Abstract
Background:
Traumatic brain injury (TBI) is accompanied by substantial accumulation of biomarkers of oxidative stress and depletion of antioxidants reserve which initiate chain reactions that damage brain cells. The present study investigated the role of ascorbic acid and α-tocopherol on the severity and management of TBI in rats.
Materials and Methods:
Wistar rats were subjected to closed head injury using an accelerated impact device. Rats were administered 45 mg/kg and 60 mg/kg body weight of ascorbic acid, α-tocopherol or a combination of the two vitamins for 2 weeks pre- and post injury. Blood and brain tissue homogenates were analyzed for vitamin C, vitamin E, malondialdehyde, superoxide dismutase, and creatine kinase activities.
Results:
The results indicated that TBI caused significant (P < 0.05) decreased in vitamins C and E levels in the blood and brain tissue of TBI-untreated rats. The activities of superoxide dismutase in TBI rats were markedly reduced when compared with non traumatized control and showed a tendency to increased following supplementation with vitamins C and E. Supplementation of the vitamins significantly (P < 0.05) reduced malondialdehyde in the treatment groups compared with the TBI-untreated group.
Conclusion:
The study indicated that pre and post treatment with ascorbic acid and α-tocopherol reduced oxidative stress induced by brain injury and effectively reduced mortality rate in rats.
Keywords: Antioxidant, oxidative stress, supplementation, traumatic brain injury
Introduction
Head injury is a trauma to the head that may or may not include injury to the brain. Road traffic accidents (RTAs) constitute a considerable concern to the general public and may be a major cause of traumatic head injury in Nigeria. Mortality rate from RTAs in Nigeria is higher than those of both the industrialized and other developing countries[1] probably due to the poor road network. Head injury is a major cause of morbidity in survivors and a major cause of traumatic brain injury.[2] Traumatic brain injury (TBI) is the leading cause of death and severe disability in people under 45 years of age in Western industrialized countries[3] affecting the young and adults in the most productive years of their lives.[4] Although TBI is a problem of major medical and socioeconomic significance, its pathogenesis is not well understood[5] and is often difficult to manage that may lead to primary and secondary lesions of varying severity.[6] The immune system response to damage done by the closed head injury as a result of free radicals produced neutrophils to remove the damaged tissue. The cells of the immune system such as T-cells, B-cells, and macrophages have membranes that are particularly rich in long-chain unsaturated fatty acids, thus making them more vulnerable to free radicals oxidation initiated by the closed head injury than other cells. The brain itself is rich in unsaturated fatty acids and consumes large amounts of oxygen that may generate excess free radicals which could overwhelm the antioxidant defense systems that can exacerbate the brain injury. The antioxidants vitamin E and vitamin C protect the brain from oxidative stress by directly scavenging the free radicals. Increased intake of vitamins C and E may positively influence the healing processes by maintaining the immune system, lymphocytes proliferation and decrease production of immunosuppressive prostaglandin E2, and suppressing neuroinflammatory processes.[7] Decreased levels of antioxidants or inhibition of the antioxidant enzymes cause oxidative stress that may damage cells.[8] The current work was designed to study the effect of α-tocopherol and ascorbic acid on the severity and management of traumatized brain injury in albino rats.
Materials and Methods
Chemicals
Analytical graded chemicals were used for the study.
Vitamins
Ascorbic acid (vitamin C) was obtained from PAL Pharmaceutical industries Ltd., Kano, Nigeria, while α- tocopherol (vitamin E) was obtained from Pharco Pharmaceuticals, Bolkely Alexandria, Egypt.
Head trauma model and management
The experimental protocol was approved by the Ethical and Animal Care and Usage Committee of the Usmanu Danfodiyo University, Sokoto Nigeria. Seventy-two (72) apparently healthy albino rats of Wistar strain, weighing between 170 and 200 g, were randomly divided into eight groups of nine rats per group.
Group A: Non-TBI-untreated group,
Group B: TBI untreated,
Group C: TBI treated with 45 mg/kg of vitamin C,
Group D: TBI treated with 45 mg/kg of vitamin E,
Group E: TBI treated with 45 mg/kg each of vitamins C and E,
Group F: TBI treated with 60 mg/kg of vitamin C,
Group G: TBI treated with 60 mg/kg of vitamin E,
Group H: TBI treated with 60 mg/kg each of vitamins C and E.
