Abstract
Background
We previously reported that the Ki-67 labeling index (LI) in primary tumors and the thyroglobulin (Tg)-doubling time (DT) were potent prognostic indicators in patients with papillary thyroid carcinoma (PTC).
Objectives
To elucidate the relationship between these two factors.
Methods
A total of 390 patients with PTC who underwent total thyroidectomy between 1998 and 2004 and in whom the Tg-DT was calculated were enrolled. We determined the Ki-67 LI in primary tumors and compared these values with the patients' clinicopathological factors, postoperative Tg status, Tg-DT, and prognosis. Tg status was categorized by postoperative serum Tg values: biochemically persistent disease (BPD), equivocal state, and biochemical remission.
Results
The Ki-67 LI was ≤5% in 312 patients (80%), 5%-10% in 48 patients (12%), and >10% in 30 patients (8%). Ki-67 LI was significantly associated with BPD (p < 0.0001). The proportion of BPD patients increased with the higher Ki-67 LI category: 24, 67, and 87%, respectively. The Ki-67 LI had a significant inverse correlation with the Tg-DT (Spearman's ρ = −0.5267, p < 0.0001). Of the 378 patients without distant metastasis at surgery, 68 patients had recurrence, and 6 of the 390 patients died of PTC during the follow-up (mean 88 months). On multivariate analyses, the Ki-67 LI remained an independent predictor of disease-free survival and disease-specific survival when Tg-DT and Tg status were excluded from the analyses.
Conclusions
Evaluation of the Ki-67 LI in primary tumors may allow the prediction of the postoperative Tg status, Tg-DT and prognosis of patients with PTC.
Published by S. Karger AG, Basel
Key Words : Papillary thyroid carcinoma, Ki-67 labeling index, Thyroglobulin-doubling time, Biochemically persistent disease, Prognosis
Introduction
Thyroid cancer is the most common malignancy in the endocrine organs. Papillary thyroid carcinoma is the representative pathological type, constituting about 90% of thyroid cancers. Papillary carcinoma generally shows an indolent nature despite frequent metastasis to regional lymph nodes, which is typically seen in young adults. However, some patients with this cancer, typically elderly patients, have poor clinical courses. Male gender, age, tumor size, extrathyroid extension, node metastasis, and distant metastases are well-established prognostic factors in papillary carcinoma that can be evaluated preoperatively [1,2,3]. Postoperatively, pathological findings such as pathological extrathyroid extension, node metastasis, and histological variants add further information for evaluations of the risks of cancer recurrence and death related to the cancer [4,5]. We previously reported that thyroglobulin (Tg)-doubling time (DT) was a very potent prognostic indicator in patients with papillary carcinoma and undetectable Tg antibody (TgAb) who underwent total thyroidectomy, when Tg-DT was calculated using serum Tg values measured under thyrotropin (TSH)-suppressed conditions [6].
Ki-67 is a cell proliferation-associated antigen that is expressed in all stages of the cell proliferative cycle except the G0 phase [7]. The expression of Ki-67 is generally evaluated immunohistochemically as a labeling index (LI) in tissue specimens. A high Ki-67 LI was associated with poor prognosis in patients with breast cancer and prostate cancer [8,9]. We conducted studies for this issue on 371 patients with papillary carcinoma, and previously reported that the Ki-67 LI was an independent prognostic factor for disease-free survival, and that patients with high Ki-67 LI values had a significantly worse disease-specific survival than patients with low Ki-67 LI values [10]. We hypothesized that the Ki-67 LI might have a strong correlation with Tg-DT, since both factors are related to tumor growth. In the present study, therefore, we investigated the Ki-67 LI in patients with papillary carcinoma in whom the Tg-DT was calculated. We also reevaluated the prognostic significance of Ki-67 LI in a different cohort of patients with the cancer.
Patients and Methods
Patients
Patients for the immunohistochemical study of the Ki-67 LI were selected from 426 patients with papillary carcinoma who underwent total thyroidectomy at Kuma Hospital between January 1998 and December 2004 and for whom the Tg-DT was calculated. None of these patients had other thyroid malignancies, and all of them had a negative TgAb test result. 36 of the patients had calcification in their primary thyroid tumors requiring decalcification for the preparation of paraffin-embedded blocks, and they were excluded from the study, leaving 390 patients for the present investigation. All of them had papillary carcinoma by WHO criteria, while 52 of them showed aggressive features including tall cell variant, columnar cell variant, diffuse sclerosing variant, and presence of focal poorly differentiated features.
