Abstract
Increased reactive oxygen species (ROS) generation and the consequent oxidative damage are involved in the development of several diseases, including autoimmune diseases. Graves' disease is an autoimmune disorder characterized by hyperthyroidism and, less frequently, orbitopathy. Hyperthyroidism is characterized by increased oxidative stress. Untreated hyperthyroidism is associated with an increase of several parameters of oxidative stress and in most studies (but not all) by an increase of antioxidant defense enzymes. Restoration of euthyroidism with antithyroid drug is associated with a reversal of the biochemical abnormalities associated with oxidative stress. Animal and human studies suggest that increased ROS may directly contribute to some clinical manifestation of the disease, including orbitopathy. Antioxidants administered alone improve some clinical signs and symptoms of hyperthyroidism and, when associated with antithyroid drugs, induce a more rapid control of clinical manifestations and a faster achievement of euthyroidism. A large randomized clinical trial has shown that antioxidant supplementation (selenium) may also be beneficial for mild Graves' orbitopathy.
Key Words: Hyperthyroidism, Graves' orbitopathy, Antithyroid drugs, Selenium
Introduction
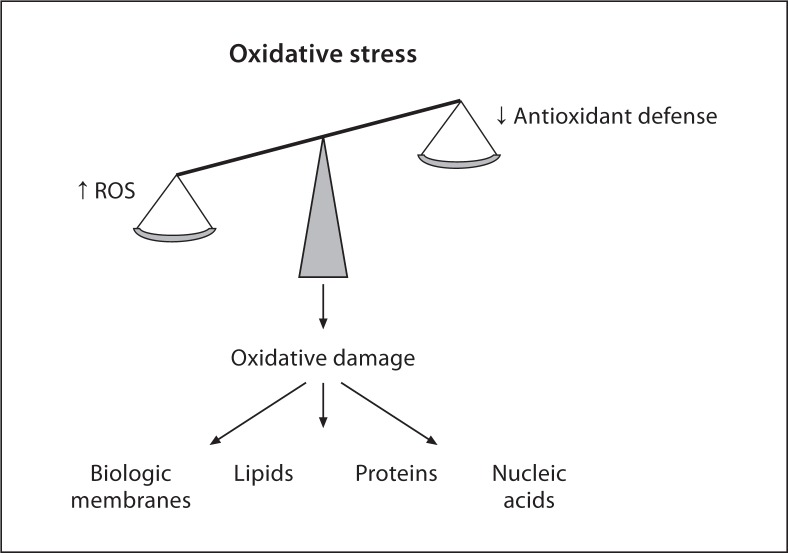
The preservation of a cell redox state is of great importance for maintaining cellular homeostasis within certain boundaries. Generation of reactive oxygen species (ROS) occurs as a consequence of the oxidative cell metabolisms [1,2]. ROS are molecules highly reactive because they contain unpaired electron(s). They include partially reduced forms of oxygen, such as hydrogen peroxide (H2O2), hydroxyl radicals (OH°) and superoxide anions (O2–), and lipid peroxides. ROS act as oxidizing agents, which may perturb intracellular reactions and damage cell structures, including membranes and cellular proteins, lipids, and nucleic acids. ROS may also regulate gene expression [3]. Under physiological conditions, an antioxidant system defends the cells form the ROS-induced damage. This system includes enzymes, such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and glutathione reductase, as well as small nonenzymatic molecules, such as glutathione (GSH) and vitamins (ascorbic acid and tocopherol). Any increase in the rate of ROS production or decrease in their scavenging ability will disrupt the oxidative stability of the cell, resulting in oxidative stress (fig. 1) [4].
Fig. 1.
Mechanisms responsible for oxidative stress and cell damage. An increased production of ROS and inactivation or excessive consumption of antioxidant defenses will be responsible for oxidative damage of biological membranes and molecules, such as lipids, proteins and nucleic acids.
Increased ROS generation and the consequent oxidative damage are believed to contribute to the development of several diseases (cardiovascular, neurodegenerative, neoplastic, endocrine) [4]. Moreover, excessive oxidative stress is also thought to be involved in the control of the immune system and in the pathogenesis of autoimmune diseases by several mechanisms (increased inflammation, proapoptotic effect, and breaking down the immunological tolerance). In recent years there has been a growing interest in the possibility that antioxidants may counteract the disease-promoting effects of oxidative stress.
