Abstract
Hakaru Hashimoto described 4 patients with a hitherto unknown cause for goitre, struma lymphomatosa, a century ago. He was careful to distinguish this from Riedel thyroiditis but it has become clear that fibrosis and atrophy of the thyroid are indeed components of Hashimoto thyroiditis, and in rare cases IgG4-related sclerosing disease may be an outcome. Although the cause of the lymphocytic infiltration was unknown to Hashimoto, we now know through the pioneering studies of N.R. Rose and E. Witebsky [J Immunol 1956;76:417–427] that this condition is the archetype for autoimmune destruction as a disease mechanism. In the last two decades in particular, there has been huge interest in unravelling the genetic basis for this and related autoimmune disorders. The list of polymorphisms associated with autoimmune thyroid disease grows each year, and in the case of vitiligo, which is frequently found in association with thyroid autoimmunity, we know that 27 separate susceptibility loci account for less than 20% of the heritability of this condition. Environmental and existential factors may turn out to be just as complex in number and in interactions. We can thus imagine a ‘Swiss cheese’ model for the causation of autoimmune thyroid disease, in which the effects of cumulative weaknesses line up – like the holes in slices of cheese – to allow the catastrophic event of autoimmune destruction to occur.
Key Words: Hashimoto thyroiditis, Genetics, Environmental factors, Autoimmunity
It is a century since Dr. Hakaru Hashimoto (1881–1934) described the thyroid condition that still bears his name. In that time, considerable efforts have been made to understand the pathogenesis of this common disease and, since 1956, Hashimoto thyroiditis has become the archetype of autoimmune destructive disorders and autoantibody production. This lecture honours Professor Aldo Pinchera, who has made many important contributions to our understanding of thyroid autoimmunity; remarkably, his first publication in the field dates back almost 50 years [1] and the most recent one has appeared this year [2]. Although covering a notably diverse range of topics, the most important of Professor Pinchera's autoimmunity papers concern the nature of the predisposing factors in autoimmune thyroid disease, and these will therefore form the basis of this review. But to start with, it is worth reminding ourselves of what Hashimoto described and how this fits historically into the general understanding of thyroiditis and hypothyroidism.
Hashimoto Legacy
Although goitre had been a well-known and depicted entity for centuries, especially in endemic areas, Hashimoto produced a detailed histopathological study of the thyroidectomy specimens of 4 women which were clearly not the colloid goitre that had been expected prior to surgery. Instead, they consisted of ‘a massive growth of lymphatic elements, primarily lymphoid follicles’ [3,4]. At least 1 of the patients had hypothyroidism. Hashimoto carefully distinguished this condition from Riedel thyroiditis and speculated whether or not it was related to Mikulicz disease, which was remarkably prescient: we now know that patients with this entity, better known as Sjögren syndrome, have a high risk of developing autoimmune thyroiditis [5].
Twenty years later, the spectrum of this new disorder was extended by the description of atrophy and fibrosis associated with Hashimoto struma lymphomatosa [6], although many abortive attempts have been made subsequently to separate atrophic and goitrous thyroiditis as discrete nosological entities based on genetic predisposition and autoantibody profile. Hashimoto had approached his eponymous disease as a cause of goitre, but taken from the stand point of myxoedema, it had been known since 1888 that this condition was a ‘destructive affectation of the thyroid … here and there infiltrated with clumps of cells … which are found to be composed of leucocytes’ [7]. By 1900, Dr. George Murray was writing confidently in his description of the successful treatment of myxoedema with thyroid extract that the thyroid changes consist of ‘fibrosis … occurring as a result of chronic inflammation leading to a secondary atrophy of the epithelial cells’; he also remarked on the familial predisposition of this disorder and its occurrence ‘particularly in women who have borne children’ [8].
The basic mechanism underlying the infiltration by lymphocytes remained unclear for over 40 years, when the studies of Rose and Witebsky [9] first convincingly showed that rabbits immunized with thyroglobulin produced autoantibodies to this self-protein, accompanied by lymphocytic thyroiditis. (Incidentally, this was not the first description of autoantibodies; Picado and Rotter [10] had published a paper in 1936 entitled ‘Precipitines seriques antithyroidiennes chez le goitreux’ and had also found cases in which pituitary autoantibodies were present [11].) At the time when these experiments were conducted, the prevailing dogma was that of an ‘instructional’ theory of antibody formation, whereby an antigen instructed cells to produce the corresponding antibody. However and Rose and Witebsky [9] realised that this theory could not explain why rabbits did not spontaneously produce thyroglobulin antibodies: autoantigen was always there to instruct the B cells. This ultimately led to the theory of clonal selection and the realisation that autoimmunity is normally prevented by deletion of self-reactive clones. Rose's pathology collaborators at the University of Buffalo spotted that the histology of the thyroglobulin-immunized rabbits resembled Hashimoto thyroiditis, but it took them 2 years to collect 4 samples from patients with chronic thyroiditis and test these for thyroglobulin autoantibodies (3 were positive) [12], and by then, Campbell et al. [13] had described the first thyroglobulin autoantibodies in Hashimoto thyroiditis.