There were 322 females and 68 males from 14 to 81 years of age (mean 50.8, median 52). Distant metastases were detected in 12 patients at the time of surgery who were classified as M1 in the UICC TNM classification system [11]. Radioiodine had been administered to 153 patients, including patients with distant metastases as a treatment or to ablate possible thyroid remnants. Metastatic foci were detected with radioiodine scintigraphy in 17 patients, and radioiodine treatments were repeated in these patients.
The patients had 4 or more serum Tg measurements with serum TSH suppressed to <0.1 mIU/l. Tg-DT was calculated in those patients who had 4 or more detectable Tg measurements. These patients were regarded as having ‘biochemically persistent disease’. In the present paper, this category included the 17 patients with distant metastases on radioiodine scintigraphy, however the other patients in this category had no structural diseases postoperatively. The patients who had 4 or more undetectable Tg measurements only were regarded as being in ‘biochemical remission’. In the patients who had 1-3 detectable Tg values and undetectable Tg measurements, Tg-DT was not calculated. These patients were regarded as having an ‘equivocal status’. None of the patients in the latter two categories had structural diseases on physical examination or imaging studies postoperatively.
The patients were followed for a median of 88 months. In this series, when carcinoma recurrence was detected on imaging studies other than radioiodine scintigraphy, the patient was regarded as having a recurrence. During the study period, 56 patients developed locoregional recurrences, 26 patients developed distant metastases, 13 of these patients had both locoregional recurrence and distant metastases, and 6 patients died of the disease. Details of the measurements of TSH, Tg, and TgAb and Tg-DT calculation are described elsewhere [6].
Ki-67 Immunohistochemistry and Labeling Index
We performed immunostaining using 4-µm-thick sections of formalin-fixed and paraffin-embedded tissue of the primary thyroid tumors. Anti-Ki-67 antibody (clone MIB1, 1:200 dilution; Dako, Carpinteria, Calif., USA) was used as a primary antibody. The staining was carried out using the Dako Cytomation Autostainer Universal Staining System (Dako) and the Envision kit (Dako) according to the manufacturer's recommendation. A pathologist (M.H.) evaluated the specimens without information of the clinical outcome of the patients. To estimate the Ki-67 LI, he observed the hot area under ×400 magnification, and calculated the percentage of cancer cell nuclei stained positively. Staining results were classified into three classes: ≤5%, >5%-10%, and >10% of the carcinoma cells were positive.
Statistical Analyses
The significance of differences in the variables among groups was calculated using the χ2 test. The Kaplan-Meier method and log-rank tests were used to compare survival between groups. The Cox proportional hazard regression model with stepwise variable selection techniques was adopted for the multivariate analysis. Spearman's rank correlation coefficient was used to evaluate correlations between two ordinal numeral variables. All statistical tests were two-sided, with the level of significance established at p < 0.05. Statistical analyses were performed using StatFlex V6.0.
Results
Ki-67 LI and Its Relationship with Clinicopathological Features
We investigated the Ki-67 LI in the primary thyroid tumors of the 390 patients with papillary carcinoma. The patients were categorized into three groups according to the results as described above: Ki-LI ≤5%: 312 patients (80%); Ki-LI >5%-10%: 48 patients (12%), and Ki-LI >10%: 30 patients (8%). We then compared the Ki-67 LI and clinicopathological features of the patients (table 1). We found that the Ki-67 LI was associated with patient's age, tumor size, extrathyroid extension, pathological lateral node metastasis (pN1b) and histological aggressive type of papillary carcinoma.
Table 1.
