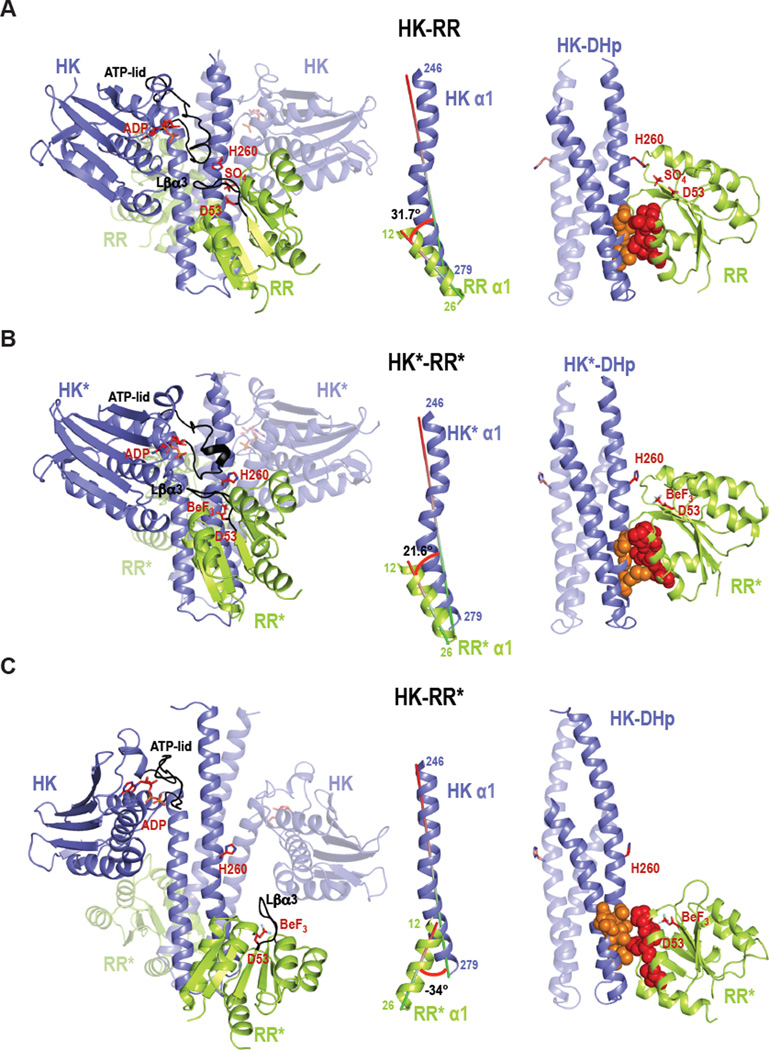
Figure 3. Crystal structures of the wild-type complex HK-RR, the rewired and functional HK*-RR* complex, and the impaired HK-RR* complex.
Cartoon representation of (A) HK-RR complex with HK853 bound to ADP and RR468 D53 bound to SO4, (B) HK*-RR* complex with HK853* bound to ADP and RR468* D53 bound to BeF3− and (C) HK-RR* complex with HK853 bound to ADP and RR468* D53 bound to BeF3−. Left; cartoon representations of the overall structure of the three complexes formed by a homodimeric HK (blue colored with one subunit transparent) bound to two molecules of RR (yellow-green colored with one molecule transparent). In each complex, the ATP-lid in the HK and the β3-α3 linker in the RR are colored in black; the phosphorylatable residues H260 and D53 as well as bound ligands ADP, sulfate (SO4) and beryllium trifluoride (BeF3) are shown as sticks. Middle; the angle formed between the interacting helices HK α1 (246–279) and RR α1 (12–26) for each complex is shown. Right; HK-RR interface shown by the DHp domain (with one subunit transparent) bound to one RR with the critical specificity residues (13, 14, 17 and 21 in red for RR468 and 268, 271, 275, 294 and 297 in orange for HK853) highlighted in space-filling spheres. Also see Figures S4–S6.