The vitamins were suspended in 0.9% NaCl and administered to the rats for 2 weeks before induction of the trauma. The control groups (A and B) received equal volumes of the vehicle.[9]
The animals were then anesthetized with 7.5 mg/kg propofol intraperitoneally and were incubated and ventilated on a Harvard Rodent ventilator. The body temperature of the animals was maintained throughout with a thermostatically controlled heating pad set at 37 °C. The skull was exposed by midline incision and a stainless steel disk measuring 10 mm in diameter and 3 mm in depth was cemented centrally along the control suture between the lambda and the bregma with a polyacrylamide adhesive. The animals were secured in the prone position on a 10 cm deep foam bed. Injury was induced by dropping 80 g brass weight from a distance of 1 m. The stainless steel disk was immediately removed from the skull and the animals were weaned off the ventilator and recover in the cages.[10] The treatment continued for additional period of 2 weeks during which the rats were followed up for recovery and survival from the trauma.
The animals were then killed by decapitation and blood was collected in labeled centrifuge tubes, then centrifuged, and serum collected was used for estimation of oxidative stress indices. Brain tissues were taken from frontal cortex, hippocampus, and cerebellum[11] and perfused with normal saline followed by 4% para-formaldehyde. The brain tissue samples were homogenized in four volumes of 0.2 M phosphate buffer, pH 7.8 and centrifuged for 2 minutes at 1,000 rpm. The samples were frozen immediately on dry ice and stored at −20 °C until required.
Biochemical estimations
Vitamin E
Vitamin E was estimated by the colorimetric method.[12] The tocopherols in the sample were oxidized to tocopheryl quinine by FeCl3 and Fe2 + and the resultant FeCl2 formed a red-colored complex with α,α-dipyridyl which was estimated at 520 nm using a spectrophotometer.
Vitamin C
Estimation of vitamin C was done by the colorimetric method.[13] The ascorbic acid in the samples was oxidized to dehydroascorbic acid which in the acid solution reacts with 2,4-dinitrophenylhydrazine to form a corresponding hydrazine and after treated with sulfuric acid developed an orange red-colored complex which was measured spectrophotometrically at 520 nm. Copper sulfate was then added to enhance the ascorbic acid and to prevent the effect of interfering compound in 2,4-dinitrophenyl hydrazine. Trichloroacetic acid was added to serum and brain tissue homogenate to precipitate the protein content to avoid its interference with the process.
Superoxide dismutase activity
Serum superoxide dismutase (SOD) activity was assayed by the colorimetric method.[14] Potassium trioxochlorate IV and manganese IV oxide reaction was used to generate oxygen and flavin adenine dinucleotide accepts an electron from oxygen produced to form superoxide anion. In the absence of superoxide dismutase, the radical reacts with the detector nitroblue tetrazolium salt. In the event when SOD is present it competes with the detector for superoxide radicals. FAD reduces oxygen to generate superoxide radicals and nitroblue tetrazolium salt scavenges the free radicals.
Brain creatine kinase (CK-BB)
Creatine kinase activity (CK-BB) was estimated by the enzymatic method[15] using a standard enzyme kit supplied by AGAPPE Diagnostics. The procedure involves measurement of CK-activity in the presence of an antibody to the CK-B monomer. This antibody completely inhibits the activity of CK-BB and half of the activity of CK-MB without affecting the M subunit activity of CK-MB and CK-MM. The CK-BB activity is obtained by multiplying the CK-B by 2.
Malondialdehyde (MDA)
The colorimetric method[16] was adopted for the analysis of MDA. Lipid peroxidation generates peroxide intermediates which upon cleavage releases MDA, a product that reacts with thiobarbituric acid (TBA) forming a colored complex, which is measured at 532 nm.
Statistical analysis
Results were expressed as means ± SEM for five rats. All data were analyzed by one-way analysis of variance (ANOVA). Dunnett's test was used for multiple comparisons and values were considered statistically significant at P < 0.05.