Evaluation of Oxidative Status
Several plasma and/or erythrocyte markers are available for evaluating the oxidant/antioxidant status. In healthy conditions, markers of oxidative stress and antioxidant defense are low [4]. The induction of a state of oxidative stress will be characterized by an increase of oxidative markers, which will be also accompanied, at least initially, by an increase of antioxidant markers [4]. The latter represent a homeostatic response in an attempt to attenuate the damage induced by oxidative stress. On the other hand, a long-lasting state of oxidative stress may exhaust the antioxidant defense and is characterized by low levels of antioxidant markers.
Thyroid Function and Oxidative Stress
Hyperthyroidism is associated with increased oxygen consumption, dysfunction in the mitochondrial respiratory chain, elevated intracellular ATP consumption, and increased ROS production [5,6]. Moreover, there is evidence supporting a role of oxidative processes in the genesis of Graves' disease (GD) [7], hyperthyroidism-induced damage such as thyrotoxic myopathy and cardiomyopathy [8,9], and Graves' orbitopathy (GO) [7] (see below).
Animal Studies
In rats rendered thyrotoxic by L-thyroxine administration, plasma malondialdehyde (MDA), a marker of lipid peroxidation, was significantly higher than in control euthyroid animals [10]. In the same animals, erythrocyte antioxidant parameters (SOD, GPx) were also increased as a response to the sustained oxidative stress induced by thyrotoxicosis. Interestingly, the concomitant administration of vitamin E – a potent inhibitor of lipid peroxidation and therefore of the burden of the oxidative stress – significantly decreased plasma MDA as well as erythrocyte SOD and GPx levels. Increased myocardial [11] and aortic wall [12] oxidative stress have also been observed in thyorotoxic rats. Vitamin E was also found to protect against a thyroxine-induced increase of lipid peroxidation in cardiac and skeletal muscles in rats [13].
Human Studies
The majority of studies evaluating the effect of thyroid status in humans have been performed in hyperthyroid patients with GD, either under basal conditions or after restoration of euthyroidism with antithyroid drug (ATD) or radioiodine [14,15,16,17,18,19,20,21,22,23,24].
Untreated hyperthyroidism is associated with an increase of several parameters of oxidative stress in serum/plasma and erythrocytes compared with normal euthyroid subjects, including hydrogen peroxide, lipid peroxides, conjugated dienes, thiobarbituric acid-reacting substances (TBARS), MDA, and tert-butyl hydroperoxide-initiated chemiluminescence. The levels of lipid peroxidation products correlate with serum concentration of thyroid hormones in patients with symptomatic hyperthyroidism, but significantly higher values have also been observed in patients with subclinical hyperthyroidism due to multinodular goiter [25]. A state of oxidative stress in hyperthyroid patients has recently been confirmed evaluating the total oxidant status using a novel automated measurement method [24]. Interestingly, in this study no difference was observed between patients with GD and toxic multinodular goiter, or between Graves' patients with and without orbitopathy.
Treatment with methimazole or propylthyouracil is followed by a decline in the levels of these parameters, which may be related to the restoration of euthyroidism as well as to the antioxidant properties of these drugs [26,27].
Contradictory results have been reported regarding the activity of the antioxidant defense system in hyperthyroid patients. Most studies have shown an increase of antioxidant defense enzymes, which represent a homeostatic response to balance the hyperthyroidism-induced increased ROS generation [16,18,20]. Komosinska-Vassev et al. [18] found an increase of erythrocyte SOD, CAT, and GPx, but not of serum glutathione reductase and total antioxidant status, in 30 patients with GD compared with age-matched controls. The authors suggested that the discrepant results between erythrocyte and serum antioxidant activities likely results in a more rapid exhaustion of the latter antioxidant system. Bednarek et al. [21] measured antioxidant activities in plasma of 47 patients with GD of short duration (1–2 months) with and without GO and found an increase compared with healthy controls of SOD and catalase, but not of GPx and glutathione reductase, which were actually decreased.
Conversely, other studies have shown no evidence of increased antioxidant defense in patients with untreated hyperthyroidism. Abalovich et al. [20] evaluated 69 hyperthyroid patients with GD, lasting more than 6 months. In this study erythrocyte SOD and catalase activities were significantly decreased compared to controls, whereas no difference was observed for erythrocyte GPx and plasma total reactive antioxidant potential. More recently, Aslan et al. [24] reported a significant decrease compared with controls of serum total antioxidant capacity in 36 hyperthyroid patients with an average duration of hyperthyroidism of 2.3 ± 1.5 months.
