Abstract
Objective
To assess the impact on cord blood (CB) thyroglobulin (Tg) of early iodine supplementation during pregnancy.
Methods
A total of 111 healthy pregnant women with normal thyroid function were included in a prospective randomized study and divided into two groups with (150 μg/day) or without iodine supplementation started during the first trimester. Maternal smoking was assessed qualitatively by self-reported statements and quantitatively by cotininuria. Exhaustive thyroid tests were performed at delivery in the mother and in CB.
Results
Third-trimester ioduria documented compliance with iodine supplementation (160 vs. 76 μg/l in controls). CB Tg was not different between the iodine and control groups (median 77 vs. 79.5 ng/ml, respectively) and did not correlate with maternal ioduria. CB Tg was higher in newborns from smoking mothers (114 vs. 64.7 ng/ml) and correlated with self-reported smoking status more than with maternal cotininuria. Nonsmokers had no difference in CB Tg whether they took iodine supplementation or not, as opposed to smokers, who tended to benefit from supplementation.
Conclusions
Iodine supplementation does not significantly impact CB Tg in healthy nonsmoker pregnant women selected for normal thyroid function, as opposed to maternal smoking. CB Tg appears to be a marker of in utero tobacco exposure. In areas of mild iodine deficiency, iodine supplementation could especially benefit the fetuses of smokers.
Key Words: Iodine, Smoking, Thyroglobulin, Cord blood, Pregnancy, Thyroid tests
Introduction
Mild iodine deficiency (ID) is still common in Western Europe, despite government programs aiming at its eradication [1]. Although spot ioduria (urinary iodine excretion, UIE) is widely used to diagnose ID at the population level [1,2], serum thyroglobulin (Tg) has been proposed as a marker of ID [3], including in newborns [4,5]. Maternal iodine supplementation has been shown to improve maternal and cord blood (CB) thyroid tests in areas of moderate and severe ID [6,7]. Despite WHO recommendations for universal iodine prophylaxis in pregnancy, this is not endorsed by the health authorities in France, an area of mild ID or borderline iodine status [8], where prescription of supplementation is left to the discretion of the physician. Data about the effect of such iodine prophylaxis on maternal and fetal thyroid function in pregnant women in areas of mild ID are scarce [9,10], and it remains to be assessed whether such supplementation is beneficial for pregnant women with initial normal thyroid function. In addition, smoking has a complex impact on thyroid function [11,12]. During pregnancy, it has been reported that maternal smoking may affect fetal thyroid function [13,14,15,16,17,18,19]. Despite recommendations, many women continue to smoke during pregnancy. Thus, the relative impact of iodine supplementation and maternal smoking deserves a closer look.
We designed a prospective study to assess the impact on CB Tg of early iodine supplementation in healthy pregnant women with special attention to maternal smoking status.
Patients and Methods
Women without a personal history of thyroid disease attending the obstetric clinic before 12 weeks of amenorrhea with a singleton pregnancy between July 2007 and July 2008 were eligible if they had strictly normal thyroid tests on their initial screening, i.e. free thyroxine (fT4) >12 pmol/l (10th percentile for first trimester in our laboratory), thyroid-stimulating hormone (TSH) <2.5 mUI/l (recommended upper limit of normal in pregnancy [20]) and negative anti-thyroid peroxidase antibodies below 100 UI/ml.
Design of the Study
After signing a consent form, 111 women were included in this study. At baseline, in addition to measurement of fT4, TSH and anti-thyroid peroxidase antibodies, they underwent comprehensive thyroid testing, including Tg, anti-Tg antibodies, free triiodothyronine (fT3), reverse T3 (rT3), total T4, spot ioduria and thyroid ultrasound. They were then randomized into two groups, one group receiving iodine-enriched pregnancy vitamins (150 μg/day, Oligobs Maxiode, Laboratoire C.C.D., Paris, France) and the other group receiving the same vitamin mix but without iodine (Oligobs grossesse, Laboratoire C.C.D.). The table of randomization (list of correspondence between the number of a patient and the study arm, i.e. iodine or not) was obtained by a method of drawing of lots by blocks, using tables of permutation. This was an open study.
Treatment was given from the day of enrolment throughout pregnancy and until 3 months postpartum. Compliance with pregnancy vitamin administration was assessed by the hospital pharmacist, based on the number of pills brought back at each visit (patients were asked to bring back all blisters, empty or not). One woman from the iodine-supplemented group was excluded shortly after inclusion when her baseline ioduria was discovered to be over 400 μg/l, suggesting possible iodine excess before supplementation.
Comprehensive thyroid tests were performed in each trimester, at delivery and at the 3-month postpartum visit. At delivery, CB samples were collected and analyzed for thyroid tests, including Tg, anti-Tg antibodies, fT4, fT3, TSH and rT3.
Assays
Spot UIE was measured by inductively coupled plasma mass spectrometry [Pasteur-Cerba Laboratory, Cergy Pontoise, France; detection threshold 15 μg/l; intra- and interseries coefficient of variation (CV) <10%]. Tg was measured by immunoradiometric assay (Thyroglobulin IRMA, Cis bio International, Gif-sur-Yvette, France). fT4, fT3, total T4, TSH and anti-Tg antibodies were measured by chemiluminescence (ADVIA Centaur, Siemens Healthcare Diagnostics, France), while rT3 was measured by radioimmunoassay (Pasteur-Cerba Laboratory). Reference ranges were established in our laboratory for fT4 and TSH during the first trimester of pregnancy (2.5 and 97.5 percentiles): fT4 11.47–19.23 pmol/l, and TSH 0.053–3.23 mUI/l. The other (nonpregnancy) reference ranges were provided by the manufacturers: Tg 5–50 ng/ml; anti-Tg antibodies <60 UI/ml; fT3 3–7 pmol/l, and rT3 0.14–0.54 nmol/l.
