Abstract
Background:
Depression is a neurological disorder characterized by sad mood, loss of pleasure, agitation and retardation. Though most relevant neuronal pathophysiology is characterized by decrease in monoamine namely; serotonin (5-HT), dopamine, noradrenaline level in central areas regulating mood and behavior, it inadequately explains the exact mechanism involved. Buspirone (BUS), a partial 5-HT1A receptor agonist has shown promising anti-depressant and anxiolytic properties in various pre-clinical and clinical studies, but the molecular and cellular mechanisms are still unclear.
Objective:
The aim of this study was to investigate, in vivo, the role of hypothalamic-pituitary-adrenal (HPA) axis dysregulation in pathophysiology of depression-related disorders and the anti-depressant like activity of BUS. To simulate HPA axis dysregulation, rats were subjected to bilateral adrenalectomy (ADX).
Materials and Methods:
We have analyzed effect of BUS (10 mg/kg, i.p.) in ADX and sham rats using open field, sucrose consumption, elevated plus maze and hyper-emotionality tests.
Results:
In all animal models tested, ADX rats exhibited significant depressive and anxiogenic states while BUS was effective in reversing the psychological diseased condition developed.
Conclusion:
Taken together, these data showed a prominent role of HPA axis in depression and neuronal mechanism of BUS as anti-depressant and anxiolytic agent. Moreover, our findings suggest that BUS can be a better candidate for stress related depression and anxiety.
Keywords: 5-HT1A partial agonist, adrenalectomy, depression
INTRODUCTION
Depression is one of the major psychological disorders prevalent in almost every part of the world. According to the World Health Organization reports, depression is expected to be the second major cause of disability adjusted life years, following ischemic heart disease by the year 2020. Twenty percent of the population develops a depressive episode at some point in life, 85% face experience more than one episode of depression, one in ten patients commit suicide and 20% suffer from chronic depression.[1] The exact mechanism of how depression develops in persons is still unclear though, monoamine hypothesis suggests that a functional deficit in level of monoamines in central areas particularly in limbic system (hippocampus, thalamus, amygdala) some part of brain stem and basal ganglia which regulate the emotional behavior results in depressive behavior.[2,3,4,5,6] Considering above, wide category of anti-depressants have been devised and marketed such as, tricyclic anti-depressants, monoamine oxidase inhibitors, selective anti-depressants like selective serotonin reuptake inhibitors (e.g., fluoxetine), serotonin-norepinephrine reuptake inhibitors (e.g., venlafaxine), nor epinephrine-dopamine reuptake inhibitors (e.g., bupropion). To continue with the hypothetical statement monoamine receptor agonists and antagonists were devised to increase the functional activity of the deficient monoamines at synaptic system. However, monoamine hypothesis is insufficient to provide adequate knowledge about the exact mechanism of anti-depressants and the pathophysiology involved in the depression. Furthermore, it fails to explain the therapeutic effect of anti-depressants in other psychological disorders such as social phobia,[7] obsessive-compulsive disorder[8] and other generalized anxiety disorders such as agoraphobia, panic disorder.[9] Furthermore, it fails to explain the anti-depressant effect of compounds like tianeptine, which increases the serotonin reuptake instead of reducing it.[10,11] This inadequacy of monoamine hypothesis has led to the search of other physiological system associated with the psychological disorders, such as the role of hypothalamic-pituitary-adrenal (HPA) axis in depression.[12,13] Buspirone (BUS), a potential 5-HT1A receptor partial agonist has been widely pre-clinically screened in various rodent models of depression and anxiety. BUS showed reversal of stress induced deficit in the sucrose intake (anhedonia), a major symptom of depression.[14] Further it exhibited significant anxiolytic activity in Vogel's and the open-field tests and novelty-suppressed feeding in food-deprived rats.[15,16] It indicated significant mood regulating activity of BUS possibly by modulating 5-HT1A receptors in brain areas. Clinically, it is popularly used as anti-anxiety agent in patients with psychiatric disturbances.[17,18] Further, evidenced by few double-blind clinical trials with standard rating scales such as total Hamilton Anxiety and Clinical Global Impression-Global Improvement scale, BUS improves symptoms in patients with mixed anxiety/depression, although the number of patients studied to date is small.[19,20] Furthermore, several studies suggest its better patient compliance with no sedation, psychomotor or cognitive function, and no additive effect with alcohol.[18] However, there exist a little data elucidating the exact pharmacological mechanism of BUS and there is increasing evidence for multiple psychological mechanisms, which may be able to explain differential patterns of the drug effects. Thus, we aimed to study the effect of BUS on HPA axis and its link to affective disorders, like depression and anxiety.
In the present study, the possible action of BUS as anti-depressant and anxiolytic was assessed. Further, reversal of HPA axis dysregulation induced behavioral deficits as an alternate mechanism of BUS was analyzed.
