms that underlie the recruitment of target cells to the GI tract will likely lead to a better understanding of immune modulations that can protect both infants and adults. It is likely that such studies of basic mechanisms of mucosal HIV transmission (both at the oral and other sites) will be necessary to provide a foundation for the development of immune therapeutic and vaccine strategies that can effectively protect both infants and adults from HIV transmission.
Acknowledgements
We would like to thank Doug Short for critically reading the manuscript. Some of the work described in this review was supported by grants to DLS (R01 DE017541), AC (CFAR03 Development Award), HBJ (K08 HD069201 and CFAR AI027757 Early Investigator award) and LFW (F30 ES022535 and CFAR AI027757 Trainee award). The authors have no conflicts of interest to declare.
References
- 1.McElrath MJ. Standing guard at the mucosa. Immunity. 2011;34:146–148. doi: 10.1016/j.immuni.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Spetz AL, Chiodi F. Reduction of HIV-1 Load in Semen During Follow-up Study of RV144 Vaccine Trial Boosts Interest for Novel Correlates of Immune Protection in Genital Mucosa. The Journal of infectious diseases. 2012 doi: 10.1093/infdis/jis477. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2012. 2012 [Google Scholar]
- 4.Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV-1: timing and implications for prevention. The Lancet infectious diseases. 2006;6:726–732. doi: 10.1016/S1473-3099(06)70629-6. [DOI] [PubMed] [Google Scholar]
- 5.WHO. HIV Transmission Through Breastfeeding: A Review of the Available Evidence: 2007 Update. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 6.Coates RA, et al. Risk factors for HIV infection in male sexual contacts of men with AIDS or an AIDS-related condition. American journal of epidemiology. 1988;128:729–739. doi: 10.1093/oxfordjournals.aje.a115026. [DOI] [PubMed] [Google Scholar]
- 7.Darrow WW, et al. Risk factors for human immunodeficiency virus (HIV) infections in homosexual men. American journal of public health. 1987;77:479–483. doi: 10.2105/ajph.77.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King LA, et al. Risk factors for seroconversion to human immunodeficiency virus among male homosexuals. Results from the Multicenter AIDS Cohort Study. Lancet. 1987;1:345–349. doi: 10.1016/s0140-6736(87)91725-9. [DOI] [PubMed] [Google Scholar]
- 9.Lyman D, Winkelstein W, Ascher M, Levy JA. Minimal risk of transmission of AIDS-associated retrovirus infection by oral-genital contact. JAMA : the journal of the American Medical Association. 1986;255:1703. [PubMed] [Google Scholar]
- 10.Mayer KH, Ayotte D, Groopman JE, Stoddard AM, Sarngadharan M, Gallo R. Association of human T lymphotropic virus type III antibodies with sexual and other behaviors in a cohort of homosexual men from Boston with and without generalized lymphadenopathy. The American journal of medicine. 1986;80:357–363. doi: 10.1016/0002-9343(86)90706-0. [DOI] [PubMed] [Google Scholar]
- 11.McCusker J, Stoddard AM, Mayer KH, Cowan DN, Groopman JE. Behavioral risk factors for HIV infection among homosexual men at a Boston community health center. American journal of public health. 1988;78:68–71. doi: 10.2105/ajph.78.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrow DG, DiFranceisco WJ, Chmiel JS, Wagstaff DA, Wesch J. A case-control study of human immunodeficiency virus type 1 seroconversion and risk-related behaviors in the Chicago MACS/CCS Cohort, 1984–1992. Multicenter AIDS Cohort Study. Coping and Change Study. American journal of epidemiology. 1995;142:875–883. doi: 10.1093/oxfordjournals.aje.a117727. [DOI] [PubMed] [Google Scholar]
- 13.Schechter MT, et al. The Vancouver Lymphadenopathy-AIDS Study: 6. HIV seroconversion in a cohort of homosexual men. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 1986;135:1355–1360. [PMC free article] [PubMed] [Google Scholar]
- 14.van Griensven GJ, et al. Risk factors and prevalence of HIV antibodies in homosexual men in the Netherlands. American journal of epidemiology. 1987;125:1048–1057. doi: 10.1093/oxfordjournals.aje.a114620. [DOI] [PubMed] [Google Scholar]
- 15.Winkelstein W, Jr., et al. Sexual practices and risk of infection by the human immunodeficiency virus. The San Francisco Men’s Health Study. JAMA : the journal of the American Medical Association. 1987;257:321–325. [PubMed] [Google Scholar]
- 16.Gilbart VL, Evans BG, Dougan S. HIV transmission among men who have sex with men through oral sex. Sexually transmitted infections. 2004;80:324. doi: 10.1136/sti.2004.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page-Shafer K, et al. Risk of HIV infection attributable to oral sex among men who have sex with men and in the population of men who have sex with men. AIDS. 2002;16:2350–2352. doi: 10.1097/00002030-200211220-00022. [DOI] [PubMed] [Google Scholar]
- 18.Page-Shafer K, Veugelers PJ, Moss AR, Strathdee S, Kaldor JM, van Griensven GJ. Sexual risk behavior and risk factors for HIV-1 seroconversion in homosexual men participating in the Tricontinental Seroconverter Study, 1982–1994. American journal of epidemiology. 1997;146:531–542. doi: 10.1093/oxfordjournals.aje.a009311. [DOI] [PubMed] [Google Scholar]
- 19.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. American journal of epidemiology. 1999;150:306–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JI, Porter J, Weiner A, Steinberg A. Oral sex, crack smoking, and HIV infection among female sex workers who do not inject drugs. American journal of public health. 1997;87:470. doi: 10.2105/ajph.87.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba TW, et al. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS research and human retroviruses. 1994;10:351–357. doi: 10.1089/aid.1994.10.351. [DOI] [PubMed] [Google Scholar]
- 22.Baba TW, et al. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;272:1486–1489. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- 23.Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. Journal of virology. 2009;83:12229–12240. doi: 10.1128/JVI.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milush JM, et al. Rapid dissemination of SIV following oral inoculation. AIDS. 2004;18:2371–2380. [PubMed] [Google Scholar]
- 25.Milush JM, Stefano-Cole K, Schmidt K, Durudas A, Pandrea I, Sodora DL. Mucosal innate immune response associated with a timely humoral immune response and slower disease progression after oral transmission of simian immunodeficiency virus to rhesus macaques. Journal of virology. 2007;81:6175–6186. doi: 10.1128/JVI.00042-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegler JB, Cooper DA, Johnson RO, Gold J. Postnatal transmission of AIDS-associated retrovirus from mother to infant. Lancet. 1985;1:896–898. doi: 10.1016/s0140-6736(85)91673-3. [DOI] [PubMed] [Google Scholar]
- 27.Nduati R, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 28.Kilewo C, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. Journal of acquired immune deficiency syndromes. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 29.Gaur AH, et al. Practice of feeding premasticated food to infants: a potential risk factor for HIV transmission. Pediatrics. 2009;124:658–666. doi: 10.1542/peds.2008-3614. [DOI] [PubMed] [Google Scholar]