Results
Upon TBI insult the animals initially became aggressive, lost control of urination, and then became lethargic and ultimately lost control and fainted which lasted for up to a quarter of an hour. The animals remained lethargic with disheveled hair and shallow and infrequent respiration. In the following days, continuous head scratching and loss of appetite were observed in TBI-untreated rats which indicated a more severe injury. Improved appetite, decreased scratching, increased hair growth, and reversed behavioral changes to normal were observed in supplemented groups. The improvement in rats’ health was found to be dose dependent in which rats treated with combined 60 mg/kg of vitamins C and E however showed a better health condition and shorter fainting time after injury when compared with rats treated with combined 45 mg/kg of vitamins C and E.
Percentage survival of TBI rats treated with α-tocopherol and ascorbic acid is presented in Figure 1. The results indicated that treatment of traumatized rats with vitamins C and E reduced significantly the mortality rates of the rats. Increased doses do not appear to confer any significant advantage with respect to survival and combination of the two vitamins appears to have a synergistic effect.
Figure 1.
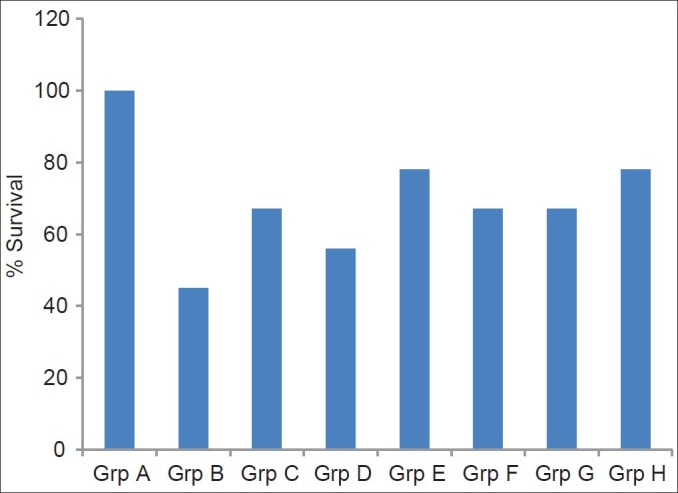
Percentage survival of tramautized brain injury rat treated with α-tocopherol and ascorbic acid
Grp A = Non-TBI-untreated group; Grp B = TBI untreated; Grp C = TBI treated with 45 mg/kg vitamin C; Grp D = TBI treated with 45 mg/kg vitamin E; Grp E = TBI treated with 45 mg/kg each of combined vitamins C and E; Grp F = TBI treated with 60 mg/kg vitamin C; Grp G = TBI treated with 60 mg/kg vitamin E; Grp H = TBI treated with 60 mg/kg each of combined vitamins C and E
Figure 2 showed that serum vitamin C level significantly (P < 0.01) increased in the treatment groups compared with the TBI-untreated group with the exception of the group treated with only vitamin C. Treatment with 45 mg/kg and 60 mg/kg each of combined vitamins C and E respectively significantly (P < 0.05) increases the level of vitamin E when compared with the TBI-untreated group while the remaining groups showed no significant (P > 0.05) difference.
Figure 2.
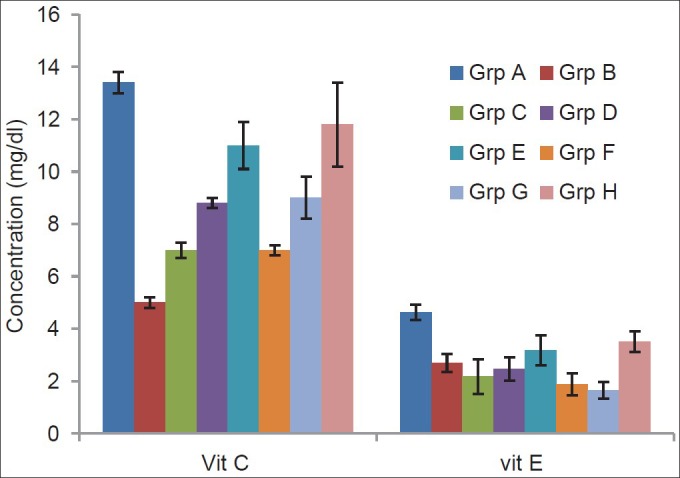
Effects of α-tocopherol and ascorbic acid on serum levels of nonenzymatic antioxidants of traumatized rats
Grp A = Non-TBI-untreated group; Grp B = TBI untreated; Grp C = TBI treated with 45 mg/kg vitamin C; Grp D = TBI treated with 45 mg/kg vitamin E; Grp E = TBI treated with 45 mg/kg each of combined vitamins C and E; Grp F = TBI treated with 60 mg/kg vitamin C; Grp G = TBI treated with 60 mg/kg vitamin E; Grp H = TBI treated with 60 mg/kg each of combined vitamins C and E
The effect of α-tocopherol and ascorbic acid on the brain tissue of traumatized rats is presented in Figure 3. The results indicated that supplementation with combined 45 mg/kg of vitamins C and E significantly (P < 0.01) increased the tissue vitamin C compared with the TBI-untreated group. The groups treated with combined 45 mg/kg and 60 mg/kg each of vitamins C and E showed a significant (P < 0.01) increase in tissue vitamin E compared with the TBI-untreated group while the group treated with 60 mg/kg of vitamin E also showed an increase significantly (P < 0.05).