MATERIALS AND METHODS
Animals
Male Wistar rats (350-400 g) were obtained from Hissar Agricultural University, Haryana, India. All animals were maintained under standard laboratory conditions (12 h light/dark cycle [lights on at 7:00 AM]; temperature 23°C±2°C; relative humidity; 60% ±5%) in the Central Animal Facility. All animals were given sterilized food (standard pellet chow feed) and filtered water ad libitum. All the experiments on animal were conducted in adherence to the approved protocol of the Institutional Animal Ethics Committee (IAEC) of Birla Institute of Technology and Science, Pilani, India (Protocol No. IAEC/RES/04/01).
Grouping of animals and treatment
There were two treatment schedules (7 day and 14 day treatment regimen) each consisted of four groups, namely; Sham + Control: Sham rats given vehicle treatment, Sham + BUS (10): Sham rats treated with 10 mg/kg of BUS, adrenalectomy (ADX) Control: adrenalectomized rats given vehicle treatment, ADX + BUS (10): adrenalectomized rats given 10 mg/ kg of BUS, with 7 animals randomly divided in each group on the day of surgery. There was overall 92.85% survival of the ADX treated animals whereas, all sham treated animals survived till end of the study. Animals with abnormal behavior and/or diseased/mortality condition were discarded from the study. Dosing schedule and behavioral tests were decided based on the time taken for recovery from surgery [Tables 1a and b].
Table 1a.
The schedule for drug administration and behavioral test
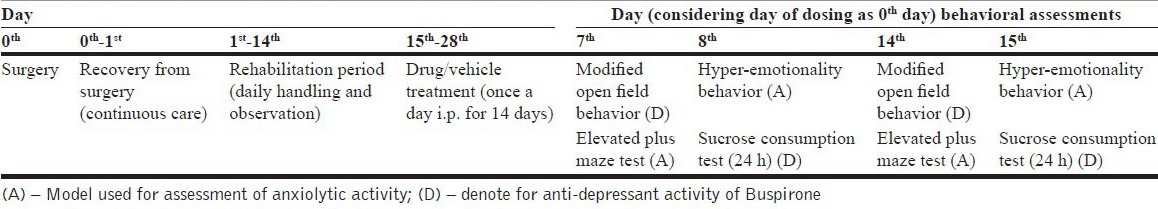
Table 1b.
Timeline showing the animal survival during the study
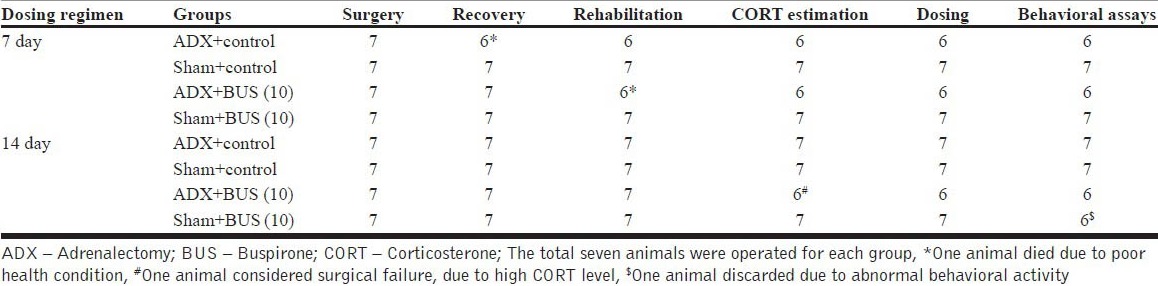
Drugs
BUS was obtained from Astron Research Ltd Ahmedabad, Gujarat as a generous gift sample. A dose of 10 mg/kg was selected on the basis of literature survey and preliminary testing done in the laboratory.[21,22] The drug solution was freshly prepared in distilled water and administered intraperitoneally (i.p.) in a constant volume of 1 ml/kg body weight of rat. The drug administration and behavioral screenings were performed between 0900 h and 1500 h. The drug/vehicle treated animals were acclimatized to the experimentation room for 1 h before testing.
