Abstract
A 32-year-old with no pre-existing liver disease was diagnosed with Graves' disease at week 4 of pregnancy. Thyroid-stimulating hormone was undetectable with elevated free thyroxine levels and positive thyroid receptor antibodies. She was started on a reducing regime of propylthiouracil (PTU). At week 20 in pregnancy, she became jaundiced. Initial bloods revealed: bilirubin 91 μmol/l, alanine aminotransferase 1,796 IU/l, alkaline phosphatase 200 IU/l, international normalized ratio 1.2, and albumin 33 g/l. A presumptive diagnosis of PTU-induced hepatitis was made. PTU was immediately discontinued and best supportive care instigated. Serum markers for autoimmune and viral hepatitis were negative, abdomen ultrasound, ferritin and caeruloplasmin were normal. Although her alanine aminotransferase began to fall, her bilirubin continued to rise, peaking at 378. Two weeks after PTU cessation she became thyrotoxic and was started on a reducing regime of carbimazole. Her thyroid function stabilized and liver function tests continued to improve with carbimazole stopped at week 32. Growth scans remained normal with delivery of a healthy baby at 38 weeks. This report highlights that good outcomes can be achieved in PTU-induced hepatitis in pregnancy. Patients on PTU should be warned of the potential risk of hepatic failure and advised to seek medical advice immediately if they develop jaundice.
Key Words: Propylthiouracil, Pregnancy, Hepatitis, Carbimazole
What Is Known about This Topic
The prevalence of hyperthyroidism in pregnancy has been estimated to range between 0.1 and 1% [1,2,3,4,5] and if untreated or poorly treated there is an increased risk of adverse outcomes including fetal loss [4,6].
Anti-thyroid drugs are the mainstay of treatment throughout pregnancy [7,8].
Propylthiouracil (PTU) is generally used first line in pregnancy following an increased incidence of scalp defects such as aplasia cutis [9] and a link with fetal choanal atresia and oesophageal atresia [10] in carbimazole and methimazole exposed pregnancies.
The optimal treatment of hyperthyroidism in pregnancy is now being actively debated following recent concerns regarding the risk of severe liver injury with PTU, including liver failure and death.
What This Case Report Adds
To our knowledge, this is the first reported case of PTU-induced hepatitis occurring in the second trimester with a subsequent successful outcome following best supportive care and carbimazole therapy for the inevitable relapse of hyperthyroidism.
It highlights that altering anti-thyroid drugs in PTU-induced hepatitis may be a viable therapeutic strategy if radioiodine and thyroidectomy are contraindicated.
Case Report
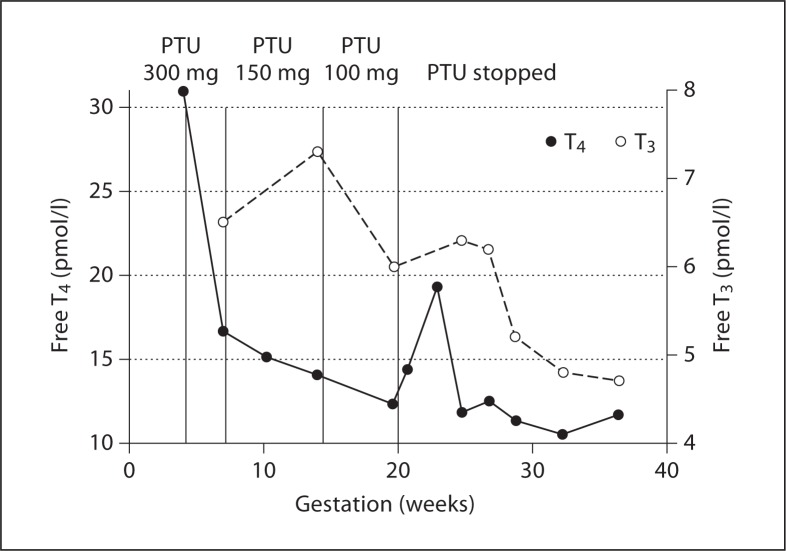
A 32-year-old with no clinical risk factors or evidence of pre-existing liver disease was diagnosed with Graves' disease at week 4 in pregnancy. Her thyroid function tests revealed thyroid-stimulating hormone (TSH) <0.02 mU/l (normal range 0.30–5.50 mU/l) and free thyroxine (free T4) 31.8 pmol/l (normal range 10–22 pmol/l), with positive TSH receptor antibodies of 1.6 U/l (normal range <1 U/l, borderline 1.0–1.4 U/l, >1.4 positive) using the RSR RIA™ assay (FIRS Laboratory, Cardiff, UK) [11] which identifies both stimulating and blocking antibodies. She was started on a reducing regime of propylthiouracil (PTU), initial dose 300 mg and followed up in the joint endocrine antenatal clinic 3 weeks later. Her thyroid function had then come under control and showed a TSH <0.02 mU/l, free T3 7.7 pmol/l (normal range 4.05–6.5 pmol/l) and free T4 16.6 pmol/l (fig. 1). Her PTU was reduced to 150 mg at this point and was reduced to 100 mg at week 14 of pregnancy, with ongoing thyroid function monitoring. No other problems were identified.
