Abstract
Background
Alcohol consumption has been identified as a protective factor for some autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus.
Objective
We hypothesized that alcohol consumption would reduce the risk of developing autoimmune thyroid disease (AITD).
Study design
Two nested case-control studies in the prospective Amsterdam AITD cohort. Follow-up was 5 years, with annual assessments. In study A, we compared alcohol consumption between cases (subjects who during follow-up remained euthyroid but developed thyroid peroxidase antibodies (TPO-Ab), called event) and controls (subjects who remained euthyroid and TPO-Ab-negative). In study B, we compared alcohol consumption between cases (subjects who during follow-up developed overt hypothyroidism, called event) and controls (subjects who did not develop overt hypothyroidism). For each case, 2 controls were selected, matched for age, duration of follow-up and smoking behavior at baseline and at the time of event.
Results
In study A, alcohol consumption did not differ between cases and controls at any time point. In study B, the number of subjects consuming >10 units of alcohol per week was not different between cases and controls at study entrance (8.3 vs. 14.5%, NS), but lower at 1 year before (5.3 vs. 19.7%, p = 0.041) and at the time of event (6.7 vs. 23.7%, p = 0.044); respective odds ratios are 0.54 (0.14–2.06), 0.23 (0.05–1.04) and 0.23 (0.05–1.06).
Conclusion
Alcohol consumption is not associated with de novo development of TPO-Ab, but is lower in subjects who developed overt hypothyroidism. The data suggest alcohol consumption may protect against overt autoimmune hypothyroidism.
Key Words: Autoimmune thyroid disease, Alcohol, Hypothyroidism, Thyroid peroxidase antibodies
Introduction
Autoimmune thyroid disease (AITD) is a multifactorial condition and genetic factors as well as environmental factors are thought to play a role in its pathogenesis. Twin studies suggest that genetic factors account for about 70% of the risk to get AITD [1,2,3]. The remaining 30% is likely due to environmental factors which may provoke autoimmune reactions. Iodine intake, stress, smoking, drugs (e.g. estrogens), pregnancy and infections have all been investigated as putative determinants of AITD.
Recent studies suggest a protective effect of alcohol on the development of rheumatoid arthritis and systemic lupus erythematosus [4,5,6]. Available literature data indeed support the possibility that alcohol is a modulator of the immune system [7].
In the present study we hypothesized that alcohol consumption would reduce the risk of developing AITD. We examined the effect of alcohol on both early and late stages of AITD. The occurrence of thyroid peroxidase antibodies (TPO-Ab) in serum represents an early stage of AITD, and the occurrence of overt hypothyroidism may represent the late final stage of AITD [8]. Thus we performed two nested case-control studies in the prospective Amsterdam AITD cohort. In study A, we compared alcohol consumption in euthyroid subjects who did or did not develop TPO-Ab. In study B, we compared alcohol consumption in subjects who did or did not progress to overt hypothyroidism.
Subjects and Methods
Participants
The present study was carried out among the 803 subjects from the Amsterdam AITD cohort. The cohort has previously been described in detail [9]. In short, the cohort consisted of women between 18 and 65 years of age in self-proclaimed good health without a history of thyroid disease, who had at least one first- or second-degree relative with documented autoimmune hyper- or hypothyroidism. Subjects were followed for 5 years, or shorter when overt hyper- or hypothyroidism had occurred (defined as TSH <0.4 mU/l in combination with fT4 >20.1 pmol/l, or TSH >5.7 mU/l in combination with fT4 <9.3 pmol/l, respectively). Results of thyroid function tests at study entrance revealed overt hypothyroidism in 10 subjects and overt hyperthyroidism in 3 subjects, leaving 790 subjects to be included in the present study.
At each annual visit, blood samples were collected to measure TSH, fT4, TPO-Ab, thyroglobulin antibodies (Tg-Ab) and TSH-binding inhibitory immunoglobulins (TBII), and subjects were asked to fill in questionnaires on alcohol consumption (number of alcoholic drinks per week) and smoking habits (current smoking defined as smoking now or stopped smoking in the last year). All subjects gave informed written consent and the medical ethics committee of the Academic Medical Center in Amsterdam approved the study.
