Abstract
Background and Purpose:
Stroke, which remains the third leading cause of death after heart disease and cancer in developed countries, is a disorder causing permanent neurologic disability. Even though, hemorrhagic strokes are seen less than the ischemic type, they are more fatal. We studied the risk factors for spontaneous intra-cerebral hemorrhage (ICH) to direct the proper preventive treatment modalities and the effects of these factors on mortality as well as applied therapeutic strategies on survival.
Materials and Methods:
The archive records of 106 patients (60 male, 46 female) who were diagnosed with spontaneous ICH in Baskent University Hospital, Ankara, between January 2003 and September 2008, were assessed retrospectively.
Results:
The mean age was found as 62.5. The most frequent risk factor was hypertension (73.5%); 69.2% of these hypertensive patients had uncontrolled blood pressure levels. The mortality rate was detected as 34.9% and patients were found to die approximately within 9 days after ICH. Older age, increased hemorrhage volume, ventricular extension of hemorrhage, and the presence of midline shift were found to significantly correlate with increased mortality (P < 0.05). Patients who underwent surgical therapy showed a longer survival rate (P = 0.016); however, no association was found between medical and surgical therapy in terms of mortality (P = 0.555).
Conclusion:
The results of this study suggest that effective control of blood pressure is important in the prevention of spontaneous ICH; clinical and radiological findings with treatment modalities influencing mortality should be carefully managed.
Keywords: Mortality, risk factors, spontaneous intra-cerebral hemorrhage, treatment
Introduction
Cerebrovascular diseases are the third leading cause of death after heart disease and cancer in developed countries. They also come first in terms of causing death and disability in neurologic diseases in adults.[1]
Spontaneous intra-cerebral hemorrhage (ICH), which is defined as spontaneous rupture of the intra-cerebral small vessels following cerebral vessel wall degeneration due to frequent chronic hypertension or rarely to cerebral amyloid angiopathy, has an incidence of 15-19/100,000/year and a 30-day mortality of 40-50%.[2] The risk factors for ICH are identified as hypertension, advancing age, male sex, excessive alcohol intake, anticoagulation therapy, smoking, and diabetes.[2,3,4,5] To determine these risk factors is very important in terms of developing preventative measures.
We aimed to determine the risk factors for spontaneous ICH in a Turkish population retrospectively, and study the effects of these factors on mortality as well as applied therapeutic strategies on survival.
Materials and Methods
Study population
In this study, the archive records of total 135 patients who were diagnosed with spontaneous ICH in Baskent University Hospital, Ankara, between January 2003 and September 2008, were assessed retrospectively. A total of 29 patients; under the age of 18, those with ICH secondary to primary or secondary intracranial tumor, those with traumatic or hemorrhagic ischemic (arterial/venous) etiologies and those with subarachnoid or subdural hemorrhages were excluded from the study. Finally, 106 patients were included in our study.
The following data were collected: Age, sex, cigarette, and alcohol (≥210 g/week),[6] intake or not, systemic diseases of the patient, anti-aggregant (acetyl salicylic acid, clopidogrel) and/or anticoagulant (warfarin, low molecular weight heparin) use on admission, family history of first-degree relative with ICH, bleeding volumes and localizations scanned by computerized tomography (CT), extension of hemorrhage to the ventricle or subarachnoid area, whether it caused shift or not, what kind of treatment modality (medical/surgery) was applied to patients and survival times. The effects of parameters such as older age, bleeding volume, subarachnoidal and ventricular extension of hemorrhage, the presence of midline shift secondary to the hemorrhage, and usage of anti-aggregant/anti-coagulant medicines, on mortality were examined. The association between anti-aggregant/anti-coagulant drug usage and bleeding volume was also investigated.
