Abstract
We aimed to investigate the diagnostic accuracy of ultrasound (US)-guided fine-needle aspirates (FNAs) obtained from 854 consecutive Danish patients with a scintigraphically cold thyroid nodule in a borderline iodine-deficient area. Clinical, sonographic, and pathological findings in patients with a cold thyroid nodule undergoing US-guided FNA were prospectively registered. 408 patients underwent thyroid surgery, resulting in 50 cancers and in addition 37 patients had an incidental finding of papillary thyroid microcarcinomas. Based on the diagnostic FNA, we found sensitivity and specificity for malignancy of 73.9 and 99.2%, respectively. The positive and negative predictive values of a diagnostic FNA for malignancy were 89.5 and 97.7%. We identified 6 false-negative and 2 false-positive diagnoses. Solid versus cystic feature of the nodule, as well as >2 high-risk US features, were predictive for malignancy. Cancer incidence was 13% among females and 9% among males. The accuracy of a diagnostic set-up based on clinical examination, scintigraphy, US, and US-guided FNA was determined with a 48% rate of histopathological validation in the cohort. The overall thyroid cancer incidence has increased worldwide, but our results suggest that the most frequent occurring cancer is an incidental papillary thyroid microcarcinoma of which the clinical significance has yet to be established.
Key Words: Cold thyroid nodules, Fine-needle aspiration, Ultrasound, Papillary thyroid microcarcinoma, Borderline iodine-deficient area
Introduction
Palpable thyroid nodules are frequent with a prevalence of 4–7% in the Western population [1]. The majority of the nodules are benign and only around 5% are malignant. Given this, great efforts are being undertaken to aim at a cost-effective assessment of thyroid nodules to reduce superfluous surgery; however, preoperative assessment of thyroid nodules is still a diagnostic challenge.
Recent guidelines recommend that a thyroid ultrasound (US) should be performed in all patients with known or suspected nodular thyroid disease and a US-guided fine-needle aspirate (FNA) is considered the gold standard when evaluating cold (without uptake on a technetium-99m scintigraphy) thyroid nodules [1]. In a borderline iodine-deficient area, European guidelines recommend a thyroid scintigraphy when one or more nodules are present, regardless of a normal or suppressed TSH level [1]. FNA is not recommended if TSH is suppressed or the nodule has iodine uptake [1,2]. In cold thyroid nodules, FNA is the single best test for preoperative selection, but the issues of non-diagnostic and suspicious cytopathological findings remain to be solved. Other studies have evaluated the accuracy of FNA and conclude that sensitivity and specificity for malignancies are above 90% if they exclude the non-diagnostic and the suspicious cytological results [3,4,5,6]. One attempt to separate the suspicious FNA in benign and malignant nodules is to add additional diagnostic features to the preoperative evaluation by the use of US. Several US characteristics have been studied as potential predictors of thyroid malignancy, [7]. Although certain findings are suggestive of malignancy, their predictive value is inconsistent, and in the study by Frates et al. [7] the accumulated predicted value of US characteristics and basic clinical features ranged between 3 and 48%.
The papillary thyroid microcarcinoma (PTMC) is a papillary thyroid carcinoma with a size of ≤1 cm. The overall incidence of thyroid cancer has increased worldwide, including Denmark, over the last decades [8,9]. Recent studies have shown that PTMC accounts for a significant proportion of this increase [10]. Recently, Hughes et al. [11] showed that PMTC is now the most common thyroid cancer in patients older than 45 years, based on the United States population.
In this study, we prospectively collected baseline clinical features, US characteristics, and cytopathological diagnosis from patients with cold thyroid nodules undergoing US-guided FNA. These results were correlated with the final histopathological diagnosis. Moreover, we considered the value of a repeat FNA in case the first FNA was non-diagnostic.