- 30.Ivy W, 3rd, et al. Premastication as a route of pediatric HIV transmission: case-control and cross-sectional investigations. Journal of acquired immune deficiency syndromes. 2012;59:207–212. doi: 10.1097/QAI.0b013e31823b4554. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn L, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PloS one. 2007;2:e1363. doi: 10.1371/journal.pone.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliff PJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 33.Kafulafula G, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. Journal of acquired immune deficiency syndromes. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 34.Newell ML. Infant feeding and HIV-1 transmission. Lancet. 1999;354:442–443. doi: 10.1016/S0140-6736(99)90118-6. [DOI] [PubMed] [Google Scholar]
- 35.Richardson BA, Hughes JP. Modeling breastmilk infectivity in HIV-1 infected mothers. Biometrics. 2003;59:179–185. doi: 10.1111/1541-0420.00021. [DOI] [PubMed] [Google Scholar]
- 36.Coovadia HM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 37.Taha TE, et al. Late postnatal transmission of HIV-1 and associated factors. The Journal of infectious diseases. 2007;196:10–14. doi: 10.1086/518511. [DOI] [PubMed] [Google Scholar]
- 38.Lunney KM, et al. Associations between breast milk viral load, mastitis, exclusive breast-feeding, and postnatal transmission of HIV. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:762–769. doi: 10.1086/650535. [DOI] [PubMed] [Google Scholar]
- 39.Neveu D, et al. Cumulative exposure to cell-free HIV in breast milk, rather than feeding pattern per se, identifies postnatally infected infants. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:819–825. doi: 10.1093/cid/ciq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gantt S, et al. Genetic analyses of HIV-1 env sequences demonstrate limited compartmentalization in breast milk and suggest viral replication within the breast that increases with mastitis. Journal of virology. 2010;84:10812–10819. doi: 10.1128/JVI.00543-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koulinska IN, et al. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. Journal of acquired immune deficiency syndromes. 2006;41:93–99. doi: 10.1097/01.qai.0000179424.19413.24. [DOI] [PubMed] [Google Scholar]
- 42.Phiri W, et al. Factors influencing breast milk HIV RNA viral load among Zambian women. AIDS research and human retroviruses. 2006;22:607–614. doi: 10.1089/aid.2006.22.607. [DOI] [PubMed] [Google Scholar]
- 43.Semba RD, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet. 1994;343:1593–1597. doi: 10.1016/s0140-6736(94)93056-2. [DOI] [PubMed] [Google Scholar]
- 44.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South African Vitamin A Study Group. Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 45.Kumwenda NI, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. The New England journal of medicine. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 46.de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. The Lancet infectious diseases. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 47.Bedri A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 48.Moorthy A, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PloS one. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeh C, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS medicine. 2011;8:e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marmor M, et al. Possible female-to-female transmission of human immunodeficiency virus. Annals of internal medicine. 1986;105:969. doi: 10.7326/0003-4819-105-6-969_1. [DOI] [PubMed] [Google Scholar]
- 51.Monzon OT, Capellan JM. Female-to-female transmission of HIV. Lancet. 1987;2:40–41. doi: 10.1016/s0140-6736(87)93071-6. [DOI] [PubMed] [Google Scholar]
- 52.Perry S, Jacobsberg L, Fogel K. Orogenital transmission of human immunodeficiency virus (HIV) Annals of internal medicine. 1989;111:951–952. doi: 10.7326/0003-4819-111-11-951_2. [DOI] [PubMed] [Google Scholar]
- 53.Rich JD, Buck A, Tuomala RE, Kazanjian PH. Transmission of human immunodeficiency virus infection presumed to have occurred via female homosexual contact. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1993;17:1003–1005. doi: 10.1093/clinids/17.6.1003. [DOI] [PubMed] [Google Scholar]
- 54.Spitzer PG, Weiner NJ. Transmission of HIV infection from a woman to a man by oral sex. The New England journal of medicine. 1989;320:251. doi: 10.1056/nejm198901263200414. [DOI] [PubMed] [Google Scholar]
- 55.Berrey MM, Shea T. Oral sex and HIV transmission. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;14:475. doi: 10.1097/00042560-199704150-00012. [DOI] [PubMed] [Google Scholar]
- 56.Chen W, Samarasinghe PL. Allergy, oral sex, and HIV. Lancet. 1992;339:627–628. doi: 10.1016/0140-6736(92)90923-q. [DOI] [PubMed] [Google Scholar]
- 57.DeGruttola V, Mayer KH. Human immunodeficiency virus and oral intercourse. Annals of internal medicine. 1987;107:428–429. doi: 10.7326/0003-4819-107-2-428_2. [DOI] [PubMed] [Google Scholar]
- 58.Detels R, et al. Seroconversion, sexual activity, and condom use among 2915 HIV seronegative men followed for up to 2 years. Journal of acquired immune deficiency syndromes. 1989;2:77–83. [PubMed] [Google Scholar]
- 59.Edwards SK, White C. HIV seroconversion illness after orogenital contact with successful contact tracing. International journal of STD & AIDS. 1995;6:50–51. doi: 10.1177/095646249500600111. [DOI] [PubMed] [Google Scholar]
- 60.Goldberg DJ, Green ST, Kennedy DH, Emslie JA, Black JD. HIV and orogenital transmission. Lancet. 1988;2:1363. doi: 10.1016/s0140-6736(88)90894-x. [DOI] [PubMed] [Google Scholar]
- 61.Keet IP, Albrecht van Lent N, Sandfort TG, Coutinho RA, van Griensven GJ. Orogenital sex and the transmission of HIV among homosexual men. AIDS. 1992;6:223–226. doi: 10.1097/00002030-199202000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Lane HC, Holmberg SD, Jaffe HW. HIV seroconversion and oral intercourse. American journal of public health. 1991;81:658. doi: 10.2105/ajph.81.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lifson AR, O’Malley PM, Hessol NA, Buchbinder SP, Cannon L, Rutherford GW. HIV seroconversion in two homosexual men after receptive oral intercourse with ejaculation: implications for counseling concerning safe sexual practices. American journal of public health. 1990;80:1509–1511. doi: 10.2105/ajph.80.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray AB, Greenhouse PR, Nelson WL, Norman JE, Jeffries DJ, Anderson J. Coincident acquisition of Neisseria gonorrhoeae and HIV from fellatio. Lancet. 1991;338:830. doi: 10.1016/0140-6736(91)90726-6. [DOI] [PubMed] [Google Scholar]
- 65.Richters J, Grulich A, Ellard J, Hendry O, Kippax S. HIV transmission among gay men through oral sex and other uncommon routes: case series of HIV seroconverters, Sydney. AIDS. 2003;17:2269–2271. doi: 10.1097/00002030-200310170-00020. [DOI] [PubMed] [Google Scholar]
- 66.Robinson EK, Evans BG. Oral sex and HIV transmission. AIDS. 1999;13:737–738. doi: 10.1097/00002030-199904160-00021. [DOI] [PubMed] [Google Scholar]
- 67.Rozenbaum W, Gharakhanian S, Cardon B, Duval E, Coulaud JP. HIV transmission by oral sex. Lancet. 1988;1:1395. doi: 10.1016/s0140-6736(88)92205-2. [DOI] [PubMed] [Google Scholar]
- 68.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Annals of internal medicine. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 69.Truong HM, Berrey MM, Shea T, Diem K, Corey L. Concordance between HIV source partner identification and molecular confirmation in acute retroviral syndrome. Journal of acquired immune deficiency syndromes. 2002;29:232–243. doi: 10.1097/00042560-200203010-00003. [DOI] [PubMed] [Google Scholar]