The intra- and interassay CVs, respectively, were as follows: fT4, 2.31 and 1.95%; TSH, 2.67 and 3.97%; Tg, 2.4 and 4.5%; anti-Tg, 5.5 and 1.8%; fT3, intra-assay CV 2.35%, and rT3, 8.54 and 6.21%.
Smoking Status
Maternal smoking was assessed qualitatively at baseline by self-reported statement as never smoked, current smokers and former smokers (women who quit smoking before pregnancy or when pregnancy was diagnosed) and quantitatively during the third trimester by measuring cotininuria. A simple reverse-phase high-performance liquid chromatographic method with UV detection adapted from Oddoze et al. [21] was used for the quantification of urinary cotinine. After a one-step solid-liquid extraction on Clean-Screen, cotinine was quantified by a Waters 2695 apparatus (Guyancourt, France) and Waters 996 photodiode array detector. The threshold of detection was 10 μg/l.
Statistics
The primary end-point of our study was the comparison of maternal Tg concentrations at delivery between the two groups, according to iodine supplementation. The sample size (number of patients to be included) was determined by our methodologist (F.B.) based on the paper by Glinoer et al. [7]. Additional end-points were the comparison and evolution of the other thyroid tests in the mother and CB, including CB Tg. Further analyses of the interaction of iodine supplementation and maternal smoking were post hoc analyses.
Quantitative variables are expressed as means and standard deviation or medians and range. Qualitative variables are expressed as counts and percentages. Student's t test or Mann-Whitney's U test were used to compare continuous variables. The χ2 test or Fisher's exact test were used for categorical variables. The correlations between variables were determined using Pearson's test or Spearman's rank test. The nonparametric Kruskal-Wallis test was used to examine associations between maternal smoking and thyroid function tests.
Statistical analyses were performed using the software SAS version 9.1. Values were considered significant at p < 0.05. All tests were two sided.
The study was approved by the ethics committee of our institution.
Results
Clinical Results
Out of the 110 women, 86 could be followed until delivery with CB measurement, making 86 mother/fetus pairs. Twenty-four women did not complete the study (20 in the iodine group and 4 in the control group). In the iodine group, there were 4 miscarriages, 1 induced abortion (trisomy 18), 10 women with digestive intolerance (nausea/vomiting), 3 consent withdrawals and 2 missing in collection; in the control group, there were 3 miscarriages and 1 woman with digestive intolerance. Clinical characteristics of the 86 pairs (32 in the iodine group and 54 in the control group) are shown in table 1 and did not differ. One quarter of the women were current smokers; the others were referred to as ‘nonsmokers’ (never smokers and former smokers together). Babies from women who took iodine had a lower birth weight and smaller length with similar duration of gestation.
Table 1.
Clinical characteristics at inclusion of 86 mother/newborn pairs
| Iodine supplementation (n = 32) | Controls (n = 54) | p | |
|---|---|---|---|
| Mothers | |||
| Age, years | 28 (21–37) | 27 (18–39) | NS |
| GA at first visit, weeks | 8 (5–12) | 8 (5–12) | NS |
| BMI | 21.9 (17.9–30.8) | 22.3 (16–39.5) | NS |
| Parity, n | 0 (0–2) | 0 (0–4) | NS |
| Smoking status at first visit, n | |||
| Nonsmokers1 | 23 (74.2%) | 39 (72.2%) | NS |
| Smokers | 8 (25.8%) | 15 (27.8%) | NS |
| Newborns | |||
| Term, weeks | 39.4 (35–41.4) | 40 (33–41.9) | NS |
| Mode of delivery, n | NS | ||
| Vaginal | 24 (75%) | 46 (85%) | |
| Cesarian section | 8 (25%) | 8 (15%) | |
| Birth weight, g | 3,160 (2,170–3,780) | 3,420 (2,050–4,770) | 0.017 |
| Birth length, cm | 48 (45.5–51.5) | 49 (45–55) | 0.04 |
Results are expressed as medians (range), except where indicated otherwise. GA = Gestational age; BMI = body mass index; NS = not significant.
The category of nonsmokers at inclusion comprises women who declared they had never smoked and those who quit smoking before or at the diagnosis of pregnancy.
Biological Results
Results in mothers and in CB are shown in table 2 according to iodine supplementation and in table 3 according to maternal smoking status.
Table 2.
Biological results according to iodine supplementation in pregnant women and in CB
| Iodine | Controls | p | |
|---|---|---|---|
| Mothers: V1 | (n = 32) | (n = 54) | |
| Tg, ng/ml | 17 (2.4–45.1) | 17.5 (1.3–84.1) | NS |
| Anti-Tg, UI/ml | 20.9 (6.4–235.2) | 19.5 (0–164.7) | NS |
| Anti-Tg >60 UI/ml, n | 2 | 5 | NS |
| fT4, pmol/l | 14.1 (12–17.2) | 14.8 (12–18.5) | NS |
| fT3, pmol/l | 5.1 (3.8–6.7) | 5 (4.1–6.2) | NS |
| TSH, mUI/l | 1.2 (0.1–2.4) | 1.2 (0.2–2.4) | NS |
| UIE, μg/l | 111 (28–399) | 103 (14–355) | NS |
| Mothers: V4 | (n = 27) | (n = 49) | |
| Tg, ng/ml | 15.7 (2.7–62) | 25 (2–112) | <0.02 |
| Anti-Tg, UI/ml | 24 (0–154) | 24 (8–43) | NS |
| Anti-Tg >60 UI/ml, n | 1 | 0 | NS |
| fT4, pmol/l | 11 (8–15.5) | 11.7 (7.4–14.2) | NS |
| TSH, mUI/l | 1.6 (0.7–4.2) | 2 (0.2–5.4) | 0.06 |
| fT3, pmol/l | 4 (3–5) | 4 (3–5) | NS |
| UIE (3rd trimester), μg/l | 160 (18–358) | 76 (16–303) | <0.0001 |
| CB | (n = 27) | (n = 47) | |
| Tg, ng/ml | 77 (10–176) | 79.5 (15–336) | NS |
| Anti-Tg, UI/ml | 13.5 (0–65) | 16 (5.5–29.5) | NS |
| Anti-Tg >60 UI/ml, n | 1 | 0 | NS |
| fT4, pmol/l | 13.2 (10.6–17) | 12.6 (9.5–17) | NS |
| TSH, mUI/l | 7.5 (2.5–19) | 6.2 (1.8–24.5) | 0.1 |
| fT3, pmol/l | 2.2 (1.4–4.8) | 2.2 (1.4–4.6) | NS |
| rT3, nmol/l | 3.2 (0.3–8) | 3.8 (1.5–16.4) | 0.05 |
Results are expressed as medians (range), except where indicated otherwise. V1: data from assessment at inclusion (first trimester); V4: data from assessment at delivery.