We performed two nested case-control studies (A and B).
Study A: Alcohol Consumption and de novo Development of TPO-Ab
In order to evaluate the relationship between alcohol consumption and the de novo occurrence of TPO-Ab, we selected participants from the inception cohort of the 790 euthyroid subjects, excluding those women who had thyroid antibodies at baseline (i.e. serum concentrations of either TPO-Ab ≥100 kU/l, Tg-Ab ≥100 kU/l or TBII ≥12 U/l), those who had subclinical hyper- or hypothyroidism at baseline, and those who had no follow-up. Consequently, 521 euthyroid participants without any serological sign of AITD at baseline were enrolled. A subject was recruited as a case when she had remained euthyroid but had developed TPO-Ab during follow-up. The end-point for a case was the time at which she had become positive for the first time for TPO-Ab without developing abnormal TSH (called event). This happened in 81 subjects.
Subjects from the Amsterdam AITD cohort qualified to act as controls if they remained euthyroid and seronegative for TPO-Ab up to the time at which the case they were matched to had received her end-point.
Study B: Alcohol Consumption and Development of Overt Hypothyroidism
In order to evaluate the relationship between alcohol consumption and the development of overt hypothyroidism, we designed a nested case-control study in the inception cohort as follows. A subject was recruited as a case when she had developed overt hypothyroidism during follow-up (called event). The end-point for a case was the time at which she had developed overt hypothyroidism. Subjects qualified as controls if they had not progressed to overt hypo- or hyperthyroidism up to the time at which the case they were matched to had received her end-point.
In both studies A and B for each case, 2 controls were selected. A subject could only be sampled once as control. Controls for both studies were matched for age, duration of follow-up and smoking status at baseline and at the time of event. Alcohol consumption at baseline, 1 year before the occurrence of the event and at the time of the event was compared between cases and controls.
Laboratory Measurements
Serum TSH and fT4 were measured using time-resolved fluoroimmunoassay (Delphia, Turku, Finland). Reference values are for TSH 0.4–5.7 mU/l and for fT4 9.3–20.1 pmol/l. Thyroid peroxidase (TPO) antibodies and thyroglobulin (Tg) antibodies were measured by chemiluminescence immunoassays (LumiTest anti-TPO and LumiTest anti-Tg, respectively; Brahms GmbH, Berlin, Germany). Improved versions of both assays became available during follow-up: detection limits of these new assays were for TPO-Ab 30 kU/l and for Tg-Ab 20 kU/l. TPO-Ab concentrations obtained with the old assay were multiplied by a factor 0.72 to obtain comparative values in the new assay. TPO-Ab and Tg-Ab concentrations were considered to be positive at values ≥100 kU/l. TSH receptor antibodies were determined as TBII using the TRAK assay (Brahms GmbH), detection limits in the first- and second-generation TRAK assays were 5 and 1 IU/l, respectively, and values >12 and 1.5 U/l, respectively, were considered as positive.
Statistical Analysis
Normally distributed data are presented as mean ± SD and group differences were analyzed by Student's t test. Data that are not distributed normally are expressed as median and 25th and 75th percentiles, and were analyzed by Mann-Whitney U test. Categorical data are expressed as percentages. The significance of differences between groups was analyzed with the χ2 test or with Fisher's exact test in the case of small numbers. Statistical significance was set at 5%.
Results
Study A: Alcohol Consumption and de novo Development of TPO-Ab
During the 5-year follow-up period, 81 of the 521 subjects (15.5%) had developed TPO-Ab under maintenance of a normal TSH, and they were considered cases. 162 matched controls could be selected. Cases and controls did not differ in age (at baseline 36 ± 12 and 36 ± 12 years, respectively), or in duration of follow-up (2.8 ± 1.3 and 2.8 ± 1.3 years, respectively). The same was true for the proportion of current smokers. Thyroid function did not change during follow-up, neither in cases nor in controls. At the time of seroconversion the TPO-Ab concentration had a median value of 140 kU/l (interquartile range 110–160 kU/l) (table 1).