- 70.Transmission of HIV possibly associated with exposure of mucous membrane to contaminated blood. MMWR Morbidity and mortality weekly report. 1997;46:620–623. [PubMed] [Google Scholar]
- 71.HIV and orogenital transmission. Lancet. 1988;2:1023–1024. [PubMed] [Google Scholar]
- 72.Greenhouse P. Female-to-female transmission of HIV. Lancet. 1987;2:401–402. doi: 10.1016/s0140-6736(87)92425-1. [DOI] [PubMed] [Google Scholar]
- 73.Spencer B. Orogenital sex and risk of transmission of HIV. Lancet. 1993;341:441. doi: 10.1016/0140-6736(93)93041-x. [DOI] [PubMed] [Google Scholar]
- 74.Ou CY, et al. Molecular epidemiology of HIV transmission in a dental practice. Science. 1992;256:1165–1171. doi: 10.1126/science.256.5060.1165. [DOI] [PubMed] [Google Scholar]
- 75.Fischl MA, Dickinson GM, Scott GB, Klimas N, Fletcher MA, Parks W. Evaluation of heterosexual partners, children, and household contacts of adults with AIDS. JAMA : the journal of the American Medical Association. 1987;257:640–644. [PubMed] [Google Scholar]
- 76.Samuel MC, Hessol N, Shiboski S, Engel RR, Speed TP, Winkelstein W., Jr. Factors associated with human immunodeficiency virus seroconversion in homosexual men in three San Francisco cohort studies, 1984–1989. Journal of acquired immune deficiency syndromes. 1993;6:303–312. [PubMed] [Google Scholar]
- 77.de Vincenzi I. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. European Study Group on Heterosexual Transmission of HIV. The New England journal of medicine. 1994;331:341–346. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 78.del Romero J, et al. Evaluating the risk of HIV transmission through unprotected orogenital sex. AIDS. 2002;16:1296–1297. doi: 10.1097/00002030-200206140-00017. [DOI] [PubMed] [Google Scholar]
- 79.Schwarcz SK, Kellogg TA, Kohn RP, Katz MH, Lemp GF, Bolan GA. Temporal trends in human immunodeficiency virus seroprevalence and sexual behavior at the San Francisco municipal sexually transmitted disease clinic, 1989–1992. American journal of epidemiology. 1995;142:314–322. doi: 10.1093/oxfordjournals.aje.a117637. [DOI] [PubMed] [Google Scholar]
- 80.Silvestre AJ, Kingsley LA, Wehman P, Dappen R, Ho M, Rinaldo CR. Changes in HIV rates and sexual behavior among homosexual men, 1984 to 1988/92. American journal of public health. 1993;83:578–580. doi: 10.2105/ajph.83.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. International journal of epidemiology. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boily MC, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. The Lancet infectious diseases. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hughes JP, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. The Journal of infectious diseases. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keele BF, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. The Journal of experimental medicine. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma ZM, et al. SIVmac251 is inefficiently transmitted to rhesus macaques by penile inoculation with a single SIVenv variant found in ramp-up phase plasma. AIDS research and human retroviruses. 2011;27:1259–1269. doi: 10.1089/aid.2011.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stone M, et al. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. Journal of virology. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chenine AL, et al. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. The Journal of infectious diseases. 2010;201:1155–1163. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Rompay KK, et al. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. The Journal of infectious diseases. 1998;177:1247–1259. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]
- 89.Stahl-Hennig C, et al. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 90.Tenner-Racz K, et al. Early protection against pathogenic virus infection at a mucosal challenge site after vaccination with attenuated simian immunodeficiency virus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3017–3022. doi: 10.1073/pnas.0308677101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abel K, et al. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. Journal of virology. 2006;80:6357–6367. doi: 10.1128/JVI.02240-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kosub DA, et al. Gamma/Delta T cell mRNA levels decrease at mucosal sites and increase at lymphoid sites following an oral SIV infection of macaques. Current HIV research. 2008;6:520–530. doi: 10.2174/157016208786501490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Durudas A, et al. Differential innate immune responses to low or high dose oral SIV challenge in Rhesus macaques. Current HIV research. 2011;9:276–288. doi: 10.2174/157016211797635928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Z, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 95.Zhang ZQ, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Q, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller CJ, Abel K. Immune mechanisms associated with protection from vaginal SIV challenge in rhesus monkeys infected with virulence-attenuated SHIV 89.6. Journal of medical primatology. 2005;34:271–281. doi: 10.1111/j.1600-0684.2005.00125.x. [DOI] [PubMed] [Google Scholar]
- 98.Pudney J, Oneta M, Mayer K, Seage G, 3rd, Anderson D. Pre-ejaculatory fluid as potential vector for sexual transmission of HIV-1. Lancet. 1992;340:1470. doi: 10.1016/0140-6736(92)92659-4. [DOI] [PubMed] [Google Scholar]
- 99.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. The Journal of infectious diseases. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 100.Satomi M, et al. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. The Journal of infectious diseases. 2005;191:174–181. doi: 10.1086/426829. [DOI] [PubMed] [Google Scholar]
- 101.Heath L, et al. Restriction of HIV-1 genotypes in breast milk does not account for the population transmission genetic bottleneck that occurs following transmission. PloS one. 2010;5:e10213. doi: 10.1371/journal.pone.0010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lewis P, et al. Cell-free human immunodeficiency virus type 1 in breast milk. The Journal of infectious diseases. 1998;177:34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pillay K, Coutsoudis A, York D, Kuhn L, Coovadia HM. Cell-free virus in breast milk of HIV-1-seropositive women. Journal of acquired immune deficiency syndromes. 2000;24:330–336. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- 104.Rousseau CM, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. The Journal of infectious diseases. 2003;187:741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salazar-Gonzalez JF, et al. Origin and evolution of HIV-1 in breast milk determined by single-genome amplification and sequencing. Journal of virology. 2011;85:2751–2763. doi: 10.1128/JVI.02316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thiry L, et al. Isolation of AIDS virus from cell-free breast milk of three healthy virus carriers. Lancet. 1985;2:891–892. doi: 10.1016/s0140-6736(85)90156-4. [DOI] [PubMed] [Google Scholar]
- 107.Ongradi J, Ceccherini-Nelli L, Pistello M, Specter S, Bendinelli M. Acid sensitivity of cell-free and cell-associated HIV-1: clinical implications. AIDS research and human retroviruses. 1990;6:1433–1436. doi: 10.1089/aid.1990.6.1433. [DOI] [PubMed] [Google Scholar]