The discrepant data on antioxidant activity in patients with Graves' hyperthyroidism may be due, in addition to differences in assessment methods, to the duration of the hyperthyroid status at the time of evaluation. Indeed, in patients with short-lasting hyperthyroidism, we might expect an increment in the antioxidant defense mechanisms in order to balance the increased oxidative stress. On the other hand, in patients with a more prolonged duration of the hyperthyroid state, the exhaustion of the antioxidant defense system may account for the decreased cellular and serum antioxidant activities.
Despite the above discrepancies, restoration of euthyroidsim with ATD is associated with a reversal of the abnormalities of the intra- and extracellular antioxidant defense system [16,18,20,21]. These effects of ATD are likely due to the restoration of the euthyroid state. A scavenging effect of ATD may also contribute to these changes [26,27].
Evaluation of the oxidant/antioxidant status in the thyroid tissue of patients undergoing thyroidectomy for GD has shown increased levels of free radicals and their scavengers compared with normal thyroid [28]. More recently, this issue has been re-evaluated in 25 consecutive patients with GD who underwent thyroidectomy by comparing markers of oxidative status between the thyroid tissue and plasma [29]. Mean duration of hyperthyroidism before surgery was 29 months. Plasma TBARS was significantly increased compared with controls, whereas the concentration of SOD and GPx did not differ from controls. Values were significantly higher in patients who had been treated with ATD for less than 6 months compared with those treated for a longer period. No difference was observed between patients with and without GO. Tissue TBARS, total thiol, SOD, and GPx were significantly higher compared with plasma levels. Patients who underwent surgery because of relapsed hyperthyroidism after ATD therapy had significantly higher plasma and tissue TBARS and GPx, and lower plasma and tissue total thiol, compared with patients who had received a first course of ATD therapy. The latter finding might be related to the ongoing autoimmune reactions in patients with relapsing GD despite being euthyroid under ATD.
Antioxidants as Treatment for Hyperthyroidism
Hyperthyroidism is characterized by modifications of the whole body homeostasis and it has been suggested that metabolic oxidation might contribute to generate some signs and symptoms. Therefore, it could be speculated that antioxidant supplementation might be of benefit either alone or in combination with ATD in the management of hyperthyroidism.
The first study aimed at investigating a possible role of antioxidant therapy in patients with hyperthyroidism was performed by Seven et al. [30], who treated 24 patients with propylthiouracil for 5 days combined with ascorbic acid for 1 month. The addition of the latter treatment resulted in a potentiation of the antioxidant defense system and a relief of the oxidative stress. On the other hand, Adali et al. [16] failed to demonstrate a benefit on the oxidative status of adding 300 mg/day vitamin E to propylthiouracil (300 mg/day) and propranolol (40 mg/day) in 44 hyperthyroid patients followed for 3–6 months.
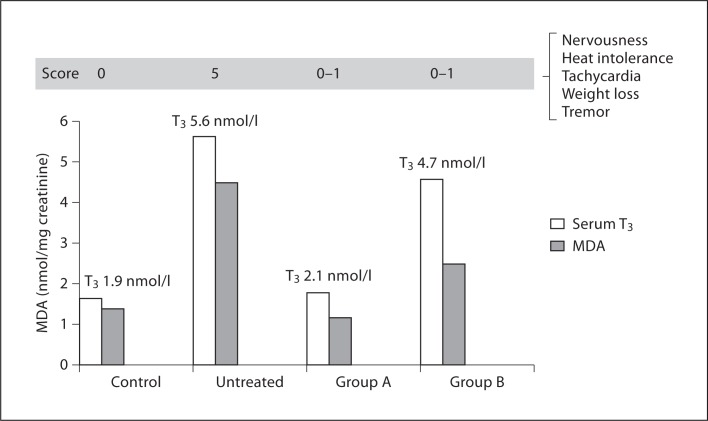
Two studies have extended these observations. Guerra et al. [31] treated 56 patients with hyperthyroid GD with different treatment schedules: (1) methimazole alone, (2) an antioxidant mixture (Larotabe®, containing vitamin E, β-carotene, vitamin C, Cu, Zn, Mn, and selenium) alone, or (3) a combination of methimazole and Larotabe (fig. 2). Serum concentration of total thyroid hormones, TSH, MDA, and erythrocyte SOD and CAT were measured before and after completion of therapy. A clinical score based on common symptoms and signs of hyperthyroidism was also recorded. Treatment with methimazole normalized thyroid function and MDA levels and improved the clinical score. Antioxidant treatment alone did not affect serum concentration of thyroid hormones, which remained elevated but significantly decreased the clinical score and normalized MDA levels. Combined treatment shortened the time required to normalize thyroid hormones and the clinical score. These data suggest that increased ROS generation may contribute to generate some clinical manifestations of thyrotoxicosis and that antioxidant treatment may improve the clinical picture, even if serum thyroid hormones remain elevated. The faster control of hyperthyroidism in patients treated with methimazole and Larotabe suggest a synergistic mechanism at the level of thyroid hormone synthesis.