Table 1.
Comparison of characteristics between cases and controls (matched for age, duration of follow-up and smoking habits) at baseline, 1 year prior to event and at the time of event in study A (de novo occurrence of TPO-Ab) and in study B (development of overt hypothyroidism)
| Study A: development of TPO-Ab, 81 cases and 162 controls |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline |
1 year before event |
at event |
p value |
|||||||||||||||
| cases | controls | cases | controls | cases | controls | baseline | 1 year before event | at event | ||||||||||
| TSH, mU/1 | 1.5 (1.2–2.2) | 1.5 (1.1–1.9) | 1.4 (1.1–2.2) | 1.4 (1.0–1.9) | 1.5 (1.1–2.2) | 1.4 (1.0–2.0) | 0.88 | 0.69 | 0.44 | |||||||||
| fT4, pmol/1 | 13.1 (11.6–14.5) | 13.4 (12.0–14.9) | 13.0 (11.7–14.4) | 13.6 (12.1–14.8) | 13.1 (11.9–14.5) | 13.3 (12.0–14.3) | 0.94 | 0.71 | 0.27 | |||||||||
| TPO-Ab, kU/1 | <25 (<25–<25) | <25 (<25–<25) | <25 (<25–50) | <25 (<25–50) | 140 (110–160) | <25 (<25–50) | 0.48 | 0.45 | <0.001 | |||||||||
| Tg-Ab, kU/1 | 15 (<10–25) | <10 (<10–15) | 18 (<10–35) | <10 (<10–14) | 17 (<10–43) | <10 (<10–20) | 0.19 | 0.01 | 0.01 | |||||||||
| Current smokers | 38% | 38% | 31% | 30% | 31% | 31% | NS | NS | NS | |||||||||
| Alcohol consumers* | 14.5% | 13.4% | 14.1% | 15.1% | 11.3% | 13.7% | 0.82 | 0.84 | 0.60 | |||||||||
| Study B: development of overt hypothyroidism, 38 cases and 76 controls |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline |
1 year before event |
at event |
p value |
|||||||||||||||
| cases | controls | cases | controls | cases | controls | baseline | 1 year before event | at event | ||||||||||
| TSH, mU/1 | 4.2 (2.7–5.7) | 1.5 (1.1–2.5) | 4.0 (2.6–6.1) | 1.5 (0.9–2.4) | 14.8 (6.3–16.9) | 1.5 (1.0–2.4) | 0.06 | 0.03 | 0.005 | |||||||||
| fT4, pmol/1 | 10.7 (9.7–11.6) | 13.0 (11.3–15.1) | 10.6 (9.5–12.0) | 13.4 (11.9–14.3) | 7.1 (4.8–8.4) | 13.0 (11.5–14.5) | 0.38 | 0.17 | 0.02 | |||||||||
| TPO-Ab, kU/1 | 1,123 (304–2,239) | <25 (<25–150) | 1,076 (666–2,326) | 50 (<25–320) | 3,000 (1,620–6,670) | <25 (<25–180) | 0.004 | 0.008 | <0.001 | |||||||||
| Tg-Ab, kU/1 | 75 (40–238) | 15 (<5–40) | 123 (42–235) | 15 (<10–40) | 140 (100–500) | 10 (<10–50) | 0.03 | 0.02 | 0.001 | |||||||||
| Current smokers | 18% | 18% | 18% | 18% | 13% | 13% | NS | NS | NS | |||||||||
| Alcohol consumers* | 8.3% | 14.5% | 5.3% | 19.7% | 6.7% | 23.7% | 0.36 | 0.04 | 0.04 | |||||||||
>10 units/week. Data given as median (interquartile range).