- 108.Tugizov SM, et al. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. Journal of virology. 2012;86:2556–2570. doi: 10.1128/JVI.06578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature medicine. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 110.Bourinbaiar AS, Phillips DM. Transmission of human immunodeficiency virus from monocytes to epithelia. Journal of acquired immune deficiency syndromes. 1991;4:56–63. [PubMed] [Google Scholar]
- 111.Phillips DM, Bourinbaiar AS. Mechanism of HIV spread from lymphocytes to epithelia. Virology. 1992;186:261–273. doi: 10.1016/0042-6822(92)90080-9. [DOI] [PubMed] [Google Scholar]
- 112.Tugizov SM, et al. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology. 2011;409:211–222. doi: 10.1016/j.virol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andreotti M, et al. Comparison of HIV type 1 sequences from plasma, cell-free breast milk, and cell-associated breast milk viral populations in treated and untreated women in Mozambique. AIDS research and human retroviruses. 2009;25:707–711. doi: 10.1089/aid.2008.0276. [DOI] [PubMed] [Google Scholar]
- 114.Lehman DA, et al. HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS. 2008;22:1475–1485. doi: 10.1097/QAD.0b013e328302cc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shapiro RL, et al. Highly active antiretroviral therapy started during pregnancy or postpartum suppresses HIV-1 RNA, but not DNA, in breast milk. The Journal of infectious diseases. 2005;192:713–719. doi: 10.1086/432489. [DOI] [PubMed] [Google Scholar]
- 116.Valea D, et al. CD4+ T cells spontaneously producing human immunodeficiency virus type I in breast milk from women with or without antiretroviral drugs. Retrovirology. 2011;8:34. doi: 10.1186/1742-4690-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rousseau CM, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. The Journal of infectious diseases. 2004;190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar RB, Maher DM, Herzberg MC, Southern PJ. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithelium: implications for HIV binding and primary oral infection. Virology journal. 2006;3:25. doi: 10.1186/1743-422X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maher D, Wu X, Schacker T, Larson M, Southern P. A model system of oral HIV exposure, using human palatine tonsil, reveals extensive binding of HIV infectivity, with limited progression to primary infection. The Journal of infectious diseases. 2004;190:1989–1997. doi: 10.1086/425423. [DOI] [PubMed] [Google Scholar]
- 120.Maher DM, Zhang ZQ, Schacker TW, Southern PJ. Ex vivo modeling of oral HIV transmission in human palatine tonsil. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2005;53:631–642. doi: 10.1369/jhc.4A6534.2005. [DOI] [PubMed] [Google Scholar]
- 121.Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Molecular biology of the cell. 2005;16:4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han Y, Ventura CL, Black KP, Cummins JE, Jr., Hall SD, Jackson S. Productive human immunodeficiency virus-1 infection of epithelial cell lines of salivary gland origin. Oral microbiology and immunology. 2000;15:82–88. doi: 10.1034/j.1399-302x.2000.150203.x. [DOI] [PubMed] [Google Scholar]
- 123.Liu X, et al. Human immunodeficiency virus type 1 infection and replication in normal human oral keratinocytes. Journal of virology. 2003;77:3470–3476. doi: 10.1128/JVI.77.6.3470-3476.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meng G, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nature medicine. 2002;8:150–156. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 125.Delezay O, et al. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS. 1997;11:1311–1318. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 126.Kage A, et al. Epithelial uptake and transport of cell-free human immunodeficiency virus type 1 and gp120-coated microparticles. Journal of virology. 1998;72:4231–4236. doi: 10.1128/jvi.72.5.4231-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moore JS, et al. Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virology. 2003;313:343–353. doi: 10.1016/s0042-6822(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 128.Colasante A, Rosini S, Piattelli A, Artese L, Aiello FB, Musiani P. Distribution and phenotype of immune cells in normal human gingiva: active immune response versus unresponsiveness. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 1992;21:12–16. doi: 10.1111/j.1600-0714.1992.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 129.Hussain LA, Lehner T. Comparative investigation of Langerhans’ cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology. 1995;85:475–484. [PMC free article] [PubMed] [Google Scholar]
- 130.van Loon LA, Krieg SR, Davidson CL, Bos JD. Quantification and distribution of lymphocyte subsets and Langerhans cells in normal human oral mucosa and skin. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 1989;18:197–201. doi: 10.1111/j.1600-0714.1989.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 131.Jotwani R, Cutler CW. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. Journal of dental research. 2003;82:736–741. doi: 10.1177/154405910308200915. [DOI] [PubMed] [Google Scholar]
- 132.Kawamura T, et al. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8401–8406. doi: 10.1073/pnas.1432450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ballweber L, et al. Vaginal langerhans cells nonproductively transporting HIV-1 mediate infection of T cells. Journal of virology. 2011;85:13443–13447. doi: 10.1128/JVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hladik F, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Jong MA, et al. Mutz-3-derived Langerhans cells are a model to study HIV-1 transmission and potential inhibitors. Journal of leukocyte biology. 2010;87:637–643. doi: 10.1189/jlb.0809577. [DOI] [PubMed] [Google Scholar]
- 136.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 137.Russell ES, et al. Short Communication: HIV Type 1 Subtype C Variants Transmitted Through the Bottleneck of Breastfeeding Are Sensitive to New Generation Broadly Neutralizing Antibodies Directed Against Quaternary and CD4-Binding Site Epitopes. AIDS research and human retroviruses. 2013;29:511–515. doi: 10.1089/aid.2012.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rychert J, Lacour N, Amedee AM. Genetic analysis of simian immunodeficiency virus expressed in milk and selectively transmitted through breastfeeding. Journal of virology. 2006;80:3721–3731. doi: 10.1128/JVI.80.8.3721-3731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Greenier JL, Van Rompay KK, Montefiori D, Earl P, Moss B, Marthas ML. Simian immunodeficiency virus (SIV) envelope quasispecies transmission and evolution in infant rhesus macaques after oral challenge with uncloned SIVmac251: increased diversity is associated with neutralizing antibodies and improved survival in previously immunized animals. Virology journal. 2005;2:11. doi: 10.1186/1743-422X-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu J, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. Journal of virology. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Varela M, et al. Molecular evolution analysis of the human immunodeficiency virus type 1 envelope in simian/human immunodeficiency virus-infected macaques: implications for challenge dose selection. Journal of virology. 2011;85:10332–10345. doi: 10.1128/JVI.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ceballos A, et al. Lack of viral selection in human immunodeficiency virus type 1 mother-to-child transmission with primary infection during late pregnancy and/or breastfeeding. The Journal of general virology. 2008;89:2773–2782. doi: 10.1099/vir.0.83697-0. [DOI] [PubMed] [Google Scholar]