Relationship between the Ki-67 LI and clinicopathological features
| Total | Ki-67 LI |
||||
|---|---|---|---|---|---|
| number | ≤5 % | >5–10% | >10% | p values | |
| Patients, n | 390 | 312 | 48 | 30 | |
| Gender | 0.5308 | ||||
| Female | 322 | 256 | 39 | 27 | |
| Male | 68 | 56 | 9 | 3 | |
| Age | |||||
| Age ≥60 years | 104 | 73 | 17 | 14 | 0.0077 |
| Age <60 years | 286 | 239 | 31 | 16 | |
| Tumor size | |||||
| ≤4 cm | 311 | 256 | 36 | 19 | 0.0351 |
| >4 cm | 79 | 56 | 12 | 11 | |
| Extrathyroid | |||||
| extension | |||||
| No or minimal | 276 | 231 | 27 | 18 | 0.0167 |
| Significant | 114 | 81 | 21 | 12 | |
| cN status | |||||
| cN0 or cN1a | 303 | 249 | 35 | 19 | 0.0818 |
| cN1b | 87 | 63 | 13 | 11 | |
| M status | |||||
| M0 | 379 | 304 | 45 | 30 | 0.3863 |
| M1 | 12 | 8 | 3 | 1 | |
| pN status | |||||
| pN0 or pN1a | 209 | 174 | 27 | 8 | 0.0088 |
| pN1b | 181 | 138 | 21 | 22 | |
| Histological type | |||||
| Common type | 338 | 291 | 34 | 13 | 0.0000 |
| Aggressive type | 52 | 21 | 14 | 17 | |
Relationship between the Ki-67 LI and Postoperative Serum Tg Status
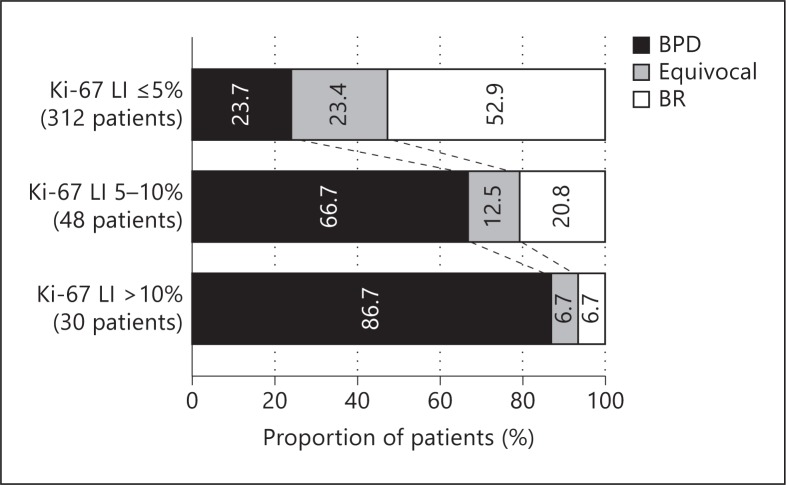
We analyzed the relationship between the Ki-67 LI and postoperative serum Tg status (fig. 1). The postoperative serum Tg status was categorized into three classes as described above: biochemically persistent disease, equivocal status, and biochemical remission. The Ki-67 LI was significantly associated with postoperative serum Tg status (p < 0.0001). The proportion of patients with biochemically persistent disease increased with higher Ki-67 LI category: 24, 67, and 87%, respectively for the three Ki-67 LI categories. Conversely, the proportion of patients with biochemical remission had the opposite relation to the Ki-67 LI categories, being 53, 21, and 7%, respectively.
Fig. 1.
Postoperative serum Tg status and Ki-67 LI values. The patients' Tg status was categorized by postoperative serum Tg values: biochemically persistent disease (BPD), equivocal status, and biochemical remission (BR). Tg status was significantly associated with the Ki-67 LI (p < 0.0001).
Relationship between the Ki-67 LI and the Tg-DT
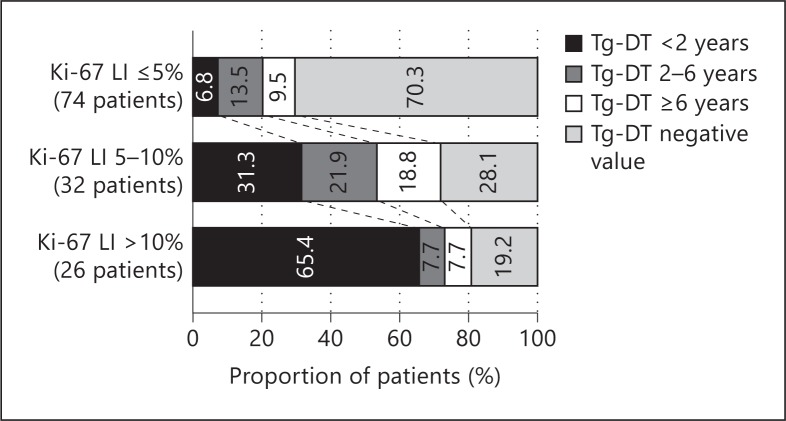
Since both the Ki-67 LI and the Tg-DT are related to tumor growth, we investigated the relationship between these factors in patients with biochemically persistent disease. Since all patients who died of the disease in the present cohort had Tg-DT <2 years, and since Tg-DT of 6 years divided patients with Tg-DT longer than 2 years into two patient groups of similar numbers, in the present study, the patients were grouped according to Tg-DT using these cut-off points. Tg-DT was of negative value in patients had decease in Tg values over time. The Ki-67 LI values were significantly associated with the Tg-DT (p < 0.0001) (fig. 2). Only 7% of our Ki-67 LI ≤5% patients had Tg-DT <2 years, whereas 31% of the Ki-67 LI 5-10% patients and 65% of the Ki-67 LI >10% patients had Tg-DT <2 years. Patients who had Tg-DT of negative values had excellent outcomes, although they were regarded as having biochemically persistent disease [6,12]. In the present study, the proportions of patients with a negative Tg-DT value were the opposite of Tg-DT <2 years, being 70, 28, and 19%, respectively. When the correlation of these ordinal numeral variables was analyzed using Spearman's rank correlation coefficient test, there was a significant inverse correlation between the Ki-67 LI and the Tg-DT (Spearman's ρ = −0.5267, p < 0.0001).