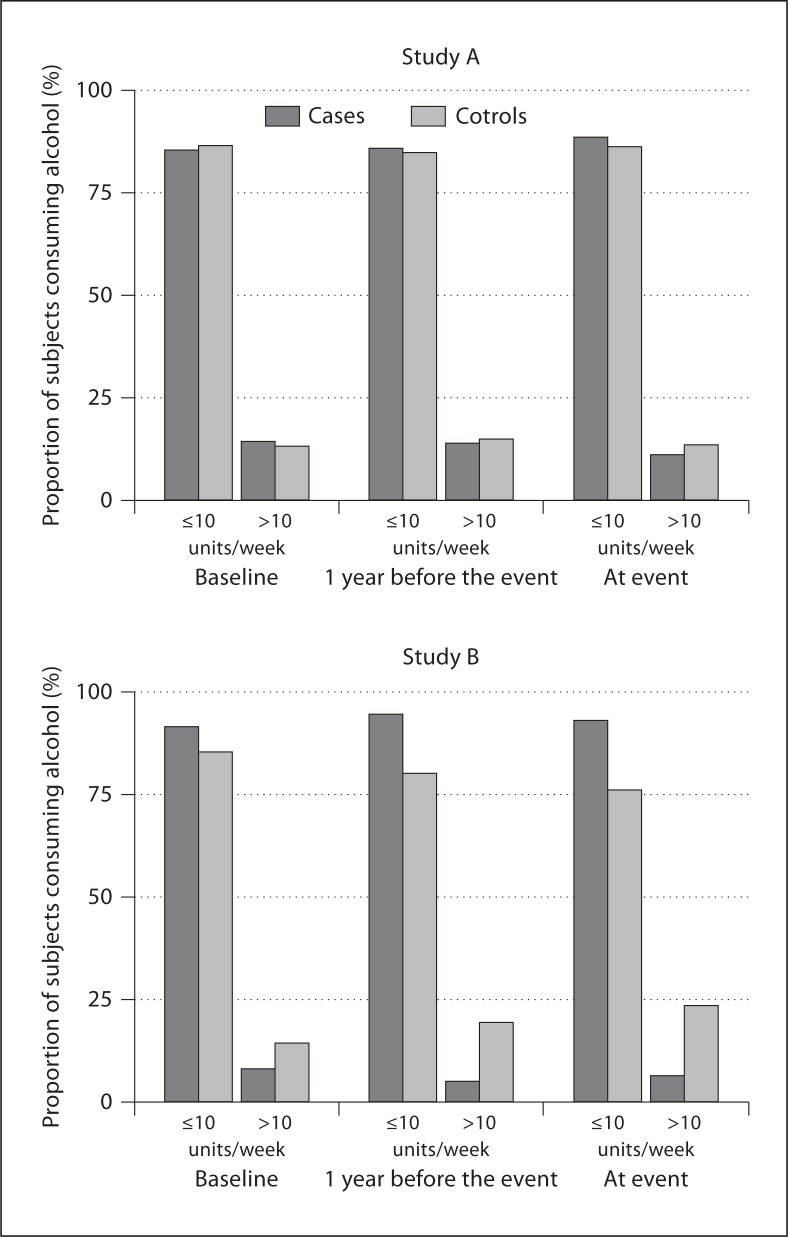
At study entrance, the frequency of consuming >10 units of alcohol per week among cases was comparable to that in controls (14.5 vs. 13.4%, NS). The same was true at 1 year before seroconversion (14.1 vs. 15.1%, NS) and at the time of seroconversion (11.3 vs. 13.7%, NS) (fig. 1, study A).
Fig. 1.
Frequency of alcohol consumption in cases (dark gray bars) and in controls (matched for age, duration of follow-up and smoking behavior – light gray bars) at baseline, 1 year prior to event and at the time of event in study A (de novo development of TPO-Ab) and in study B (development of overt hypothyroidism).
Study B: Alcohol Consumption and Development of Overt Hypothyroidism
During the 5-year follow-up period, 38 cases of overt autoimmune hypothyroidism occurred, as reported elsewhere [10]. 76 matched controls could be selected in whom overt hypo- or hyperthyroidism had not occurred. Cases and controls did not differ with regard to mean age (at baseline 38 ± 12 and 38 ± 12 years, respectively), mean follow-up (3.2 ± 1.3 and 3.2 ± 1.2 years, respectively) and the proportion of current smoking at baseline, 1 year before the event and at the time of event. Cases had already higher serum TSH and lower serum fT4 concentrations than controls at baseline (table 1).