Revolutionary as this work truly was, it also added new layers of uncertainty as to why only some people develop self-reactivity and others do not. It quickly became apparent that clonal elimination of self-reactive lymphocytes during fetal development is an important mechanism to prevent autoimmunity, but that this elimination is typically incomplete. Those cells which escape ‘central’ deletion in the thymus are held in check by a variety of ‘peripheral’ mechanisms that maintain self-tolerance. Over the last 3 decades, much effort has gone into identifying these mechanisms, moving from an era of suppressor T cells to one in which regulatory T cells, tolerogenic dendritic cells and cytokine networks interact to prevent autoimmune disease.
Rose's group were the first to show that susceptibility to the production of autoantibodies and thyroiditis are under independent genetic control, and that environmental factors could induce thyroid autoimmunity in genetically susceptible strains of animals [14,15]. Understanding how these genetic and environmental factors influence thyroid autoimmunity offers us the best opportunity to fully understand disease pathogenesis and, in turn, how this might be influenced for the benefit of patients.
Genetic Factors
Monogenic Autoimmune Thyroiditis
Two rare monogenic disorders provide outstanding examples of the importance of thymic tolerance and regulatory T cells in preventing thyroid autoimmunity from arising spontaneously. Autoimmune polyglandular syndrome type 1 is the result of mutations in the autoimmune regulator AIRE gene. As well as the cardinal childhood manifestations of autoimmune hypoparathyroidism, Addison's disease and chronic mucocutaneous candidiasis, many other autoimmune diseases including hypothyroidism appear in these patients, albeit not in all populations [16]. AIRE is expressed in thymic medullary epithelial cells and plays a key role in presenting thousands of different, ectopically expressed self-antigens to immature T cells, leading to their deletion. At least 60 AIRE mutations have been described, and in patients with these mutations, a failure to present self-antigen correctly in the thymus leads to loss of self-tolerance. The chronic candidiasis component of the syndrome is most likely the result of the development of neutralizing autoantibodies against both type 1 interferons and T helper cell cytokines related to interleukin-17 [17]. Emerging models suggest that the abnormal thymic microenvironment in this syndrome may lead to the production of cytokine autoantibodies as an initiating step, which in turn may permit less-well tolerized T cells to emerge, with a greater susceptibility to activation by autoantigens in the periphery.
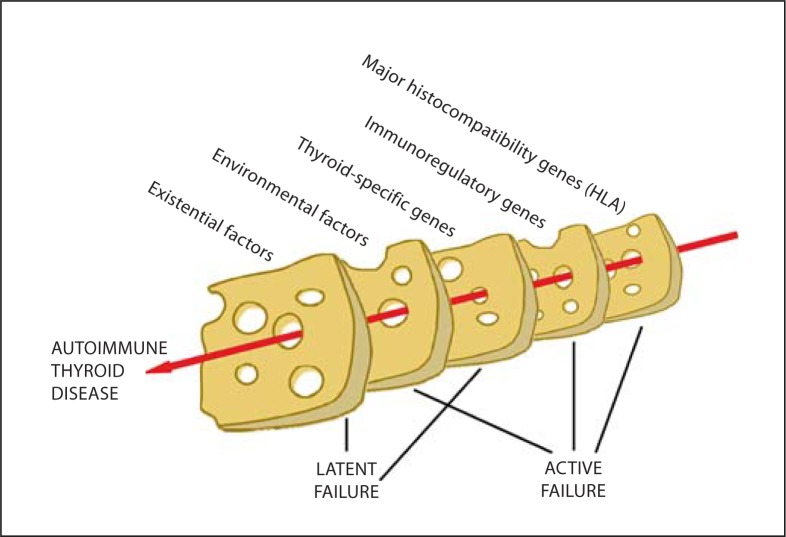
In the second of these monogenic disorders, IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome, there is a fatal, neonatal onset of autoimmune diseases including thyroiditis. These patients have mutations in the FOXP3 gene, which is responsible for the normal functioning of regulatory T cells [18]. Whilst these two monogenic disorders are rare, and polymorphisms of the genes responsible have no known role in more typical forms of autoimmune thyroiditis, they serve to underline how either disordered thymic development or the failure of peripheral tolerance can result in autoimmunity. They may be regarded as single-order catastrophes. Typical autoimmune thyroiditis conforms to the Swiss-cheese model of catastrophe in which multiple small genetic (and non-genetic) events, none in themselves sufficient to precipitate autoimmune destruction, line up like the holes in slices of such cheese to allow the untoward event to occur (fig. 1).
Fig. 1.