Patients using antihypertensive drug on admission or whose systolic blood pressure was measured as ≥140 mmHg or diastolic blood pressure as ≥90 mmHg in serial measurements in follow-up, were defined as “hypertensive.” Those whose blood pressure measurement was <120 mmHg systolic, <80 mmHg diastolic, were referred to as having controlled levels.[7] Patients taking anti-lipidemic medication or those whose fasting total cholesterol level was found as >200 mg/dL were assessed as “hyperlipidemic.”[7] Similarly, patients using the anti-diabetic drug before or whose fasting blood glucose was determined as >126 mg/dL or post-prandial blood glucose (after oral intake) was >200 mg/dL or serial high measurements above these values in follow-up were defined as “diabetic.”[7]
Bleeding localization
In the study, ICH was divided into two groups according to their localizations, either supratentorial or infratentorial. Supratentorial hemorrhages were divided into subgroups as either lobar (cortex-subcortical white matter) or deep (capsula interna, basal ganglia, and periventricular white matter) hemorrhages and infratentorial ones were referred to as cerebellar or brainstem hemorrhages.
Bleeding volumes
Bleeding volumes were calculated by using the ellipsoidal method (A × B × C × 1/2),[8] = A: Wideness, B: Length, C: Height (determined according to section number found in CT). Thus, the volumes were defined as small (0-9.9 cm3), medium (10-29.9 cm3), large (30-59.9 cm3), and very large (≥60 cm3).[9]
Treatment method
Surgical treatment was applied to patients with the following: Gradual disturbances of consciousness; cerebral hematomas greater than a volume of 15 cm3 and cerebellar hematomas greater than 3 cm in diameter; brainstem compression or shift; risk of obstructive hydrocephalus; lobar, external capsule, cerebellar hematomas, and localized non-dominant hemisphere hematomas. On the other hand, medical treatment was given to patients with the following: Small hematomas; minor or major neurological deficits; deep-seated hemorrhage such as basal ganglia or thalamic ones; pons hematomas; and to older patients (>75 years) who could not tolerate surgical treatment.
Statistical analysis
Shapiro-Wilk's test was used to assess the data normality. To compare the differences between the treatment groups, Chi-square analysis was used for categorical variables and independent samples t-test and Mann-Whitney U-tests were used for continuous variables. Values are expressed as frequencies and percentages, mean and standard deviation or median and 25th-75th percentiles. Moreover, univariate and multivariate binary logistic regression analysis were performed to determine the risk factors of mortality in ICH patients. Odds ratios and 95% confidence intervals were calculated for each factor. Also an adjusted model is built beside the crude model due to the significant effect of age on mortality. To identify the independent determinants, variables significant at P < 0.25 in univariate analysis were included to multivariate model and backward elimination was performed using Wald statistic at P < 0.05 stringency level. IBM SPSS software (release 20.0, IBM, SPSS Inc., Chicago, IL, USA) was used for data analysis and P < 0.05 was considered as statistically significant.
Results
The demographic and clinical features of the patients are summarized in Table 1. Bleeding localization in the patients was determined as 95 (89.6%) for supratentorial and 11 (10.4%) for infratentorial. In the supratentorial hemorrhage group, 51 (48.1%) patients bled in the cortex, 1 (0.9%) bled in the subcortical white matter, 42 (39.6%) bled in the basal ganglions and 1 (0.9%) in the capsula interna while in the infratentorial group, 10 (9.4%) patients bled in the cerebellum and 1 (0.9%) in the brainstem. In terms of bleeding volumes 50 (47.2%) patients had small (0-9.9 cm3), 37 (34.9%) had medium (10-29.9 cm3), 15 (14.2%) had large (30-59.9 cm3), and 4 (3.8%) patients had very large (≥60 cm3) hemorrhages.
Table 1.