Fig. 2.
Relationship between the Tg-DT and the Ki-67 LI in patients with biochemically persistent disease. The Tg-DT was significantly associated with the Ki-67 LI (p < 0.0001). There was a significant inverse correlation between these factors on Spearman's rank correlation coefficient test (Spearman's ρ = −0.5267, p < 0.0001).
Relationship between Age and the Ki-67 LI
The patient's age at surgery was significantly associated with the Ki-67 LI, and the Ki-67 LI was inversely correlated with the Tg-DT, as described above. When the patients were divided into three age groups (≤40, >40-60, and >60 years), the distribution of Tg-DT values varied with age in our recent study [13]. Thus, we looked at the relationship between these age groups and the Ki-67 LI (table 2). We found that the Ki-67 LI was significantly associated with age at surgery (p = 0.0013). The proportion of patients with Ki-67 LI >10% increased with age, being 4, 6, and 13%, respectively. However, the proportion of patients with Ki-67 LI 5-10% showed a biphasic pattern: high (20%) in young patients, low (7%) in middle-aged patients, and high (16%) in elderly patients. The proportion of patients with Ki-67 LI ≤5% was highest in the middle-aged patients, being 77, 86, and 70%, respectively for the age groups. Thus, each age group had a different distribution pattern of Ki-67 LI, which was similar to our recent observations regarding Tg status and Tg-DT [13].
Table 2.
Relationship between patient age at surgery and the Ki-67 LI
| Age | Patients | Ki-67 LI |
|||
|---|---|---|---|---|---|
| n | ≤5% | 5–10% | >10% | p values | |
| ≤40 | 82 | 63 | 16 | 3 | 0.0013 |
| >40–60 | 204 | 176 | 15 | 13 | |
| >60 | 104 | 73 | 17 | 14 | |
Survival and the Ki-67 LI
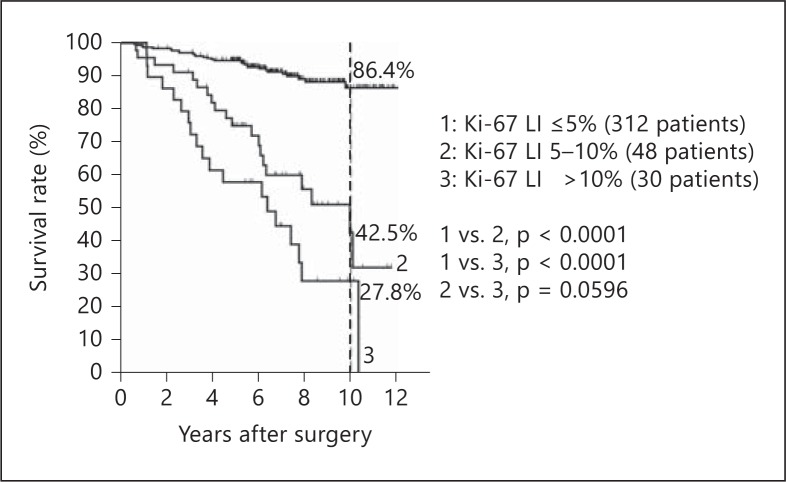
During the study period, 56 patients developed locoregional recurrences, 26 patients developed distant metastases, 13 of these patients had both locoregional recurrences and distant metastases, and 6 patients died of the disease. Higher Ki-67 LI values were significantly associated with both poorer disease-free survival (p < 0.0001) and disease-specific survival (p < 0.0001) (fig. 3, 4). The rates of disease-free survival at 10 years were 86.4, 42.5, and 27.8% in the patients with Ki-67 LI ≤5, 5-10, and 10%, respectively (Ki-67 LI ≤5 vs. 5-10%, p < 0.0001; ≤5 vs. >10%, p < 0.0001; 5-10 vs. >10%, p = 0.0596).