NS = Not significant.
Table 3.
Biological results according to smoking status in pregnant women and in CB
| Smokers | Nonsmokers | p | |
|---|---|---|---|
| Mothers | (n = 21) | (n = 54) | |
| Ioduria | |||
| (3rd trimester), μg/l | 69 (16–193) | 125 (18–358) | 0.007 |
| Iodine supplementation | 28.6% | 40.4% | NS |
| Cotininuria | |||
| (3rd trimester), μg/l | 129 (0–364) | 0 (0–77) | <0.0001 |
| Mothers: V4 | |||
| Tg, ng/ml | 26.9 (5.4–112) | 19.7 (2.1–107) | NS |
| Anti-Tg, UI/ml | 25 (9–154) | 23 (0–45) | NS |
| Anti-Tg >60 UI/ml, n | 1 | 0 | NS |
| fT4, pmol/l | 10.9 (8.1–14.4) | 11.7 (7.4–15.5) | 0.02 |
| TSH, mUI/l | 2 (0.4–3.2) | 1.8 (0.2–5.4) | NS |
| fT3, pmol/l | 4.2 (3.5–4.8) | 4 (3–5) | NS |
| CB | (n = 21) | (n = 52) | |
| Tg, ng/ml | 114 (29–336) | 64.7 (10–176) | <0.005 |
| Anti-Tg, UI/ml | 18 (5.5–65) | 14 (0–29.5) | NS |
| fT4, pmol/l | 14.7 (9.8–16.6) | 12.7 (9.5–17.4) | 0.09 |
| TSH, mUI/l | 6.4 (1.8–19) | 6.3 (2.5–24.5) | NS |
| fT3, pmol/l | 2.2 (1.8–4) | 2.2 (1.4–4.8) | NS |
| rT3, nmol/l | 4 (2–16.4) | 3.3 (0.3–8.8) | NS |
Results are expressed as medians (range), except where indicated otherwise. V4: data from assessment at delivery. NS = Not significant.
Maternal Results
Ioduria and thyroid assessment at inclusion were similar in the iodine and control groups, with UIE below the recommended threshold in pregnancy of 150 μg/l [2]. Ioduria in the third trimester was higher in the iodine group (160 vs. 76 μg/l), in agreement with the good compliance reported by our pharmacist (data not shown). At delivery, in the iodine group, Tg was significantly lower and TSH tended to be lower, but there was no difference in fT4 or other thyroid tests.
Cotininuria in the third trimester was higher in the smoking group, correlating well with self-reported smoking status (p < 0.0001). Of note, 5 nonsmokers had positive cotininuria, while 4 smokers had undetectable levels. fT4 was lower in smokers, but there was no significant difference for Tg or TSH.
CB Results
CB Tg was similar in the iodine and control groups (median 77 vs. 79.5 ng/ml, respectively), while it was significantly higher in newborns from smoking mothers versus newborns from nonsmoking mothers (114 vs. 64.7 ng/ml; p < 0.005). CB anti-Tg antibodies were negative, except for 1 sample from the iodine group at a very low titer. Results of thyroid testing in CB showed no difference in fT4 and fT3, with a trend in the iodine group for higher TSH (7.5 vs. 6.2 mUI/l) and a significantly lower rT3 (3.2 vs. 3.8 nmol/l). fT4 tended to be higher in newborns from smoking mothers.
Table 4 shows the correlations between CB Tg and the various parameters studied. CB Tg correlated with birth size and maternal Tg at delivery. Of interest, it also correlated with self-reported smoking status and to a lesser degree with third-trimester maternal cotininuria, but not with maternal ioduria.
Table 4.