Fig. 2.
Effect of treatment with methimazole (Group A) or Larotabe® (an antioxidant mixture containing vitamin E, β-carotene, vitamin C, Cu, Zn, Mn, selenium; Group B) on serum T3 levels and urinary MDA levels and clinical score (based on symptoms and signs of hyperthyroidism) in 36 patients with GD (derived from data of Guerra et al. [31]).
Vrca et al. [32] confirmed a beneficial effect of antioxidant supplementation to methimazole. These authors evaluated 57 patients with GD randomly treated for 2 months with methimazole alone or combined with a mixture of antioxidant containing β-carotene, vitamin C, vitamin E, and selenium (Symbion®). Patients who were treated with methimazole plus antiodixant supplementation reached euthyroidism earlier than those given methimazole alone. Whole blood GPx activity increased in both groups, but a significantly greater increase was observed at 1 month in patients who received the combined therapy compared with those treated with methimazole alone.
Based on the latter findings it might be reasonable to consider antioxidant supplementation in the early phase of ADT therapy in order to obtain a more rapid control of clinical manifestations and a faster achievement of euthyroidism. Further studies on larger series of hyperthyroid patients are needed to confirm these preliminary results.
Oxidative Stress and Graves' Orbitopathy
GO, the most common extrathyroidal manifestation of GD, is an autoimmune disorder in which endogenous and environmental factors are involved [33]. According to the most widely accepted hypothesis, after recognition of antigen(s) shared by the thyroid and the orbit, activated T lymphocytes infiltrating the orbit initiate a cascade of events leading to increased production of cytokines, growth factor, and ROS. Increased proliferation of orbital fibroblast and adipocytes and overproduction of glycosaminoglycans, which attract water, will increase the volume of orbital structures (fibroadipose tissue and extraocular muscles) [33]. The contribution of ROS in the changes occurring in the orbit is underscored by in vitro studies in orbital fibroblasts as well as in human studies [34]. Moreover, cigarette smoking, which is the most important environmental factor associated with GO, might act, among other mechanisms, by enhancing the generation of ROS and reducing the antioxidant defense [34].
In vitro Studies
Superoxide radicals increase orbital fibroblast proliferation and glycosaminoglycan production [35]. H2O2 induces the expression of HLA-DR and heat shock protein-72, which is involved in antigen recognition and T lymphocyte recruitment [36]. IL-1β increases ROS production in GO and controls orbital fibroblasts, and is associated with an increase of intracellular SOD activity [37]. Antioxidants (nicotinamide and allopurinol), methimazole, and propylthiouracil reduced superoxide- and hydrogen peroxide-induced fibroblast proliferation, glycosaminoglycan production, and HDL-DR and heat shock protein-72 expression in GO orbital fibroblasts, likely by increasing the ROS scavenging activity [36]. Recent studies by Hondur et al. [38] and Tsai et al. [39,40] have extended these observations. Oxidative DNA damage, lipid peroxidation, H2O2, and SOD activity, as well as decreased GPx activity and reduced glutathione/oxidized glutathione ratio were found in orbital fibroblasts from GO patients compared with orbital fibroblasts from normal controls. The addition of H2O2 elicited a more pronounced effect on the oxidant/antioxidant balance in GO fibroblasts than in normal fibroblasts.