The demographic and clinical features of patients
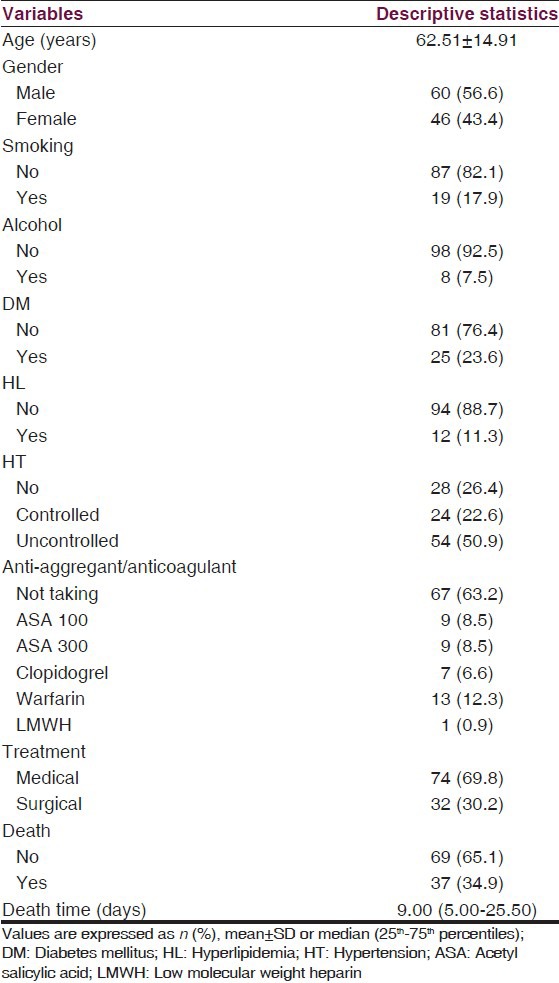
We found that mortality increased with older age (P = 0.043), advancing bleeding volume (P < 0.001), development of ventricular extension of hemorrhage (P = 0.001), the presence of midline shift (P < 0.001), but not with gender (P > 0.05) or the development of subarachnoidal extension of hemorrhage (P > 0.05). However, there was no significant association between mortality and anti-aggregant and/or anticoagulant drug usage (P > 0.05) [Table 2].
Table 2.
Determinative factors affecting mortality
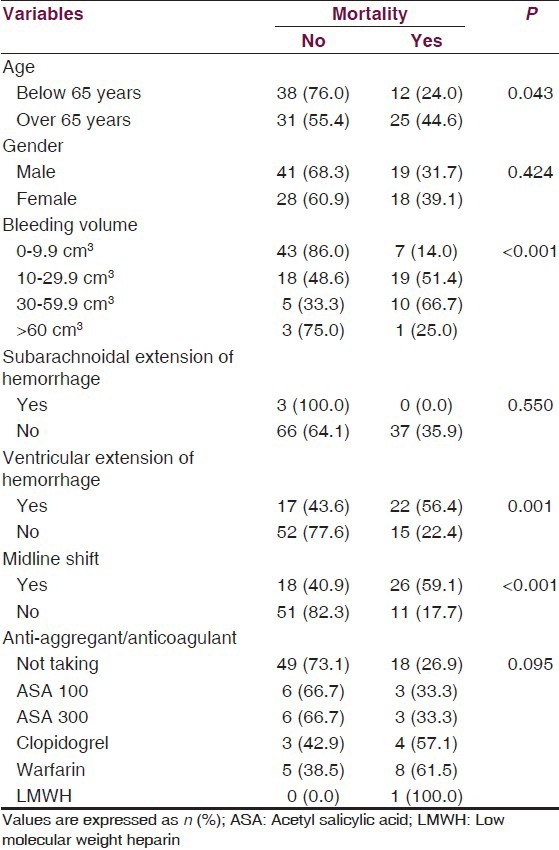
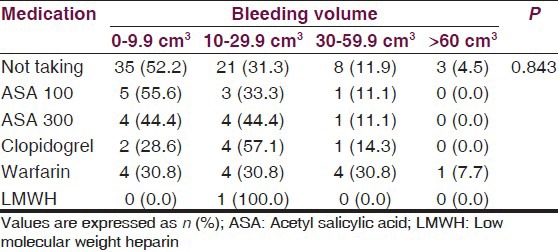
Due to the significant effect on mortality, we adjusted the logistic regression models for age variable. In univariate analysis, we found increased mortality with advancing bleeding volume (P = 0.001), development of ventricular extension of hemorrhage (P < 0.001), the presence of midline shift (P < 0.001), and warfarin usage (P < 0.05). In multivariate analysis, being over 65 years (P < 0.05), advancing bleeding volume (P < 0.05) and the presence of midline shift (P < 0.05) were found as independent factors on mortality. Both univariate and multivariate analyses were summarized in Table 3. There was also no significant difference between anti-aggregant/anti-coagulant medication and bleeding volume (P > 0.05) [Table 4].