Patients and Methods
The consecutive patients were all referred to a university outpatient clinic specialized in thyroid diseases due to goiter with a solitary or dominant nodule without uptake on a technetium-99m scintigraphy. All patients underwent clinical examination and thyroid function test and TPO antibodies. Familial disposition, present when one or more first-degree relatives were affected with thyroid disease (goiter, thyroid cancer and/or thyroid dysfunction), and basic parameters of patient characteristics (age, gender, and smoking habits) were listed prospectively. Sonographic features of the neck US were registered including detailing number and position of the nodule(s), size, shape, margins, content, and vascularity focusing on risk stratification for nodule malignancy. Sonographic high-risk signs were defined as poorly defined margins, irregular shape, presence of microcalcifications, hypoechoic, solid, increased or chaotic intranodular flow on color Doppler flow, and presence of pathologic lymph nodes. Moreover, we evaluated if any of the nodules were taller than wide, but this was found in none of the examined nodules in our cohort and therefore not further addressed. US examination was performed using a Logiq 5 US scanner (GE Medical Systems, Milwaukee, Wisc., USA) with a 12-MHz linear transducer.
Only the nodules that underwent FNA were registered for this study and only nodules of ≥1 cm in at least one dimension would undergo FNA, except for 3 of the included nodules. FNA examinations involved a minimum of three needle passes of solid and cystic-solid nodules. In purely cystic nodules, defined as cysts without any evident solid component present after aspiration, the fluid was aspirated and cytospin and two air-dried smears were prepared. Cytology was evaluated by specialized cytopathologists and classified as either benign, non-diagnostic, suspicious, including follicular neoplasia, and malignant. The criteria for a sufficient FNA were a minimum of six groups of follicular cells per slide on at least two slides. A group of cells had to contain ≥10 cells.
Patients not undergoing surgery were reexamined after 6 or 12 months by the same endocrinologist (F.N.B., B.N.) and included neck US and re-FNA if US revealed any of the high-risk signs other than hypoechoic and solid feature or nodule growth documented by increase in size of ≥2 mm in one dimension.
The criteria for recommending surgery were FNA cytology compatible with malignancy, suspicious, or non-diagnostic cytology on repeat FNA or patient decision (pressure symptoms and/or cosmetic complaints).
Statistics
The χ2 test was used for univariate analysis and non-parametric Mann-Whitney test was used to compare quantitative variables of two groups. Statistical analysis was performed using the PASW statistics 18.0.
Results
In a 33-month period (June 2008–February 2011) 854 consecutive patients with a scintigraphically cold (solitary or dominant) thyroid nodule all underwent US-guided FNA. All patients were biochemically euthyroid except for 1 patient with mild thyrotoxicosis presenting with a dominant cold thyroid nodule. The baseline characteristics of the 854 patients undergoing US-guided FNA are listed in table 1, and findings are in concordance with larger epidemiological studies regarding age and gender [12].
Table 1.
Patient characteristics of 854 patients undergoing FNA
| Patient characteristics | % | n | |
|---|---|---|---|
| Mean age, years | 52.3 (16–93) | ||
| Female | 52.0 (16–93) | ||
| Male | 54.4 (21–88) | ||
| Gender | |||
| Female | 85 | 726 | |
| Male | 15 | 128 | |
| Familial disposition | |||
| Benign thyroid disease | 27.9 | 238 | |
| Thyroid cancer | <1 | 5 | |
| None | 42.4 | 362 | |
| Information not acquired | 29.2 | 249 | |
| Smoking | |||
| Smoker | 17.6 | 150 | |
| Ex-smoker | 11.6 | 99 | |
| Non-smoker | 58.8 | 502 | |
| Information not acquired | 12.1 | 103 |
Of the 854 patients undergoing FNA, 408 were subsequently operated on (48%), resulting in 358 histopathologically verified benign nodules and 50 cancers – 6 of the cancers were metastases from non-thyroid cancers (table 2). In addition, 37 patients with a benign index nodule were also diagnosed with an incidental PTMC. The prevalence of overall thyroid malignancies among the patients undergoing surgery was 21.3% (87/408).