ADX
Bilateral adrenal glands removal was performed as described elsewhere,[23] with slight modifications as mentioned below. All the surgical equipments were sterilized before use. The rat was anesthetized with ketamine (75 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) and dorsal fur was shaved. It was then placed on a block (8 cm × 3 cm × 2 cm) in order to elevate the viscera. A transverse incision (6-8 mm long) was made to the ventral side of the costovertebral angle. To remove the left adrenal gland, the skin was retracted and the lumbar muscles incised just superior and anterior to the splenic shadow. The periadrenal tissue between the kidney and the adrenal by was grasped small curved forceps and the intact gland together with the periadrenal fat was removed. All remnants of the capsule to which cortical tissue may adhere were removed. After ablation of the left adrenal, the rat was turned around and another incision was made through the lumbar muscles. The curved forceps were inserted over the kidney and by elevating the liver, (which covers the adrenal on right side) the gland was grasped removing again the intact gland with the periadrenal fat. The incisions made in the lumbar muscles and skins were sutured. To prevent post-surgical infection, the rats were given Sulprim injection (each mL containing 200 mg of sulfadiazine and 40 mg of trimethoprim) intramuscularly (0.2 mL/300 g) once a day for 3 days, post-surgery. Sham operated rats were treated in the same way, including gentle mobilization of both glands without removal. After surgery all animals were kept in the cages with one in each. They were given proper diet and saline (0.9% w/v) as drinking water throughout the study so as to restore the disturbed homeostasis. The ADX operated rats were given corticosterone (CORT) (250 μg/ml) in their drinking water for 1st 3 days to prevent the death of animal from immediate homeostatic shock. All the operated rats were allowed a 14 days of rehabilitation. During this period the animals were handled by the experimenter and were kept in keen observation. After 14th day the animals were treated with drug/vehicle followed by behavioral assays as shown in Table 1a.
Body weights and plasma CORT estimations
Body weight of sham and ADX rats was continuously observed till the behavioural tests were performed. The animals were weighed individualy on 1st, 2nd, 3rd, 4th, 8th, 14th and on the 21st day. The plasma CORT was measured using fluorimetric assay of each animal irrespective of the type of surgery performed (whether ADX or sham).
Preliminary behavioral assays
Open field exploration
The ADX and sham operated rats were subjected to the open field test. The general design was essential as reported by Kelly et al.[24] with slight modifications. The apparatus consisted of a circular (90-cm diameter) arena with 75-cm high polished aluminum walls and floor equally divided into 10 cm squares. A 60 W light bulb was positioned 90 cm above the base of the arena and the test was performed in a quiet room without previous habituation. Each rat was individually placed in the center of the open field apparatus and the following parameters were observed for 5 min by a trained observer. The ambulation scores (the number of crossings, as horizontal activity) and number of rearings (when rat stand upright on its hind paws, as vertical activity) were measured. Other parameters observed were defecation, percentage time spent at the periphery and freezing time (which indicate time duration during which the animal was immobile with stable at one point). The number of fecal pellets was counted at the end of each 5 min trial and mean value of each experimental group was obtained. The apparatus was cleaned with ethyl alcohol and dried between each trial to remove any residual odor, which would have affected the exploratory behavior of animals otherwise.
Sucrose consumption test
The method was adopted elsewhere with slight modifications.[25] The rats had free access to sucrose solution (1% w/v) for 5 days from the commencement of dosing for habituation. The test was performed by presenting sucrose solution bottles in the morning on the day of experiment (0900 h) followed by measuring the volume after 24 h (in the next morning, 0900 h). The sucrose solution consumed was calculated as the difference between initial volume in bottles and the volume (ml) left after 24 h.
Hyper-emotionality response
Hyper-emotionality response was measured by adopting the procedure described by Shibata et al.[26] with slight modifications. Briefly, hyper-emotionality in rats was measured by scoring the responses to the stimuli, namely (i) Bite response: The response to a rod (4-5 cm) presented in front of the snout, (ii) Startle response: the response to a stream of air (using 10-ml syringe) directed at the dorsum (iii) Struggle response: The response was scored by handling the animal with a gloved hand (iv) Fight response: The response was scored by pinching the tail with forceps. These responses were graded as follows: 0 - no reaction, 1 - slight, 2 - moderate, 3 - marked, 4 - extreme response. The scores for emotional response were given for 5 min and the results were expressed as the sum of the individual scores. The animals were brought in the experimental room 1 day prior to the experiment to avoid the novel area induced behavior. The rats from the different treatment groups were observed on the same day.
Elevated plus maze
The procedure was adopted same as described elsewhere.[27] The elevated plus-maze consisted of two open (50 cm × 10 cm) arms and two enclosed (50 cm × 10 cm) arms surrounded by 30-cm high walls. The four arms were joined by a central platform (10 cm × 10 cm) open to all the arms, to form a plus shape. The entire apparatus was elevated to a height of 60 cm above the floor. The apparatus was indirectly illuminated with a ceiling mounted lamp (60 W) which was placed 90 cm above the apparatus. At the beginning of the test, the animal was placed in the central platform facing an open arm and allowed to freely explore the apparatus. The number of entries and time spent in open arms was recorded for 5 min. The apparatus was cleaned as mentioned above.
Statistical analysis
The data was analyzed by Graph pad PRISM software (version 4.03). The data from the single drug treatment studies were subjected to one-way ANOVA followed by post-hoc Dunnett's test. All the comparisons were made against the control (vehicle treatment) or as specified otherwise. The level of statistical significance was fixed at P<0.05 and the results were expressed as mean±SEM.