Fig. 1.
Overview of thyroid function by week of gestation.
At her 20 week antenatal endocrine appointment she highlighted that the British Thyroid Foundation guidance was that PTU should be used in the first trimester of pregnancy only and that carbimazole (CBZ)/methimazole (MMI) should then be used instead. This strategy is also recommended for consideration by the latest American Thyroid Association guidelines [8] to reduce the risk of PTU-induced hepatitis. She was advised that current practice at the Royal United Hospital was for PTU to be used throughout pregnancy, because of the concerns of increased risks of congenital abnormalities with CBZ such as aplasia cutis [12].
Three days later, she became noticeably jaundiced and contacted the endocrine department for advice. She was seen that day on the medical take by the endocrine registrar and was reviewed that evening by a consultant gastroenterologist. No liver function tests had been performed prior to the development of jaundice and her initial blood tests revealed bilirubin 91 μmol/l (normal 1–17 μmol/l), alanine aminotransferase (ALT) 1,796 IU/l (normal 7–55 IU/l), alkaline phosphatase (ALP) 200 IU/l (normal 35–104 IU/l), albumin 42 g/l (normal 34–48 g/l), prothrombin time 12.4 s (normal 9.5–11.7 s), international normalized ratio 1.2, and a presumptive diagnosis of PTU-induced hepatitis was made. Symptomatic hepatic injury usually develops within the first few months of PTU administration and the clinical course is often relatively benign once the drug is withdrawn [13]. However, rare severe complications do occur and PTU is the third commonest cause of drug-induced liver injury requiring liver transplantation, in the USA, after paracetamol and isoniazid [14]. The development of PTU-induced hepatitis is difficult to predict and no differences have been observed in age, sex, PTU dose, or T4 and T3 levels at initial diagnosis between patients with and without hepatic injury [13]. It has therefore been suggested that all patients should be monitored for rise in liver function tests at regular intervals, especially during the early period [13]. Prompt discontinuation of PTU is vital in PTU-induced hepatitis; 2 cases of PTU-induced hepatitis requiring liver transplantation had key delays in PTU cessation despite clinical and biochemical evidence of liver derangement [15].
The PTU was immediately discontinued and best supportive care instigated. We undertook a full liver screen including viral serology for hepatitis A IgM, B surface antigen, C antibody, E IgM and CMV IgM were negative, with evidence of past EBV exposure (capsid and nuclear antigen antibodies positive, capsid IgM negative, EBV PCR negative). Autoimmune profile screen was negative, with normal IgG level, ferritin and caeruloplasmin. An upper abdomen ultrasound demonstrated normal liver appearance with no evidence of biliary dilatation, ascites or splenomegaly and normal portal blood flow.