Table 2.
Distribution of all thyroid cancers found in patients undergoing surgery
| Classification | n |
|---|---|
| Papillary carcinoma, total | 62 |
| Papillary carcinoma | 25 |
| Papillary microcarcinoma | 37 |
| Follicular carcinoma | 18 |
| Anaplastic carcinoma | 1 |
| Metastasis from other cancer | 6 |
Value of Patient Characteristics
Of the 340 (83%) nodules originating from females, 213 were benign (predominantly colloid nodules), 84 follicular adenomas (FA), and 43 cancers (15 follicular carcinomas (FC), 23 papillary thyroid carcinomas (PTC), 1 anaplastic carcinoma (ATC) and 4 metastases), and of the 68 (17%) nodules originating from males there were 46 benign, 16 FA and 6 cancers (3 FC, 2 PTC and 1 metastasis), resulting in 13% of nodules in females and 9% of nodules in males were malignant. When comparing malignant (metastases from non-thyroid cancers excluded) and benign thyroid nodules, neither gender nor familial dispositions differed, p = 0.3 and 0.9. Patients with a verified malignant nodule tended to be younger (mean age of 46.6 vs. 50.5, p = 0.04). All of the 5 patients providing information of a familial disposition to thyroid cancer had benign cytology. However, 2 predisposed patients underwent surgery resulting in 1 benign nodule and 1 follicular variant of PTC, as well as a 5-mm PTMC in the opposite thyroid lobe. Furthermore, there was an almost even distribution of smoking habits in the groups diagnosed with a benign (40% smokers) or malignant (37% smokers) nodule, p = 0.2.
Value of Sonographic Findings
Of 854 nodules, 436 were solitary (51%) and 418 (49%) were dominant in a multinodular goiter (MNG) (table 3). The distribution was similar in the patients undergoing surgery, as 53% had a solitary nodule and 47% a dominant nodule in a MNG. We found no significant difference when comparing size and volume of benign and malignant nodules (36.0 vs. 35.6 mm, p = 0.5, and 17.3 vs. 20.1 ml, p = 0.3), as well as the distribution of malignant nodules when comparing a solitary nodule versus a dominant nodule in a MNG (p = 0.1). Malignant nodules were more frequent solid (p = 0.002) than cystic-solid and purely cystic. One of the purely cystic nodules had a malignant FNA and the histopathological diagnoses revealed a metastasis of unknown origin.
Table 3.
Sonographic features of the index nodule in 854 patients undergoing FNA
| Sonographic features | % | n |
|---|---|---|
| Sonographic findings | ||
| Solitary nodule | 51.1 | 436 |
| Dominant in MNG1 | 48.9 | 418 |
| Solid vs. cystic | ||
| Solid | 54.4 | 465 |
| Cystic/solid2 | 36.7 | 313 |
| Cystic3 | 8.9 | 76 |
| Size | ||
| Size3, mm | 31.7 (7–212) | |
| Volume4, ml | 12.5 (0.3–210) | |
| Nodule morphology5 | ||
| Hypoechoic | 90.6 | 705 |
| Isoechoic | 1.8 | 14 |
| Microcalcifications | 3.1 | 24 |
| Coarse calcifications | 2.7 | 21 |
| Increased intranodular vascularization | <1 | 5 |
| Poorly defined margins | <1 | 1 |
| Irregular shape | <1 | 0 |
| Enlarged lymph nodes | 1.5 | 12 |
MNG = one or more coexisting nodules (>10 mm).
≥10% cystic component.
No evident solid component present after aspiration.
Largest diameter/volume (1 × w × d × π/6).
Morphology in 778 solid or cystic/solid nodules (cysts excluded).