- 143.Becquart P, et al. Compartmentalization of HIV-1 between breast milk and blood of HIV-infected mothers. Virology. 2002;300:109–117. doi: 10.1006/viro.2002.1537. [DOI] [PubMed] [Google Scholar]
- 144.Becquart P, Courgnaud V, Willumsen J, Van de Perre P. Diversity of HIV-1 RNA and DNA in breast milk from HIV-1-infected mothers. Virology. 2007;363:256–260. doi: 10.1016/j.virol.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 145.Gray RR, et al. Multiple independent lineages of HIV-1 persist in breast milk and plasma. AIDS. 2011;25:143–152. doi: 10.1097/QAD.0b013e328340fdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Henderson GJ, et al. HIV-1 populations in blood and breast milk are similar. Virology. 2004;330:295–303. doi: 10.1016/j.virol.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 147.Permar SR, et al. Local replication of simian immunodeficiency virus in the breast milk compartment of chronically-infected, lactating rhesus monkeys. Retrovirology. 2010;7:7. doi: 10.1186/1742-4690-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Haaland RE, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS pathogens. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Archibald DW, Cole GA. In vitro inhibition of HIV-1 infectivity by human salivas. AIDS research and human retroviruses. 1990;6:1425–1432. doi: 10.1089/aid.1990.6.1425. [DOI] [PubMed] [Google Scholar]
- 150.Baron S, Poast J, Cloyd MW. Why is HIV rarely transmitted by oral secretions? Saliva can disrupt orally shed, infected leukocytes. Archives of internal medicine. 1999;159:303–310. doi: 10.1001/archinte.159.3.303. [DOI] [PubMed] [Google Scholar]
- 151.Bolscher JG, et al. Inhibition of HIV-1 IIIB and clinical isolates by human parotid, submandibular, sublingual and palatine saliva. European journal of oral sciences. 2002;110:149–156. doi: 10.1034/j.1600-0722.2002.11175.x. [DOI] [PubMed] [Google Scholar]
- 152.Fultz PN. Components of saliva inactivate human immunodeficiency virus. Lancet. 1986;2:1215. doi: 10.1016/s0140-6736(86)92218-x. [DOI] [PubMed] [Google Scholar]
- 153.Malamud D, Davis C, Berthold P, Roth E, Friedman H. Human submandibular saliva aggregates HIV. AIDS research and human retroviruses. 1993;9:633–637. doi: 10.1089/aid.1993.9.633. [DOI] [PubMed] [Google Scholar]
- 154.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. The Journal of clinical investigation. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nagashunmugam T, Friedman HM, Davis C, Kennedy S, Goldstein LT, Malamud D. Human submandibular saliva specifically inhibits HIV type 1. AIDS research and human retroviruses. 1997;13:371–376. doi: 10.1089/aid.1997.13.371. [DOI] [PubMed] [Google Scholar]
- 156.Robinovitch MR, Ashley RL, Iversen JM, Vigoren EM, Oppenheim FG, Lamkin M. Parotid salivary basic proline-rich proteins inhibit HIV-I infectivity. Oral diseases. 2001;7:86–93. [PubMed] [Google Scholar]
- 157.Robinovitch MR, Iversen JM, Resnick L. Anti-infectivity activity of human salivary secretions toward human immunodeficiency virus. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1993;4:455–459. doi: 10.1177/10454411930040032801. [DOI] [PubMed] [Google Scholar]
- 158.Yeh CK, Handelman B, Fox PC, Baum BJ. Further studies of salivary inhibition of HIV-1 infectivity. Journal of acquired immune deficiency syndromes. 1992;5:898–903. [PubMed] [Google Scholar]
- 159.Bergey EJ, et al. Aggregation of human immunodeficiency virus type 1 by human salivary secretions. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1993;4:467–474. doi: 10.1177/10454411930040033001. [DOI] [PubMed] [Google Scholar]
- 160.Crombie R. Mechanism of thrombospondin-1 anti-HIV-1 activity. AIDS patient care and STDs. 2000;14:211–214. doi: 10.1089/108729100317821. [DOI] [PubMed] [Google Scholar]
- 161.Crombie R, Silverstein RL, MacLow C, Pearce SF, Nachman RL, Laurence J. Identification of a CD36-related thrombospondin 1-binding domain in HIV-1 envelope glycoprotein gp120: relationship to HIV-1-specific inhibitory factors in human saliva. The Journal of experimental medicine. 1998;187:25–35. doi: 10.1084/jem.187.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bergey EJ, et al. Interaction of HIV-1 and human salivary mucins. Journal of acquired immune deficiency syndromes. 1994;7:995–1002. [PubMed] [Google Scholar]
- 163.Habte HH, Mall AS, de Beer C, Lotz ZE, Kahn D. The role of crude human saliva and purified salivary MUC5B and MUC7 mucins in the inhibition of Human Immunodeficiency Virus type 1 in an inhibition assay. Virology journal. 2006;3:99. doi: 10.1186/1743-422X-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Saeland E, de Jong MA, Nabatov AA, Kalay H, Geijtenbeek TB, van Kooyk Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Molecular immunology. 2009;46:2309–2316. doi: 10.1016/j.molimm.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 165.Nagashunmugam T, Malamud D, Davis C, Abrams WR, Friedman HM. Human submandibular saliva inhibits human immunodeficiency virus type 1 infection by displacing envelope glycoprotein gp120 from the virus. The Journal of infectious diseases. 1998;178:1635–1641. doi: 10.1086/314511. [DOI] [PubMed] [Google Scholar]
- 166.Malamud D, et al. Inhibition of HIV infectivity by human saliva. Oral diseases. 1997;(3 suppl):S58–S63. doi: 10.1111/j.1601-0825.1997.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 167.Wu Z, et al. Salivary agglutinin inhibits HIV type 1 infectivity through interaction with viral glycoprotein 120. AIDS research and human retroviruses. 2003;19:201–209. doi: 10.1089/088922203763315704. [DOI] [PubMed] [Google Scholar]
- 168.Harmsen MC, et al. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. The Journal of infectious diseases. 1995;172:380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- 169.Kazmi SH, et al. Comparison of human immunodeficiency virus type 1-specific inhibitory activities in saliva and other human mucosal fluids. Clinical and vaccine immunology : CVI. 2006;13:1111–1118. doi: 10.1128/CDLI.00426-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Swart PJ, et al. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vitro. AIDS research and human retroviruses. 1996;12:769–775. doi: 10.1089/aid.1996.12.769. [DOI] [PubMed] [Google Scholar]
- 171.Henrick BM, Nag K, Yao XD, Drannik AG, Aldrovandi GM, Rosenthal KL. Milk matters: soluble Toll-like receptor 2 (sTLR2) in breast milk significantly inhibits HIV-1 infection and inflammation. PloS one. 2012;7:e40138. doi: 10.1371/journal.pone.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lohman-Payne B, et al. Breast milk cellular HIV-specific interferon gamma responses are associated with protection from peripartum HIV transmission. AIDS. 2012;26:2007–2016. doi: 10.1097/QAD.0b013e328359b7e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arsenault JE, Webb AL, Koulinska IN, Aboud S, Fawzi WW, Villamor E. Association between breast milk erythropoietin and reduced risk of mother-to-child transmission of HIV. The Journal of infectious diseases. 2010;202:370–373. doi: 10.1086/653706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walter J, et al. High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. The Journal of infectious diseases. 2009;200:1498–1502. doi: 10.1086/644603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Villamor E, et al. Long-chain n-6 polyunsaturated fatty acids in breast milk decrease the risk of HIV transmission through breastfeeding. The American journal of clinical nutrition. 2007;86:682–689. doi: 10.1093/ajcn/86.3.682. [DOI] [PubMed] [Google Scholar]