A Swiss cheese model for the causation of autoimmune thyroid disease, showing the effect of cumulative weaknesses lining up to allow a catastrophic event to occur, like the holes in slices of cheese. Each of the broad slices represented is composed of many individual components (tables 1, 2) which also have to line up. The Swiss cheese model for human accident causation incorporates active failures (e.g. pilot error) and latent failures (which lie dormant until the accident e.g. aircraft maintenance deficiencies). Some factors contributing to the initiation of Hashimoto thyroiditis may be regarded as latent (e.g. ageing, growing up in a hygienic environment) and others as active (e.g. possession of an HLA allele which permits presentation of a thyroid autoantigen).
Multigenic Autoimmune Thyroiditis
Observations in the early models of experimental autoimmune thyroiditis quickly demonstrated the importance of the major histocompatibility complex [MHC; in humans, this is the well-known human leukocyte antigen (HLA) system] in the predisposition of animals to this disorder, with the influence of additional non-MHC genes quickly becoming apparent [14]. At the same time, family studies in humans, such as those by my mentor Reg Hall and colleagues in Newcastle-upon-Tyne [19] confirmed that there was a genetic component to Hashimoto thyroiditis. More recently, Brix et al. [20] have used extensive twin studies in Denmark to identify the importance of genetic factors in Hashimoto thyroiditis and thyroid autoantibody formation. The last two decades have seen the application of increasingly sophisticated molecular genetics approaches to identify the genes responsible for this predisposition; I have summarized this work elsewhere [21]. What follows is an update with specific reference to the period since that review.
Perhaps the most telling overall comment is that of Davies [22] who has written that ‘really significant genes for autoimmune thyroid disease do not exist’. In other words, there is no dominant gene conferring major susceptibility to Hashimoto thyroiditis; even the importance of the HLA system in susceptibility is less obvious than in related disorders such as type 1 diabetes mellitus and coeliac disease. Four different types of genetic influence have been identified in autoimmune thyroid disease, as in many other autoimmune disorders (table 1). It must be acknowledged that the majority of genetic studies have focussed on Graves disease rather than Hashimoto thyroiditis, and in some studies these two disorders are bundled together as ‘autoimmune thyroid disease’, which has dubious nosological credibility. As just one example, disease associations with these two disorders, which are very likely to have a genetic basis, are rather distinct [23].
Table 1.
Genetic polymorphisms associated with thyroid disease
| Possible effect | |
|---|---|
| Major histocompatibility complex genes (HLA in man) | May determine cell-mediated destruction (HLA class I) or auto-antigen presentation (HLA class II) |
| T cell immune response genes | CTLA-4 and PTPN22 regulate T cell activation; IL2RA encodes the interleukin-2 receptor; FCRL3 affects regulatory T cell function |
| Other immune response genes | CD40 encodes a co-stimulator of antigen-presenting cells; CD226 encodes own activating receptor on NK cells |
| Thyroid-specific genes | Genes encoding two thyroid auto antigens, thyroglobulin and the TSH-receptor, may regulate availability of these to the immune system |
In the most recent discoveries of genetic polymorphisms associated with Hashimoto thyroiditis, there is a focus on possible genetic heterogeneity across different ethnic groups, which compounds the difficulty of interpretation. For instance, single nucleotide polymorphisms (SNPs) in the PTPN22 gene have been associated with several autoimmune disorders in discrete ethnic groups. In Koreans, the association appears to be with Hashimoto thyroiditis but not Graves disease whereas in Caucasians, there is evidence of an association with the latter [24]. In Koreans, SNPs of the STAT4 gene, involved in interleukin signalling, are associated differentially with these two autoimmune thyroid diseases [25]. There may also be sex differences in the influence of genetic polymorphisms. In a recent study of SNPs in the IL12B gene, a common variant in the coding region was differentially associated between Hashimoto thyroiditis and Graves disease, but only in men [26].
In all 3 of these studies, relatively small populations were studied, albeit with replication in the last. Identification of new risk loci by genome-wide association methods typically requires enormous populations: the recognition of new loci at 6q27 and 4p14 in Graves disease recently required an initial set of 1,516 patients, with replication in a further 3,994 along with similar numbers of controls [27]. This is not work for the faint-hearted or inadequately funded. To date there have been no similar mammoth undertakings in Hashimoto thyroiditis. Genome-wide approaches cannot readily identify the rare alleles (<5% of the population) that associate with disease, and the loci found are often located in regions that affect gene expression, which in turn makes interpretation of the underlying mechanism of disease very difficult, although this is essential to understanding how environmental factors interact with genetic susceptibility [28]. Copy number variation is also likely to contribute to susceptibility to autoimmune disease and thus add even greater complexity [29].
