Abstract
Background:
Symptomatic vasospasm (SV) is often seen after aneurysmal subarachnoid hemorrhage (aSAH). The pathophysiology suggests that platelets initiate the process and are consumed. This is likely to result in thrombocytopenia. The objective of this study was to find out if thrombocytopenia preceded or followed SV and to analyze the relationship between the two.
Materials and Methods:
The platelet counts of 74 patients were studied on day 1, 3, 5, 7, 9, 11, and 14 following aSAH. Clinical symptoms and raised velocities on transcranial Doppler were studied on the same days to determine SV. The relationship of platelet counts and SV were analyzed.
Results:
Thirty-nine (52.7%) patients developed SV. Platelet counts dropped on postictal day (PID) 3-7 and SV was commonly seen on PID 5-9. The median platelet counts were significantly lower in patients with SV when compared to those without SV. Platelet count <150,000/mm3 on PID 1 and 7 had statistically significant association (P < 0.001) with SV. The odds ratio was 5.1, 6.9, and 5.1 on PID 5, 7, and 9, respectively, for patients with relative thrombocytopenia (P < 0.001).
Conclusions:
There is a strong correlation between thrombocytopenia and SV. A platelet count < 150,000/mm3 on PID 1 and 7 predicts presence of SV. The relative risk of developing SV is >5 times for a patient with relative thrombocytopenia especially on PID 5-9. Additionally, it appears that thrombocytopenia precedes vasospasm and may be an independent predictor. However, this requires further studies for validation.
Keywords: Subarachnoid hemorrhage, thrombocytopenia, vasospasm
Introduction
Ruptured cerebral aneurysms are the most frequent cause (~80%) of spontaneous subarachnoid hemorrhage (SAH).[1,2] Delayed ischemic deficits (DIDs) due to cerebral vasospasm are still the major cause of death and disability following aneurysmal subarachnoid hemorrhage (aSAH).[3] “Symptomatic vasospasm (SV)” and “DIDs” are considered as synonymous, referring to the clinical syndrome, wherein the narrowing of the arteries is severe enough to cause ischemic clinical symptoms.
The incidence of angiographic vasospasm ranges between 40% and 97% of affected individuals, with an average of 67.3%.[4] SV occurs in 20-30% of patients affected by SAH and leads to a 15-20% risk of stroke or death.[5] Indeed, progression to cerebral infarction occurs in approximately 50% of symptomatic cases; recovery without deficit in the remaining individuals may occur despite the persistence of angiographic vasospasm.[6]
Many factors may contribute to circulatory pathology at any level of the vasculature in patients with vasospasm after SAH, including infiltration of leukocytes into the wall of arteries together with injury of the endothelium, aggregation of platelets on the injured endothelium of arteries, release of substances from leukocytes and platelets with vasoactive or aggregation-inducing effects, and microthrombi in peripheral vessels.[3,7,8] Subsequently, intraluminal platelet consumption may occur in patients with cerebral vasospasm.[7] Theoretically, the platelet counts would reduce during vasospasm. It is still unclear whether thrombocytopenia precedes or follows vasospasm.
The onset, severity, and duration of vasospasm can have a very unpredictable nature. There has been a long overdue wish for a marker, which can help clinicians pre-empt vasospasm consistently. The purpose of this study was to analyze the relationship between thrombocytopenia and vasospasm, if any. If yes, what is the temporal course of events?
Materials and Methods
Patients of aneurysmal SAH presenting within 72 h of ictus were considered for study. Of these, patients with poor grade SAH (H and H grade 3 or 4 and World Federation of Neurosurgical Societes (WFNS) grade 3 or 4), non-aneurysmal SAH, those on antiplatelet medication (aspirin and/or clopidogrel), antibiotics causing thrombocytopenia, and those who received heparin (prophylactic or therapeutic) were excluded. These patients either underwent clipping or coiling to obliterate the aneurysm. The subgroup of patients who died within 14 days of ictus was excluded. After excluding these, 74 consecutive patients of aSAH were studied.