The vast majority of nodules (n = 854) were hypoechoic (n = 705, 91% of the solid and cystic/solid) and calcifications were rarely seen (5.8%). Of the 408 patients undergoing surgery, 25 had US-verified calcifications of which 17 (68%) were benign and 8 (32%) malignant based on histopathology. All but 1 of the 8 malignant nodules with calcifications had a malignant or suspicious finding on FNA.
Enlarged lymph nodes were identified in 9 of the 408 histopathologically verified nodules – of these 5 were benign, 2 FC, 1 ATC and 1 metastasis from non-thyroid cancer. When excluding the metastasis from non-thyroid cancer, the finding of enlarged lymph nodes was overrepresented in the malignant nodules (p = 0.014) making this finding a significant predictor for thyroid malignancy. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of enlarged lymph nodes was 7, 99, 38, and 90% for malignancy. In line with this result, the finding of >2 high-risk signs based on US was predictive for malignancy (p = 0.011). The sensitivity, specificity, PPV, and NPV of >2 high-risk signs was 20, 94, 30, and 91% for malignancy.
Initial FNA (FNA1) and Repeat FNA (FNA2/FNA3)
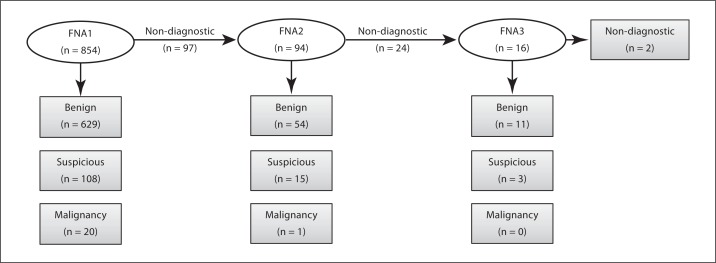
The cytopathological findings are depicted in figure 1. Of the 629 nodules with a benign cytology (124 were classified as cysts based on the FNA), 237 were referred to surgery resulting in 203 benign, 27 FA, 2 FC, 4 PTC, and 1 metastasis from a non-thyroid cancer. None of the malignant nodules originated from nodules with a cystic cytology. Of the 124 cysts, 34 were operated on, resulting in 32 benign nodules and 2 FA. A false-negative finding in 6 patients with a primary benign cytology resulted in a final diagnosis of cancer (1 FC, 4 PTC, and 1 metastasis). Noteworthy, 3 of the 4 unidentified PTC were of the follicular variant and the 1 unidentified FC was a part of a toxic MNG with a dominant low-uptake area on scintigraphy.
Fig. 1.
Cytologic diagnosis. Based on the primary FNA, a diagnostic result was obtained in 88.6% (629 + 108 + 20/854) of patients and a non-diagnostic result in 11.4% (97/854). The number of non-diagnostic FNA was further reduced after repeat FNA in 94 patients resulting in an overall diagnostic result of 96.8% (629 + 108 + 20 + 54 + 15 + 1/854) and further reduced after a third FNA in 16 patients to 98.5% (629 + 108 + 20 + 54 + 15 + 1 + 11 + 3/854). A total of 11 patients that were scheduled for a repeat FNA either refused or did not attend the appointment as scheduled.
97 cytological specimens from the initial FNA were classified as non-diagnostic and on repeat FNA (FNA2, n = 94) 54 were classified as benign, 24 still non-diagnostic, 15 suspicious, and 1 as malignant. 16 of the non-diagnostic had a third FNA (FNA3) within 2 weeks resulting in 11 compatible with a benign lesion, 2 still non-diagnostic, and 3 suspicious. In summary, the cytological results upon 110 repeat FNAs were: 65 benign, 26 non-diagnostic, 18 suspicious, and 1 malignant. A non-diagnostic FNA was converted to a diagnostic FNA by a repeat FNA in 76.4% (84/110) of the cases. 24 of the 65 nodules classified as benign on repeat FNA (FNA2/FNA3), were operated on and resulted in 19 with a benign nodule, 3 FA, 1 FC, and 1 PTC.