Table 3.
Comparison of variables on mortality
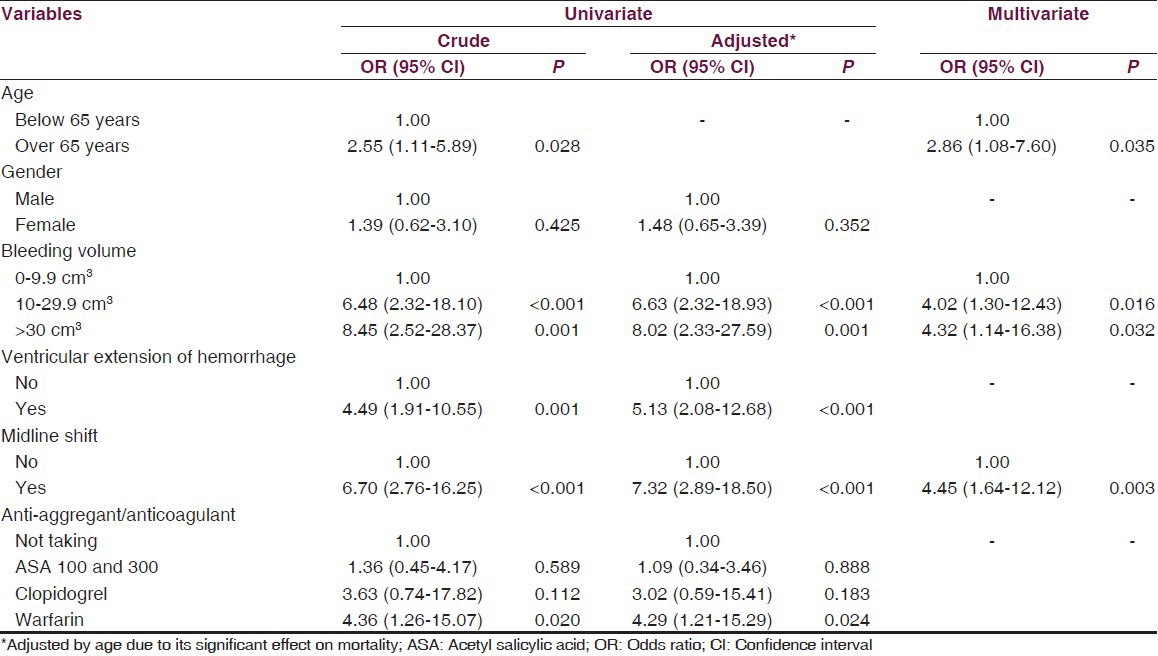
Table 4.
Association between medication and bleeding volume
In a total of 106 patients, medical treatment was applied to 74 (69.8%) patients, whereas surgical treatment was given to 32 (30.2%). Thirty seven (34.9%) patients in total died in hospital. In addition, 30 (81%) of the deaths were in the 1st month (early mortality) and 7 (18.9%) occurred after 1 month (late mortality). Seven of nine patients with complications of systemic and infectious diseases, died in the 1st month. We also found that patients who were given surgical therapy showed a longer survival rate (P = 0.016); however, no association was found between medical and surgical therapy in terms of mortality (P = 0.555) [Figure 1a and b] and clinical differences (P > 0.05) [Table 5].