- 176.Hocini H, Becquart P, Bouhlal H, Adle-Biassette H, Kazatchkine MD, Belec L. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clinical and diagnostic laboratory immunology. 2000;7:515–518. doi: 10.1128/cdli.7.3.515-518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 178.Skott P, Lucht E, Ehnlund M, Bjorling E. Inhibitory function of secretory leukocyte proteinase inhibitor (SLPI) in human saliva is HIV-1 specific and varies with virus tropism. Oral diseases. 2002;8:160–167. doi: 10.1034/j.1601-0825.2002.01807.x. [DOI] [PubMed] [Google Scholar]
- 179.Farquhar C, et al. Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. The Journal of infectious diseases. 2002;186:1173–1176. doi: 10.1086/343805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Becquart P, Gresenguet G, Hocini H, Kazatchkine MD, Belec L. Secretory leukocyte protease inhibitor in colostrum and breast milk is not a major determinant of the protection of early postnatal transmission of HIV. AIDS. 1999;13:2599–2602. doi: 10.1097/00002030-199912240-00018. [DOI] [PubMed] [Google Scholar]
- 181.Ma G, et al. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. The Journal of experimental medicine. 2004;200:1337–1346. doi: 10.1084/jem.20041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bonass WA, High AS, Owen PJ, Devine DA. Expression of beta-defensin genes by human salivary glands. Oral microbiology and immunology. 1999;14:371–374. doi: 10.1034/j.1399-302x.1999.140607.x. [DOI] [PubMed] [Google Scholar]
- 183.Dale BA, et al. Localized antimicrobial peptide expression in human gingiva. Journal of periodontal research. 2001;36:285–294. doi: 10.1034/j.1600-0765.2001.360503.x. [DOI] [PubMed] [Google Scholar]
- 184.Dunsche A, Acil Y, Dommisch H, Siebert R, Schroder JM, Jepsen S. The novel human beta-defensin-3 is widely expressed in oral tissues. European journal of oral sciences. 2002;110:121–124. doi: 10.1034/j.1600-0722.2002.11186.x. [DOI] [PubMed] [Google Scholar]
- 185.Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infection and immunity. 1998;66:4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mathews M, et al. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infection and immunity. 1999;67:2740–2745. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.O’Neil DA, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. Journal of immunology. 1999;163:6718–6724. [PubMed] [Google Scholar]
- 188.Sahasrabudhe KS, Kimball JR, Morton TH, Weinberg A, Dale BA. Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. Journal of dental research. 2000;79:1669–1674. doi: 10.1177/00220345000790090601. [DOI] [PubMed] [Google Scholar]
- 189.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS letters. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 190.Quinones-Mateu ME, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 191.Sun L, et al. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. Journal of virology. 2005;79:14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mackewicz CE, et al. alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS. 2003;17:F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 193.Zhang L, et al. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 194.Kuhn L, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. Journal of acquired immune deficiency syndromes. 2005;39:138–142. [PMC free article] [PubMed] [Google Scholar]
- 195.Cole AM, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1813–1818. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. Journal of immunology. 2003;170:4708–4716. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- 197.Wang W, et al. Activity of alpha- and theta-defensins against primary isolates of HIV-1. Journal of immunology. 2004;173:515–520. doi: 10.4049/jimmunol.173.1.515. [DOI] [PubMed] [Google Scholar]
- 198.Martin V, Maldonado A, Fernandez L, Rodriguez JM, Connor RI. Inhibition of human immunodeficiency virus type 1 by lactic acid bacteria from human breastmilk. Breastfeeding medicine : the official journal of the Academy of Breastfeeding Medicine. 2010;5:153–158. doi: 10.1089/bfm.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tomas MS, Claudia Otero M, Ocana V, Elena Nader-Macias M. Production of antimicrobial substances by lactic acid bacteria I: determination of hydrogen peroxide. Methods in molecular biology. 2004;268:337–346. doi: 10.1385/1-59259-766-1:337. [DOI] [PubMed] [Google Scholar]
- 200.Pourtois M, Binet C, Van Tieghem N, Courtois PR, Vandenabbeele A, Thirty L. Saliva can contribute in quick inhibition of HIV infectivity. AIDS. 1991;5:598–600. [PubMed] [Google Scholar]
- 201.Iqbal SM, et al. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. The Journal of infectious diseases. 2005;192:728–738. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 202.Taborda-Vanegas N, Zapata W, Rugeles MT. Genetic and Immunological Factors Involved in Natural Resistance to HIV-1 Infection. The open virology journal. 2011;5:35–43. doi: 10.2174/1874357901105010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mackelprang RD, et al. Maternal HLA homozygosity and mother-child HLA concordance increase the risk of vertical transmission of HIV-1. The Journal of infectious diseases. 2008;197:1156–1161. doi: 10.1086/529528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature medicine. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 205.Hofmann-Lehmann R, et al. Postnatal pre- and postexposure passive immunization strategies: protection of neonatal macaques against oral simian-human immunodeficiency virus challenge. Journal of medical primatology. 2002;31:109–119. doi: 10.1034/j.1600-0684.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 206.Van de Perre P, et al. Infective and anti-infective properties of breastmilk from HIV-1-infected women. Lancet. 1993;341:914–918. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 207.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS pathogens. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hasselrot K, et al. Oral HIV-exposure elicits mucosal HIV-neutralizing antibodies in uninfected men who have sex with men. AIDS. 2009;23:329–333. doi: 10.1097/QAD.0b013e32831f924c. [DOI] [PubMed] [Google Scholar]
- 209.Farquhar C, et al. Salivary human immunodeficiency virus (HIV)-1-specific immunoglobulin A in HIV-1-exposed infants in Kenya. Clinical and experimental immunology. 2008;153:37–43. doi: 10.1111/j.1365-2249.2008.03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kuhn L, et al. Hiv-specific secretory IgA in breast milk of HIV-positive mothers is not associated with protection against HIV transmission among breast-fed infants. The Journal of pediatrics. 2006;149:611–616. doi: 10.1016/j.jpeds.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Becquart P, Hocini H, Levy M, Sepou A, Kazatchkine MD, Belec L. Secretory anti-human immunodeficiency virus (HIV) antibodies in colostrum and breast milk are not a major determinant of the protection of early postnatal transmission of HIV. The Journal of infectious diseases. 2000;181:532–539. doi: 10.1086/315255. [DOI] [PubMed] [Google Scholar]