In the study, blood was drawn from a peripheral vein. The WBC count, Hb concentration, and platelet count were noted in postictal day (PID) 1, 3, 5, 7, 9, 11, and 14. Platelet counts were specifically monitored on a fully automated blood cell counter. Peripheral blood smears were examined by hematologists to confirm the blood counts by microscopic examination of Leishman-stained blood films. The baseline platelet count was defined as the platelet count done within 24 h of ictus. Platelet report from the referring hospital was considered in case the patient came after 24 h of ictus.
SV was defined by the following combined clinical and/or radiological criteria: (1) onset of confusion, disorientation, and/or decline in level of consciousness with or without focal deficit(s); (2) a head computed tomography (CT) scan excluding other causes of neurological worsening such as hydrocephalus, intracranial hemorrhage, and/or focal brain swelling; (3) no other identifiable cause of neurological worsening such as electrolyte disturbance, hypoxia, or seizures; and (4) vasospasm found on transcranial Doppler (TCD) studies along with above features. Vasospasm on TCD was defined as mean flow velocity >120 cm/s in any vessel.[9] TCD was performed on same days as mentioned above.
Both absolute and relative thrombocytopenia were studied. Absolute thrombocytopenia was defined as platelet count ≤100,000/mm3. Patients who developed SV received triple “H” therapy. (hypervolumia, hemodilution, and hypertension). As a part of hemodilution, additional 3 l of fluid was given. This would itself cause a decrease in platelet count (0.8 times the baseline platelet count) due to dilution. A platelet count of less than 0.8 times the baseline platelet count could be attributed to vasospasm per se rather than its treatment. Such decrease in platelet count, even if >100,000/mm3 but less than 0.8 times the baseline platelet count would qualify as relative thrombocytopenia.
Statistical analysis was done using Chi square and Mann–Whitney tests.
Results
Out of 74 patients, 39 (52.7%) developed SV. Mean age of the group which develops SV (cases) was 53.72 years (range of 33-75 years) and that of other group (non-vasospasm or controls) was 48.80 years (19-75 years).
There were 10 patients (6 were SV positive) in H and H grade 1, 41 (21 were SV positive) in grade 2, 11 (5 were SV positive) in grade 3, and 12 (7 were SV positive) in grade 4.
There were 41 patients (18 were SV positive) in WFNS grade 1, 17 (12 were SV positive) in grade 2, 4 (3 were SV positive) in grade 3, and 12 (6 were SV positive) in grade 4.
Further, there were 2 patients (none was SV positive) in Fisher grade 1, 6 (5 were SV positive) in grade 2, 33 (20 were SV positive) in grade 3, and 28 (13 were SV positive) in grade 4.
Anterior communicating artery (A.com artery) aneurysm was present in 34 patients (21 were SV positive), distal anterior cerebral artery (DACA) aneurysm was present in 6 patients (4 were SV positive), middle cerebral artery (MCA) aneurysm was present in 25 patients (12 were SV positive), and internal cerebral artery aneurysm was present in 9 patients (3 were SV positive).
Age, H and H grade, WFNS grade, Fischer grade, and site of aneurysm were not found to statistically influence the development of vasospasm.
Using the receiver operating characteristic curve, we found that platelet count <150,000/mm3 had statistically significant association (P < 0.001) with SV especially on PID 1 and 7. This (platelet count) had a sensitivity and specificity of 100% and 82% on PID 1 and 78% and 85% on PID 7, respectively. This connotes that a patient with platelet count <150,000/mm3 on PID 1 and 7 was likely to have SV.
When relative thrombocytopenia (platelet count on that day <0.8 times the baseline value) was analyzed with SV, the odds ratio was 5.1, 6.9, and 5.1 on PID 5, 7, and 9, respectively. This was statistically highly significant (P < 0.001). This indicates that a patient with relative thrombocytopenia on PID 5-9 had a relative risk of >5 times of having SV as compared to those who had no thrombocytopenia.
The median platelet counts of SV and non-SV group were compared [Figure 1]. The difference was statistically significant (P < 0.001). The median platelet counts in SV group showed absolute thrombocytopenia on PID 5 and 7.