Analysis of CB Tg by linear regression
| p | R2 | |
|---|---|---|
| Univariate analysis | ||
| Significant correlations | ||
| Maternal smoking status | 0.0029 | 0.13 |
| Third-trimester cotininuria | 0.007 | 0.24 |
| Birth size | 0.0037 | 0.14 |
| Maternal Tg at delivery | 0.03 | 0.07 |
| Nonsignificant correlations | ||
| Iodine supplementation | 0.2 | |
| Maternal ioduria in first, second and | respectively: 0.25, | |
| third trimester | 0.28, 0.14 | |
| Mode of delivery | 0.23 | |
| Term | 0.97 | |
| Birth weight | 0.26 | |
| Gender of newborn | 0.19 | |
| Newborn thyroid tests | respectively: 0.4, | |
| (fT4, fT3, rT3, TSH) | 0.67, 0.16, 0.4 | |
| Multivariate analysis | ||
| Maternal smoking | 0.001 | 0.18 |
| Birth size and maternal smoking | 0.003 | 0.3 |
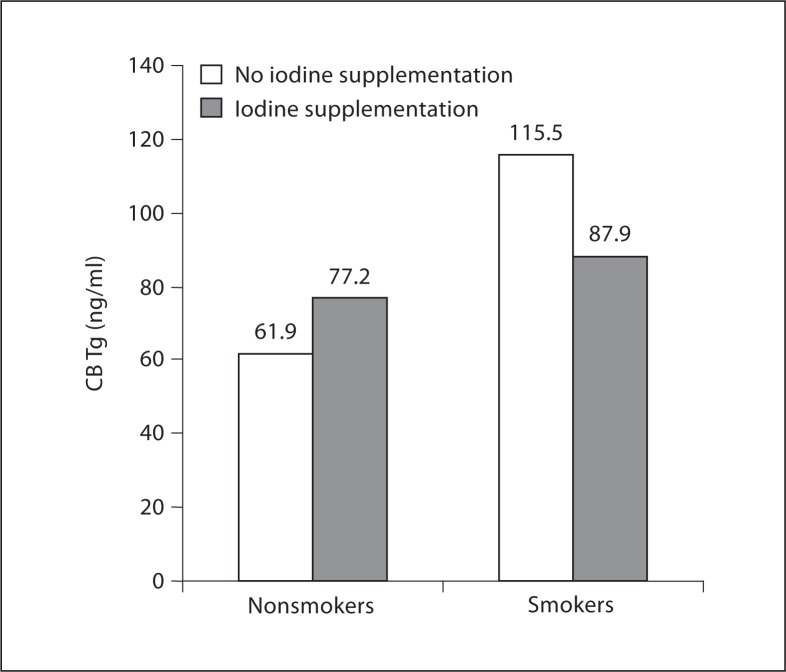
Figure 1 shows that newborns from nonsmokers had no difference in CB Tg whether their mother took iodine supplementation or not (77.2 vs. 61.9 ng/ml), as opposed to newborns from smokers, who tended to benefit from maternal iodine supplementation (87.9 ng/ml in the iodine group vs. 115.5 ng/ml in the control group; p = 0.1). Tg of smokers from the control group was significantly higher than the Tg from nonsmokers whether they took iodine or not (p < 0.01). It tended to be higher than the Tg of smokers from the group taking iodine supplementation (p = 0.1). On the other hand, Tg of nonsmokers was not different whether they took iodine or not, nor from the Tg of smokers who took iodine.
Fig. 1.
CB Tg according to maternal smoking and iodine supplementation. Results are expressed as medians. The CB Tg of smokers from the control group was significantly higher than the CB Tg from nonsmokers whether they took iodine (p = 0.007) or not (p = 0.002). It also tended to be higher than the CB Tg of smokers from the group taking iodine supplementation (p = 0.1). On the other hand, the Tg of nonsmokers was not different whether they took iodine or not, nor from the Tg of smokers who took iodine.
Discussion
We present data from our interventional, randomized study of iodine supplementation from the first trimester throughout pregnancy in 111 women carefully selected for initial strictly normal thyroid tests and their newborns (86 pairs at delivery). With such a design, it is unavoidable that some women do not complete the study. Women were enrolled very early (8 weeks of gestation), with the risk of first-trimester miscarriage. The imbalance in numbers at delivery between the iodine and control groups is explained by the fact that most women who did not complete the study belonged to the iodine group, mainly due to digestive intolerance to iodinated vitamins. There was no difference in miscarriage rates between the two groups.
Mild ID was present at the first-trimester visit in our population of healthy women without a personal history of thyroid disease. This ID shifted from the mild to moderate ID range in the third trimester in the group that did not receive iodine supplementation, while in the group receiving 150 μg of iodine/day, the median UIE rose to 160 μg/l, in the range of adequate iodine nutrition in pregnancy [2]. This result supports iodine supplementation throughout pregnancy. Of note, third-trimester UIE in women from the control group was similar to results we reported earlier in a third-trimester cross-sectional study of 330 unselected pregnant women [22].
Effect of Iodine and Tobacco on CB Tg
CB Tg of newborns from women with iodine supplementation was not different from CB Tg of newborns from women without iodine supplementation. Anti-Tg antibodies were negative, which eliminates assay interference for the level of Tg, thus validating the results of Tg measurements. As shown in table 5, which summarizes the literature on the impact of iodine supplementation and maternal smoking on Tg and other thyroid tests, there are very few studies on CB Tg and iodine supplementation [6,7,9]. In an observational study, Nohr and Laurberg [9] reported that newborns from unselected women, studied only at delivery and who declared that they took iodinated vitamins during pregnancy, had CB Tg lower than that from women who did not take iodinated vitamins. Our data are apparently at odds with the studies of Glinoer et al. [7] and Pedersen et al. [6], who reported higher CB Tg in newborns from women who did not take iodine supplementation. This might be explained by a difference in the enrolled women. Indeed, our patients were selected for their strictly normal initial thyroid function, while women from the other studies were either unselected [6] or were selected because of excessive thyroid stimulation based on elevated Tg, a low normal fT4 index and an elevated molar ratio of T3 to T4 [7]. Furthermore, they had much lower UIE (median 50 μg/l in Pedersen et al. [6] and 36 μg/l in Glinoer et al. [7]), reflecting more severe ID in these populations.
Table 5.