Figure 3.
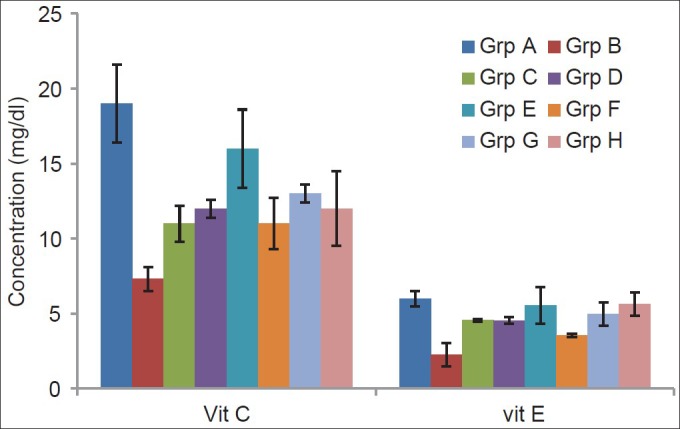
Effects of α-tocopherol and ascorbic acid on brain tissue of nonenzymatic antioxidants of traumatized rats
Grp A = Non-TBI-untreated group; Grp B = TBI untreated; Grp C = TBI treated with 45 mg/kg vitamin C; Grp D = TBI treated with 45 mg/kg vitamin E; Grp E = TBI treated with 45 mg/kg each of combined vitamins C and E; Grp F= TBI treated with 60 mg/kg vitamin C; Grp G = TBI treated with 60 mg/kg vitamin E; Grp H = TBI treated with 60 mg/kg each of combined vitamins C and E
The serum SOD, MDA, and CK-BB of TBI rats are presented in Table 1. The result indicated a significant (P < 0.01) decrease in the activity of SOD of the traumatized untreated group compared with the normal control. Treatment with vitamins significantly (P < 0.01) increased the activities of SOD compared with the traumatized untreated group. The result also indicated a significant (P < 0.01) decrease in the activity of CK-BB in the treatment groups compared with the TBI-untreated group while the group treated with 45 mg/kg of vitamin C showed a significant (P < 0.05) decrease in the activity. There was no significant (P > 0.05) decrease in the activity of the group treated with 60 mg/kg of vitamin C.
Table 1.
Effects of α-tocopherol and ascorbic acid on serum SOD, MDA and CK-BB of traumatized rats
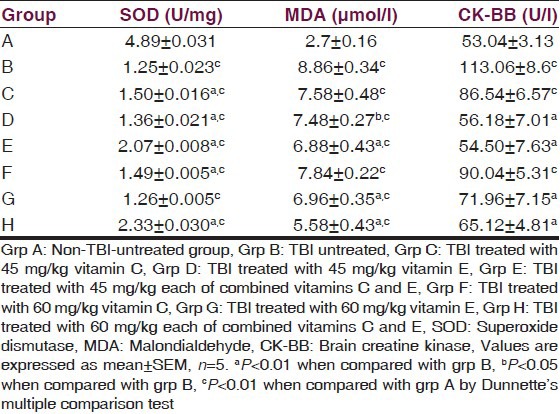
The level of MDA of the TBI-untreated group increased significantly (P < 0.01) compared with the nontraumatized control. Treatment with either 45 mg/kg or 60 mg/kg of vitamin C had no significant (P > 0.05) effect on the level of serum MDA compared with the TBI-untreated control but treatment with 45 mg/kg (P < 0.05) of vitamin E, 60 mg/kg (P < 0.01) of vitamin E and 45 mg/kg and 60 mg/kg (P < 0.01) each of combined vitamins C and E respectively significantly reduced serum MDA.