RESULTS
Body weights and plasma CORT estimations
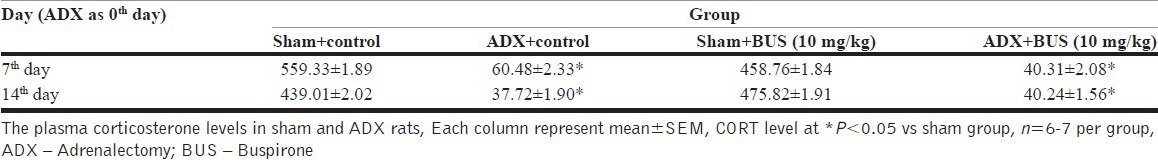
ADX rats exhibited a decreased in body weight for a few days after the surgery. Later all the animals showed recovery and weight gain. Statistical analysis revealed that weight gained by ADX rats was significanlty (P<0.05) less as compared to sham rats [Figure 1]. The plasma CORT of ADX rats was less than that of sham operated rats [Table 2]. ADX rats with heigher plasma CORT were discarded for the study.
Figure 1.
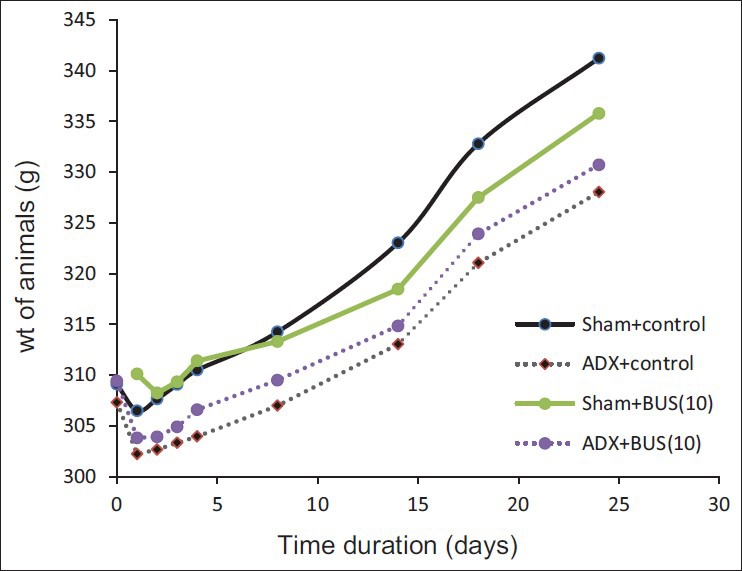
Effect of adrenalectomy on body weight (g)
Table 2.
Plasma corticosterone level in adrenalectomy and sham treated rats
Open field exploration
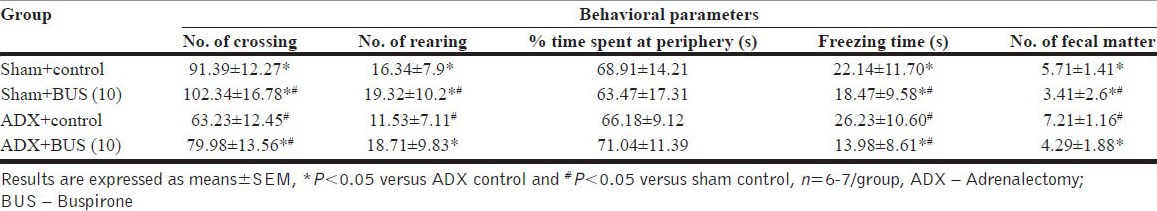
ADX rats exhibited depressive behavior in open field test. ADX rats showed decreased ambulation (P<0.05) and rearing behavior (P<0.05) and increased fecal pellets (P<0.05) behavior after being put into the open field arena compared to sham operated rats. The results revealed that, both decreased frequencies of ambulation and rearing were significantly reversed by chronic BUS (10 mg/kg, i.p.) treatment. The % time spent at the periphery was slightly affected however; the freezing time was significantly reduced by the BUS treatment. The data summarized in [Tables 3a and b], indicate behavioral assessment after 7 days and 14 days treatment regimen from the recovery period.
Table 3a.
Effect of buspirone on open field test of sham and adrenalectomy rats on 7 days treatment regimen
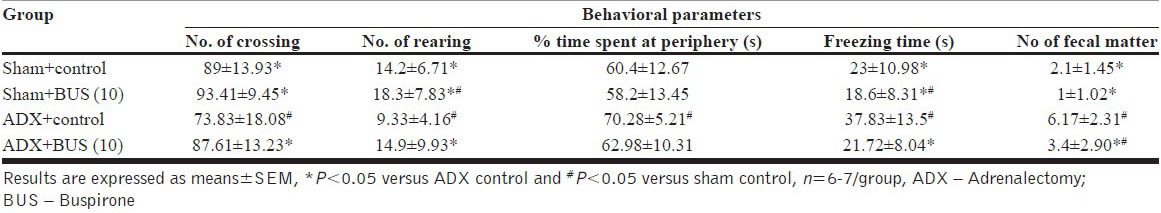
Table 3b.