Human Studies
As mentioned before, patients with hyperthyroid GD have an imbalance in the prooxidant/antioxidant status and ATD therapy may reverse these abnormalities. Interestingly, in the study by Bednarek et al. [21], which also included Graves' patients with active GO, increased peripheral ROS and antioxidant activity normalized only in patients without GO, suggesting that orbital inflammation contributed to the increased circulating markers of the oxidative status. Tsai et al. [41] showed increased oxidative DNA damage, as evaluated by measurement of urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) levels, compared with controls, in 8 patients with active GO who had been rendered euthyroid for at least 6 months with ATD therapy. Treatment with oral glucocorticoids (GC) was associated with a significant decrease of urinary 8-OHdG compared with pretreatment levels, which paralleled changes in the clinical activity score and ophthalmopathy index. The urinary 8-OHdG increased again in 2 patients who had a relapse of GO during GC therapy or after its withdrawal. These data were extended in a large series (n = 25) of euthryoid Graves' patients, which included patients with active and inactive GO [42]. Mean urinary 8-OHdG values were greater in GO patients compared with controls, and, among GO patients, in smokers compared with never smokers as well as those with active GO compared with those with inactive GO.
Recently, Akarsu et al. [43] further evaluated the relationship between GO and the effect of GC therapy (intravenous vs. oral) on serum of MDA and GSH. The study group included 33 recently diagnosed euthyroid GD patients with moderately severe and active GO, 20 euthyroid GD patients without GO, and 15 healthy controls. Serum levels of MDA were significantly higher and those of GSH significantly lower in Graves' patients with GO compared to those without GO or healthy controls. Moreover, in patients with GO, MDA levels were positively correlated with the clinical activity score. Patients with GO were randomly assigned to therapy with intravenous or oral GC and re-evaluated during and after withdrawal of therapy. Serum MDA levels decreased significantly in both treatment groups, and at the end of GC therapy did not differ from those of GD patients with GD or healthy controls. Abalovich et al. [20] and Bednarek et al. [21] reported similar data. On the other hand, variations of serum GSH during treatment were not significant. The levels of serum MDA during oral and intravenous GC therapy were positively correlated with the clinical activity scores.
Taken together, these studies indicate that in euthyroid patients with GD and active GO, markers of oxidative status remain elevated compared to GD patients without GO, suggesting that the active orbital inflammatory process accounts for this finding and that oxidative stress is involved in the pathogenesis of GO. Moreover, parameters of the oxidative status might become useful markers in the follow-up of patients with GO.
The potential role of the oxidative stress in the pathogenesis of GO has been the rationale for the use of antioxidants in the management of GO. A small nonrandomized study investigated the effects of 3-month treatment with antioxidants (nicotinamide and allopurinol) in patients with moderately severe GO [44]. Favorable results, particularly on soft tissue involvement, were reported. Recently, a large multicenter, randomized, double-blind, placebo-controlled study evaluated the effect of selenium on the course of mild GO [45]. Selenium is a trace mineral which is incorporated as selenocysteine into several selenoproteins, mostly enzymes, in which selenium acts as a reduction-oxidation center and functions as an antioxidant. This study showed that a 6-month course with sodium selenite (100 μg twice daily) was associated with a significant improvement in the quality of life and eye manifestations compared with patients treated with placebo (fig. 3). In addition, a lower rate of worsening was also observed in patients given selenium. The beneficial effect of selenium persisted in the 6 months after treatment withdrawal. The patients included in the study were living in marginally selenium-deficient areas. Whether selenium administration will be of benefit to patients living in selenium-sufficient areas and whether patients with more severe GO will also benefit from selenium supplementation associated with immunosuppressive therapy remain to be established.
Fig. 3.
Changes of GO-specific quality of life score questionnaire (a) and overall eye evaluation (b) at 6 and 12 months in patients with mild GO randomly assigned to treatment with sodium selenite (100 μg twice daily) or placebo (twice daily). Changes of quality of life and overall eye evaluation were scored as improved, unchanged, or worsened according to predefined criteria (derived from data of Marcocci et al [45]).
Conclusions
There is a body of evidence indicating Graves' hyperthyroidism is characterized by increased oxidative stress and that increased free radicals might be responsible for some symptoms and signs of hyperthyroidism. Indeed, antioxidant treatment, without changing the serum concentrations of thyroid hormones, may improve some clinical manifestations of hyperthyroidism. Restoration of euthyroidism with ATD improves and often normalizes the oxidative status. Oxidative stress is also involved in the pathogenesis of GO and evidence for an imbalance in the oxidative status has been provided in patients with GO. A beneficial effect of antioxidant therapy has been shown in mild GO. Future studies should evaluate whether antioxidant therapy may have a role in association with ATD for the control of symptoms and signs related to increased thyroid hormone levels as well as influence the rate of relapse after ATD therapy. Further studies should evaluate whether selenium supplementation may also be beneficial in patients with moderately severe GO (in association with immunosuppressive therapy).