A non-diagnostic result after repeat FNAs (FNA2 and FNA3) was still found in 26 non-diagnostic, and 14 of these underwent surgery resulting in 8 benign nodules, 5 FA, and 1 FC. Suspicious cytology after repeat FNA (FNA2 and FNA3) was found in 18 patients resulting in 3 benign nodules, 12 FA, 1 PTC, and 1 FC after surgery. One patient with a malignant cytology based on repeat FNA died due to competing gynecologic cancer before thyroid surgery. The NPV for cancer in a repeat FNA as a result of a non-diagnostic initial FNA1 was 90.5%.
The 97 non-diagnostic FNAs from the initial FNA accounts for 11.4% (97/854). The diagnostic rate of FNA increased from 88.6% (757/854) to 98.5% (757 + 84/854) after repeat FNA.
Predictive Value of FNA
The cytopathological classification was compared with the final histopathological diagnosis in the 408 cases where the patient underwent surgery and listed in table 4. Evaluating the diagnostic outcome of the initial FNA together with the repeat FNAs, hereby leaving out the suspicious and the non-diagnostic, we found sensitivity and specificity for malignancy of 73.9 and 99.2%, respectively. The PPV and NPV of FNA for malignancy was 89.5 and 97.7% (table 5).
Table 4.
FNA (n = 408) with final histopathological diagnosis
| FNA result | Histopathological diagnosis |
||||||
|---|---|---|---|---|---|---|---|
| benign1 | FA | FC | PTC | ATC | metastasis2 | total | |
| Benign | 215 | 29 | 1 | 4 | 0 | 1 | 250 |
| Suspicious | 26 | 67 | 16 | 8 | 0 | 0 | 117 |
| Malignancy | 2 | 0 | 0 | 11 | 1 | 5 | 19 |
| Non-diagnostic | 15 | 4 | 1 | 2 | 0 | 0 | 22 |
| Total | 258 | 100 | 18 | 25 | 1 | 6 | 408 |
1 Dominantly colloid nodules and cysts.
2 Metastasis from non-thyroid cancer.
Table 5.
Predictive value of all diagnostic FNAs for malignancies
| Predictive value of FNA | % |
|---|---|
| Sensitivity | 73.9 |
| Specificity | 99.2 |
| Positive predictive value | 89.5 |
| Negative predictive value | 97.7 |
A total of 6 (5 initial FNA and 1 from the repeat FNA) false-negative biopsies resulted in a false-negative rate of 2.3% (6/259). The false-positive rate of FNA was 10.5% (2/19) all originating from the initial FNA.
Follow-Up FNA
A total of 223 patients with an initial benign result on FNA were re-examined with US and US-guided follow-up FNA 6 or 12 months after the first FNA. 196 (88%) of these still had a benign cytology at follow-up. A non-significant decrease in mean nodule volume from 11 to 10 ml was found at the follow-up examination. Of the initial benign FNA results, 11 were classified as suspicious at follow-up and in these nodules a mean increase in volume of 2 ml (9–11 ml) was found. Follow-up FNA was classified as non-diagnostic in 16 patients and none were malignant based on the follow-up cytology. Subsequently, 1 of the benign follow-up FNAs was histopathologically classified as a PTC and 1 of the non-diagnostic FNAs showed to be a follicular variant of PTC. Of the suspicious findings on follow-up FNA (n = 11), 7 were FA, 1 FC, and 1 benign nodule upon surgery (2 patients refused surgery).
Papillary Thyroid Microcarcinoma
We identified PTMC in 37 (9.1% of 408) of the patients operated on (table 6). Of the microcarcinomas, 45% were found in patients with a solitary thyroid nodule and 55% in patients with a dominant thyroid nodule (p = 0.3). 84% of the microcarcinomas were found in surgical resections with a benign index nodule and the remaining 16% were found in resections where the index nodule was either a PTC or a FC. Microcarcinomas were found more frequently in women (33/37, 89.2%) and this corresponded with the overall distribution of gender (p = 0.6). Index nodules tended to be smaller in patients with coexisting microcarcinomas, 12.5 versus 18.2 ml (p = 0.4); however, this difference did not reach statistical significance. Comparing features as age, presence/absence of family history, smoking habits, solitary versus dominant nodule, solid versus cystic morphology and other high-risk vs. low-risk US features, no differences were found between patients with and without an incidental papillary microcarcinoma.