As of yet, we have no real idea of the number of genetic loci which contribute to the development of Hashimoto thyroiditis, but an insight into this may be gleaned from studies of vitiligo. This disorder of skin pigmentation is well known to be associated with autoimmune thyroid diseases and other autoimmune disorders, based on the sharing of genetic susceptibility [30]. An international consortium has recently published two genome-wide association studies with a meta-analysis, and this has revealed the existence of 27 separate susceptibility loci, an unexpectedly large number when it is noted that these loci account in total for less than 20% of the heritability of vitiligo [31,32]. Most of these loci encode genes which can be identified as having a broadly immunoregulatory function or encoding melanocyte proteins; many of the former are shared with other autoimmune disorders, including thyroiditis.
In summary, autoimmune thyroid disease is not a discrete genetic entity: we now know that although some genetic susceptibility is shared by Graves disease, Hashimoto thyroiditis and other related disorders (HLA-DR3 allele and CTLA4), there are immunoregulatory genetic differences. Thyroid-specific genes such as TSHR[33] play a possibly decisive role in determining the precise disease outcome from an underlying thyroid-centred autoimmune diathesis in which thyroglobulin and thyroid peroxidase autoantibody formation feature. As in vitiligo, it is likely that heritability is determined by an eye-watering number of genetic loci, some of which are ethnically distinct, and these in turn will interact with each other and environmental factors in ways which may be unique to very small cohorts of patients. We are currently in a position in which it is clear that ‘the complexity of the etiology of autoimmune thyroid diseases is gravely underestimated’ [34].
Non-Genetic Factors
The best evidence for the importance of environmental factors in the aetiology of Hashimoto thyroiditis comes from epidemiological studies. The 10-fold increase in the incidence of Hashimoto thyroiditis over three decades in Sicily can only be the result of environmental changes [35]. A histological survey of both thyroid infiltration lymphocytic and Hashimoto thyroiditis over 30 years in Austria has also shown an 8-fold increase in prevalence in the specimens from surgery for benign goitre [36].
Table 2 summarises the non-genetic factors which have been associated with Hashimoto thyroiditis: this list is undoubtedly incomplete. Broadly speaking, these factors can be grouped as dietary factors, toxins (including drugs) and a more poorly defined group of factors that one could consider as ‘existential’ i.e. the consequence of humans living life to the full. One way in which some environmental and many existential factors may operate is through epigenetic modifications, such as changes in DNA methylation and histone modification, at the tissue level. Indeed, remarkable epigenetic differences have been demonstrated in monozygotic twins, beautifully illustrating how the same genotype can give rise to different phenotypes. As an excellent review was published 2 years ago on the impact of environmental exposures, the following summary is limited to papers which have appeared since or were not covered in depth [37].
Table 2.
Possible non-genetic factors associated with Hashimoto thyroiditis
| Factor | |
|---|---|
| Dietary | Iodine excess |
| Alcohol (protective) | |
| Vitamin D deficiency | |
| Selenium deficiency | |
| Toxins | Environmental pollutants |
| Low-dose irradiation | |
| Smoking (protective) | |
| Drugs (lithium, immunomodulators and | |
| antineoplastic agents) | |
| Existential | Female sex |
| Parity | |
| Age | |
| Hygienic environment | |
| Increased affluence | |
Dietary Factors
The best evidence for a direct effect of dietary iodine on autoimmune thyroiditis has come from animal models. Excess iodine intake affects thyroid gene expression differentially in thyroiditis-prone BioBreeding (BB) rats, compared to thyroiditis-resistant BB rats, and some of these changes occurred early enough in the disease to suggest that such alterations may play an initiating role [38]. But this can only be part of the explanation, as the incidence of insulitis is also increased by iodine, an observation which implies that iodine also has an effect on immune cells. Although there are conflicting studies on the influence of dietary iodine in human thyroiditis, the weight of evidence is now in favour of a more than adequate iodine intake being a risk factor for the appearance of both thyroid autoantibodies and subclinical hypothyroidism [39].
A meta-analysis of trials of selenium supplementation has shown that this element lowers thyroid autoantibody levels but does not alter the level of hypothyroidism as determined by levothyroxine requirements [40]. Whether selenium deficiency is a true risk factor for Hashimoto thyroiditis is unclear. Similarly, there is only equivocal evidence for an effect of low vitamin D levels on autoimmune thyroiditis [41]. Another dietary factor has recently been identified, namely alcohol consumption, in a study from Amsterdam which has looked at the development of thyroid autoimmunity in a genetically predisposed group of women [42]. Although alcohol consumption had no association with the development of thyroid autoantibodies, there was a protective effect with regard to the development of hypothyroidism, an observation which parallels such an effect in rheumatoid arthritis.