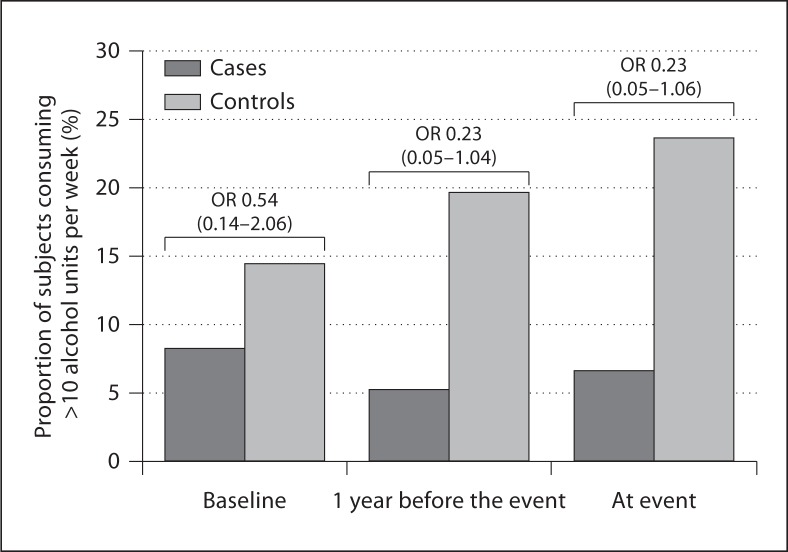
At study entrance, the frequency of consumers of >10 units of alcohol per week among cases was not significantly different from that in controls (8.3 vs. 14.5%, NS). At 1 year before the occurrence of overt hypothyroidism, there were less consumers of >10 units of alcohol per week in cases than in controls (5.3 vs. 19.7%, p = 0.041), and this was still true at the time of overt hypothyroidism (6.7 vs. 23.7%, p = 0.044) (fig. 1, study B). The odds of consuming >10 units of alcohol per week for developing overt hypothyroidism were 0.54 (95% CI 0.14–2.06) at baseline, 0.23 (95% CI 0.05–1.04) 1 year before the event, and 0.23 (95% CI 0.05–1.06) at the event (fig. 2).
Fig. 2.
Proportion of subjects consuming >10 units/week in cases (dark gray bars) and in controls (matched for age, duration of follow-up and smoking behavior – light gray bars) at baseline, 1 year prior to event and at the time of event in study B (development of overt hypothyroidism). OR denotes odds ratio and the 95% confidence intervals are in parentheses.
Discussion
The aim of the present case-control studies nested in the observational Amsterdam AITD cohort study was to evaluate in a prospective manner the involvement of alcohol in both the early stages (when thyroid antibodies develop but thyroid function is still normal) and late stages (when overt thyroid dysfunction emerges) of the natural course of AITD. We find that alcohol consumption is not associated with the de novo occurrence of TPO-Ab. However, we observed that alcohol consumption decreases the risk of the development of overt hypothyroidism in subjects susceptible for developing AITD. At study entrance, alcohol consumption did not differ between those who subsequently developed overt hypothyroidism and those who did not; the effect of alcohol consumption became obvious during follow-up, although the odds ratio at baseline was already 0.54.
The present study is the first to evaluate the relationship between alcohol consumption and thyroid autoimmunity in a prospective manner. Its prospective nature guarantees solid evidence. Cases and controls were matched for age, duration of follow-up and smoking behavior as higher age and longer exposure time increase while smoking decreases the likelihood of developing autoimmune hypothyroidism and thyroid antibodies [11,12,13]. The perfect matching procedure between cases and controls effectively exclude the potential biases and enhance the validity of our results. A weakness of our study is the limited number of subjects who converted from euthyroidism to overt hypothyroidism (n = 38), but even with this small sample size we find evidence that alcohol consumption exhibits an inverse association with overt autoimmune hypothyroidism. However, the limited sample size may have precluded to find a dose-response relationship in our study. We did not observe differences between cases and controls when we categorized alcohol consumption into three groups: non-drinkers, low consumption (>0 but ≤10 units/week) and high consumption (>10 units/week).
