Abstract
Background
Hashimoto's encephalopathy (HE) is a rare immune-mediated encephalopathy associated with autoimmune Hashimoto's thyroiditis.
Objectives and Methods
We report on a patient with HE and significant clinical improvement correlating with an increase in cerebral blood flow demonstrated by hexamethylpropyleneamine oxime (HMPAO) single-photon emission computed tomography (SPECT). HMPAO-SPECT was performed with 740 MBq of technetium-99m-HMPAO. To demonstrate the improvement in regional cerebral blood flow, individual regions of interest were drawn around visually diminished HMPAO uptake, the lesion to reference region ratio was calculated and transverse section images and semi-quantitative measurements were performed.
Results
We show a 5-year follow-up with significant clinical improvement, a 10-fold reduction in autoantibodies to thyroid peroxidase and an approximately 20% improvement in cerebral blood flow with HMPAO-SPECT.
Conclusion
Adequate levothyroxine treatment achieving and maintaining euthyroidism should be considered as therapy to lower autoantibodies and improve clinical outcome in patients with Hashimoto's thyroiditis and encephalopathy.
Key Words : Hashimoto's encephalopathy, Hashimoto's thyroiditis, Hypothyroidism, Levothyroxine, Hexamethylpropyleneamine oxime single-photon emission computed tomography
Introduction
Hashimoto's encephalopathy (HE) is a rare immune-mediated encephalopathy associated with Hashimoto's thyroiditis. The relationship between Hashimoto's thyroiditis and HE is unclear. However, most patients respond to corticosteroids in the short term, and the term ‘steroid-responsive encephalopathy associated with autoimmune thyroiditis’ is also used to describe this disease [1]. We report on a patient with HE with a significant clinical improvement correlating with a 20% increase in cerebral blood flow on 99mTc-hexamethylpropyleneamine oxime (HMPAO)single-photon emission computed tomography (SPECT) under levothyroxine therapy. This was accompanied by a more than 10-fold decrease in autoantibodies to thyroid peroxidase (TPO-Abs) during the follow-up of 5 years.
Case Presentation
A 52-year-old female Caucasian patient presented with increasing cognitive impairment and seizures for 26 years. In the year 1985, due to partial seizures, a diagnosis of partial epilepsy was based on an electroencephalogram. A subcortical frontal potential and a few subcortical lesions were suspected, although many muscle-related artifacts were described. At this time, computed tomography of the brain was normal. A first attempt to control her seizures with carbamazepine was started. This therapy was continued until the year 1999, although without clinical benefit. Therefore, the patient underwent another examination. During an electroencephalogram with sleep deprivation, again with many muscle-related artifacts, a right-sided frontal-temporal lesion with theta-delta activity was seen, and complex partial seizures were suspected. However, brain magnetic resonance imaging was normal. Psychic seizures were also included and discussed in the differential diagnosis. The therapy was changed to lamotrigine and primidone, again without clinical benefit.
At presentation in our institution, the patient experienced increasing frequency and intensity of daily partial and generalized seizures, some recognized by the patient but all including fluctuations in the level of consciousness and mood disturbances, as noted by her husband. Her cognitive impairment involved problems with memory, thinking and judgment, which were greater than age-related changes. Six months previously, an updated consultation suspected cryptogenic epilepsy with simple focal and rare complex focal seizures, and therapy with levetiracetam was initiated but also failed to improve seizures. During the patient's work-up, the Mini-Mental State Examination score was 6/30. However, brain magnetic resonance imaging and a repeat electroencephalogram were normal. Evaluation of cerebrospinal fluid after lumbar puncture revealed high protein levels; TPO-Abs were not determined, and no explanation for her symptoms was revealed.
Laboratory examination showed elevated thyroid-stimulating hormone of 10.9 mU/l (normal range 0.2-3.8). Autoimmune Hashimoto's thyroiditis was then diagnosed on the basis of excessively elevated levels of TPO-Abs (6,296 U/l, normal <5) and a diffuse reduction in thyroid echogenicity on ultrasonography. All the other routine laboratory parameters, including free triiodothyronine, free thyroxine and thyrotropin receptor antibodies, were within normal limits.