Toxins
The early observation of experimental autoimmune thyroiditis being precipitated by 3-methylcholanthrene [15] has been reprised by the demonstration of thyroid peroxidase autoantibodies and lymphocytic thyroiditis in rats given polychlorinated biphenyls, widely used compounds which have known effects as endocrine disruptors [43]. Much less work has been done on environmental pollutants in human disease, although the striking recent epidemiology of Hashimoto thyroiditis [35,36] tends to point to these as a possible explanation. Residents living in areas close to a petrochemical complex may have a higher prevalence and risk of developing thyroiditis and thyroid autoantibodies [44], and the spouses of licensed pesticide applicators have a higher risk of developing hypothyroidism than controls, although an autoimmune basis for this disordered thyroid function has not been examined [45]. Somewhat surprisingly, given the known adverse effect of smoking on thyroid eye disease [46], smoking appears to be protective for the development of thyroid autoantibodies [47].
Sublethal irradiation has long been used to create experimental autoimmune thyroiditis in animals, acting in concert with thymectomy to adversely affect the immune system. The effect of smaller doses of irradiation, arising typically from the fallout from nuclear reactor disasters, is less clear: in adolescents there appears to have been a transient autoimmune thyroiditis up to 15 years after the Chernobyl nuclear accident, without triggering clinically significant Hashimoto thyroiditis [48]. Such effects have been postulated to be related to thyroid damage as a result of its sensitivity to radiation from iodine isotopes. However, a single low-dose (0.5 Gy) of entire body irradiation increases the severity of thyroiditis and the frequency of thyroglobulin autoantibodies in thyroiditis-prone non-obese diabetic mice for up to 15 months, consistent with a more general effect of low-dose irradiation on the immune system which results in thyroid autoimmunity in genetically predisposed animals [49]. Antineoplastic agents, especially interferon-α, frequently precipitate autoimmune thyroiditis [50]. Multiple immunomodulatory mechanisms underlie these effects, but recently interferon-α has been shown to have a thyroid-specific action by increasing thyroglobulin promoter activity via an interferon-induced transcription factor only when the promoter has a thyroid disease-associated polymorphism [51].
Existential Factors
Being a woman rather than a man increases the risk of Hashimoto thyroiditis 8-fold, making this the greatest known risk factor by some margin. It is still not clear why this is so. The effects of sex hormones, X-chromosome-encoded susceptibility, skewed X-chromosome inactivation, pregnancy and fetal microchimerism have all been postulated: the first three would be expected to affect all women, the last two only those who have been pregnant (of course, these mechanisms are not mutually exclusive). In a large Danish survey (45.5 million person-years of follow-up), parity was associated with only an 11% increased risk of autoimmune diseases in general, and an identical risk of Hashimoto thyroiditis [52]. Although therefore suggesting only a small role for fetal microchimerism, patients with Hashimoto thyroiditis have significantly more circulatory fetal CD8+ T cells than healthy controls within 5 years of pregnancy, compatible with such a role [53].
Stress in various forms has been associated with an increased risk of Graves disease, but there is not strong evidence in favour of such a role in Hashimoto thyroiditis [54]. Exposure to an environment of inferior prosperity and hygiene is protective against the development of thyroid autoimmunity, compatible with the ‘hygiene hypothesis’ in which enhanced exposure to microbes in general skews the immune system away from the tendency to produce autoreactivity [55]. Another hint that certain environments can increase the risk of thyroid autoimmunity comes from a survey of the Yakut in Siberia; women there have an unexpectedly high frequency of thyroid autoantibodies which could be linked to circumpolar adaptation and recent changes in lifestyle [56]. Such analyses of the geoepidemiology of thyroid autoimmunity are few and far between, but this is a field that is ripe for development and with the potential to provide fresh insights into disease aetiology and pathogenesis [57]. Ageing is of course another obvious risk factor for the development of thyroid autoimmunity, given that the prevalence of Hashimoto thyroiditis increases with age up to 70 years; it then declines and healthy centenarians have a lower than predicted prevalence of thyroid autoimmunity [58]. This observation is somewhat at odds with the finding of a lower risk of frailty in older adults who have thyroid autoantibodies [59], and it is still unclear what is cause and what is effect in these associations.
Conclusion
Although huge strides have been made in our understanding of the causes of Hashimoto thyroiditis since its original description, the genetic and non-genetic factors which are involved in its aetiology are far more complex than could be imagined even a decade ago. Dissecting their interaction looks set to keep thyroidologists occupied for many years to come. This disorder is part of a spectrum of autoimmune thyroid diseases which cluster in families and even evolve from one to another within the same individual. These relationships also require better classification.
We now understand that Hashimoto thyroiditis itself is heterogeneous. One significant recent advance is the description of a distinctive subtype of Hashimoto thyroiditis characterized by an increased number of IgG4+ plasma cells, high serum IgG4 levels and thyroid fibrosis; this entity is associated with more rapid progression to hypothyroidism and high levels of circulating thyroid autoantibodies [60]. In turn there may be a systemic form of IgG4-related sclerosing disease which, when it affects the thyroid, is Riedel thyroiditis, the condition Hashimoto took great care to distinguish from his new form of thyroiditis. As we move rapidly into a new era of molecular diagnostics and disease classification, further such exciting and unexpected developments are inevitable.