The external validity of the present study is limited because we studied only women who had a family history of AITD. Our findings are in an agreement with a recent case-control study from Denmark [14] in which evidence that alcohol consumption is protective for the development of autoimmune hypothyroidism was also obtained. Cases were 140 patients with incident autoimmune overt hypothyroidism identified in a Danish population-based study and they were matched for age and sex with 560 controls recruited from the same population who had normal thyroid function and no history of thyroid disease. The odds ratio for developing hypothyroidism for alcohol consumers of 1–10 units/week was 0.58 (95% CI 0.35–0.96) and for alcohol consumers of >11 units/week was 0.40 (95% CI 0.21–0.78) while non-drinkers (abstainers) were used as the reference group. The observed odds ratios did not change after multivariate adjustment for smoking habits and family history of hypothyroidism (0.59 (95% CI 0.35–0.99) and 0.41 (95% CI 0.21–0.79), respectively). Our data on a protective effect of alcohol on autoimmune hypothyroidism are reminiscent of the protective effect of alcohol on the development of systemic lupus erythematosus as found in a meta-analysis [6]. Alcohol also protects against rheumatoid arthritis: non-drinkers have an odds ratio of 4.17 compared with subjects consuming alcohol >10 days/month [5], and in another study the odds ratio is 0.5–0.6 for subjects in the quartile with the highest alcohol consumption compared with the lowest quartile [4].
The mechanism behind the effect of alcohol on thyroid autoimmunity is not clear. Although experimental and clinical data suggest that alcohol is a potential modulator of the immune system, our findings do not suggest any association between alcohol and the immune response at early stages of thyroid autoimmunity. One may thus ask the question if our findings regarding the development of overt hypothyroidism are due to a direct effect of alcohol on the thyroid gland. Studies in the past have reported decreased serum thyroid hormones, normal TSH and a blunted TSH response to TRH among alcohol-dependent patients [15]. A direct and irreversible toxic effect of alcohol on the thyroid gland has been proposed as explanation for the reduction of serum thyroid hormones. A direct toxic effect could also explain that alcohol protects against the development of goiter, as evident from a significant decrease of thyroid volume observed in alcohol-dependent patients [16,17]. However, in our study, alcohol consumption protected against thyroid hormone deficiency, thereby rendering a direct toxic effect of alcohol on the thyroid gland in our study highly unlikely.
Recently, we [12] and others [13] have reported that smoking has a protective effect on the development of thyroid antibodies and also on the development of Hashimoto's hypothyroidism. The mechanisms behind the protective effect of smoking on AITD are also not well understood so far. Because both smoking and alcohol seem to protect against autoimmune hypothyroidism, it may be hypothesized that smoking and alcohol act via the same immune pathways; however, this seems to be less likely if one realizes that the association between alcohol consumption and overt hypothyroidism in our study was independent of smoking. An alternative hypothesis could be based on genetics. Twin studies [18,19,20,21,22] and studies on selected strains of laboratory animals [23] suggest a strong genetic influence on the liability to both alcohol and nicotine dependence. From that perspective it can be hypothesized that genetic factors which predispose to addiction behavior are also involved in the pathogenesis of thyroid autoimmunity.
In conclusion, alcohol consumption of >10 units/week may protect against the development of overt hypothyroidism, independent of smoking. The mechanism behind this phenomenon remains to be clarified.