Table 6.
Number of patients with incidentally found papillary mi-crocarcinomas
| Microcarcinoma | n |
|---|---|
| One microcarcinoma | 25 |
| Two microcarcinomas | 8 |
| Three microcarcinomas | 3 |
| Four microcarcinomas | 1 |
Two or more microcarcinomas were found in 12 patients. A microcarcinoma was defined as an incidental finding of a 10-mm (or less) papillary thyroid carcinoma found in adjacent apparently normal thyroid tissue resections. The prevalence of microcarcinoma in the present study was 37/408 (9.1%).
Of the 37 patients, 12 had at least two PTMC and were thus classified as ‘high-risk’ patients according to the guidelines, and in these patients treatment recommendations comprise total thyroidectomy and radioiodine.
Discussion
In this study we evaluated the predictive value of US features and US-guided FNA in consecutive patients with a solitary or dominant and scintigraphically cold thyroid nodule. So far, reports on the validity of FNA have been confirmed based on repeat FNA and limited surgical controls (less than 20–30% of patients operated on). However, the power of the present study is a final histological diagnosis confirmed in 48% of patients. The main reason for this high percentage of patients undergoing surgery is that patients were selected upon scintigraphy and US-guided FNA performed in scintigraphically cold thyroid nodules only.
The cytopathological classification of FNA used in the present study is in concordance with the guidelines by the European Thyroid Association, the American Association of Clinical Endocrinologist, and the American Thyroid Association, as they recommend five categories: non-diagnostic, benign, follicular lesion, suspicious, and malignant [13]. However, in our study we classified the follicular lesions as suspicious resulting in four cytopathological categories. This change has no clinical impact because the surgical approach still comprises removal, and in cases other than follicular neoplasia, intraoperative frozen-section evaluation is carried out to determine the extent of the subsequent surgical procedure.
Based on our results it is evident that the value of clinical and baseline features was limited. Patients with a subsequent diagnosis of thyroid cancer were slightly younger (∼4 years) and this was the only statistically significant characteristic when comparing patients with benign and malignant thyroid nodules and interestingly, this finding opposes general beliefs. This could in part be explained by the high percentage of patients being operated on in the present study (48%), keeping the criteria for recommending surgery in mind: FNA cytology compatible with malignancy, suspicious, or non-diagnostic cytology on repeat FNA or patient decision (pressure symptoms and/or cosmetic complaints). Moreover, in contrast to other studies, the incidence of thyroid cancer among males was not higher than in females in our cohort [12].
It is generally agreed that no single sonographic criterion distinguishes benign from malignant thyroid nodules with complete reliability, for example most thyroid carcinomas appear hypoechoic compared with the remaining gland but so do the majority of benign thyroid nodules. This was confirmed in our study, demonstrated by the fact that 91% of the examined nodules were hypoechoic and hypoechogenicity is listed as a high-risk sign and of high clinical utility in recent guidelines [2,11]. However, we found enlargement of lymph nodes and the presence of two or more high-risk signs to be a significant predictor for malignancy, and this result is in line with recent guidelines [13].