Disclosure Statement
The author declares no conflict of interest relevant to this article.
References
- 1.Pinchera A, Pinchera MG, Stanbury JB. Thyrotropin and long-acting thyroid stimulator assays in thyroid disease. J Clin Endocrinol Metab. 1965;25:189–208. doi: 10.1210/jcem-25-2-189. [DOI] [PubMed] [Google Scholar]
- 2.Latrofa F, Ricci D, Montanelli L, Rocchi R, Piaggi P, Sisti E, et al. Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab. 2012;97:2380–2387. doi: 10.1210/jc.2011-2812. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto H. Zur Kenntis der lymphomatosen Veranderung der Schilddruse (Struma lymphomatosa) Arch Klin Chir. 1912;97:219–248. [Google Scholar]
- 4.Sawin CT. The heritage of Dr. Hakaru Hashimoto (1881–1934) Endocr J. 2002;49:399–403. doi: 10.1507/endocrj.49.399. [DOI] [PubMed] [Google Scholar]
- 5.Zeher M, Horvath IF, Szanto A, Szodoray P. Autoimmune thyroid diseases in a large group of Hungarian patients with primary Sjögren's syndrome. Thyroid. 2009;19:39–45. doi: 10.1089/thy.2007.0398. [DOI] [PubMed] [Google Scholar]
- 6.Graham A, McCullagh E. Atrophy and fibrosis associated with lymphoid tissue in the thyroid; struma lymphomatosa (Hashimoto) Arch Surg. 1931;22:548–567. [Google Scholar]
- 7.Ord WM. Report of a committee of the Clinical Society of London nominated December 14, 1883, to investigate the subject of myxoedema. Trans Clin Soc Lond. 1888;21 [Google Scholar]
- 8.Murray GR. Diseases of the Thyroid Gland. London: HK Lewis; 1900. [Google Scholar]
- 9.Rose NR, Witebsky E. Studies in organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol. 1956;76:417–427. [PubMed] [Google Scholar]
- 10.Picado C, Rotter W. Precipitines seriques antithyroidiennes chez le goitreux. Compte Rend Soc Biol. 1936;123:1111. [Google Scholar]
- 11.Stuart A. An origin of the term autoantibody. JR Coll Phys Edinb. 2007;37:92–93. [Google Scholar]
- 12.Rose NR. Autoimmunity: a personal memoir. Autoimmun. 1988;1:15–21. doi: 10.3109/08916938808997172. [DOI] [PubMed] [Google Scholar]
- 13.Campbell PN, Doniach D, Hudson RV, Roitt IM. Auto-antibodies in Hashimoto's disease (lymphadenoid goitre) Lancet. 1956;271:820–821. doi: 10.1016/s0140-6736(56)92249-8. [DOI] [PubMed] [Google Scholar]
- 14.Rose NR. The genetics of autoimmune thyroiditis: the first decade. J Autoimmun. 2011;37:88–94. doi: 10.1016/j.jaut.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman DA, Rose NR. Autoimmunity in methylcholanthrene-induced and spontaneous thyroiditis in Buffalo strain rats. Proc Soc Exp Biol Med. 1971;138:579–584. doi: 10.3181/00379727-138-35945. [DOI] [PubMed] [Google Scholar]
- 16.Meloni A, Willcox N, Meager A, Atzeni M, Wolff AS, Husebye ES, et al. Autoimmune polyendocrine syndrome type 1: an extensive longitudinal study in Sardinian patients. J Clin Endocrinol Metab. 2012;97:1114–1124. doi: 10.1210/jc.2011-2461. [DOI] [PubMed] [Google Scholar]
- 17.Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: known and novel aspects of the syndrome. Ann NY Acad Sci. 2011;1246:77–91. doi: 10.1111/j.1749-6632.2011.06308.x. [DOI] [PubMed] [Google Scholar]
- 18.d'Hennezel E, Bin Dhuban K, Torgerson T, Piccirillo C. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2012;49:291–230. doi: 10.1136/jmedgenet-2012-100759. [DOI] [PubMed] [Google Scholar]
- 19.Hall R, Owen SG, Smart GA. Evidence for genetic predisposition to formation of thyroid autoantibodies. Lancet. 1960;2:187. doi: 10.1016/s0140-6736(60)91330-1. [DOI] [PubMed] [Google Scholar]
- 20.Brix TH, Kyvik KO, Hegedüs L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85:536–539. doi: 10.1210/jcem.85.2.6385. [DOI] [PubMed] [Google Scholar]
- 21.Weetman AP. The genetics of autoimmune thyroid disease. Horm Metab Res. 2009;41:421–425. doi: 10.1055/s-0029-1214415. [DOI] [PubMed] [Google Scholar]
- 22.Davies TF. Really significant genes for autoimmune thyroid disease do not exist – so how can we predict disease? Thyroid. 2007;17:1027–1029. doi: 10.1089/thy.2007.1526. [DOI] [PubMed] [Google Scholar]
- 23.Wiebolt J, Achterbergh R, den Boer A, van der Leij S, Marsch E, Suelmann B, et al. Clustering of additional autoimmunity behaves differently in Hashimoto's patients compared with Graves patients. Eur J Endocrinol. 2011;164:789–794. doi: 10.1530/EJE-10-1172. [DOI] [PubMed] [Google Scholar]