Effect of buspirone on open field test of sham and adrenalectomy rats on 14 days treatment regimen
Sucrose consumption test
A significant decrease in sucrose consumption (P<0.05) was observed in ADX rats as compared to sham rats. Further, chronic BUS treatment significantly (P<0.05) increased the sucrose consumption in ADX rats as compared to vehicle treatment [Figure 2].
Figure 2.
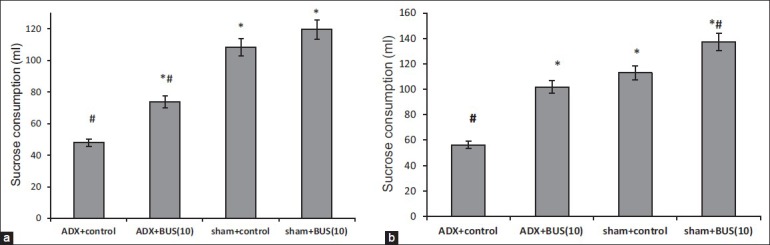
Effect of buspirone (10 mg/kg) on the sucrose (1% w/v) consumption of sham/adrenalectomy (ADX) rats. The column bar represent the mean sucrose consumption. BUS/vehicle is administered i.p. once a day for 7 days (a) and 14 days; (b) Results are expressed as mean ± SEM *P<0.05 versus ADX control, #P<0.05 versus sham control. n=6‑7 per group
Hyper-emotionality behavior
ADX rats exhibited significantly increased hyper-emotionality scores (P<0.05), as compared to sham group. Treatment with BUS (10 mg/kg, i.p.) significantly (P<0.05) decreased the hyper-emotionality as compared to the vehicle treated ADX group by 7 day and 14 day regimen [Figure 3].
Figure 3.
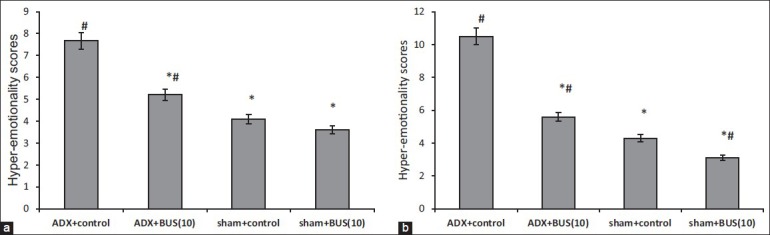
Effect of buspirone (10 mg/kg) on hyper‑emotionality scores of adrenalectomy and sham rats. All drugs/vehicle were administered once a day i.p. for 7 (a) and 14 days; (b) respectively. Results are expressed as mean hyper‑emotionality scores. Error bars represent mean SEM *P<0.05 versus adrenalectomy control, #P<0.05 versus sham control. n=6‑7 per group
Elevated plus maze test
The results for the elevated plus maze test are shown in [Figure 4]. The % time spent and % number of entries in open arms by sham and ADX rats, cumulated over the 5-min test is displayed. ADX rats exhibited a significant (P<0.05) reduction in the percent entries and percent time spent in the open arms as compared to sham rats. Further analysis revealed that chronic BUS (10 mg/kg, i.p.) significantly increased the percent entries (P<0.05) and percent time spent (P<0.05) in open arms compared to ADX control in elevated plus maze task.
Figure 4a.
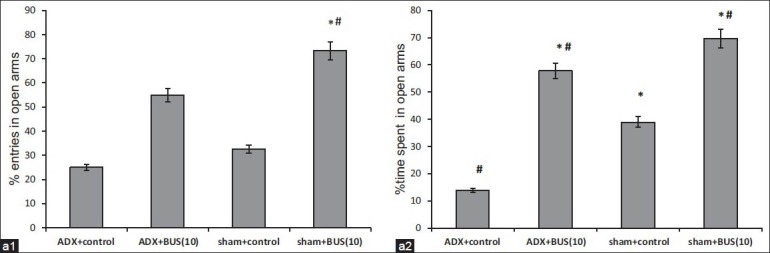
Effect of buspirone (10 mg/kg) on (a. 1) percent entries and (a. 2) percent time spent in open arms in elevated plus maze test. BUS/vehicle is administered i. p. once a day for 7 days. The column bar represent mean percentage entries (a. 1) and percent time spent (a. 2) in open arms. Results were expressed in mean±SEM *P<0.05 versus adrenalectomy control, #P<0.05 versus sham control. n=6-7 per group
Figure 4b.