Figure 1.
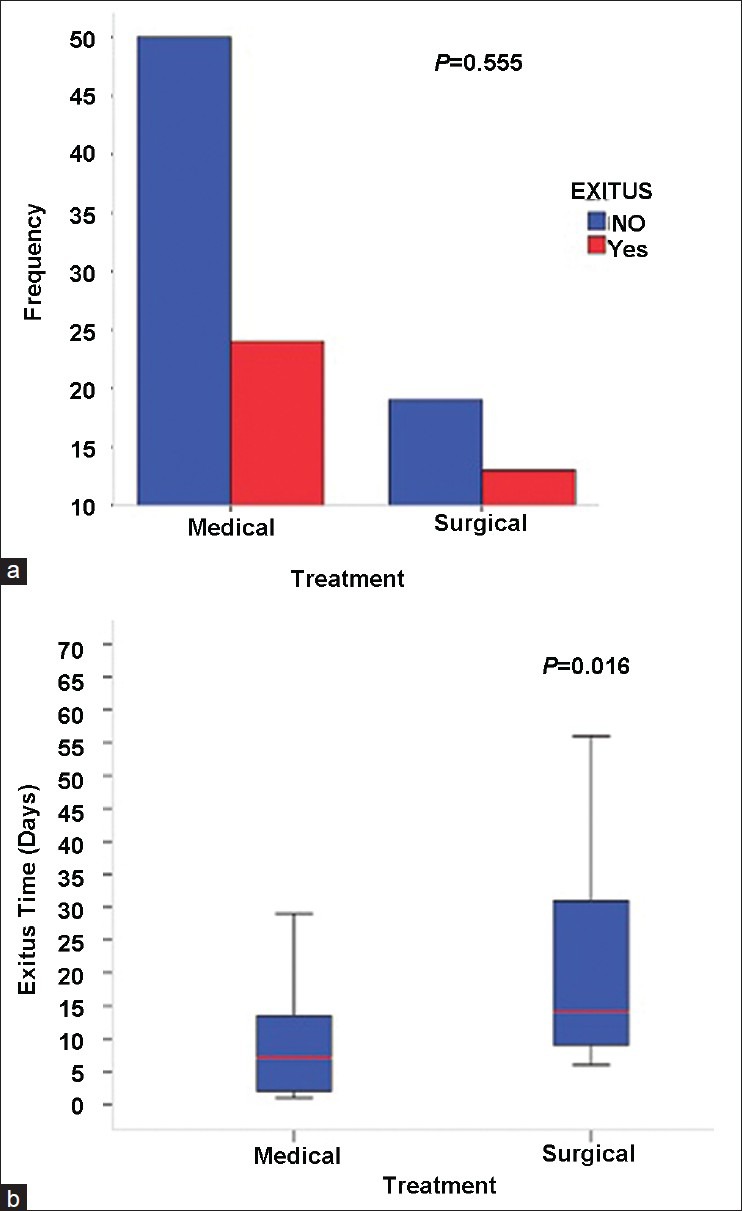
Comparison of exitus status (a) and exitus time (b) between treatment groups
Table 5.
Clinical differences between treatment groups
Discussion
There have been several publications on the hemorrhagic strokes; however, we aimed to determine the risk factors for spontaneous ICH in a Turkish population retrospectively. The salient findings of our study were as follows: (i) In those over the age of 65, increased hemorrhage volume, having ventricular extension of hemorrhage and the presence of midline shift were demonstrated as the determinative factors affecting mortality; (ii) uncontrolled blood pressure levels were found to be the most common risk factor leading to cerebral hemorrhage; (iii) the surgical treatment group showed a longer survival rate compared to patients who were given medical treatment.