The value of repeat FNAs, when the initial FNA was non-diagnostic, was evident as our results revealed an overall diagnostic cytology in 98.5% of the 854 patients undergoing US-guided FNA. For comparison, we obtained 88.6% of diagnostic FNAs from the initial FNAs alone. The percentages of non-diagnostic FNAs from the initial FNA and the repeat FNAs were 11.4 and 23.6%, respectively. The value of US guidance to reduce the number of non-diagnostic specimens has been confirmed in numerous studies [13]. An American study by Richards et al. [14] showed a high non-diagnostic rate between 21 and 29% from the initial FNA and repeat FNAs, respectively, and 14% of patients with a non-diagnostic FNA had a malignancy stressing the importance of diagnostic surgery in case of uncertainty. All in all, the rate of non-diagnostic specimens in our study is acceptable comparing similar studies showing a non-diagnostic rate of the initial FNA of 3.5–29.5% [3,5,6,15,16].
Our results revealed a false-negative rate of 2.3% of FNA samples as 6 patients were classified as benign and subsequently diagnosed with a malignant thyroid nodule on histopathology. Three (50%) of the false-negative FNAs resulted in PTC of the follicular variant based on histology and this proves the difficulties of identifying this variant as malignant on cytology alone. One of the false-negative FNAs turned out to be a metastasis from a non-thyroid cancer. The final two false-negative FNAs were diagnosed as one PTC and one FC, both were found in thyroid glands with multiple nodules. The nodules were all low-uptake lesions on scintigraphy, US morphology was similar and all nodules ≥1 cm underwent FNA. Based on the histopathological report it is not clear whether the PTC corresponded with the largest nodule undergoing FNA. However, this stresses the importance that nodule size cannot be the single determinant of which nodule to biopsy, and furthermore the need to biopsy more than one nodule in case of ‘high-risk’ features found in additional nodules on US, according to guidelines [11]. However, a false-negative rate of 2.3% is still acceptable when compared to other studies reporting a false-negative rate between 4 and 17% [3,5,16,17,18].
The sensitivity and specificity of the 408 histopathologically verified FNAs in our study was 73.9 and 99.2%, respectively. Apart from the sensitivity, our findings are in accordance with previous studies reporting of a sensitivity and specificity between 86–94 and 93–98.5%, respectively [3,4,6]. The low sensitivity in the present study could in part be caused by the relative low threshold for recommending or agree on surgical intervention in our cohort and as mentioned above, half of our false-negative FNAs resulted in PTC of the follicular variant, known to cause misclassification based on cytology alone.
The reported number of microcarcinomas in our study population was surprisingly high (9.1%) and noteworthy as this prevalence is close to the prevalence of all the cancers with a diameter >1 cm (12.3%). Looking at the PTC alone, the prevalence was just 6.1%, which stands in contrast to the high prevalence of microcarcinomas and which evidently was the most frequent cancer of the thyroid in our cohort. This is to some extent in concordance with the finding by Harach et al. [19] where the prevalence of microcarcinomas was close to 10% in thyroid autopsies in a Finnish population. However, as these were autopsies, the mean age was decades above the one in our study [19]. A more recent study based on a US cohort resulted in a prevalence of PTMC of 4.1% [20]. The long-term clinical significance of the high prevalence of microcarcinomas is still an unresolved issue. Nevertheless, the finding of a PTMC has an immense impact on the affected patients since a diagnosis of cancer involves a close follow-up in an outpatient setting with oncological expertise. Moreover, the patients with multifocal (two or more microcarcinomas) PMTC are considered as high-risk patients and therefore the need of total thyroidectomy and ablation with radioiodine according to present guidelines [2]. One third (12/37) of the patients with PMTC in our series had two or more foci and were treated as high-risk patients.
Our findings regarding the high incidence of PMTC support the state of an overall increase in thyroid cancer as a result of an increased number of PMTC. Davies and Welch [21] showed a 2.4-fold increase in thyroid cancer from 1973 to 2002 in the United States and 87% of this increase was due to the diagnosis of PMTC. They further concluded that due to the high rate of PTMC at autopsy, the increase is caused by increased diagnostic scrutiny rather than a real increase in cancer emphasized by the stable rate in mortality in this period. However, recent statistics from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute revealed a 6.4 (1997–2008) and 0.7 (1988–2008) significant increase in the annual percentage change in thyroid cancer incidence and mortality [22].