References
- 1.Brix TH, Kyvik KO, Hegedüs L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85:536–539. doi: 10.1210/jcem.85.2.6385. [DOI] [PubMed] [Google Scholar]
- 2.Brix TH, Kyvik KO, Christensen K, Hegedüs L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86:930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 3.Ringold DA, Nicoloff JT, Kesler M, Davis H, Hamilton A, Mack T. Further evidence for a strong genetic influence on the development of autoimmune thyroid disease: the California twin study. Thyroid. 2002;12:647–653. doi: 10.1089/105072502760258613. [DOI] [PubMed] [Google Scholar]
- 4.Kallberg H, Jacobsen S, Bengtsson C, Pedersen M, Padyukov L, Garred P, et al. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: results from two Scandinavian case-control studies. Ann Rheum Dis. 2009;68:222–227. doi: 10.1136/ard.2007.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell JR, Gowers IR, Moore DJ, Wilson AG. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology. 2010;49:2140–2146. doi: 10.1093/rheumatology/keq202. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Pan H-F, Ye D-Q, Su H, Li X-P. Moderate alcohol drinking might be protective for systemic lupus erythematosus: a systematic review and meta-analysis. Clin Rheumatol. 2008;27:1557–1563. doi: 10.1007/s10067-008-1004-z. [DOI] [PubMed] [Google Scholar]
- 7.Romeo J, Wärnberg J, Nova E, Díaz LE, Gómez-Martinez S, Marcos A. Moderate alcohol consumption and the immune system: a review. Br J Nutr. 2007;98(suppl 1):S111–S115. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- 8.Effraimidis G, Strieder TGA, Tijssen JGP, Wiersinga WM. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur J Endocrinol. 2011;164:107–113. doi: 10.1530/EJE-10-0785. [DOI] [PubMed] [Google Scholar]
- 9.Strieder TGA, Prummel MF, Tijssen JGP, Endert E, Wiersinga WM. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol. 2003;59:396–401. doi: 10.1046/j.1365-2265.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 10.Strieder TGA, Tijssen JGP, Wenzel BE, Endert E, Wiersinga WM. Prediction of progression to overt hypothyroidism or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Intern Med. 2008;168:1657–1663. doi: 10.1001/archinte.168.15.1657. [DOI] [PubMed] [Google Scholar]
- 11.Hollowell J, Staehling N, Flanders W. Serum TSH, T4, and thyroid antibodies in the United States population (1988–1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 12.Effraimidis G, Tijssen JGP, Wiersinga WM. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab. 2009;94:1324–1328. doi: 10.1210/jc.2008-1548. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen IB, Laurberg P, Knudsen N, Jørgensen T, Perrild H, Ovesen L, et al. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol. 2008;158:367–373. doi: 10.1530/EJE-07-0595. [DOI] [PubMed] [Google Scholar]
- 14.Carle A, Pedersen I, Knudsen N, Perrild H, Ovesen L, Rasmussen L, et al. Alcohol consumption is protective for development of autoimmune hypothyroidism – a population-based study. Eur Thyroid J 2011(Sept 1):82–83.
- 15.Hermann D, Heinz A, Mann K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction. 2002;97:1369–1381. doi: 10.1046/j.1360-0443.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 16.Hegedüs L. Decreased thyroid gland volume in alcoholic cirrhosis of the liver. J Clin Endocrinol Metab. 1984;58:930–933. doi: 10.1210/jcem-58-5-930. [DOI] [PubMed] [Google Scholar]
- 17.Hegedüs L, Rasmussen N, Ravn V, Kastrup J, Krogsgaard K, Aldershvile J. Independent effects of liver disease and chronic alcoholism on thyroid function and size: the possibility of a toxic effect of alcohol on the thyroid gland. Metabolism. 1988;37:229–233. doi: 10.1016/0026-0495(88)90100-x. [DOI] [PubMed] [Google Scholar]
- 18.Carmelli D, Swan GE, Robinette D. The relationship between quitting smoking and changes in drinking in World War II veteran twins. J Subst Abuse. 1993;5:103–116. doi: 10.1016/0899-3289(93)90055-g. [DOI] [PubMed] [Google Scholar]
- 19.Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep. 2001;3:144–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- 20.Swan GE, Carmelli D, Cardon LR. Heavy consumption of cigarettes, alcohol and coffee in male twins. J Stud Alcohol. 1997;58:182–190. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- 21.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 22.Bierut LJ, Rice JP, Goate A, Hinrichs AL, Saccone NL, Foroud T, et al. A genomic scan for habitual smoking in families of alcoholics: common and specific genetic factors in substance dependence. Am J Med Genet. 2003;124A:19–27. doi: 10.1002/ajmg.a.20329. [DOI] [PubMed] [Google Scholar]
- 23.Le AD. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]