Impact of iodine supplementation and maternal smoking on Tg and other thyroid tests
| a Thyroglobulin | |||||
|---|---|---|---|---|---|
| Type of study | Iodine impact | Dose, μg/day | Tobacco impact | ||
| CB | |||||
| Our study | interventional | (n = 86) | similar | 150 | higher |
| Nohr and Laurberg [9] | observational | (n = 139) | lower | 150 | – |
| Pedersen et al. [6] | interventional | (n = 54) | lower | 200 | – |
| Glinoer et al. [7] | interventional | (n = 181) | lower | 100 ± LT4 | – |
| Ericsson et al. [14] | observational | (n = 160) | – | higher | |
| Gasparoni et al. [15] | observational | (n = 54) | – | higher | |
| Maternal | |||||
| Our study | interventional | (n = 86) | lower | 150 | similar |
| Nohr and Laurberg [9] | observational | (n = 144) | lower | 150 | – |
| Pedersen et al. [6] | interventional | (n = 54) | lower | 200 | – |
| Glinoer et al. [7] | interventional | (n = 181) | lower | 100 ± LT4 | – |
| Antonangeli et al. [10] | interventional | (n = 86) | similar | 50 or 200 | – |
| Liesenkötter et al. [28] | interventional | (n = 108) | similar | 300 | – |
| b Other thyroid tests | |||||
| CB | |||||
| TSH | |||||
| Our study | interventional | (n = 86) | trend higher | 150 | similar |
| Nohr and Laurberg [9] | observational | (n = 139) | higher | 150 | – |
| Pedersen et al. [6] | interventional | (n = 54) | similar | 200 | – |
| Glinoer et al. [7] | interventional | (n = 181) | similar | 100 ± LT4 | – |
| Velasco et al. [30] | observational | (n = 194) | higher | 300 | – |
| Ericsson et al. [14] | observational | (n = 160) | – | similar | |
| Gasparoni et al. [15] | observational | (n = 54) | – | similar | |
| McDonald et al. [16] | observational | (n = 104) | – | similar | |
| Meberg and Marstein [17] | observational | (n = 95) | – | lower | |
| Shields et al. [18] | observational | (n = 618) | – | lower | |
| fT4 | |||||
| Our study | interventional | (n = 86) | similar | 150 | similar |
| Nohr and Laurberg [9] | observational | (n = 139) | higher | 150 | – |
| Pedersen et al. [6] | interventional | (n = 54) | similar | 200 | – |
| Glinoer et al. [7] | interventional | (n = 181) | similar | 100 ± LT4 | – |
| Gasparoni et al. [15] | observational | (n = 54) | – | similar | |
| McDonald et al. [16] | observational | (n = 104) | – | similar | |
| Meberg and Marstein [17] | observational | (n = 95) | – | trend higher (fT4I) | |
| Shields et al. [18] | observational | (n = 618) | – | similar | |
| rT3 | |||||
| Our study | interventional | (n = 86) | lower | 150 | similar |
| Maternal | |||||
| TSH | |||||
| Our study | interventional | (n = 86) | trend lower | 150 | similar |
| Nohr and Laurberg [9] | observational | (n = 144) | lower | 150 | – |
| Glinoer et al. [7] | interventional | (n = 181) | lower | 100 ± LT4 | – |
| Antonangeli et al. [10] | interventional | (n = 86) | similar | 50 or 200 | – |
| Velasco et al. [30] | |||||
| (3rd trimester) | observational | (n = 194) | lower | 300 | – |
| McDonald et al. [16] | observational | (n = 104) | – | lower | |
| Shields et al. [18] | |||||
| (3rd trimester) | observational | (n = 927) | – | lower (NS in TPO−) | |
| Liesenkötter et al. [28] | interventional | (n = 108) | similar | 300 | – |
| Pearce et al. [29] | |||||
| (1st trimester) | observational | (n = 585) | similar | unknown | similar |
| fT4 | |||||
| Our study | interventional | (n = 86) | similar | 150 | lower |
| Nohr and Laurberg [9] | observational | (n = 144) | higher | 150 | – |
| Pedersen et al. [6] | interventional | (n = 54) | similar | 200 | |
| Glinoer et al. [7] | interventional | (n = 181) | similar if I alone | 100 ± LT4 | – |
| Antonangeli et al. [10] | interventional | (n = 86) | similar | 50 or 200 | – |
| Velasco et al. [30] | |||||
| (3rd trimester) | observational | (n=194) | lower | 300 | |
| Pearce et al. [29] | |||||
| (1st trimester) | observational | (n = 585) | similar (fT4I) | unknown | lower (fT4I) |
| McDonald et al. [16] | observational | (n = 104) | – | similar | |
| Shields et al. [18] | |||||
| (3rd trimester) | observational | (n = 927) | – | similar | |
| fT3 | |||||
| Our study | interventional | (n = 86) | similar | similar | |
| Antonangeli et al. [10] | interventional | (n = 86) | similar | 50 or 200 | – |
| Velasco et al. [30] | |||||
| (3rd trimester) | observational | (n = 194) | lower | 300 | – |
| Shields et al. [18] | |||||
| (3rd trimester) | observational | (n = 927) | – | higher | |
LT4 = Levothyroxine; fT4I = freeT4 index; NS = not significant; TPO– = negative anti TPO antibodies.