- 212.Hocini H, et al. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS research and human retroviruses. 1997;13:1179–1185. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 213.Chomont N, et al. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology. 2008;370:246–254. doi: 10.1016/j.virol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 214.Bomsel M, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 215.John-Stewart GC, et al. HV-1-specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. The Journal of infectious diseases. 2009;199:889–898. doi: 10.1086/597120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lohman-Payne B, et al. Infants with late breast milk acquisition of HIV-1 generate interferon-gamma responses more rapidly than infants with early peripartum acquisition. Clinical and experimental immunology. 2009;156:511–517. doi: 10.1111/j.1365-2249.2009.03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kuhn L, et al. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS. 2001;15:1–9. doi: 10.1097/00002030-200101050-00003. [DOI] [PubMed] [Google Scholar]
- 218.Taha TE. Mother-to-child transmission of HIV-1 in sub-Saharan Africa: past, present and future challenges. Life sciences. 2011;88:917–921. doi: 10.1016/j.lfs.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 219.Averbach SH, et al. The association between cervical human papillomavirus infection and HIV acquisition among women in Zimbabwe. AIDS. 2010;24:1035–1042. doi: 10.1097/qad.0b013e3283377973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnson KE, et al. Effects of HIV-1 and herpes simplex virus type 2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. The Journal of infectious diseases. 2011;203:602–609. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Johnson KE, et al. Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. AIDS. 2009;23:1807–1815. doi: 10.1097/QAD.0b013e32832efdf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Laga M, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344:246–248. doi: 10.1016/s0140-6736(94)93005-8. [DOI] [PubMed] [Google Scholar]
- 223.Laga M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 224.Reynolds SJ, et al. High rates of syphilis among STI patients are contributing to the spread of HIV-1 in India. Sexually transmitted infections. 2006;82:121–126. doi: 10.1136/sti.2005.015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Reynolds SJ, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. The Journal of infectious diseases. 2003;187:1513–1521. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 226.van de Wijgert JH, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African Women. Sexually transmitted diseases. 2009;36:357–364. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 227.Van Der Pol B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. The Journal of infectious diseases. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 228.Embree JE, et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–2541. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]
- 229.Faruque S, et al. Crack cocaine smoking and oral sores in three inner-city neighborhoods. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1996;13:87–92. doi: 10.1097/00042560-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 230.Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. The Journal of infectious diseases. 2009;199:1318–1322. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- 231.Koning FA, et al. Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. Journal of immunology. 2005;175:6117–6122. doi: 10.4049/jimmunol.175.9.6117. [DOI] [PubMed] [Google Scholar]
- 232.Lajoie J, et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal immunology. 2012;5:277–287. doi: 10.1038/mi.2012.7. [DOI] [PubMed] [Google Scholar]
- 233.McLaren PJ, et al. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. The Journal of infectious diseases. 2010;2023:S339–S344. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]
- 234.Lane BR, et al. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. Journal of virology. 2001;75:8195–8202. doi: 10.1128/JVI.75.17.8195-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Poli G, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. The Journal of experimental medicine. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Poli G, et al. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Giavedoni LD, et al. Impact of mucosal inflammation on oral simian immunodeficiency virus transmission. Journal of virology. 2013;87:1750–1758. doi: 10.1128/JVI.02079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rollins NC, Filteau SM, Coutsoudis A, Tomkins AM. Feeding mode, intestinal permeability, and neopterin excretion: a longitudinal study in infants of HIV-infected South African women. Journal of acquired immune deficiency syndromes. 2001;28:132–139. doi: 10.1097/00042560-200110010-00004. [DOI] [PubMed] [Google Scholar]
- 241.Rossenkhan R, et al. Infant feeding practices were not associated with breast milk HIV-1 RNA levels in a randomized clinical trial in Botswana. AIDS and behavior. 2012;16:1260–1264. doi: 10.1007/s10461-011-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fiocchi A, Assa’ad A, Bahna S. Food allergy and the introduction of solid foods to infants: a consensus document. Adverse Reactions to Foods Committee, American College of Allergy, Asthma and Immunology; Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, &. Immunology. 2006;97:10–20. doi: 10.1016/s1081-1206(10)61364-6. quiz 1, 77. [DOI] [PubMed] [Google Scholar]
- 243.Kramer MS, Kakuma R. The optimal duration of exclusive breastfeeding: a systematic review. Advances in experimental medicine and biology. 2004;554:63–77. doi: 10.1007/978-1-4757-4242-8_7. [DOI] [PubMed] [Google Scholar]
- 244.Scariati PD, Grummer-Strawn LM, Fein SB. A longitudinal analysis of infant morbidity and the extent of breastfeeding in the United States. Pediatrics. 1997;99:E5. doi: 10.1542/peds.99.6.e5. [DOI] [PubMed] [Google Scholar]
- 245.Silvestri G, et al. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. Journal of virology. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Silvestri G, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 247.Sumpter B, et al. Correlates of preserved CD4(+) T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. Journal of immunology. 2007;178:1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- 248.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 249.Gordon SN, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. Journal of immunology. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. The Journal of clinical investigation. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Estes JD, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. Journal of immunology. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Okoye A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. The Journal of experimental medicine. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 254.Chahroudi A, Meeker T, Lawson B, Ratcliffe S, Else J, Silvestri G. Mother-to-infant transmission of simian immunodeficiency virus is rare in sooty mangabeys and is associated with low viremia. Journal of virology. 2011;85:5757–5763. doi: 10.1128/JVI.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Otsyula MG, Gettie A, Suleman M, Tarara R, Mohamed I, Marx P. Apparent lack of vertical transmission of simian immunodeficiency virus (SIV) in naturally infected African green monkeys, Cercopithecus aethiops. Annals of tropical medicine and parasitology. 1995;89:573–576. doi: 10.1080/00034983.1995.11812990. [DOI] [PubMed] [Google Scholar]