Therapy with levetiracetam was continued at a stable dosage throughout the whole 5-year observational period. Although the diagnosis of HE and/or steroid-responsive encephalopathy was made and thoroughly explained to the patient, she refused cortisone and immunosuppressive therapy. Therefore, levothyroxine therapy only was introduced, with 100 μg of levothyroxine daily. Initially, the patient described worsening of seizures for a few days; subsequently, gradual improvement occurred. Within 1 month the patient was euthyroid, and during the following 5 years a thyroid-stimulating hormone value within the normal range (mean 2.1 ± 0.9 mU/l) was measured repeatedly. Unfortunately, at the time of diagnosis the patient refused to undergo HMPAO-SPECT. Finally, due to improvement of the seizures after 2 and 5 years of euthyroidism an HMPAO-SPECT was performed. However, before the first and second HMPAO-SPECT, TPO-Abs results were 3,846 and 414 U/l, respectively. Imaging was performed 60 min after intravenous injection of 740 MBq of 99mTc-HMPAO, and brain SPECT images were obtained using a double-head gamma camera (GE, Millennium HE, Haifa, Israel) equipped with a fan beam collimator. Data were acquired in a 128 × 128 matrix through 180° rotation at 3° intervals for 40 s per view. Data reconstruction of transverse sections was performed by filtered back projection. A uniform ellipse was placed around each slice in order to apply Chang's first order correction method for photon attenuation.
The cerebral distribution of 99mTc-HMPAO is dependent on regional blood flow and is used to evaluate the cerebral hemodynamic changes in cerebrovascular diseases and other neuro-psychiatric disorders. This lipophilic radiocompound is trapped by cerebral cells and can be assessed semi-quantitatively by the definition of a region of interest (ROI) representing the affected cerebral cortex as compared to a background ROI of the same size on tomographic SPECT recordings.
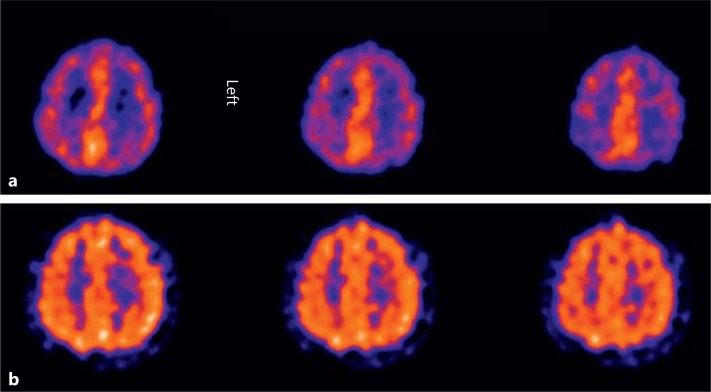
The first examination demonstrated frontal and parietal cerebral hypoperfusion as demonstrated in 3 transverse sections (fig. 1a). The patient's seizures improved clinically significantly such that the frequency and intensity were reduced to one partial seizure in a time period of 4 weeks and the Mini-Mental State Examination score increased to 26/30. To demonstrate improvement of regional cerebral function, individual ROIs were drawn around visually diminished parietal HMPAO uptake on both sides. This was compared to an ROI of the same size drawn with normal occipital HMPAO uptake as a reference region. In addition, a background region was drawn in a small subcortical area. Finally, a lesion to reference region ratio was calculated (as a percentage) according to the following formula: ratio = (parietal ROI – background ROI)/(occipital ROI – background ROI). This resulted in an improvement of parietal perfusion from 49 to 72% on the left side and from 57 to 67% on the right side. The semi-quantitative measurements using ROIs were performed on a Hermes workstation (Hermes Medical Solutions, Sweden). The significant improvement in the perfusion after 5 years is shown in 3 transverse sections (fig. 1b). Written informed consent was obtained for all procedures, which were in accordance with the Declaration of Helsinki and the recommendations of the local ethics committee.