The main correlation for CB Tg in our population was with maternal smoking, while there was no correlation with maternal UIE in the third trimester, nor with other parameters that have been proposed by some authors, including mode of delivery [14] and gestational age [5]. Thus, it appears that smoking is the main factor explaining CB Tg in our population. In 1987, Ericsson et al. [14] already showed that CB Tg concentrations were higher in newborns from smokers. Chanoine et al. [13], in 1991, showed a positive correlation between CB Tg and CB thiocyanate, with an increase in the ratio of fetal thyroid volume to fetal birth weight. A larger neonatal thyroid volume linked to maternal smoking was also reported by Klett et al. [23]. In 1998, Gasparoni et al. [15] reported that CB Tg was higher in newborns from smoking parents (mother and/or father) than in those from nonsmoking parents; these results were confirmed at 1 year of age. This study underlined the effect of passive smoking in babies, their results being confirmed by the level of thiocyanate. We did not measure thiocyanate, but we used maternal cotininuria as an objective short-term marker of active or even passive smoking. Cotininuria usually correlated very well with self-reported maternal smoking. Occasional discrepancies between spot cotininuria and self-reported smoking status could be explained by the fact that cotininuria only reflects smoking habits within the last 48 h. A false negative result (negative cotininuria in smokers) could reflect a transient change in smoking habits. On the other hand, the presence of positive cotininuria in self-reported nonsmokers (false positive) could reflect passive smoking or inaccuracy of self-report of smoking status. Of note, in our population maternal smoking was also associated with lower birth weight (3,060 vs. 3,345 g; p < 0.005), as reported elsewhere [17].
Iodine supplementation seems to mitigate the negative impact of tobacco on Tg. Indeed, CB Tg from newborns of smoking mothers who took iodine supplementation tended to be lower than CB Tg from newborns of control smoking mothers, though the numbers were small; furthermore, the CB Tg was not different from nonsmokers. This effect of iodine in smokers has also been suggested to impact thyroid volume or goiter in adults of the general population [24,25]. In addition, two studies of large nonpregnant cohorts, one in Korea [26] and one in the USA [27], have brought different results about the interaction between smoking and iodine nutrition. Cho et al. [26] suggest that in the case of iodine excess, smoking decreases the risk of subclinical hypothyroidism in an iodine-sufficient area. Vanderver et al. [27] report that the impact of smoking on the prevalence of hypothyroxinemia is limited to women of childbearing age with the highest UIE. Thus, the respective impact of iodine supplementation and maternal smoking on Tg that we report here in healthy pregnant women should be confirmed in a larger series.
Effect of Iodine Supplementation on Other Thyroid Tests
Though our study focused on CB Tg, we also report interesting results of the impact of iodine supplementation on other thyroid tests, both in mothers and newborns.
Firstly, there was a positive effect on maternal Tg (lower Tg in the iodine group), contrasting with the absence of an effect on CB Tg. Similar results in mothers have been found by others [6,7,9], but not all [10,28] (table 5). This shows that, even in normal women, iodine may have a beneficial effect on maternal thyroid economy, as assessed by Tg. However, we only found a trend for lower maternal TSH and no effect on fT4. Of note, in a large cohort studied during the first trimester, Pearce et al. [29] found no correlation of TSH and fT4 with the use of iodine-containing multivitamins.
In the newborns, we mainly observed a lower CB rT3 and a trend for a higher TSH with similar fT4 in newborns of women taking iodine supplementation. Nohr and Laurberg [9] reported higher CB TSH and fT4 in newborns from women taking iodinated vitamins, noting an opposite effect on maternal and CB TSH and suggesting that ‘at least in areas of mild ID, fetal thyroid is more sensitive to the inhibitory effect of iodine than anticipated hitherto’. In addition, Velasco et al. [30] reported CB TSH levels 2-fold higher in the group of newborns from women who took 300 μg of iodine/day (above the recommended supplementation) compared to controls; however, they did not measure fT4. The significance of higher TSH in CB from infants of women taking iodine supplementation is uncertain, and paradoxically proposed by some as beneficial [31], but probably depending on the dosage administered. Data on CB TSH seem to be in contrast with those of neonatal TSH measured at 3–4 days of life for the screening of congenital hypothyroidism and which are considered by some authors as a sensitive indicator of iodine nutrition during pregnancy [32,33]. Data on rT3 are lacking. Our results showing lower rT3 with iodine supplementation could suggest an effect of iodine supplementation on placental type 3 deiodinase activity.
Effect of Maternal Smoking on Other Thyroid Tests
In CB, we found no significant difference in the results of thyroid tests. Review of the literature shows scarce and sometimes conflicting results [14,15,16,17,18] (table 5), usually with no information provided on iodine status.
In the mothers, we found a significant negative impact of smoking on maternal fT4 at delivery, but no detectable effect on TSH or other thyroid tests, results similar to those of Pearce et al. [29] during the first trimester, while others have reported similar fT4 and lower TSH [16,18] (table 5). Outside pregnancy, TSH is usually reported to be lower in smokers [24,26], and fT4 is variably reported as lower [19,27], higher [24] or similar [26].
The action of tobacco on the thyroid is known to be complex, with discrepancies likely due to different factors. Tobacco components may have different effects (thiocyanate, nicotine and others), with the intensity of smoking found to be significant by some authors [27], but not all [26]. For example, thiocyanate is a potent antithyroid compound in vitro [34], while in vivo studies suggest a possible stimulating effect [12]. On the other hand, Gondou et al. [35] have reported an effect of nicotine on type 3 deiodinase activity in vitro. Other environmental factors may mitigate the effect of smoking, such as ID and exposure to thyroid disruptors [36,37]. In addition, some factors contributing to discrepancies are linked to the population studied, e.g. pregnancy status or individual susceptibility to thyroid disease. Tobacco is supposed to have both a stimulating effect on thyroid function in normal patients and a hypothyroid and/or goitrogenic effect on patients with a predisposition to hypothyroidism [11,12,17]. Pregnancy is an additional stressor on the maternal thyroid and could reveal hypothyroxinemia in smokers in a context of ID.
Conclusion
Iodine supplementation did not seem to significantly impact CB Tg in our population of normal pregnant women. By contrast, self-reported maternal smoking is associated with a stimulation of the fetal thyroid as assessed by CB Tg, which appears to be a marker of in utero exposure to tobacco. Iodine supplementation during pregnancy could especially benefit the fetuses of mothers addicted to tobacco in areas of ID. Along with optimization of iodine nutrition at the population level [38], public health policies to protect fetal thyroid function should include encouragement and help for mothers to quit smoking.