Fig. 3.
Kaplan-Meier curves of disease-free survival by Ki-67 LI groups.
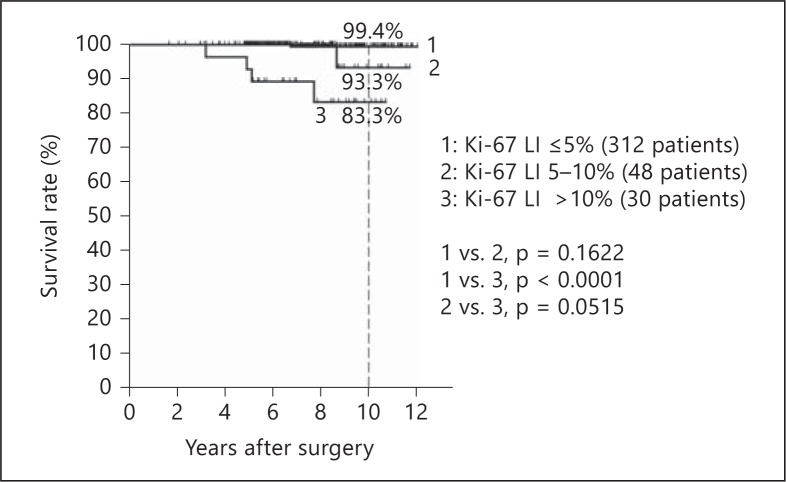
Fig. 4.
Kaplan-Meier curves of disease-specific survival by Ki-67 LI groups.
The disease-specific survival rates at 10 years were 99.4, 93.3, and 83.3% for the Ki-67 LI groups, respectively. In the present study, only 6 patients (1 of the 312 patients with Ki-67 LI ≤5%, 1 of the 48 patients with Ki-67 LI 5-10%, and 4 of the 30 patients with Ki-67 LI >10%) died of the disease. However, the difference in survival between the Ki-67 LI ≤5% patients and the Ki-67 LI >10% patients reached significance on log-rank test (p < 0.0001).
Univariate and Multivariate Analyses of Factors Associated with Disease-Free Survival
We evaluated the prognostic significance of clinicopathological factors for disease-free survival in the 378 patients without distant metastases at the time of surgery. Age <40 years, age ≥60 years, tumor >4 cm, significant extrathyroid extension (Ex2), clinical lateral node metastasis (cN1b), aggressive histological type, Ki-67 LI >10%, Ki-LI 5-10%, biochemically persistent disease, and Tg-DT ≤2 years were all significantly associated with disease-free survival (table 3). Since the latter two factors were strongly associated with the Ki-67 LI as described above, we performed a multivariate analysis using the Cox proportional hazard regression model with stepwise variable selection techniques excluding these two factors. In this analysis, Ki-67 LI >10%, Ki-LI 5-10%, age <40 years, age ≥60 years, and clinical lateral node metastasis (cN1b) remained significant independent factors. The hazard ratios for these factors were 15.33, 9.33, 5.23, 4.10, and 2.82, respectively. Thus, high Ki-67 LI categories, young and old age at surgery, and cN1b were independent factors for tumor recurrence.
Table 3.
Univariate and multivariate Cox regression analysis of factors associated with disease-free survival of patients with papillary thyroid carcinoma
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p values | HR | 95% CI | p values | |
| Male | 1.47 | 0.83–2.61 | 0.1900 | |||
| Age <40 years | 1.97 | 1.18–3.31 | 0.0095 | 5.23 | 1.46–18.77 | 0.0112 |
| Age ≥60 years | 2.62 | 1.61–4.24 | 0.0001 | 4.10 | 1.41–11.89 | 0.0094 |
| Tumor >4 cm | 2.73 | 1.68–4.46 | 0.0001 | |||
| Ex2 | 2.58 | 1.60–4.15 | 0.0001 | |||
| cN1b | 3.63 | 2.25–5.84 | <0.0001 | 2.82 | 1.20–6.65 | 0.0178 |
| Aggressive type | 5.81 | 3.58–9.43 | <0.0001 | 2.13 | 0.84–5.41 | 0.1100 |
| Ki-67 LI >10% | 6.34 | 3.72–10.79 | <0.0001 | 15.33 | 4.13–56.96 | 0.00005 |
| Ki-67 LI 5–10% | 3.47 | 2.06–5.85 | <0.0001 | 9.33 | 2.98–29.21 | 0.00013 |
| Biochemically PD | 12.04 | 6.31–22.99 | <0.0001 | excluded | ||
| Tg-DT <2 years | 13.54 | 6.16–29.76 | <0.0001 | excluded | ||
HR = Hazard ratio; CI = confidence interval; Ex2 = significant extrathyroid extension; Biochemically PD = biochemically persistent disease; Tg-DT = thyroglobulin doubling time. For the multivariate analysis, biochemically PD and Tg-DT were excluded from the analysis because these factors were strongly associated with Ki-67 LI.