Fig. 1.
Transverse sections of HMPAO-SPECT images after 2 (a) and 5 (b) years of levothyroxine therapy demonstrating improvement of cerebral blood flow within the fronto-parietal region on both sides.
Discussion
HE or steroid-responsive encephalopathy associated with autoimmune thyroiditis is a rare condition and is still problematic in terms of pathophysiology, diagnosis and treatment. However, initially most patients respond to short-term high-dose corticosteroids, and the term ‘steroid-responsive encephalopathy associated with autoimmune thyroiditis’ is also used to describe this disorder [2,3]. It is generally accepted that the diagnosis of HE must include encephalopathy, characterized by cognitive impairment and seizures, and may also be associated with psychiatric features such as hallucinations, delusions or paranoia. Seizures are noted in more than 60% of affected patients and include both partial and generalized seizures [4,5]. Autoimmune thyroiditis is diagnosed on the basis of elevated levels of TPO-Abs and usually a diffuse reduction in thyroid echogenicity on ultrasonography.
The nature of the relationship between Hashimoto's thyroiditis and HE is unclear. The most broadly accepted hypothesis of the pathogenesis of HE describes an autoimmune aetiology resulting in either cerebral vasculitis or direct injury from autoantibodies. However, the histologic finding of perivascular lymphocytic infiltration, the female preponderance and the response to corticosteroid and immunosuppressive therapy suggest an autoimmune aetiology [2,5]. An increased prevalence of other additional antibodies in the central nervous system tissue and gangliosides was recently demonstrated in Hashimoto's thyroiditis, and their relation to HE is under investigation [6]. We linked Hashimoto's thyroiditis and HE in this patient since none of the initiated therapies were successful in controlling seizures, Hashimoto's thyroiditis was present and the patient responded clinically to levothyroxine treatment.
Impaired brain perfusion in patients with known autoimmune thyroiditis has been demonstrated and supports a hypothesis of cerebral involvement in autoimmune thyroiditis [7,8,9]. However, a recent study showed that a 6-month levothyroxine treatment reduces systemic inflammation in patients with Hashimoto's thyroiditis. This anti-inflammatory effect was described to be clinically relevant and to delay the progression of Hashimoto's thyroiditis even in euthyroid women [10]. A long-term follow-up of TPO-Abs in patients with Hashimoto's thyroiditis treated with levothyroxine demonstrated a 70% decrease in TPO-Abs after 5 years [11].
In HE with initially high-dose cortisone therapy, clinical improvement and improved HMPAO-SPECT is described within a few days [12], and approximately 80% of patients show complete remission for up to 2 years. Relapses can occur after improvement of symptoms or reduction in corticosteroid therapy. Some patients relapsed after an asymptomatic period, prompting other therapeutic measures such as plasmapheresis, immunoglobulins and change of immunosuppressant medication [13].
Levothyroxine monotherapy has become the mainstay in treating hypothyroidism but has not been studied in HE. We speculate that in this patient, the improvement in frontal lobe perfusion seen on HMPAO-SPECT may have been the reason for the clinical improvement. However, it is not known if levothyroxine therapy induces changes in cerebral blood flow in hypothyroid patients without encephalopathy. Therefore, studies investigating cerebral blood flow in patients with and without Hashimoto's thyroiditis, both euthyroid and hypothyroid, with levothyroxine therapy could clarify if improvement of cerebral blood flow is due to this therapy.