The epidemiology and the phenotypic presentation of a patient with a palpable thyroid nodule vary with the population in focus. The relative risk of harboring thyroid malignancy is still low, especially in iodine-deplete (Denmark being borderline iodine-deficient) areas. For targeting nodules at the highest risk of being malignant, guidelines recommend functional and morphological characterization and US-guided FNA. Using this strategy the risk of overlooking clinical significant cancer can be reduced to less than 2–3%, which is confirmed in our study with the so far largest number of histopathological controls reported (48%). Finally, our results revealed that papillary thyroid microcarcinoma is now the most frequent cancer of the thyroid, emphasizing the need for future characterization of this group of patients.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, Vitti P. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest. 2010;33(suppl):1–50. [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine-needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125:484–488. doi: 10.5858/2001-125-0484-AOFNAO. [DOI] [PubMed] [Google Scholar]
- 4.Boyd LA, Earnhardt RC, Dunn JT, Frierson HF, Hanks JB. Preoperative evaluation and predictive value of fine-needle aspiration and frozen section of thyroid nodules. J Am Coll Surg. 1998;187:494–502. doi: 10.1016/s1072-7515(98)00221-x. [DOI] [PubMed] [Google Scholar]
- 5.Lew JI, Snyder RA, Sanchez YM, Solorzano CC. Fine needle aspiration of the thyroid: correlation with final histopathology in a surgical series of 797 patients. J Am Coll Surg. 2011;213:188–194. doi: 10.1016/j.jamcollsurg.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4,703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 7.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD, Jr, Larsen PR, Marqusee E, Alexander EK. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 8.Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer 2012 (E-pub ahead of print). [DOI] [PubMed]
- 9.Bores-Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype – papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18:1–7. doi: 10.1007/s12022-007-0002-z. [DOI] [PubMed] [Google Scholar]
- 10.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653–660. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 11.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–236. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C, Vigneri R. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med. 1992;93:363–369. doi: 10.1016/0002-9343(92)90164-7. [DOI] [PubMed] [Google Scholar]
- 13.Paschke R, Hegedus L, Alexander E, Valcavi R, Papini E, Gharib H. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol. 2011;7:354–361. doi: 10.1038/nrendo.2011.1. [DOI] [PubMed] [Google Scholar]
- 14.Richards ML, Bohnenblust E, Sirinek K, Bingener J. Nondiagnostic thyroid fine-needle aspiration biopsies are no longer a dilemma. Am J Surg. 2008;196:398–402. doi: 10.1016/j.amjsurg.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8:15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 16.Sclabas GM, Staerkel GA, Shapiro SE, Fornage BD, Sherman SI, Vassillopoulou-Sellin R, Lee JE, Evans DB. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am J Surg. 2003;186:702–709. doi: 10.1016/j.amjsurg.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Blansfield JA, Sack MJ, Kukora JS. Recent experience with preoperative fine-needle aspiration biopsy of thyroid nodules in a community hospital. Arch Surg. 2002;137:818–821. doi: 10.1001/archsurg.137.7.818. [DOI] [PubMed] [Google Scholar]
- 18.Meko JB, Norton JA. Large cystic/solid thyroid nodules: a potential false-negative fine-needle aspiration. Surgery. 1995;118:996–1003. doi: 10.1016/s0039-6060(05)80105-9. [DOI] [PubMed] [Google Scholar]
- 19.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A ‘normal’ finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Roti E, Rossi R, Trasforini G, Bertelli F, Ambrosio MR, Busutti L, Pearce EN, Braverman LE, Degli Uberti EC. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91:2171–2178. doi: 10.1210/jc.2005-2372. [DOI] [PubMed] [Google Scholar]
- 21.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 22.Howlader N, et al. SEER Cancer Statistics Review, 1975–2008. Bethesda: National Cancer Institute; 2011. [Google Scholar]