Disclosure Statement
There is no conflict of interest.
Acknowledgements
We want to thank the women for their participation, Eva Baez, our Data Manager, and the obstetric staff who performed the deliveries. This study was funded by a grant from the French Health Ministry (PHRC 2005) and was promoted by the Direction de la Recherche Clinique of the University Hospital of Nice.
References
- 1.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 2.WHO Secretariat. Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women, and in children less than 2 years of age: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–1611. doi: 10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- 3.Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Carlé A, Bülow Pedersen I, Rasmussen L, Ovesen L, Jorgensen T. Thyroglobulin as a marker of iodine nutrition status in the general population. Eur J Endocrinol. 2009;161:475–481. doi: 10.1530/EJE-09-0262. [DOI] [PubMed] [Google Scholar]
- 4.Sava L, Tomaselli L, Runello F, Belfiore A, Vigneri R. Serum thyroglobulin levels are elevated in newborns from iodine-deficient areas. J Clin Endocrinol Metab. 1986;62:429–432. doi: 10.1210/jcem-62-2-429. [DOI] [PubMed] [Google Scholar]
- 5.Pacini F, Lari R, La Ricca P, Grasso L, Taddei D, Bardini N, Fenzi GF, Di Bartolo F, Baschieri L, Pinchera A. Serum thyroglobulin in newborns’ cord blood, in childhood and adolescence: a physiological indicator of thyroidal status. J Endocrinol Invest. 1984;7:467–471. doi: 10.1007/BF03348452. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS, Larsen KR, Eriksen GM, Johannesen PL. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab. 1993;77:1078–1083. doi: 10.1210/jcem.77.4.8408456. [DOI] [PubMed] [Google Scholar]
- 7.Glinoer, D, De Nayer P, Delange F, Lemone M, Toppet V, Spehl M, Grün JP, Kinthaert J, Lejeune B. A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab. 1995;80:258–269. doi: 10.1210/jcem.80.1.7829623. [DOI] [PubMed] [Google Scholar]
- 8.Institut de Veille Sanitaire L’état de santé de la population en France. Suivi des objectifs annexés à la Loi de Santé Publique. Rapport 2009–2010.
- 9.Nohr SB, Laurberg P. Opposite variations in maternal and neonatal thyroid function induced by iodine supplementation during pregnancy. J Clin Endocrinol Metab. 2000;85:623–627. doi: 10.1210/jcem.85.2.6391. [DOI] [PubMed] [Google Scholar]
- 10.Antonangeli L, Maccherini D, Cavaliere R, Di Giulio C, Reinhardt B, Pinchera A, Aghini-Lombardi F. Comparison of two different doses of iodide in the prevention of gestational goiter in marginal iodine deficiency: a longitudinal study. Eur J Endocrinol. 2002;147:29–34. doi: 10.1530/eje.0.1470029. [DOI] [PubMed] [Google Scholar]
- 11.Bartalena L, Bogazzi F, Tanda ML, Manetti L, Dell'Unto E, Martino E. Cigarette smoking and the thyroid. Eur J Endocrinol. 1995;133:507–512. doi: 10.1530/eje.0.1330507. [DOI] [PubMed] [Google Scholar]
- 12.Utiger RD. Effects of smoking on thyroid function. Eur J Endocrinol. 1998;138:368–369. doi: 10.1530/eje.0.1380368. [DOI] [PubMed] [Google Scholar]
- 13.Chanoine JP, Toppet V, Bourdoux P, Spehl M, Delange F. Smoking during pregnancy: a significant cause of neonatal thyroid enlargement. Br J Obstet Gynaecol. 1991;98:65–68. doi: 10.1111/j.1471-0528.1991.tb10313.x. [DOI] [PubMed] [Google Scholar]
- 14.Ericsson UB, Ivarsson SA, Persson PH. Thyroglobulin in cord blood. The influence of the mode of delivery and the smoking habits of the mother. Eur J Pediatr. 1987;146:44–47. doi: 10.1007/BF00647282. [DOI] [PubMed] [Google Scholar]
- 15.Gasparoni A, Autelli M, Ravagni-Probizer MF, Bartoli A, Regazzi-Bonora M, Chirico G, Rondini G. Effect of passive smoking on thyroid function in infants. Eur J Endocrinol. 1998;138:379–382. doi: 10.1530/eje.0.1380379. [DOI] [PubMed] [Google Scholar]
- 16.McDonald S, Walker M, Ohlsson A, Murphy K, Beyene J, Perkins S. The effect of tobacco exposure on maternal and fetal thyroid function. Eur J Obstet Gynecol Reprod Biol. 2008;140:38–42. doi: 10.1016/j.ejogrb.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Meberg A, Marstein S. Smoking during pregnancy. Effects on the fetal thyroid function. Acta Paediatr Scand. 1986;75:762–766. doi: 10.1111/j.1651-2227.1986.tb10287.x. [DOI] [PubMed] [Google Scholar]
- 18.Shields B, Hill A, Bilous M, Knight B, Hattersley A, Bilous R, Vaidya B. Cigarette smoking during pregnancy is associated with alterations in maternal and fetal thyroid function. J Clin Endocrinol Metab. 2009;94:570–574. doi: 10.1210/jc.2008-0380. [DOI] [PubMed] [Google Scholar]
- 19.Soldin OP, Goughenour BE, Gilbert SZ, Landy HJ, Soldin SJ. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. 2009;19:817–823. doi: 10.1089/thy.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and post partum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2007;92(8 suppl):S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 21.Oddoze C, Pauli AM, Pastor JJ. Rapid and sensitive high-performance liquid chromatographic determination of nicotine and cotinine in nonsmoker human and rat urines. J Chromatogr B Biomed Sci Appl. 1998;708:95–101. doi: 10.1016/s0378-4347(97)00632-4. [DOI] [PubMed] [Google Scholar]