- 24.Lee HS, Kang J, Yang S, Kim D, Park Y. Susceptibility influence of a PTPN22 haplotype with thyroid autoimmunity in Koreans. Diab Metab Res Rev. 2011;27:878–882. doi: 10.1002/dmrr.1265. [DOI] [PubMed] [Google Scholar]
- 25.Park Y, Lee HS, Park Y, Min D, Yang S, Kim D, et al. Evidence for the role of STAT4 as a general autoimmunity locus in the Korean population. Diab Metab Res Rev. 2011;27:867–871. doi: 10.1002/dmrr.1263. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JP, Berry J, Liu S, Panicker V, Dayan CM, Brix TH, et al. The clinical presentation of autoimmune thyroid disease in men is associated with IL12B genotype. Clin Endocrinol. 2011;74:508–512. doi: 10.1111/j.1365-2265.2010.03970.x. [DOI] [PubMed] [Google Scholar]
- 27.Chu X, Pan CM, Zhao SX, Liang J, Gao, GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves disease. Nat Genet. 2011;43:897–901. doi: 10.1038/ng.898. [DOI] [PubMed] [Google Scholar]
- 28.Hardy J, Singleton A. Genome-wide association studies and human disease. N Engl J Med. 2009;360:1759–1768. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wain LV, Armour JA, Tobin MD. Genomic copy number variation, human health, and disease. Lancet. 2009;374:340–350. doi: 10.1016/S0140-6736(09)60249-X. [DOI] [PubMed] [Google Scholar]
- 30.Spritz RA. Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid disease. Thyroid. 2010;20:745–754. doi: 10.1089/thy.2010.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–1697. doi: 10.1056/NEJMoa0908547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676–680. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand OJ, Barrett JC, Simmonds MJ, Newby PR, McCabe CJ, Bruce CK, et al. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves disease. Hum Mol Genet. 2009;18:1704–1713. doi: 10.1093/hmg/ddp087. [DOI] [PubMed] [Google Scholar]
- 34.Brix TH, Hegedüs L. The complexity of the etiology of autoimmune thyroid disease is gravely underestimated. Thyroid. 2011;21:1289–1292. doi: 10.1089/thy.2011.2112.ed. [DOI] [PubMed] [Google Scholar]
- 35.Benvenga S, Trimarchi F. Changed presentation of Hashimoto's thyroiditis in North-Eastern Sicily and Calabria (Southern Italy) based on a 31-year experience. Thyroid. 2008;18:429–441. doi: 10.1089/thy.2007.0234. [DOI] [PubMed] [Google Scholar]
- 36.Ott J, Meusel M, Schultheis A, Promberger R, Pallikunnel SJ, Neuhold N, et al. The incidence of lymphocytic thyroid infiltration and Hashimoto's thyroiditis increased in patients operated for benign goiter over a 31-year period. Virchows Arch. 2011;459:277–281. doi: 10.1007/s00428-011-1130-x. [DOI] [PubMed] [Google Scholar]
- 37.Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755–761. doi: 10.1089/thy.2010.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swist E, Chen Q, Qiao C, Caldwell D, Gruber H, Scoggan KA. Excess dietary iodine differentially affects thyroid gene expression in diabetes, thyroiditis-prone versus -resistant BioBreeding (BB) rats. Mol Nutr Food Res. 2011;55:1875–1886. doi: 10.1002/mnfr.201100299. [DOI] [PubMed] [Google Scholar]
- 39.Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–950. doi: 10.1530/EJE-10-1041. [DOI] [PubMed] [Google Scholar]
- 40.Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis. Thyroid. 2010;20:1163–1173. doi: 10.1089/thy.2009.0351. [DOI] [PubMed] [Google Scholar]
- 41.Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N, et al. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. Br J Nutr. 2009;102:382–386. doi: 10.1017/S0007114509220824. [DOI] [PubMed] [Google Scholar]
- 42.Effraimidis G, Tijssen JGP, Wiersinga WM. Alcohol consumption as a risk factor for autoimmune thyroid disease: a prospective study. Eur Thyroid J. 2012;1:99–104. doi: 10.1159/000338920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu JY, Qian CH, Tang W, Wu XH, Xu KF, Scherbaum WA, et al. Polychlorinated biphenyls affect thyroid function and induce autoimmunity in Sprague-Dawley rats. Horm Metab Res. 2009;41:471–474. doi: 10.1055/s-0029-1220768. [DOI] [PubMed] [Google Scholar]