Age was known as the most important unchangeable risk factor for spontaneous ICH. Ariesen et al., Sturgeon et al., and Efstathiou et al., have reported that older age was a major risk factor for ICH.[2,7,10] In the present study, the mean age was determined as 62.5 accompanied by the finding that mortality was observed to be higher in patients over the age of 65. This is in accordance with the literature. A decrease in vessel elasticity with aging makes the vessel wall vulnerable to the effects of hypertension; this may be the hypothetic mechanism for increased risk of cerebral hemorrhage and result in with worse outcomes. Male sex, especially being over the age of 55, has been described as another risk factor for spontaneous ICH in the literature. While the meta-analysis of Ariesen et al., supported this belief, no association was found in the reports of Sturgeon et al., and Efstathiou et al.[2,7,10] Gender was in favor of males in our study group.
The importance of hypertension in spontaneous ICH etiology has been already pointed out in the studies of Sacco, Ariesen et al., and Woo et al.[1,2,5] In one study with 152 cases, hypertension was found in 73% of patients.[11] In another series involving 141 patients, hypertension was determined in 83% of patients.[4] In our study group, hypertension was determined in 78 patients (73.5%). The number of patients with uncontrolled blood pressure levels was found as 69.2% among hypertensive patients in spite of antihypertensive treatment. This result is remarkable in that it underlines the importance of effective blood pressure control in the prevention and management of ICH. Recently, antihypertensive treatment of acute cerebral hemorrhage II study group confirmed the efficacy and safety of early, intensive acute antihypertensive treatment, providing evidence of attenuation of hematoma expansion with intensive systolic blood pressure reduction, in subjects with spontaneous ICH.[12]
With respect to mortality, Delgado et al., and Castellanos et al., found the mortality as 20% and 28.3% respectively.[13,14] In this study, the mortality rate was determined as 34.9% (37 patients) and 81% (30 patients) of deaths were observed in the 1st month (early mortality), whereas early mortality relating to ICH was about 35-50% in the literature.[15] Likewise, Hemphill et al., reported 30-day mortality as 45% in their study.[11] In our study, it was very surprising that early mortality was found at higher levels; a possible explanation may be confounding systemic diseases or opportunistic infections. As a matter of fact, 9 of the 37 patients who died during hospitalization were found to have complications with infectious, pulmonary and hematologic issues and seven of them died in the 1st month. Therefore, it should be kept in mind that these systemic pathologic courses may influence morbidity and mortality in a negative direction in the course of evaluating patients with ICH.
In the literature, bleeding volume has been shown as an important factor that affects mortality. Cheung and Zou, found the hematoma volume was statistically higher in the dead group than in the recovering group.[4] Similar results were obtained in the studies of Castellanos et al.[14] Likewise, parallel results were achieved from different studies.[11,13,16] In line with previous data, we found a significant association between bleeding volume and mortality. Another determinative factor for mortality was subarachnoidal extension of hemorrhage in ICH as reported previously.[4] However, we did not find a relation in our study. This might be due to fewer patients in defined group. Other significant factor was shown to be ventricular extension of hemorrhage. Cheung and Zou, Hemphill et al. and Delgado et al. reported concomitance of ventricular extension of hemorrhage with increased mortality radiologically in their studies.[4,11,13] Similarly, we found that the mortality rate was higher in patients with ventricular extension than in those who did not. The presence of midline shift has also been defined as another determinative factor on mortality in ICH.[17] In a series with 141 patients, the presence of shift was found in 83.9%.[4] In agreement with the literature, in the present study, 59.1% of patients with shift and 17.7% of those without shift, were found to die. In the literature, there are a few studies examining the association between mortality and anti-aggregant/anti-coagulant usage. In a study of 435 patients performed by Rosand et al., the mortality rate was found to be significantly higher in the group using warfarin (52%) than in those who did not (25.8%).[18] In contrast, we did not find a significant association between anti-aggregant/anti-coagulant medication and mortality. This might be due to another finding that indicates no significant difference between advanced bleeding volume and medication types. Another possible explanation may have been the small size of the group. In their recent study, Flaherty et al., also reported that warfarin usage caused a significant increase in hematoma volume in the group whose International Normalized Ratio values were over three among patients using warfarin.[19] In our study, among these factors being over 65 years, advancing bleeding volume and the presence of midline shift were found as independent factors on mortality. Overall, determinative factors affecting mortality were almost similar in European, Asian studies and those from United States with different ethnic backgrounds.[2,4,7] Although not unique or undescribed, our results confirm the previous reports in a Turkish perspective. Because we aimed to study the risk factors, especially related to the prognosis of ICH patients with medical and/or surgical therapy, we designed the study in a clinical manner rather than epidemiological.