Figure 1.
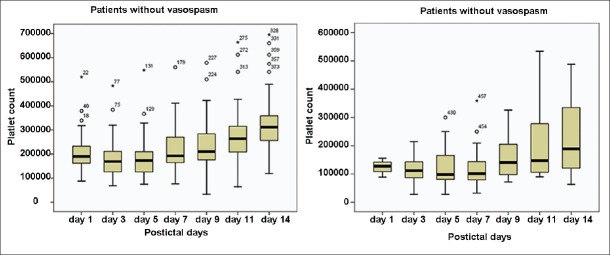
Comparison of median platelet counts in patients with and without vasospasm
The maximum decrease in platelet counts was on PID 3, 5, and 7 after which it started rising. Additionally, a number of patients with thrombocytopenia were maximum on PID 3, 5, and 7. The maximum number of patients with SV were on day 5, 7, and 9. Therefore, it appears that the maximum decrease in platelets preceded SV [Table 1].
Table 1.
Comparison of platelet counts in patients with and without SV
On correlation analysis between the platelet counts and WFNS scores in the patients in vasospasm group at various point of time in pre- and post-operative period no correlation was found between the two which could be statistically significant.
Discussion
From nearly over a century, physicians have come across development of vasospasm following SAH and a lot of research has been done related to its causes, mechanism, and treatment. More so, advances made in the field during the past 50 years have significantly improved the quality of care.
There had been a few studies which implicated role of platelets in the pathogenesis of cerebral vasospasm following aSAH directly (release of metabolites) or indirectly (consumption of platelets).[7,10,11,12] In this study, we studied the sequential changes in the pattern of platelet counts and tried to correlate it to cerebral vasospasm as a cause or effect phenomenon.
Out of total 74 individuals, 39, i.e., 52.7% developed SV diagnosed by development of new focal neurological signs, deterioration in level of consciousness, or appearance of hypodensity or new infarct on CT not attributable to other causes. This incidence is quite higher as being quoted in previous studies of 20-30%.[4] The exact cause of this could not be ascertained.
Hirashima et al. in their study found that the decrease in platelet count was more severe and the number of days to recover to normal platelet count tended to be greater in patients with SV than without SV.[7] They also found that decrease in platelet count more than 30% was as independent predictor of the occurrence of SV. In our study, this ratio was 0.8 and acted as an independent marker of SV. However, it cannot be labeled as an independent predictor of vasospasm as our study had certain limitations (vide infra).
Niikawa et al. in their study analyzed correlation between blood parameters and SV and found platelet counts were significantly higher in patients with SV than patients without; these results are in contradiction to our findings. Although the mechanism proposed by the study group is similar to our study, i.e., damage of arterial endothelium of ruptured aneurysm, adherence of platelets to subendothelial structures, platelet aggregation, and thrombus formation in the affected arteries, and finally, release of various factors such as thromoboxane, serotonin, and growth factors which cause contraction of vascular smooth muscle and resultant post-SAH vasospasm.[13] They concluded that higher platelets in early period may have an adverse influence, but they did not explain why there was more increase in platelet count in vasospasm group study, whereas decrease in platelet count as in our study can be explained by the consumption of platelets. However, the overall pathogenesis remains elusive and needs further studies.
Schebesch et al. compared postbleed platelet count with vasospasm in Caucasian population and found no decrease in platelet count.[14] Their results were opposite to those reported in Asian population. They concluded that genetic differences between the Asian and European populations may alter the pathophysiology.
It is likely that platelets are involved in the pathogenesis of vasospasm and during this phenomenon there is consumption of platelets, which causes thrombocytopenia. The process involved in the pathogenesis of consumption of platelets apparently starts before clinical vasospasm appears. The maximum fall in platelets occur during the period of SV; also the maximum dip in platelets occurs before maximum number of patients develops SV. Therefore, it appears that the maximum decrease in platelets preceded SV. However, this cannot be stated with conviction because of study limitations mentioned below.