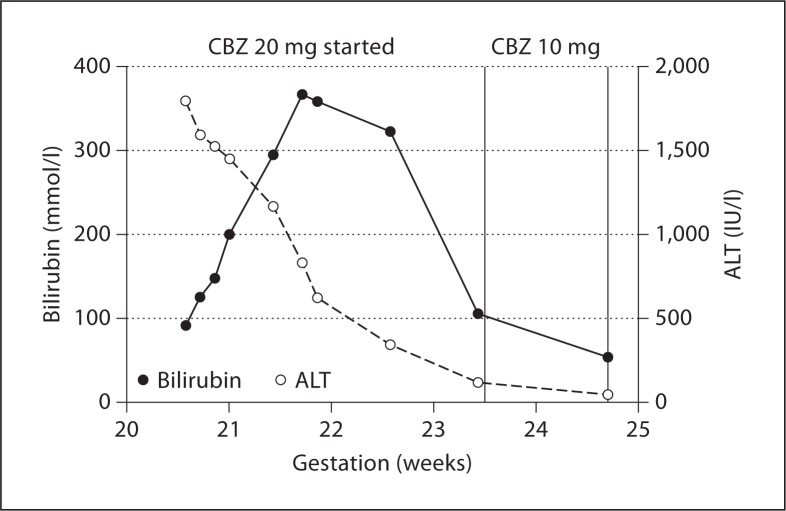
Although her ALT immediately began to fall, her bilirubin continued to rise, peaking 8 days later at 367 mmol/l (fig. 2). She was discussed with the regional transplant centre who agreed with continuation of best supportive care. Two weeks after cessation of PTU, her free T4 had risen to 19.3 pmol/l. As her hyperthyroidism was likely to continue to progress further, its treatment became essential. PTU re-introduction was absolutely contraindicated. Although both radioiodine and prolonged oral iodide therapy had been successfully used to treat hyperthyroidism or stabilize thyroid function to allow thyroidectomy after PTU-induced hepatitis [16], this approach was not possible during pregnancy, although short-term oral iodide can still be used in pregnancy prior to thyroidectomy [17].
Fig. 2.
Bilirubin ALT and ALP by week of gestation.
Thyroidectomy was considered as the optimal time for thyroidectomy is in the second trimester [8], when organogenesis is complete, the fetus is at minimal risk for teratogenic effects of medications, and the uterus is relatively resistant to contraction-stimulating events [7,18]. As well as allergies/contraindications to anti-thyroid drugs (ATDs), thyroidectomy should also be considered in pregnancy in patients requiring large doses of ATDs or patients not compliant with drug therapy [8].
The anaesthetists were however concerned that there would be impaired metabolism of anaesthetic agents due to the patient's deranged liver function. After seeking advice from national experts, the patient was offered the choice of either surgery after a delay of 2–4 weeks to allow the liver function to normalize, or to commence CBZ which could be started earlier, but may exacerbate the hepatitis. The patient declined surgery and instead opted for treatment with CBZ and was started on 20 mg during week 23 of pregnancy. Whilst CBZ/MMI has also been associated with cholestatic and fulminant hepatitis [19], this appears to be a much rarer occurrence than with PTU. Her thyroid function stabilized on a rapid reducing regime of CBZ (reduced to 10 mg during week 24 and 5 mg during week 26 of pregnancy) and her liver function test continued to improve. We were able to stop the CBZ at week 32. Growth scans throughout pregnancy remained in normal limits and a successful spontaneous delivery of a healthy baby was achieved weighing 3.86 kg with a normal Apgar score of 9 at 1 and 5 min.
Overall, patients with significant PTU hepatotoxicity present unique challenges in patient management [16] and our case was further complicated by its occurrence in the second trimester of pregnancy. To our knowledge, this is the first reported case of its occurrence in the second trimester with a subsequent successful outcome following best supportive care and CBZ therapy for the inevitable relapse of hyperthyroidism. Whilst this situation may have been potentially avoided if the new Endocrine Society recommendations had been used [7] with PTU converted to CBZ/MMI at the end of the first trimester, PTU-induced hepatitis may have occurred even within this reduced time exposure. Furthermore, many practitioners use PTU in women considering pregnancy resulting in longer exposure to PTU and increased risk of PTU-induced hepatitis during pregnancy.
This report has highlighted that good outcomes can be achieved with early and immediate cessation of PTU, best supportive care and subsequent treatment of thyrotoxicosis with CBZ. Patients started on PTU should be warned of the small but potentially serious risk of hepatic failure and advised to seek medical advice immediately should they develop jaundice and stop taking PTU. Monitoring of liver function tests in patients on PTU in the early stages of pregnancy may also be of value, although this may create unnecessary anxiety and create difficult decisions regarding alteration of ATD medication in the first trimester.