Univariate and Multivariate Analyses of Factors Associated with Disease-Specific Survival
We evaluated the prognostic significance of clinicopathological factors for disease-specific survival in the 390 patients with papillary carcinoma. The univariate analysis revealed that male gender, age ≥60 years, Ex2, cN1b, aggressive type, and Ki-67 LI >10% were significant factors for disease-specific survival, and tumor >4 cm and distant metastases (M1) were marginally significant (table 4). All 6 patients who died of papillary carcinoma had biochemically persistent disease and Tg-DT ≤2 years. Thus, these factors were excluded from the multivariate analysis, which revealed that Ki-67 LI >10%, age >60 years, and Ex2 were independent factors for disease-specific survival. The hazard ratios for these factors were 34.08, 20.78, and 13.98, respectively. The presence of distant metastases at the time of surgery did not remain significant. This may be partially due to the small number of patients (6) who died of the cancer in the present study.
Table 4.
Univariate and multivariate Cox regression analyses of factors associated with cause-specific survival of patients with papillary thyroid carcinoma
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p values | HR | 95% CI | p values | |
| Male | 5.19 | 1.04–25.83 | 0.0445 | |||
| Age ≥60 years | 15.5 | 1.81–132.7 | 0.0124 | 20.78 | 1.86–231.86 | 0.0137 |
| Tumor >4 cm | 3.84 | 0.77–19.03 | 0.0995 | |||
| Ex2 | 12.1 | 1.41–103.4 | 0.0231 | 13.98 | 1.29–151.66 | 0.0301 |
| cN1b | 6.44 | 1.18–35.2 | 0.0315 | 5.18 | 0.88–33.55 | 0.0844 |
| Ml | 7.28 | 0.845–62.87 | 0.0709 | 9.42 | 0.60–148.37 | 0.1108 |
| Aggressive type | 5.57 | 1.12–27.7 | 0.0359 | |||
| Ki-67 LI >10% | 23.58 | 4.31–128.94 | 0.0003 | 34.08 | 3.81–305.16 | 0.0016 |
| Ki-67 LI 5–10% | 1.28 | 0.149–11.01 | 0.8210 | |||
| Biochemically PD | NC | NC | NC | excluded | ||
| Tg-DT ≤2 years | NC | NC | NC | excluded | ||
HR = Hazard ratio; CI = confidence interval; Ex2 = significant extrathyroid extension; Biochemically PD = biochemically persistent disease; Tg-DT = thyroglobulin doubling time. NC = Not calculated because all of the patients who died of the cancer had biochemically PD and Tg-DT <2 years. For the multivariate analysis, these two factors were excluded from the analysis.
Discussion
Assessments of prognosis are very important in the management of patients with cancer. Many risk assessment systems for thyroid cancer have been established, including the UICC TNM classification system, AMES, AGES, MACIS, and EORTC [11,14,15,16,17]. The patient's age at the diagnosis, gender, tumor size, extrathyroid extension, lymph node and distant metastases, and pathological differentiation of cancer are well-established prognostic factors in thyroid cancer. The most commonly used system might be the TNM classification, which uses age, tumor size, extrathyroid extension, node and distant metastases for risk assessment. The TNM stage can be evaluated preoperatively, helping the planning of treatment for patients. Pathological findings give additional information for evaluating the pathological TNM stage. The growth activity of tumors is also a very important biological factor. However, none of the above systems use factors directly related to tumor growth activity for risk assessment.
We reported that the Tg-DT was a very potent prognostic indicator in patients with papillary carcinoma and undetectable TgAb who underwent total thyroidectomy [6]. When the Tg-DT was added to the classical prognostic factors mentioned above for multivariate analyses of disease-free survival and disease-specific survival, only Tg-DT remained an independent prognostic factor. Serum tumor marker DT is thought to indicate the growth rate of the tumor [18].