Studies on treatment modalities (medical/surgical) have revealed conflicting results with regard to patients with supratentorial hemorrhages. The superiority of surgical treatment over medical has been much emphasized in recent trials because of its benefits such as decreasing intracranial pressure by lessening hematoma volume; thus, restoring perfusion in the surrounding tissue and helping to remove toxic products despite the unfavorable effects such as the risk of causing parenchymal tissue damage and influencing functional recovery in a negative direction.[20] Whereas in another prospective, international, multicenter clinical study published by Mendelow et al. named surgical trial of ICH, similar results displaying no benefit for surgery were obtained in two groups receiving surgical and medical treatment for “disability” and “mortality with 6 months” end points.[21] In other randomized controlled studies in which minimal invasive surgical techniques were used, no definitive result was obtained.[22,23] In comparing treatment modalities, we found that patients who were given surgical therapy showed a longer survival rate; however, no association was found between medical and surgical therapy in terms of mortality and clinical differences. This might be related to the quality of care services after surgery during hospitalization. As a matter of fact, unconscious patients with ICH and those went to surgical treatment were hospitalized on intensive care unit of which concept was to provide systematic prevention of complications through specialized nursing care and appropriate monitoring as well as early rehabilitation.[24] Whereas the rest were followed-up on regular neurological ward settings having lower resource utilization conversely as mentioned by Epifanov et al.[25]
This study has some potential limitations. First, the sample size is relatively small, mainly due to the fact that the participants were selected from a single institute. Second, it is retrospective. Third, although Glasgow coma scale is an important determinant of outcome, we could not able to provide this data from retrospective analysis. Fourth, despite the serial high measurements of blood pressure and serum glucose levels in follow-up, hypertensive or diabetic conditions still might be masked because of low blood pressure or serum glucose measurements on admission during the acute phase. Fifth, it might be the paucity of discharge data and absence of advanced imaging.