The effect of α-tocopherol and ascorbic acid on brain tissue homogenate SOD and MDA levels of traumatized rats is presented in Table 2. The result showed that treatment with 60 mg/kg of vitamins C and E combined significantly (P < 0.01) increased the SOD activity in the brain tissue compared with traumatized untreated rats. There was no significant (P > 0.05) increase in the activity of SOD in the remaining treated groups.
Table 2.
Effect of α-tocopherol and ascorbic acid on brain tissue homogenate SOD and MDA level of traumatized rats
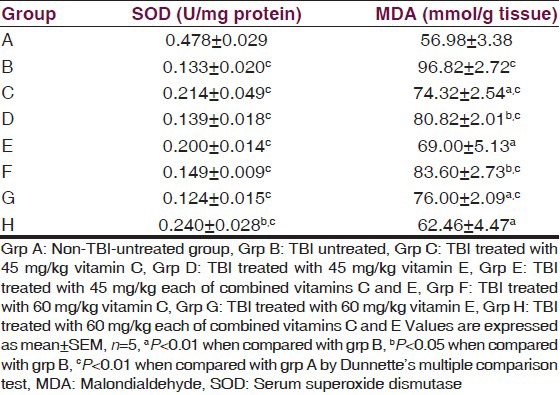
There was a significant (P < 0.05) decrease in the tissue MDA of the treatment groups compared with the TBI-untreated group.
Discussion
Traumatized brain injury is an oxidative related injury associated with biological and metabolic alteration which generates free radicals from various cellular activities. Widespread and bilateral damage of the neurons, axons, dendrites, and microvasculature have been shown to be associated with TBI. According to ref.[17] TBI induces brain edema that begins 20 minutes after injury and peaks at 24 hours after trauma. The current study indicated high mortality (55%) rate in the untreated traumatized group compared with the treatment group while treatment with combined vitamins performed better in the prevention of mortality.
Report has shown that primary injury was not adequate to explain the degeneration, rather the deterioration was caused by a complex set of biochemical cascade that occurred in hours to days following trauma.[18] Pretreatment of animals with α-tocopherol has been reported by Hall et al.[19] to lessen secondary damage in several models of ischemic or traumatic injury to the central nervous system.
Impaired antioxidant defense and increased lipid peroxidation have been reported in traumatic brain injury rats[20] and chronic schizophrenic patients.[21] Free radicals play an important role in the pathogenesis of structural and functional changes of neuronal membrane that could be responsible for the beginning or aggravation of the schizophrenic disease.[22,23]
The significant increase in the levels of serum and brain tissue homogenate of ascorbic acid and vitamin E in the treated rats when compared with TBI-untreated rats indicated that oxidative stress has been implicated in the traumatized brain injury and supplementation with antioxidant vitamins can ameliorate the oxidative stress insult. Vitamin E and ascorbic acid are important chain-breaking antioxidants, responsible for scavenging the free radicals and suppression of peroxidation in the aqueous and lipid region of the cell.[24] Studies have indicated a decrease in lipid peroxidation and an increase in the activity of antioxidant enzymes after the administration of α-tocopherol and selenium during the treatment of brain impairment.[25] Intensive pretreatment of animals with endogenous lipid peroxyl radical scavenger, α-tocopherol, with 21- aminosteroids has been shown to decrease posttraumatic spinal ischemia and enhance chronic neurological recovery.[19] Low levels of serum and brain tissue homogenate of vitamin E in the traumatized untreated control as shown in the current study may facilitate oxidative damage in the traumatized brain injury of rats.
The marked decrease in serum ascorbic acid level after TBI may have several possible implications. First TBI might disrupt the blood brain barrier and decrease the concentration gradient for ascorbate accumulation. Second movement of ascorbate from brain to blood could occur and ascorbate may have been consumed by the free radicals associated with TBI. This is the most likely scenario and one that provides a unifying explanation for the results in the current study.