Another popular method for evaluating tumor growth activity is the immunohistochemical estimation of the Ki-67 LI on tumor tissue. Ki-67 is a cell proliferation-associated antigen that is expressed in all stages of the cell proliferative cycle except the G0 phase [7]. A high Ki-67 LI was associated with poor prognosis in patients with breast cancer and those with prostate cancer [8,9]. In our previous study of 371 patients with papillary carcinoma, we found that the Ki-67 LI was an independent prognostic factor for disease-free survival, and that patients with high Ki-67 LI values had significantly worse disease-specific survival than patients with low Ki-67 LI values [10]. Our recent studies clearly indicate the importance of tumor growth activity in assessing the prognosis of patients with cancer.
In the present study, we found that the Ki-67 LI had a significant inverse correlation with the Tg-DT in patients with biochemically persistent disease postoperatively. This status suggests that the patients have hidden metastases. An inverse correlation means that a higher Ki-67 LI is associated with a shorter Tg-DT, which indicates rapid growth of the tumor. This finding was an expected result, since high Ki-67 LI values indicate high proliferation activity of the tumor. Very interestingly, the Ki-67 LI was also significantly associated with the postoperative serum Tg status, which was categorized into three classes: biochemically persistent disease, equivocal status, and biochemical remission. The proportion of patients with biochemically persistent disease increased with higher Ki-67 LI values: 24, 67, and 87%, respectively. Conversely, the proportion of patients with biochemical remission decreased with higher Ki-67 LI values, being 53, 21, and 7%, respectively. These data indicate that the Ki-67 LI was also inversely associated with the surgical cure rate when it was evaluated as the postoperative serum Tg status. Thus, the Ki-67 LI seems to be associated with metastasizing activity as well as tumor growth activity.
To the best of our knowledge, the only previous report on the prognostic significance of the Ki-67 LI in a large series of patients with papillary carcinoma is ours [10]. That study was done on 371 patients with papillary carcinoma who underwent surgery in 1996 and 1997. The immunohistochemical technique used for the study was the immunoperoxidase ABC method performed manually, as opposed to the method used in the present study, which has a much higher sensitivity. In the previous study, the cut-off points for categorizing the Ki-67 LI were 1 and 3%, whereas 5 and 10% were adopted in the present study. The 390 patients in the present study were completely different from the previous series, but low, medium, and high Ki-67 LI categories included similar proportions of patients: 57, 33, and 9%, respectively in the previous study and 80, 12, and 8%, respectively in the present study. Both studies gave similar results: the Ki-67 LI was significantly associated with disease-free survival and disease-specific survival, and the Ki-67 LI was the most potent indicator of disease-free survival and disease-specific survival on multivariate analyses when the postoperative serum Tg status and Tg-DT were excluded from the analyses. Thus, these clinically important findings were confirmed in different cohort studies.
In the present multivariate logistic regression analysis, the patient's age at surgery was the second strongest indicator of disease-free survival and disease-specific survival. For disease-free survival, age <40 years and age ≥60 years were significant factors with hazard ratios of 5.23 and 4.10, respectively, whereas age ≥60 years was a much stronger factor for disease-specific survival with a hazard ratio of 20.78, and age <40 years was not associated with cancer death. None of the 82 patients <40 years old died of the cancer, although 5 patients had M1 status at surgery and 21 of the remaining 77 M0 patients had recurrence.
Poor disease-specific survival in elderly patients with papillary carcinoma was well documented [1,2,3]. Interestingly, a high recurrence rate with excellent survival was reported in young patients [19,20]. The data of the present study are in accordance with these descriptions. However, there is no clear explanation for these confusing age-related phenomena. Regarding age-related tumor biology, we recently found that (i) the proportion of patients with a Tg-DT ≤2 years increased with age, (ii) the proportion of patients with biochemically persistent disease showed a biphasic pattern (high in patients <40 years, low in patients 40-60 years, and high in patients ≥60 years), and (iii) the proportion of patients with biochemical remission was highest in middle-aged patients [13]. These findings are in accord with the relationship between age and Ki-67 LI found in the present study: the proportion of patients with Ki-67 LI >10% increased with age; the proportion of patients with Ki-67 LI 5-10% showed a biphasic pattern (high in young patients, low in middle-aged patients, and high in elderly patients), and the proportion of patients with Ki-67 LI ≤5% was highest in the middle-aged patients. The confusing age-related phenomena on recurrence and mortality in patients with papillary carcinoma may be explained with age-related variations in Tg-DT and postoperative serum Tg status, and these factors may be explained with age-related variations in Ki-67 LI. However, the causes of age-related variations in Ki-67 LI remain to be clarified.