- 44.de Freitas CU, Grimaldi Campos RA, Rodrigues Silva MA, Panachão MR, de Moraes JC, Waissmann W, et al. Can living in the surroundings of a petrochemical complex be a risk factor for autoimmune thyroid disease? Environ Res. 2010;110:112–117. doi: 10.1016/j.envres.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Goldner WS, Sandler DP, Yu F, Hoppin JA, Kamel F, Levan TD. Pesticide use and thyroid disease among women in the Agricultural Health Study. Am J Epidemiol. 2010;171:455–464. doi: 10.1093/aje/kwp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartalena L, Martino E, Marcocci C, Bogazzi F, Panicucci M, Velluzzi F, et al. More on smoking habits and Graves ophthalmopathy. J Endocrinol Invest. 1989;12:733–737. doi: 10.1007/BF03350047. [DOI] [PubMed] [Google Scholar]
- 47.Effraimidis G, Tijssen JG, Wiersinga WM. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab. 2009;94:1324–1328. doi: 10.1210/jc.2008-1548. [DOI] [PubMed] [Google Scholar]
- 48.Agate L, Mariotti S, Elisei R, Mossa P, Pacini F, Molinaro E, et al. Thyroid autoantibodies and thyroid function in subjects exposed to Chernobyl fallout during childhood: evidence for a transient radiation-induced elevation of serum thyroid antibodies without an increase in thyroid autoimmune disease. J Clin Endocrinol Metab. 2008;93:2729–2736. doi: 10.1210/jc.2008-0060. [DOI] [PubMed] [Google Scholar]
- 49.Nagayama Y, Ichikawa T, Saitoh O, Abiru N. Induction of late-onset spontaneous autoimmune thyroiditis by a single low-dose irradiation in thyroiditis-prone non-obese diabetic-H2h4 mice. J Radiat Res. 2009;50:573–577. doi: 10.1269/jrr.09067. [DOI] [PubMed] [Google Scholar]
- 50.Hamnvik OP, Larsen PR, Marqusee E. Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst. 2011;103:1572–1587. doi: 10.1093/jnci/djr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefan M, Jacobson EM, Huber AK, Greenberg DA, Li CW, Skrabanek L, et al. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon alpha-modulated mechanism. J Biol Chem. 2011;286:31168–31179. doi: 10.1074/jbc.M111.247510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jørgensen KT, Pedersen BV, Nielsen NM, Jacobsen S, Frisch M. Childbirths and risk of female predominant and other autoimmune diseases in a population-based Danish cohort. J Autoimmun. 2011;38:J81–J87. doi: 10.1016/j.jaut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Lepez T, Vandewoestyne M, Hussain S, Van Nieuwerburgh F, Poppe K, Velkeniers B, et al. Fetal microchimeric cells in blood of women with an autoimmune thyroid disease. PLoS One. 2011;6:e29646. doi: 10.1371/journal.pone.0029646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Effraimidis G, Tijssen JG, Brosschot JF, Wiersinga WM. Involvement of stress in the pathogenesis of autoimmune thyroid disease: a prospective study. Psychoneuroendocrinol. 2012;37:1191–1198. doi: 10.1016/j.psyneuen.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Kondrashova A, Viskari H, Haapala AM, Seiskari T, Kulmala P, Ilonen J, et al. Serological evidence of thyroid autoimmunity among schoolchildren in two different socioeconomic environments. J Clin Endocrinol Metab. 2008;93:729–734. doi: 10.1210/jc.2007-1644. [DOI] [PubMed] [Google Scholar]
- 56.Cepon TJ, Snodgrass JJ, Leonard WR, Tarskaia LA, Klimova TM, Fedorova VI, et al. Circumpolar adaptation, social change, and the development of autoimmune thyroid disorders among the Yakut (Sakha) of Siberia. Am J Hum Biol. 2011;23:703–709. doi: 10.1002/ajhb.21200. [DOI] [PubMed] [Google Scholar]
- 57.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34:J168–J177. doi: 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Mariotti S, Sansoni P, Barbesino G, Caturegli P, Monti D, Cossarizza A, et al. Thyroid and other organ-specific autoantibodies in healthy centenarians. Lancet. 1992;339:1506–1508. doi: 10.1016/0140-6736(92)91265-a. [DOI] [PubMed] [Google Scholar]
- 59.Wang GC, Talor MV, Rose NR, Cappola AR, Chiou RB, Weiss C, et al. Thyroid autoantibodies are associated with a reduced prevalence of frailty in community-dwelling older women. J Clin Endocrinol Metab. 2010;95:1161–1168. doi: 10.1210/jc.2009-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kakudo K, Li Y, Hirokawa M, Ozaki T. Diagnosis of Hashimoto's thyroiditis and IgG4-related sclerosing disease. Pathol Int. 2011;61:175–183. doi: 10.1111/j.1440-1827.2011.02661.x. [DOI] [PubMed] [Google Scholar]