- 256.Pandrea I, et al. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. Journal of virology. 2008;82:5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Phillips-Conroy JE, Jolly CJ, Petros B, Allan JS, Desrosiers RC. Sexual transmission of SIVagm in wild grivet monkeys. Journal of medical primatology. 1994;23:1–7. doi: 10.1111/j.1600-0684.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 258.Amedee AM, Lacour N, Ratterree M. Mother-to-infant transmission of SIV via breast-feeding in rhesus macaques. Journal of medical primatology. 2003;32:187–193. doi: 10.1034/j.1600-0684.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 259.Amedee AM, Rychert J, Lacour N, Fresh L, Ratterree M. Viral and immunological factors associated with breast milk transmission of SIV in rhesus macaques. Retrovirology. 2004;1:17. doi: 10.1186/1742-4690-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Santiago ML, et al. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d’Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. Journal of virology. 2005;79:12515–12527. doi: 10.1128/JVI.79.19.12515-12527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wilks AB, et al. High cell-free virus load and robust autologous humoral immune responses in breast milk of simian immunodeficiency virus-infected african green monkeys. Journal of virology. 2011;85:9517–9526. doi: 10.1128/JVI.00796-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pandrea I, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Paiardini M, et al. Low levels of SIV infection in sooty mangabey central memory CD(4)(+) T cells are associated with limited CCR5 expression. Nature medicine. 2011;17:830–836. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pandrea I, et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood. 2012;120:1357–1366. doi: 10.1182/blood-2012-03-414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Beer B, et al. Simian immunodeficiency virus of African green monkeys is apathogenic in the newborn natural host. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1998;18:210–220. doi: 10.1097/00042560-199807010-00003. [DOI] [PubMed] [Google Scholar]
- 266.Mofenson LM. Antiretroviral drugs to prevent breastfeeding HIV transmission. Antiviral therapy. 2010;15:537–553. doi: 10.3851/IMP1574. [DOI] [PubMed] [Google Scholar]
- 267.Persaud D, et al. Functional HIV Cure after Very Early ART of an Infected Infant; Atlanta, Georgia. Conference on Retroviruses and Opportunistic Infections.2013. [Google Scholar]
- 268.Borkow G, Lara HH, Covington CY, Nyamathi A, Gabbay J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrobial agents and chemotherapy. 2008;52:518–525. doi: 10.1128/AAC.00899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gerrard SE, et al. Reducing infectivity of HIV upon exposure to surfaces coated with N,N-dodecyl, methyl-polyethylenimine. Biotechnology and bioengineering. 2013 doi: 10.1002/bit.24867. [DOI] [PubMed] [Google Scholar]
- 270.Israel-Ballard K, et al. Flash-heat inactivation of HIV-1 in human milk: a potential method to reduce postnatal transmission in developing countries. Journal of acquired immune deficiency syndromes. 2007;45:318–323. doi: 10.1097/QAI.0b013e318074eeca. [DOI] [PubMed] [Google Scholar]
- 271.Jeffery BS, Webber L, Mokhondo KR, Erasmus D. Determination of the effectiveness of inactivation of human immunodeficiency virus by Pretoria pasteurization. Journal of tropical pediatrics. 2001;47:345–349. doi: 10.1093/tropej/47.6.345. [DOI] [PubMed] [Google Scholar]
- 272.Urdaneta S, et al. Inactivation of HIV-1 in breast milk by treatment with the alkyl sulfate microbicide sodium dodecyl sulfate (SDS) Retrovirology. 2005;2:28. doi: 10.1186/1742-4690-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Volk ML, Hanson CV, Israel-Ballard K, Chantry CJ. Inactivation of cell-associated and cell-free HIV-1 by flash-heat treatment of breast milk. Journal of acquired immune deficiency syndromes. 2010;53:665–666. doi: 10.1097/QAI.0b013e3181ba47df. [DOI] [PubMed] [Google Scholar]
- 274.Chantry CJ, et al. Effect of flash-heat treatment on immunoglobulins in breast milk. Journal of acquired immune deficiency syndromes. 2009;51:264–267. doi: 10.1097/QAI.0b013e3181aa12f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ferrantelli F, et al. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. The Journal of infectious diseases. 2004;189:2167–2173. doi: 10.1086/420833. [DOI] [PubMed] [Google Scholar]
- 276.Van Rompay KK, et al. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. Journal of acquired immune deficiency syndromes. 2005;38:124–134. doi: 10.1097/00126334-200502010-00002. [DOI] [PubMed] [Google Scholar]
- 277.Marthas ML, et al. Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine. 2011;29:3124–3137. doi: 10.1016/j.vaccine.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Van Rompay KK, et al. Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV. Vaccine. 2010;28:1481–1492. doi: 10.1016/j.vaccine.2009.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mumcu G, et al. Salivary levels of antimicrobial peptides Hnp 1–3, Ll-37 and S100 in Behcet’s disease. Archives of oral biology. 2012;57:642–646. doi: 10.1016/j.archoralbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 280.Ghosh SK, Gerken TA, Schneider KM, Feng Z, McCormick TS, Weinberg A. Quantification of human beta-defensin-2 and −3 in body fluids: application for studies of innate immunity. Clinical chemistry. 2007;53:757–765. doi: 10.1373/clinchem.2006.081430. [DOI] [PubMed] [Google Scholar]
- 281.Hasselrot K, Bratt G, Duvefelt K, Hirbod T, Sandstrom E, Broliden K. HIV-1 exposed uninfected men who have sex with men have increased levels of salivary CC-chemokines associated with sexual behavior. AIDS. 2010;24:1569–1575. doi: 10.1097/qad.0b013e32833ac646. [DOI] [PubMed] [Google Scholar]
- 282.Hung GY, Jeng MJ, Lin CY, Soong WJ, Hwang B. The relationship between serum and saliva erythropoietin concentrations in adults, full-term and premature infants. Zhonghua Minguo xiao er ke yi xue hui za zhi [Journal] Zhonghua Minguo xiao er ke yi xue hui. 1998;39:380–385. [PubMed] [Google Scholar]
- 283.Dalvi MS, Abrams WR, Barber CA, Norman R, Malamud D. Salivary and Plasma Cytokine Alterations in HIV Infection. San Diego: IADR/AADR/CADR 89th General Session and Exhibition; 2011. [Google Scholar]
- 284.Actis AB, Perovic NR, Defago D, Beccacece C, Eynard AR. Fatty acid profile of human saliva: a possible indicator of dietary fat intake. Archives of oral biology. 2005;50:1–6. doi: 10.1016/j.archoralbio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 285.Sanchez GA, Miozza V, Delgado A, Busch L. Determination of salivary levels of mucin and amylase in chronic periodontitis patients. Journal of periodontal research. 2011;46:221–227. doi: 10.1111/j.1600-0765.2010.01332.x. [DOI] [PubMed] [Google Scholar]