References
- 1.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1:1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Driessens N, Costa M, De Deken X, Detours V, Corvilain B, Maenhaut C, Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Ann Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. ed 4. New York: Oxford University Press; 2007. Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death; pp. 187–267. [Google Scholar]
- 5.Venditti P, Di Meo S. Thyroid hormone-induced oxidative strees. Cell Mol Life Sci. 2006;63:414–434. doi: 10.1007/s00018-005-5457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miot F, Van Sande J, Many MC, Dumont JE. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007;92:3764–3773. doi: 10.1210/jc.2007-0660. [DOI] [PubMed] [Google Scholar]
- 7.Zarkovic M. The role of oxidative stress on the pathogenesis of Graves' disease. J Thyroid Res. 2012;2012:302537. doi: 10.1155/2012/302537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asayama K, Dobashi H, Hayashibe H, Megata Y, Kato K. Lipid peroxidation and free radical scavengers in thyroid dysfunction in the rat: a possible mechanism of injury to hearth and skeletal muscle in hyperthyroidism. Endocrinology. 1987;121:2112–2118. doi: 10.1210/endo-121-6-2112. [DOI] [PubMed] [Google Scholar]
- 9.Yamada T, Mishima T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M. Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J Appl Physiol. 2006;100:1520–1526. doi: 10.1152/japplphysiol.01456.2005. [DOI] [PubMed] [Google Scholar]
- 10.Seven A, Seymen O, Hatemi S, Hatemi H, Yigit G, Candan G. Antioxidant status in experimental hyperthyroidism: effect of vitamin E supplementation. Clin Chim Acta. 1996;256:65–74. doi: 10.1016/s0009-8981(96)06415-7. [DOI] [PubMed] [Google Scholar]
- 11.Araujo AS, Ribeiro MF, Enzveiler A, Schenkel P, Fernandes TR, Partata WA, Irigoyen MC, Llesuy S, Belló-Klein A. Myocardial antioxidant enzyme activities and concentration and glutathione metabolism in experimental hyperthyroidism. Mol Cell Endocrinol. 2006;249:133–139. doi: 10.1016/j.mce.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Moulakakis KG, Poulakou MV, Paraskevas KI, Dontas I, Vlachos IS, Sokolis DP, Dosios T, Karayannacos PE, Perrea D. Hyperthyroidism is associated with increased aortic oxidative DNA damage in a rat model. In Vivo. 2007;21:1021–1026. [PubMed] [Google Scholar]
- 13.Asayama K, Dobashi K, Hayashibe H, Kato K. Vitamin E protects against thyroxine-induced acceleration of lipid peroxidation in cardiac and skeletal muscles in rats. J Nutr Sci Vitaminol (Tokyo) 1989;35:407–418. doi: 10.3177/jnsv.35.407. [DOI] [PubMed] [Google Scholar]
- 14.Videla LA, Sir T, Wolff C. Increased lipid peroxidation in hyperthyroid patients: suppression by propylthiouracil treatment. Free Radic Res Commun. 1988;5:1–10. doi: 10.3109/10715768809068553. [DOI] [PubMed] [Google Scholar]
- 15.Wilson R, Chopra M, Bradley H, McKillop JH, Smith WE, Thomson JA. Free radicals and Graves' disease: the effects of therapy. Clin Endocrinol (Oxf) 1989;30:429–433. doi: 10.1111/j.1365-2265.1989.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 16.Adali M, Inal-Erden M, Akalin A, Efe B. Effects of propylthiouracil, propranolol, and vitamin E on lipid peroxidation and antioxidant status in hyperthyroid patients. Clin Biochem. 1999;32:363–367. doi: 10.1016/s0009-9120(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi G, Solaroli E, Zaccheroni V, Grossi G, Bargossi AM, Melchionda N, Marchesini G. Oxidative stress and anti-oxidant metabolites in patients with hyperthyroidism: effect of treatment. Horm Metab Res. 1999;31:620–624. doi: 10.1055/s-2007-978808. [DOI] [PubMed] [Google Scholar]