In conclusion, early detection and pharmacological rapid control of hypertension is the key intervention to decrease the incidence of this devastating type of stroke. Future genetic and epidemiologic studies will help identify at-risk populations and hopefully allow for primary prevention. Randomized, controlled studies focusing on novel therapeutics should aid in minimizing secondary injury and hopefully improve morbidity and mortality.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Sacco RL. Risk factors, outcomes, and stroke subtypes for ischemic stroke. Neurology. 1997;49:S39–44. doi: 10.1212/wnl.49.5_suppl_4.s39. [DOI] [PubMed] [Google Scholar]
- 2.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: A systematic review. Stroke. 2003;34:2060–5. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz S, Häfner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–61. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- 4.Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 2003;34:1717–22. doi: 10.1161/01.STR.0000078657.22835.B9. [DOI] [PubMed] [Google Scholar]
- 5.Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, et al. Genetic and environmental risk factors for intracerebral hemorrhage: Preliminary results of a population-based study. Stroke. 2002;33:1190–5. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- 6.Puddey IB, Beilin LJ, Vandongen R, Rouse IL, Rogers P. Evidence for a direct effect of alcohol consumption on blood pressure in normotensive men. A randomized controlled trial. Hypertension. 1985;7:707–13. doi: 10.1161/01.hyp.7.5.707. [DOI] [PubMed] [Google Scholar]
- 7.Sturgeon JD, Folsom AR, Longstreth WT, Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718–25. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 8.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 9.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 10.Efstathiou SP, Tsioulos DI, Zacharos ID, Tsiakou AG, Mitromaras AG, Mastorantonakis SE, et al. A new classification tool for clinical differentiation between haemorrhagic and ischaemic stroke. J Intern Med. 2002;252:121–9. doi: 10.1046/j.1365-2796.2002.01013.x. [DOI] [PubMed] [Google Scholar]
- 11.Hemphill JC 3 r rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi AI, Palesch YY. Antihypertensive treatment of acute cerebral hemorrhage (ATACH) II: Design, methods, and rationale. Neurocrit Care. 2011;15:559–76. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado P, Alvarez-Sabín J, Abilleira S, Santamarina E, Purroy F, Arenillas JF, et al. Plasma d-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2006;67:94–8. doi: 10.1212/01.wnl.0000223349.97278.e0. [DOI] [PubMed] [Google Scholar]
- 14.Castellanos M, Leira R, Tejada J, Gil-Peralta A, Dávalos A, Castillo J, et al. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. 2005;76:691–5. doi: 10.1136/jnnp.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santalucia P. Intracerebral hemorrhage: Medical treatment. Neurol Sci. 2008;29(Suppl 2):S271–3. doi: 10.1007/s10072-008-0961-y. [DOI] [PubMed] [Google Scholar]
- 16.Gebel JM, Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–41. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- 17.Kalita J, Misra UK, Vajpeyee A, Phadke RV, Handique A, Salwani V. Brain herniations in patients with intracerebral hemorrhage. Acta Neurol Scand. 2009;119:254–60. doi: 10.1111/j.1600-0404.2008.01095.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–4. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 19.Flaherty ML, Tao H, Haverbusch M, Sekar P, Kleindorfer D, Kissela B, et al. Warfarin use leads to larger intracerebral hematomas. Neurology. 2008;71:1084–9. doi: 10.1212/01.wnl.0000326895.58992.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi S, Sato A, Kageyama Y, Nakamura H, Watanabe Y, Yamaura A. Treatment of hypertensive cerebellar hemorrhage: Surgical or conservative management? Neurosurgery. 1994;34:246–50. [PubMed] [Google Scholar]
- 21.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the ınternational surgical trial in ıntracerebral haemorrhage (STICH): A randomised trial. Lancet. 2005;365:387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 22.Marquardt G, Wolff R, Janzen RW, Seifert V. Basal ganglia haematomas in non-comatose patients: Subacute stereotactic aspiration improves long-term outcome in comparison to purely medical treatment. Neurosurg Rev. 2005;28:64–9. doi: 10.1007/s10143-004-0355-4. [DOI] [PubMed] [Google Scholar]
- 23.Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G, et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: A multicenter randomized controlled trial (SICHPA) Stroke. 2003;34:968–74. doi: 10.1161/01.STR.0000063367.52044.40. [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen HS, Kammersgaard LP, Nakayama H, Raaschou HO, Larsen K, Hübbe P, et al. Treatment and rehabilitation on a stroke unit improves 5-year survival. A community-based study. Stroke. 1999;30:930–3. doi: 10.1161/01.str.30.5.930. [DOI] [PubMed] [Google Scholar]
- 25.Epifanov Y, Dodel R, Haacke C, Schaeg M, Schöffski O, Hennerici M, et al. Costs of acute stroke care on regular neurological wards: A comparison with stroke unit setting. Health Policy. 2007;81:339–49. doi: 10.1016/j.healthpol.2006.07.004. [DOI] [PubMed] [Google Scholar]