Conclusion
In this patient, we showed a 5-year follow-up with significant clinical improvement under levothyroxine therapy, and, from year 3-5, an up to 20% improvement of cerebral blood flow with HMPAO-SPECT was demonstrated. We conclude that adequate levothyroxine treatment achieving and maintaining euthyroidism should be considered as therapy to lower autoantibodies and improve clinical outcome in patients with Hashimoto's thyroiditis and encephalopathy.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Tamagno G, Gaoatswe G. Time for the endocrinologists to expand their awareness of and contribution to the diagnosis and management of encephalopathy associated with autoimmune thyroid disease. Hormones. 2011;10:36–38. doi: 10.14310/horm.2002.1298. [DOI] [PubMed] [Google Scholar]
- 2.Chang T, Riffsy MTM, Gunaratne PS. Hashimoto encephalopathy. Clinical and MRI improvement following high-dose corticosteroid therapy. Neurologist. 2010;16:394–396. doi: 10.1097/NRL.0b013e3181d6b6f6. [DOI] [PubMed] [Google Scholar]
- 3.Lee SW, Donlon S, Caplan JP. Steroid responsive encephalopathy associated with autoimmune thyroiditis (SREAT) or Hashimoto's encephalopathy: a case and review. Psychosomatics. 2011;52:99–108. doi: 10.1016/j.psym.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Lass P, Slawek J, Derejko M, Rubello D. Neurological and psychiatric disorders in thyroid dysfunctions. The role of nuclear medicine: SPECT and PET imaging. Minerva Endocrinol. 2008;33:75–84. [PubMed] [Google Scholar]
- 5.Zhao W, Li J, Wang J, Guo Y, Tuo H, Kang Z, Jiang B, Wang R, Wang D. A case of Hashimoto encephalopathy. Clinical manifestation, imaging, pathology, treatment, and prognosis. Neurologist. 2011;17:141–143. doi: 10.1097/NRL.0b013e3182173341. [DOI] [PubMed] [Google Scholar]
- 6.Müssig K, Leyhe T, Holzmüller S, Klein R, Weinert C, Saur R, Klingberg S, Häring HU, Gallwitz B. Increased prevalence of antibodies to central nervous system tissue and gangliosides in Hashimoto's thyroiditis compared to other thyroid illnesses. Psychoneuroendocrinology. 2009;34:1252–1256. doi: 10.1016/j.psyneuen.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Kaya M, Cermik TF, Bedel D, Kutucu Y, Tuglu C, Yigitbasi ÖN. Assessment of alterations in regional blood flow in patients with hypothyroidism due to Hashimoto's thyroiditis. J Endocrinol Invest. 2007;30:491–496. doi: 10.1007/BF03346333. [DOI] [PubMed] [Google Scholar]
- 8.Kinuya S, Michigishi T, Tonami N, Aburano T, Tsuji S, Hashimoto T. Reversible cerebral hypoperfusion observed with Tc-99 HMPAO SPECT in reversible dementia caused by hypothyroidism. Clin Nucl Med. 1999;24:666–668. doi: 10.1097/00003072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Zettinig G, Asenbaum S, Fueger BJ, Hofmann A, Diemling M, Mittlboeck M, Dudczak R. Increased prevalence of sublinical brain perfusion abnormalities in patients with autoimmune thyroiditis: evidence of Hashimoto's encephalitis? Clin Endocrinol (Oxf) 2003;59:637–643. doi: 10.1046/j.1365-2265.2003.01901.x. [DOI] [PubMed] [Google Scholar]
- 10.Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2011;96:2206–2215. doi: 10.1210/jc.2010-2986. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt M, Voell M, Rahlff I, Dietlein M, Kobe C, Faust M, Schicha H. Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto's thyroiditis) treated with levothyroxine. Thyroid. 2008;18:755–760. doi: 10.1089/thy.2008.0008. [DOI] [PubMed] [Google Scholar]
- 12.Chen PL, Wang PY, Hsu HY. Reversible electroencephalographic and single photon emission computed tomography abnormalities in Hashimoto's encephalopathy. J Chin Med Assoc. 2005;68:77–81. doi: 10.1016/S1726-4901(09)70139-X. [DOI] [PubMed] [Google Scholar]
- 13.De Holanda NCP, De Lima DD, Cavalcanti TB, Lucena CS, Bandeira F. Hashimoto's encephalopathy: systematic review of the literature and an additional case. J Neuropsychiatry Clin Neurosci. 2011;23:384–390. doi: 10.1176/jnp.23.4.jnp384. [DOI] [PubMed] [Google Scholar]