- 18.Komosinska-Vassev K, Olczyk K, Kucharz EJ, Marcisz C, Winsz-Szczotka K, Kotulska A. Free radical activity and antioxidant defense mechanisms in patients with hyperthyroidism due to Graves' disease during therapy. Clin Chim Acta. 2000;300:107–117. doi: 10.1016/s0009-8981(00)00306-5. [DOI] [PubMed] [Google Scholar]
- 19.Guerra LN, Moiguer S, Karner M, de Molina MC, Sreider CM, Burdman JA. Antioxidants in the treatment of Graves disease. IUBMB Life. 2001;51:105–109. doi: 10.1080/15216540152122102. [DOI] [PubMed] [Google Scholar]
- 20.Abalovich M, Llesuy S, Gutierrez S, Repetto M. Peripheral parameters of oxidative stress in Graves disease: the effect of methimazole and 131I iodine treatment. Clin Endocrinol (Oxf) 2003;59:321–327. doi: 10.1046/j.1365-2265.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- 21.Bednarek J, Wysocki H, Sowiński J. Oxidative stress peripheral parameters in Graves' disease: the effect of methimazole treatment in patients with and without infiltrative ophthalmopathy. Clin Biochem. 2005;38:13–18. doi: 10.1016/j.clinbiochem.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Guerra LN, Rios de Molina M del C, Miler EA, Moiguer S, Karner M, Burdman JA. Antioxidants and methimazole in the treatment of Graves' disease: effect on urinary malondialdehyde levels. Clin Chim Acta. 2005;352:115–120. doi: 10.1016/j.cccn.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Rybus-Kalinowska B, Zwirska-Korczala K, Kalinowski M, Kukla M, Birkner E, Jochem J. Activity of antioxidative enzymes and concentration of malondialdehyde as oxidative status markers in women with newly diagnosed Graves-Basedow disease and after thiamazole therapy leading to euthyroidism. Pol Arch Med Wewn. 2008;118:420–425. [PubMed] [Google Scholar]
- 24.Aslan M, Cosar N, Celik H, Aksoy N, Dulger AC, Begenik H, Soyoral YU, Kucukoglu ME, Selek S. Evaluation of oxidative status in patients with hyperthyroidism. Endocrine. 2011;40:285–289. doi: 10.1007/s12020-011-9472-3. [DOI] [PubMed] [Google Scholar]
- 25.Cetinkaya A, Kurutas EB, Buyukbese MA, Kantarceken B, Bulbuloglu E. Levels of malondialdehyde and superoxide dismutase in subclinical hyperthyroidism. Mediators Inflamm. 2005;2005:57–59. doi: 10.1155/MI.2005.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weetman AP. Effect of the anti-thyroid drug methimazole on interleukin-1 and interleukin-2 levels in vitro. Clin Endocrinol (Oxf) 1986;25:133–142. doi: 10.1111/j.1365-2265.1986.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 27.Whiteman M, Halliwell B. Thiourea and dimethylthiourea inhibit peroxynitrite-dependent damage: nonspecificity as hydroxyl radical scavengers. Free Radic Biol Med. 1997;22:1309–1312. doi: 10.1016/s0891-5849(96)00545-x. [DOI] [PubMed] [Google Scholar]
- 28.Mano T, Shinohara R, Iwase K, Kotake M, Hamada M, Uchimuro K, Hayakawa N, Hayashi R, Nakai A, Ishizuki Y, Nagasaka A. Changes in free radical scavengers and lipid peroxide in thyroid glands of various thyroid disorders. Horm Metab Res. 1997;29:351–354. doi: 10.1055/s-2007-979052. [DOI] [PubMed] [Google Scholar]
- 29.Ademoglu E, Ozbey N, Erbil Y, Tanrikulu S, Barbaros U, Yanik BT, Bozbora A, Ozarmagan S. Determination of oxidative stress in thyroid tissue and plasma of patients with Graves' disease. Eur J Intern Med. 2006;17:545–550. doi: 10.1016/j.ejim.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Seven A, Tasan E, Inci F, Hatemi H, Burçak G. Biochemical evaluation of oxidative stress in propylthiouracil treated hyperthyroid patients. Effects of vitamin C supplementation. Clin Chem Lab Med. 1998;36:767–770. doi: 10.1515/CCLM.1998.136. [DOI] [PubMed] [Google Scholar]