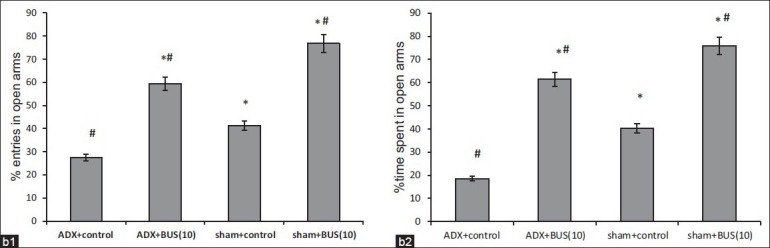
Effect of buspirone (10 mg/kg) on (b. 1) percent entries and (b. 2) percent time spent in open arms in elevated plus maze test. BUS/vehicle is administered i.p. once a day for 14 days. The column bar represent mean percentage entries (b. 1) and percent time spent (b. 2) in open arms. Results were expressed in mean±SEM *P<0.05 versus adrenalectomy control, #P<0.05 versus sham control. n=6-7 per group
DISCUSSION AND CONCLUSION
The key purpose of the work was to assess the possible involvement of HPA axis in depression and anxiety and the anti-depressant like activity BUS (10 mg/kg, i.p.). The HPA axis includes central and peripheral components namely hypothalamus, pituitary and adrenal glands that control the stress condition of an individual.[28,29] It was found that the removal of adrenal gland lead to decrease blood CORT levels resulting in the loss of negative feedback control over the hypothalamus resulting in the excess corticotrophin releasing factor (CRF) release.
In the present study, bilateral ADX in Wistar male rats was performed, with the removal of both right and left supra-renal glands resulting in decrease in blood CORT level thereby simulating HPA axis hyperactivity condition. Moreover, due to a persistent decreased level of plasma CORT, there is a loss of negative feedback system and elevation in CRF levels in blood resulting in dysregulation of whole HPA axis. For the standardization and confirmation of the surgical model plasma CORT estimation was taken into account. The ADX operated rats had significant low levels of plasma CORT than the sham operated. Though, a large decline in plasma CORT level indicate successful removal of the glands, however, literature suggest that there exists some extraadrenal glandular cortical tissue that maintains the CORT levels in rat plasma.[30] Thus, the plasma CORT concentration did not reach the baseline levels.
According to the Diagnostic and Statistical Manual of Mental Disorders-IV,[31] the weight gain/loss is one of the symptoms associated with depressed patients. The measurement of body weight of animal was considered. Results suggested that, weight gain by ADX rat were significantly less than the sham operated rats though they received the same feeding and growing condition, indicating depressive like symptoms.
A behavioral test is an important tool to simulate the symptoms of human mood disorder. The behavioral assays selected were open field, elevated plus maze, sucrose consumption and hyper-emotionality test based on the preliminary testing. Open field test is the most widely accepted indices of altered mood behavior. The exposure to a novel area leads to the exploratory behavior of the animal.[32] Precipitation of the behavioral and emotional responses is generally dependent upon high illumination and high reflective walls in the “open field” and is associated with stress-induced behaviors including thigmotaxis and defecation. ADX rats expressed depressive and axiogenic episodes in open field arena in response to stressful environment which reflect, may be in part the involvement of HPA axis dysregulation in the psychomotor retardation as one of the depressive symptoms.[31] Thigmotaxis, another commonly reported open field behavior, characterizes the natural tendency of rodents to prefer the periphery over the center space of an open field arena. Thigmotaxis is considered an index of anxiety since many anxiolytic drugs reduce thigmotaxis, and increase time spent in the central part of the field.[33] The percent time spent at the periphery did not show any significant alterations in the values. Verma et al. findings suggested, the anxiety and fear response relates to the size and illumination of open field arena used.[34] It is possible that the species differences and/or housing or handling conditions all contributed to the discrepancies between studies. BUS treated ADX rats, exhibited increase exploratory activity (number of crossings and rearings) indicating anti-depressant like activity. Similarly, freezing time (s) decreased in case of ADX rats were reversed by the BUS (7 day and 14 day treatment regimen). Also the defecation parameters obtained further confirms the above facts. The anti-depressant and anxiolytic activity of BUS (as quite established) is 5-HT1A mediated.[35] this might suggest the relationship between serotonin and HPA axis. Together, these findings show that ADX exhibited depressive and anxiety like behavior in unfamiliar open field area with significant reversal of the pathological episodes by BUS treatment.