- 22.Hieronimus S, Bec-Roche M, Ferrari P, Chevalier N, Fenichel P, Brucker-Davis F. Iodine status and thyroid function of 330 pregnant women from Nice area assessed during the second part of pregnancy. Ann Endocrinol (Paris) 2009;70:218–224. doi: 10.1016/j.ando.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Klett M, Ohlig M, Manz F, Tröger J, Heinrich U. Effect of iodine supply on neonatal thyroid volume and TSH. Acta Paediatr. 1999;88:18–20. doi: 10.1111/j.1651-2227.1999.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 24.Vejbjerg P, Knudsen N, Perrild H, Carlé A, Laurberg P, Bülow Pedersen I, Rasmussen L, Ovesen L, Jorgensen T. The impact of smoking on thyroid volume and function in relation to a shift towards iodine sufficiency. Eur J Epidemiol. 2008;23:423–429. doi: 10.1007/s10654-008-9255-1. [DOI] [PubMed] [Google Scholar]
- 25.Ittermann T, Schmidt CO, Kramer A, Below H, John U, Thamm M, Wallaschofski H, Völzke H. Smoking as a risk factor for thyroid volume progression and incident goiter in a region with improved iodine supply. Eur J Endocrinol. 2008;159:761–766. doi: 10.1530/EJE-08-0386. [DOI] [PubMed] [Google Scholar]
- 26.Cho NH, Choi HS, Kim KW, Kim HL, Lee SY, Choi SH, Lim S, Park YJ, Park Do J, Jang HC, Cho BY. Interaction between cigarette smoking and iodine intake and their impact on thyroid function. Clin Endocrinol. 2010;73:264–270. doi: 10.1111/j.1365-2265.2010.03790.x. [DOI] [PubMed] [Google Scholar]
- 27.Vanderver GB, Engel A, Lamm S. Cigarette smoking and iodine as hypothyroxinemic stressors in US women of childbearing age: a NHANES III analysis. Thyroid. 2007;17:741–746. doi: 10.1089/thy.2006.0332. [DOI] [PubMed] [Google Scholar]
- 28.Liesenkötter KP, Göpel X, Bogner U, Stach B, Grüters A. Earliest prevention of endemic goiter by iodine supplementation during pregnancy. Eur J Endocrinol. 1996;134:443–448. doi: 10.1530/eje.0.1340443. [DOI] [PubMed] [Google Scholar]
- 29.Pearce EN, Oken E, Gillman MW, Lee SL, Magnani B, Platek D, Braverman LE. Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking and multivitamin use. Endocr Pract. 2008;14:33–39. doi: 10.4158/EP.14.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velasco I, Carreira M, Santiago P, Muela JA, Garcia-Fuentes E, Sanchez-Munoz B, Garriga MJ, Gonzalez-Fernandez MC, Rodriguez A, Caballero FF, Machado A, Gonzalez-Romero S, Anarte MT, Soriguer F. Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab. 2009;94:3234–3241. doi: 10.1210/jc.2008-2652. [DOI] [PubMed] [Google Scholar]
- 31.Marco A, Vicente A, Castro E, Perez CE, Rodriguez O, Merchan MA, Sastre J, Canocas B, Maqueda E, Pena V, Lopez J. Patterns of iodine intake and urine iodine concentrations during pregnancy and blood thyroid-stimulating hormone concentrations in newborn progeny. Thyroid. 2010;20:1295–1299. doi: 10.1089/thy.2010.0046. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann MB, Aeberli I, Torresani T, Bürgi H. Increasing the iodine concentration in the Swiss iodized salt program markedly improved iodine status in pregnant women and children: a 5-y prospective national study. Am J Clin Nutr. 2005;82:388–392. doi: 10.1093/ajcn.82.2.388. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann MB. The adverse effects of mild to moderate iodine deficiency during pregnancy and childhood: a review. Thyroid. 2007;17:829–835. doi: 10.1089/thy.2007.0108. [DOI] [PubMed] [Google Scholar]
- 34.Fukayama H, Nasu M, Murakami S, Sugawara M. Examination of antithyroid effects of smoking products in cultured thyroid follicles: only thiocyanate is a potent antithyroid agent. Acta Endocrinol (Copenh) 1992;127:520–525. doi: 10.1530/acta.0.1270520. [DOI] [PubMed] [Google Scholar]
- 35.Gondou A, Toyoda N, Nishikawa M, Yonemoto T, Sakaguchi N, Tokoro T, Inada M. Effect of nicotine on type 3 deiodinase activity in cultured rat glial cells. Endocr J. 1999;46:107–112. doi: 10.1507/endocrj.46.107. [DOI] [PubMed] [Google Scholar]
- 36.Zoeller RT. Environmental chemicals impacting the thyroid: targets and consequences. Thyroid. 2007;17:811–817. doi: 10.1089/thy.2007.0107. [DOI] [PubMed] [Google Scholar]
- 37.Brucker-Davis F, Ferrari P, Boda-Buccino M, Wagner-Mahler K, Pacini P, Gal J, Azuar P, Fenichel P. Cord blood thyroid tests in boys born with and without cryptorchidism. Correlations with birth parameters and in utero xenobiotics exposure. Thyroid. 2011;21:1133–1141. doi: 10.1089/thy.2010.0459. [DOI] [PubMed] [Google Scholar]
- 38.Pearce EN. What do we know about iodine supplementation in pregnancy? J Clin Endocrinol Metab. 2009;94:3188–3190. doi: 10.1210/jc.2009-1512. [DOI] [PubMed] [Google Scholar]