- 31.Guerra LN, Moiguer S, Karner M, de Molina MC, Sreider CM, Burdman JA. Antioxidants in the treatment of Graves disease. IUBMB Life. 2001;51:105–109. doi: 10.1080/15216540152122102. [DOI] [PubMed] [Google Scholar]
- 32.Vrca VB, Skreb F, Cepelak I, Romic Z, Mayer L. Supplementation with antioxidants in the treatment of Graves' disease; the effect on glutathione peroxidase activity and concentration of selenium. Clin Chim Acta. 2004;341:55–63. doi: 10.1016/j.cccn.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Bahn RS. Graves' ophthalmopathy. New Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartalena L, Tanda ML, Piantanida E, Lai A. Oxidative stress and Graves' ophthalmopathy: in vitro studies and therapeutic implications. Biofactors. 1003;19:155–163. doi: 10.1002/biof.5520190308. [DOI] [PubMed] [Google Scholar]
- 35.Burch HB, Lahiri S, Bahn RS, Barnes S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves' ophthalmopathy. Exp Eye Res. 1997;65:311–316. doi: 10.1006/exer.1997.0353. [DOI] [PubMed] [Google Scholar]
- 36.Heufelder AE, Wenzel BE, Bahn RS. Methimazole and propylthiouracil inhibit the oxygen free radical-induced expression of a 72 kilodalton heat shock protein in Graves' retroocular fibroblasts. J Clin Endocrinol Metab. 1992;74:737–742. doi: 10.1210/jcem.74.4.1532179. [DOI] [PubMed] [Google Scholar]
- 37.Lu R, Wang P, Wartofsky L, Sutton BD, Zweier JL, Bahn RS, Garrity J, Burman KD. Oxygen free radicals in interleukin-1beta-induced glycosaminoglycan production by retro-ocular fibroblasts from normal subjects and Graves' ophthalmopathy patients. Thyroid. 1999;9:297–303. doi: 10.1089/thy.1999.9.297. [DOI] [PubMed] [Google Scholar]
- 38.Hondur A, Konuk O, Dincel AS, Bilgihan A, Unal M, Hasanreisoglu B. Oxidative stress and antioxidant activity in orbital fibroadipose tissue in Graves' ophthalmopathy. Curr Eye Res. 2008;33:421–427. doi: 10.1080/02713680802123532. [DOI] [PubMed] [Google Scholar]
- 39.Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Chiou SH, Hsu WM, Wei YH. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves' ophthalmopathy: evidence that oxidative stress has a role in this disorder. Eye (Lond) 2010;24:1520–1525. doi: 10.1038/eye.2010.31. [DOI] [PubMed] [Google Scholar]
- 40.Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Lee SM, Wei YH. Increased response to oxidative stress challenge in Graves' ophthalmopathy orbital fibroblasts. Mol Vis. 2011;17:2782–2788. [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai CC, Kao SC, Cheng CY, Kau HC, Hsu WM, Lee CF, Wei YH. Oxidative stress change by systemic corticosteroid treatment among patients having active Graves ophthalmopathy. Arch Ophthalmol. 2007;125:1652–1656. doi: 10.1001/archopht.125.12.1652. [DOI] [PubMed] [Google Scholar]
- 42.Tsai CC, Cheng CY, Liu CY, Kao SC, Kau HC, Hsu WM, Wei YH. Oxidative stress in patients with Graves' ophthalmopathy: relationship between oxidative DNA damage and clinical evolution. Eye (Lond) 2009;23:1725–1730. doi: 10.1038/eye.2008.310. [DOI] [PubMed] [Google Scholar]
- 43.Akarsu E, Buyukhatipoglu H, Aktaran S, Kurtul N. Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2011;74:118–124. doi: 10.1111/j.1365-2265.2010.03904.x. [DOI] [PubMed] [Google Scholar]
- 44.Bouzas EA, Karadimas P, Mastorakos G, Koutras DA. Antioxidant agents in the treatment of Graves' ophthalmopathy. Am J Ophthalmol. 2000;129:618–622. doi: 10.1016/s0002-9394(00)00359-7. [DOI] [PubMed] [Google Scholar]
- 45.Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, Altea MA, Nardi M, Pitz S, Boboridis K, Sivelli P, von Arx G, Mourits MP, Baldeschi L, Bencivelli W, Wiersinga W, European Group on Graves' Orbitopathy Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011;364:1920–1931. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]