Lipid peroxidation product, MDA, levels were significantly reduced in serum and brain tissue homogenate of treated rats compared with the TBI-untreated group. Reduction in MDA could be due to decreased generation of reactive oxygen species (ROS), due to its destruction by the antioxidants treatment. The raised serum and brain tissue homogenate of MDA level in TBI-untreated control reflects the oxidative injury which is attributed to free-radical formation that abstracts hydrogen atoms from lipoproteins causing lipid peroxidation.[26,27] Increased levels of thiobarbituric acid reaction products have been found in the serum of patients experiencing craniotomy[28] and also in plasma of schizophrenic patients, with or without tardive dyskinesia.[29]
The results also indicated that vitamin E and vitamin C supplementation was found to increased SOD levels in both serum and brain tissue homogenate compared with the TBI-untreated group. The role of SOD is to protect cells from the toxic effect of superoxide radicals as it catalyzes the dismutation reaction of the radicals.[30] The levels of CK-BB were found to be significantly elevated immediately after the head injury and that the greater the degree of external cranial injury inflicted the higher isoenzyme activity.[31] This seems to provide evidence that CKߝBB activity is an early indicator of brain damage and that its level may reflect the extent of cerebral damage involved.[31]
In the present study perhaps the amount of CK-BB released from the brain damage caused an appreciable rise in the serum level of CK-BB of TBI rats. This indicated that the model was good enough to cause the released of CK-BB into peripheral blood and so this may possibly offer new criteria for early assessment of the severity of brain damage.
Conclusion
The current study provides evidence of the involvement of oxidative stress in the pathogenesis of TBI as manifested in the elevation of CK-BB activity and MDA, and reduction in the serum and brain tissue homogenate of vitamin C and vitamin E. Treatment also significantly improved the serum and brain tissue antioxidant parameters an indication of the effectiveness of these vitamins in reversing the complications of TBI. Treatment does not only reduce oxidative stress in TBI but also effectively reduce the mortality rate and healing time. Thus combined therapy is more effective in protecting and reversing the effects against trauma-induced damage.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Awojebi OA. Surgery in Nigeria Health care Delivery: The ibarra experience. Nigerian Med Practi. 1987;13:49–51. [Google Scholar]
- 2.Thornhill S, Teasdale GM, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: Prospective cohort study. Br Med J. 2000:3201631–5. doi: 10.1136/bmj.320.7250.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearnside MR, Simpson DA. Epidemiology. In: Reilly P, Bullock R, editors. Head Injury. Pathophysiology and management of severe closed injury. London UK: Chapman and Hall Medical; 1997. pp. 3–24. [Google Scholar]
- 4.Graham DI, Gennarelli TA. Graham DI, Lantos PL. Greenfield's neuropathology. London: Arnold; 1997. Trauma; pp. 197–262. [Google Scholar]
- 5.Blumberg's PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–6. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 6.Graham DI, Ford I, Adams JH, Doyle D, Lawrence AE, McLellan DR, et al. Fatal head injury in children. J Clin Pathol. 1989;42:18–22. doi: 10.1136/jcp.42.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin A, Youdim K, Szprengiel A, Shukitt H, Babra J. Role of vitamins E and C on neurodegenerative diseases and cognitive performance. Nut Rev. 2002;60:308–26. doi: 10.1301/002966402320583433. [DOI] [PubMed] [Google Scholar]
- 8.Chaudière J, FerrariIliou R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37:949–62. doi: 10.1016/s0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 9.Bagchi D, Garg A, Krohn RL, Bagchi M, Bagchi DJ, Balmoori J, et al. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA induced hepatic and brain lipid peroxidation, DNA fragmentation and peritoneal macrophage activation in mice. Gen Pharmacol. 1998;30:771–6. doi: 10.1016/s0306-3623(97)00332-7. [DOI] [PubMed] [Google Scholar]