The elevated plus-maze is one of the most widely used anxiety test across several laboratories.[36] Measures of percent time spent and number of entries in open arms indicated that ADX rats were more anxious than sham rats. Though, the percent number of entries in open arms was increased after 7 days dosing of BUS, but did not reach to statistically significant level. The only significant percent time spent in open arms indicate that BUS treated ADX rats entered the open arms less frequently, but explored longer at each entry. Approach of the open arm has been interpreted as a measure of “risk assessment” in potentially stressful environment, defined as “an attempt at entry into open arms followed by avoidance responses.”[37] Risk assessment is considered to be an ethologically valid measure of fear/anxiety.[34] Interestingly, there was a significant increase in both percent time spent and number of entries in open arms by 14 days treatment regimen. This may reveal that chronic treatment is required for reversal of the anxiogenic effect produced by ADX.
Pre-clinical studies often use sucrose consumption tests as a measure of anhedonia. It is well-known fact that anhedonia is one of the core criteria for depression diagnosis.[31] It indicates the loss of pleasure activity observed in depressed patients. The cellular mechanisms underlying anhedonia in ADX rats that induces such effects were not addressed in the present study, but these effects may be mediated by brain derived neurotrophic factor (BDNF) and such effects are reversed by chronic anti-depressant treatment and may be correlated directly or in part the with HPA axis dysregulation.[25] In the current study, ADX rats consumed less sucrose solution (1% w/v) than sham operated rats reflecting anhedonia. Significantly, BUS reversed the anhedonic behavior in ADX rats over vehicle treated ADX rats by altering the threshold of reward.
To assess the hyperactivity of the animal, hyper-emotionality test was taken into account. This effect can be interpreted as a blunting of the emotional response by the animal. ADX rats exhibited hyperemotional behavior and was significantly reversed after BUS treatment (7 day and 14 day treatment regimen). The data also highlight the fact that HPA axis substantially involve in the regulation of emotional behavior.
It can be concluded that, BUS exhibit anti-depressant and anxiolytic like activity. The study suggested that HPA axis dysregulation, in addition to depletion of brain monoamines, develops depression and anxious states and endocrinal alterations may involve in the pathophysiology of these affective disorders. Thus BUS could serve as dual as regulating central neurotransmission and endocrinal (HPA axis) dysregulation induced behavioral effects in psychiatric patients. This may help in combating the refractory depressant cases, co-morbid depression and anxiety conditions and endocrinal related behavioral and mood alterations. BUS, in combination with other anti-depressant may pave the way for the development of newer therapeutic regimen with better efficacy and patient compliance. However, further studies are required to be done to understand at cellular and molecular level, the BUS modulatory effect on HPA axis.
ACKNOWLEDGMENT
The authors are thankful to Birla Institute of Technology and Science (BITS), Pilani and University Grants Commission, India for providing support and research facilities to pursue this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Keller MB, Klerman GL, Lavori PW, Coryell W, Endicott J, Taylor J. Long-term outcome of episodes of major depression. Clinical and public health significance. JAMA. 1984;252:788–92. [PubMed] [Google Scholar]
- 2.Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am J Psychiatry. 1965;122:509–22. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 3.Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–14. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Seo D, Patrick CJ, Kennealy PJ. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and its Comorbidity with other Clinical Disorders. Aggress Violent Behav. 2008;13:383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar R, Mahesh R. Assessing the neuronal serotonergic target-based antidepressant stratagem: Impact of in vivo interaction studies and knockout models. Curr Neuropharmacol. 2008;6:215–34. doi: 10.2174/157015908785777256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression: A probable neuronal target? J Psychopharmacol. 2010;24:455–69. doi: 10.1177/0269881109348161. [DOI] [PubMed] [Google Scholar]
- 7.Dunbar GC, Judge R. Long-term evaluation of paroxetine, clomipramine and placebo in panic disorder. Eur Neuropsychopharmacol. 1995;5:361–2. [Google Scholar]
- 8.Uhlenhuth EH, Balter MB, Ban TA, Yang K. International study of expert judgement on therapeutic use of benzodiazepines and other psychotherapeutic medications: II. Pharmacotherapy of anxiety disorders. J Affect Disord. 1995;35:153–62. doi: 10.1016/0165-0327(95)00064-x. [DOI] [PubMed] [Google Scholar]
- 9.Allsopp LF, Cooper GL, Poole PH. Clomipramine and diazepam in the treatment of agoraphobia and social phobia in general practice. Curr Med Res Opin. 1984;9:64–70. doi: 10.1185/03007998409109561. [DOI] [PubMed] [Google Scholar]