The study had following limitations: Both the measurement of platelet counts and TCD were not done daily. Therefore, cause and effect relationship of thrombocytopenia and SV need further evaluation. Furthermore, hematocrit values, if measured, would have been a better marker of hemodilution.
Conclusion
In conclusion, a patient with platelet count <150,000/mm3 on PID 1 and 7 was likely to have SV. There was a significant difference in the median platelet counts of patients who had SV and no SV. Relative thrombocytopenia on PID 5-9 had a relative risk of >5 times of having SV as compared to those who had no thrombocytopenia.
Additionally, it appears that thrombocytopenia precedes vasospasm and may be an independent predictor. However, this requires further studies for validation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid haemorrhage. N Engl J Med. 2000;342:29–36. doi: 10.1056/NEJM200001063420106. [DOI] [PubMed] [Google Scholar]
- 2.Edlow JA. Diagnosis of subarachnoid hemorrhage. Neurocrit Care. 2005;2:99–109. doi: 10.1385/NCC:2:2:099. [DOI] [PubMed] [Google Scholar]
- 3.Hirashima Y, Endo S, Nukui H, Kobayshi N, Takaku A. Effect of a platelet-activating factor receptor antagonist, E5880, on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurol Med Chir. 2001;41:165–76. doi: 10.2176/nmc.41.165. [DOI] [PubMed] [Google Scholar]
- 4.Dorsch NWC. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Crit Care. 2002;8:128–33. doi: 10.1097/00075198-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;28:2315–28. doi: 10.1161/01.str.25.11.2315. [DOI] [PubMed] [Google Scholar]
- 6.Heros RC, Zervas NT, Varsos V. Cerebral vasospasm after subarachnoid hemorrhage: An update. Ann Neurol. 1983;14:599–608. doi: 10.1002/ana.410140602. [DOI] [PubMed] [Google Scholar]
- 7.Hirashima Y, Hamada H, Kurimoto M, Origasa H, Endo S. Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005;102:882–7. doi: 10.3171/jns.2005.102.5.0882. [DOI] [PubMed] [Google Scholar]
- 8.McGirt MJ, Mavropoulus JC, McGirt LY, Alexander MJ, Friedmann AH, Laskowitz DT. Leukocytosis as an independent risk factor for cerebral vasospasm following subarachnoid haemorrhage. J Neurosurg. 2003;87:1222–6. doi: 10.3171/jns.2003.98.6.1222. [DOI] [PubMed] [Google Scholar]
- 9.Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM. Role of transcranial doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44:1237–48. [PubMed] [Google Scholar]
- 10.Pyne GJ, Cadoux-Hudson TA, Clark JF. Platelets play an essential role in the etiology of cerebral vasospasm after subarachnoid hemorrhage. Med Hypotheses. 2003;60:525–30. doi: 10.1016/s0306-9877(02)00452-8. [DOI] [PubMed] [Google Scholar]
- 11.Ohkuma H, Suzuki S, Kimura M, Sobota E. Role of platelet function in symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1991;22:854–9. doi: 10.1161/01.str.22.7.854. [DOI] [PubMed] [Google Scholar]
- 12.Juvela S, Ohman J, Servo A, Heiskanen O, Kaste M. Angiographic vasospasm and release of platelet thromboxane after subarachnoid hemorrhage. Stroke. 1991;22:451–5. doi: 10.1161/01.str.22.4.451. [DOI] [PubMed] [Google Scholar]
- 13.Niikawa S, Hara S, Ohe N, Miwa Y, Ohkuma A. Correlation between blood parameters and symptomatic vasospasm in subarachnoid hemorrhage patients. Neurol Med Chir (Tokyo) 1997;37:881–5. doi: 10.2176/nmc.37.881. [DOI] [PubMed] [Google Scholar]
- 14.Schebesch KM, Woertgen C, Brawanski A, Rothoerl RD. A study of possible correlation between subarachnoid haemorrhage related vasospasm and the post-bleed blood platelet count chart in a Caucasian population. Acta Neurochir (Wien) 2007;149:387–91. doi: 10.1007/s00701-007-1124-2. [DOI] [PubMed] [Google Scholar]