- 10.Heath DL, Vink R. Impact acceleration – induced severe diffuse axonal injury in rats: Characterization of phosphate metabolism and neurologic out come. J Neurotrauma. 1995;12:1027–34. doi: 10.1089/neu.1995.12.1027. [DOI] [PubMed] [Google Scholar]
- 11.Rice ME, Russo MI. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–23. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 12.Baker H, Frank O. Method and interpretation. New York: Inter science Publishers; 1968. Clinical vitaminology; pp. 172–3. [Google Scholar]
- 13.Natelson S. Determination of Ascorbic acid using 2, 4- Dinitrophenyl hydrazine. In: Thomas CC, editor. “Technique of Clinical Chemistry”. 3rd ed. USA: Spring field; 1971. pp. 165–6. [Google Scholar]
- 14.McCord JM, Fridovich I. Superoxide dismutase. An enzyme functions for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 15.Lamprecht W, Stan F, Weisser H, Heinz F. Determination of creatine phosphate and adenosine triphosphate with creatine kinase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1776–8. [Google Scholar]
- 16.Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Foda MA, Marmarou A. A new model of diffuse brain injury in rats part II: Morphological characterization. J Neurosurg. 1994;80:301–13. doi: 10.3171/jns.1994.80.2.0301. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Y, Lee CP, Peterson PL. Mitochondrial dysfunction following traumatic brain injury In Head Trauma. In: Miller LP, Hayes RL, editors. Basic, Preclinical, and Clinical Directions. New York: Co-edited by Newcomb JK John Wiley and Sons Inc; 2001. pp. 257–80. [Google Scholar]
- 19.Hall ED, Yonkers PA, Andrus PK. Biochemistry and pharmacology of lipid peroxidants in acute brain and spinal cord injury. J Neurotrauma. 1992;9:425–42. [PubMed] [Google Scholar]
- 20.IVink R, McIntosh TK, Demediuk P, Fadden AI. Decrease in total and free magnesium concentration following traumatic brain injury in rats. Biochem Biophys Res Commun. 1987;149:594–9. doi: 10.1016/0006-291x(87)90409-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang JY, Wer LL, Hung YM, Chen YT, Ku MC. Dual effects of antioxidant in neurodegeneration. Curr Pharm. 2006;12:3521–33. doi: 10.2174/138161206778343109. [DOI] [PubMed] [Google Scholar]
- 22.Valko M, Leibfritz D, Moncol J, Cronin M, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Reiter RJ. Oxidative process and antioxidative defence mechanisms in aging brain. FASEB J. 1995;9:526–33. [PubMed] [Google Scholar]
- 24.Simon JA. Vitamin C and cardiovascular disease: A review. J Am Coll Nutr. 1992;11:107–25. [PubMed] [Google Scholar]
- 25.Dzhandzhgava TG, Shakarishivilli RR. Activity of antioxidant protective enzymes and content of lipid peroxidation products in blood serum and cerebrospinal fluid in patients with ischemic heart disease. Vopr Med Khim. 1992;38:33–5. [PubMed] [Google Scholar]
- 26.Singal PK, Beamish RE, Dhalla NS. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. Adv Exp Med Biol. 1983;61:391–401. doi: 10.1007/978-1-4684-4472-8_22. [DOI] [PubMed] [Google Scholar]
- 27.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1999;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 28.Jian WN, Yi Nan Meng, Hai F, Xiang Q, Jun Iu WW. Effect of transcutaneous acupoint electrical stimulation on lipid peroxidation and cognitive function in patients experiencing craniotomy. Zhen Ci Yan Ji. 2009;34:52–61. [PubMed] [Google Scholar]
- 29.Kwiatkowski G, Piasecka MZ, Piotrowta W, Nowata D. Increased serum concentrations of conjugated diens and malondialdehyde in patients with pulmonary tuberculosis. Resp Med. 1999;93:272–6. doi: 10.1016/s0954-6111(99)90024-0. [DOI] [PubMed] [Google Scholar]
- 30.Boya P, Pena A, Belogui O, Larrea E, Conchillo M, Gastelruiz Y. Anti oxidant status and glutathione metabolism, in peripheral blood mono nuclear cells from patients with chronic hepatitis C. J Hepatol. 1999;31:809–14. doi: 10.1016/s0168-8278(99)80281-5. [DOI] [PubMed] [Google Scholar]
- 31.Anagnostopoulos DI, Dontas IA, Kotsarelis DV, Julien G, Karayannacos PE, Diakolios CE, et al. Creatine kinase (CK-BB) determination in cerebral spinal fluid after acute experimental head injury. Br J Nerosur. 1988;2:169–72. doi: 10.3109/02688698808992666. [DOI] [PubMed] [Google Scholar]