Acknowledgements
The authors acknowledge the support of Prof. Colin Dayan (Centre for Endocrine and Diabetes Science, Cardiff University School of Medicine, Cardiff, UK) and Prof. Tony Weetman (Faculty of Medicine, Dentistry and Health, University of Sheffield, Sheffield, UK).
References
- 1.Becks GP, Burrow GN. Thyroid disease and pregnancy. Med Clin North Am. 1991;75:121–150. doi: 10.1016/s0025-7125(16)30475-8. [DOI] [PubMed] [Google Scholar]
- 2.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.LeBeau SO, Mandel SJ. Thyroid disorders during pregnancy. Endocrinol Metab Clin North Am. 2006;35:117–136. doi: 10.1016/j.ecl.2005.09.009. vii. [DOI] [PubMed] [Google Scholar]
- 4.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus JH. Thyroid function in pregnancy. Br Med Bull. 2011;97:137–148. doi: 10.1093/bmb/ldq039. [DOI] [PubMed] [Google Scholar]
- 6.Millar LK, Wing DA, Leung AS, Koonings PP, Montoro MN, Mestman JH. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet Gynecol. 1994;6:946–949. [PubMed] [Google Scholar]
- 7.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 8.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milham S, Elledge W. Maternal methimazole and congenital defects in children. Teratology. 1972;5:125. [Google Scholar]
- 10.Di Gianantonio E, Schaefer C, Mastroiacovo PP, Cournot MP, Benedicenti F, Reuvers M, Occupati B, Robert E, Bellemin B, Addis A, et al. Adverse effects of prenatal methimazole exposure. Teratology. 2001;64:262–266. doi: 10.1002/tera.1072. [DOI] [PubMed] [Google Scholar]
- 11.Smith BR, Bolton J, Young S, Collyer A, Weeden A, Bradbury J, Weightman D, Perros P, Sanders J, Furmaniak J. A new assay for thyrotropin receptor autoantibodies. Thyroid. 2004;14:830–835. doi: 10.1089/thy.2004.14.830. [DOI] [PubMed] [Google Scholar]
- 12.Mandel SJ, Brent GA, Reed Larsen P. Review of antithyroid drug use during pregnancy and report of a case of aplasia cutis. Thyroid. 1994;4:129–133. doi: 10.1089/thy.1994.4.129. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Kim BH, Han YS, Yang I, Kim KJ, Dong SH, Kim HJ, Chang YW, Lee JI, Chang R. The incidence and clinical characteristics of symptomatic propylthiouracil-induced hepatic injury in patients with hyperthyroidism: a single-center retrospective study. Am J Gastroenterol. 2001;96:165–169. doi: 10.1111/j.1572-0241.2001.03469.x. [DOI] [PubMed] [Google Scholar]
- 14.Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transplant. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 15.Carrion AF, Czul F, Arosemena LR, Selvaggi G, Garcia MT, Tekin A, Tzakis AG, Martin P, Ghanta RK. Propylthiouracil-induced acute liver failure: role of liver transplantation. Int J Endocrinol. 2010;2010:910636. doi: 10.1155/2010/910636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams KV, Nayak S, Becker D, Reyes J, Burmeister LA. Fifty years of experience with propylthiouracil-associated hepatotoxicity: what have we learned? J Clin Endocrinol Metab. 1997;82:1727–1733. doi: 10.1210/jcem.82.6.4011. [DOI] [PubMed] [Google Scholar]
- 17.Momotani N, Hisaoka T, Noh J, Ishikawa N, Ito K. Effects of iodine on thyroid status of fetus versus mother in treatment of Graves' disease complicated by pregnancy. J Clin Endocrinol Metab. 1992;75:738–744. doi: 10.1210/jcem.75.3.1517362. [DOI] [PubMed] [Google Scholar]
- 18.Levinson G. Anesthesia for surgery during pregnancy. In: Hughes SC, Levinson G, Rosen MA, editors. Shnider and Levinson's Anesthesia for Obstetrics. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 249–265. [Google Scholar]
- 19.Epeirier JM, Pageaux GP, Coste V, Perrigault PF, Banc P, Larrey D, Michel H. Fulminant hepatitis after carbimazole and propranolol administration. Eur J Gastroenterol Hepatol. 1996;8:287–288. doi: 10.1097/00042737-199603000-00018. [DOI] [PubMed] [Google Scholar]