- 10.Lôo H, Saiz-Ruiz J, Costa e Silva JACE, Ansseau M, Herrington R, Vaz-Serra A, et al. Efficacy and safety of tianeptine in the treatment of depressive disorders in comparison with fluoxetine. J Affect Disord. 1999;56:109–18. doi: 10.1016/s0165-0327(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 11.Piñeyro G, Blier P. Autoregulation of serotonin neurons: Role in antidepressant drug action. Pharmacol Rev. 1999;51:533–91. [PubMed] [Google Scholar]
- 12.Redmond AP, Leonard BE. An evaluation of the role of noradrenergic system in the neurobiology of depression: a review. Hum Psychopharmacol Clin Exp. 1997;12:407–30. [Google Scholar]
- 13.Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986;314:1329–35. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- 14.Stefanski R, Pałejko W, Kostowski W, Płaznik A. The comparison of benzodiazepine derivatives and serotonergic agonists and antagonists in two animal models of anxiety. Neuropharmacology. 1992;31:1251–8. doi: 10.1016/0028-3908(92)90053-r. [DOI] [PubMed] [Google Scholar]
- 15.Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl) 1989;97:277–9. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- 16.Przegalinski E, Moryl E, Papp M. The effect of 5-HT 1A receptor ligands in a chronic mild stress model of depression. Neuropharmacology. 1995;34:1305–10. doi: 10.1016/0028-3908(95)00102-c. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg HL, Finnerty RJ. The comparative efficacy of buspirone and diazepam in the treatment of anxiety. Am J Psychiatry. 1979;136:1184–7. doi: 10.1176/ajp.136.9.1184. [DOI] [PubMed] [Google Scholar]
- 18.Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32:114–29. doi: 10.2165/00003495-198632020-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gammans RE, Stringfellow JC, Hvizdos AJ, Seidehamel RJ, Cohn JB, Wilcox CS, et al. Use of buspirone in patients with generalized anxiety disorder and coexisting depressive symptoms. A meta-analysis of eight randomized, controlled studies. Neuropsychobiology. 1992;25:193–201. doi: 10.1159/000118837. [DOI] [PubMed] [Google Scholar]
- 20.Robinson DS, Rickels K, Feighner J, Fabre LF, Jr, Gammans RE, Shrotriya RC, et al. Clinical effects of the 5-HT1A partial agonists in depression: A composite analysis of buspirone in the treatment of depression. J Clin Psychopharmacol. 1990;10:67S–76. doi: 10.1097/00004714-199006001-00013. [DOI] [PubMed] [Google Scholar]
- 21.Sato H, Skelin I, Debonnel G, Diksic M. Chronic buspirone treatment normalizes open field behavior in olfactory bulbectomized rats: Assessment with a quantitative autoradiographic evaluation of the 5-HT1A binding sites. Brain Res Bull. 2008;75:545–55. doi: 10.1016/j.brainresbull.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe A, Hasegawa S, Nishi K, Nguyen KQ, Diksic M. Chronic buspirone treatment normalizes regional serotonin synthesis in the olfactory bulbectomized rat brain: An autoradiographic study. Brain Res Bull. 2006;69:101–8. doi: 10.1016/j.brainresbull.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Grollman A. Biological assay of adrenal cortical activity. Endocrinol. 1941;29:855–61. [Google Scholar]
- 24.Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: An update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 25.Muscat R, Papp M, Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl) 1992;109:433–8. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- 26.Shibata S, Nakanishi H, Watanabe S, Ueki S. Effects of chronic administration of antidepressants on mouse-killing behavior (muricide) in olfactory bulbectomized rats. Pharmacol Biochem Behav. 1984;21:225–30. doi: 10.1016/0091-3057(84)90219-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, et al. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: Implications for emotional behavior. J Neuroimmunol. 2000;111:131–8. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]
- 28.Aguilera G. Corticotropin releasing hormone, receptor regulation and the stress response. Trends Endocrinol Metab. 1998;9:329–36. doi: 10.1016/s1043-2760(98)00079-4. [DOI] [PubMed] [Google Scholar]
- 29.Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am. 1994;23:451–66. [PubMed] [Google Scholar]
- 30.Parker GA, Valerio MG. Accessory adrenocortical tissue, rat. Endocrine Sys (Berl) 1983:16–7. [Google Scholar]
- 31.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association; 2000. American Psychiatric Association. Mood Disorders; pp. 345–70. text revision. [Google Scholar]
- 32.Rex A, Voigt JP, Voits M, Fink H. Pharmacological evaluation of a modified open-field test sensitive to anxiolytic drugs. Pharmacol Biochem Behav. 1998;59:677–83. doi: 10.1016/s0091-3057(97)00461-9. [DOI] [PubMed] [Google Scholar]
- 33.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 34.Verma P, Hellemans KG, Choi FY, Yu W, Weinberg J. Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiol Behav. 2010;99:276–85. doi: 10.1016/j.physbeh.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DP, Hyslop DK, Riblet LA. Buspirone: model for anxioselective drug action. Soc Neurosci Abstr. 1980;6:791. [Google Scholar]
- 36.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 37.Griebel G, Rodgers RJ, Perrault G, Sanger DJ. Risk assessment behaviour: Evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacol Biochem Behav. 1997;57:817–27. doi: 10.1016/s0091-3057(96)00402-9. [DOI] [PubMed] [Google Scholar]