Conclusion
The Ki-67 LI was significantly associated with the postoperative serum Tg status and Tg-DT, and it was a strong prognostic indicator in patients with papillary carcinoma. The age-related variations in the Ki-67 LI might elucidate age-related phenomena on recurrence and mortality in patients with papillary carcinoma.
Disclosure Statement
None of the authors have any conflicts of interest to disclose.
Acknowledgement
The authors thank Miss Miwa Miyauchi for preparing the manuscript.
References
- 1.Ito Y, Miyauchi A. Prognostic factors and therapeutic strategies for differentiated carcinomas of the thyroid. Endocr J. 2009;56:177–192. doi: 10.1507/endocrj.k08e-166. [DOI] [PubMed] [Google Scholar]
- 2.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–1278. doi: 10.1007/s00268-012-1423-5. [DOI] [PubMed] [Google Scholar]
- 3.Wu HS, Young MT, Ituarte PH, D'Avanzo A, Duh QY, Greenspan FS, Loh KC, Clark OH. Death from thyroid cancer of follicular cell origin. J Am Coll Surg. 2000;191:600–606. doi: 10.1016/s1072-7515(00)00731-6. [DOI] [PubMed] [Google Scholar]
- 4.Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–3562. doi: 10.1210/jcem.82.11.4373. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Hirokawa M, Fukushima M, Inoue H, Yabuta T, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Prevalence and prognostic significance of poor differentiation and tall cell variant in papillary carcinoma in Japan. World J Surg. 2008;32:1535–1543. doi: 10.1007/s00268-007-9406-7. [DOI] [PubMed] [Google Scholar]
- 6.Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Inoue H, Tomoda C, Yabuta T, Masuoka H. Prognostic impact of serum thyroglobulin-doubling time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011;21:707–716. doi: 10.1089/thy.2010.0355. [DOI] [PubMed] [Google Scholar]
- 7.Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad HD. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–873. [PMC free article] [PubMed] [Google Scholar]
- 8.Luporsi E, André F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, Egele C, Poulet B, Clough KB, Crouet H, Fourquet A, Lefranc JP, Mathelin C, Rouyer N, Serin D, Spielmann M, Haugh M, Chenard MP, Brain E, de Cremoux P, Bellocq JP. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berney DM, Gopalan A, Kudahetti S, Fisher G, Ambroisine L, Foster CS, Reuter V, Eastham J, Moller H, Kattan MW, Gerald W, Cooper C, Scardino P, Cuzick J. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study. Br J Cancer. 2009;100:888–893. doi: 10.1038/sj.bjc.6604951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito Y, Miyauchi A, Kakudo K, Hirokawa M, Kobayashi K, Miya A. Prognostic significance of Ki-67 labeling index in papillary thyroid carcinoma. World J Surg. 2010;34:3015–3021. doi: 10.1007/s00268-010-0746-3. [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Gospodarowicz MK, Wittekind CH, editors. Thyroid gland; in UICC: TNM Classification of Malignant Tumours. ed 7. Oxford: Wiley-Blackwell; 2010. pp. 58–62. [Google Scholar]
- 12.Tomoda C, Miyauchi A. Undetectable serum thyroglobulin levels in patients with medullary thyroid carcinoma after total thyroidectomy without radioiodine ablation. Thyroid. 2012;22:680–682. doi: 10.1089/thy.2011.0508. [DOI] [PubMed] [Google Scholar]
- 13.Miyauchi A, Kudo T, Kihara M, Higashiyama T, Ito Y, Kobayashi K, Miya A. Relationship of biochemically persistent disease and thyroglobulin-doubling time to age at surgery in patients with papillary thyroid carcinoma. Endocr J 2012 (E-pub ahead of print). [PubMed]
- 14.Cady B, Rosai R. An expanded view of risk group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 15.Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–1095. [PubMed] [Google Scholar]
- 16.Hay ID, Bergstrahl EJ, Goellner JR, Ebersokd JR, Grant CG. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1,779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1058. [PubMed] [Google Scholar]
- 17.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg. 2007;245:366–378. doi: 10.1097/01.sla.0000250445.92336.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyauchi A, Onishi T, Morimoto S, Takai S, Matsuzuka F, Kuma K, Maeda M, Kumahara Y. Relation of doubling time of plasma calcitonin levels to prognosis and recurrence of medullary thyroid carcinoma. Ann Surg. 1984;199:461–466. doi: 10.1097/00000658-198404000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung W, Sarlis NJ. Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: a review. Thyroid. 2002;12:683–702. doi: 10.1089/105072502760258668. [DOI] [PubMed] [Google Scholar]
- 20.Mazzaferri EL, Jhiang S. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]