Abstract
Purpose:
Racial differences exist in the incidence of prostate cancer (PCa). Although many studies have looked at the performance of prostate-specific antigen (PSA) and PSA density (PSAD) in the detection of PCa, only a few have looked at it in relation to Indonesian men. The objective of this study is to find out better PSA and PSAD cutoff point in the detection of PCa in Indonesian men.
Methods:
A total of 404 consecutive Indonesian men underwent prostate biopsy for suspicion of PCa from 2008 to 2011. The biopsy criteria include one or more of the following: serum PSA more than 10 ng/mL, PSAD more than 0.15 if PSA 4–10 ng/mL, hypoechoic lesion during transrectal sonography and/or abnormal digital rectal examination.
Results:
Forty five out of 404 (11.1%) had positive biopsies. The mean age, prostate volume, PSA and PSAD were respectively 64.06 years, 43.03 mL, 45.59 ng/mL and 1.15. Of the 404, 131 cases (32.4%) were confirmed to be urinary retention. Positive urine culture found in 182 cases (45%). The cutoff point to detect PCa as estimated by the receiver operating characteristics was 6.95 ng/mL for PSA (sensitivity 97.8%, specificity 19.6%) and 0.7072 for PSAD (sensitivity 62.2%, specificity 78.7%). Positive predictive value for this PSA and PSAD cutoff point were 11.6% and 27.5% respectively (P=0.004 and P=0.000). There was a significant correlation between hypoechoic lesion and positive biopsy results (P =0.000). Urinary retention elevates PSA cutoff point to 14.55 (sensitivity 90.9%, specificity 50%), while positive urine culture alters almost no PSA cutoff elevation.
Conclusions:
PSA and PSAD cutoff point for Indonesian men in this series is relatively different from international consensus. Furthermore, these data show that PSA and PSAD cutoff point must be adjusted to racial variation to discriminate between malignant and benign disease. Urinary retention is a significant factor for PSA cutoff increase.
Keywords: Prostate neoplasms, Prostate-specific antigen, Prostate specific antigen density, Indonesia
INTRODUCTION
Prostate cancer (PCa) is the most common estimated cancer among men. It is responsible for approximately 33% of incidental cases in men [1]. Despite its prevalence, the natural history of this disease is remarkably heterogeneous [2]. Regular serum prostate-specific antigen (PSA) evaluation and digital rectal examination (DRE) are recommended for detecting PCa [1]. The serum PSA level is among the best of the screening tools available in medicine today and is recognized as the best marker for early detection [3]. PSA testing is widely used for PCa screening and increases cancer detection by 81% over use of the DRE alone. Nearly 70% of cancers can be detected using PSA cutoff 4 ng/mL in the first 4 years of screening. However, PSA is limited by its relative lack of specificity when serum concentrations are moderately elevated (4–10 ng/mL). The choice of a PSA threshold or cut point above which one would recommend further evaluation with prostate biopsy to rule out PCa is controversial. Although the PSA threshold of 4 ng/mL has been the most commonly used, the value that most efficiently balances the dual goal of reducing cancer mortality and reducing unnecessary testing is unknown [4].
Recently, various strategies were introduced to improve the sensitivity and specificity of the PSA. Among those, we can highlight PSA density (PSAD), PSA velocity (PSAV), distribution of serum PSA levels according to age and the determination of molecular forms of PSA [5]. Because of the increasing frequency of benign prostate hyperplasia (BPH) and consequent prostate growth from 40 years on, some additional parameters were proposed in association with PSA. One of these parameters is PSAD with cutoff level of 0.15, for patients with PSA between 4–10 ng/mL, would discriminate most of the cases of carcinoma from BPH. But, other studies show that PSAD has no value for such discrimination [6]. The specific cutoff for PSA and PSAD to delineate patients at high risk who should undergo biopsy has been controversial, especially when the PSA and PSAD values are influenced by race and environment [7]. In the Western world, the reliable cutoff value of PSA to perform prostatic biopsies is still in debate: a PSA level more than 4.0 ng/mL was stated as abnormal and it is recommended to perform prostatic biopsies, but present studies reported that a cutoff point below 4 ng/mL would also produce a high positive predictive value (PPV) [8]. While in Asia, with its low rate of PCa, some authors revealed a higher cutoff of PSA and PSAD to detect PCa. Therefore, minimizing unnecessary biopsies might be a more important issue than maximizing the cancer detection rate [9]. In 2000, Rahardjo et al. [10] studied 805 consecutive patients and revealed higher PSA and PSAD cutoffs (8 ng/mL and 0.20 ng/mL) in Indonesian men. They also found that 148 biopsies (33.4%) could be saved with this cutoff level.
MATERIALS AND METHODS
The study was performed in an outpatient public reference urology service for prostatic diseases. Demographic data, comorbidities, urine culture, urinary retention, PSA, founding during transrectal ultrasonography (TRUS), pathologic data as well as clinical information from 404 patients who underwent prostate biopsy for PCa screening at the Department of Urology, University of Airlangga, Indonesia, during 2008 to 2011 were entered in a retrospective database.
The biopsy criteria include one or more of the following: PSA greater than 10 ng/mL; PSAD greater than 0.15 (in PSA 4–10 ng/mL); hypoechoic lesion during TRUS and/or; abnormal DRE. Prostate volume was measured through TRUS. The specimen of the biopsy were fixed in 10% formalin and submitted to pathological department for hematoxylin-eosin staining. The findings were classified as adenocarcinoma or nodular hyperplasia. Histopathological studies were performed by the same pathologist. PSA determination was carried out by the Immulite assay (Diagnostics Products Co., Los Angeles, CA, USA). TRUS guided systematic 10-core biopsy was performed using a biplanar technique with a 7.5 MHz probe (Hitachi EUB 405, Hitachi Medical Co., Tokyo, Japan).
For statistical analysis, the IBM SPSS ver. 10.0.1 (IBM Co., Armonk, NY, USA) was used. Since all continuous variables did not present a normal distribution, Mann-Whitney U and the Kruskal-Wallis tests was applied to compare the groups’ median. Correlation among variables was analyzed with chi-square test.
The statistical program Medcalc ver. 8.1.0.0 (Medcalc Software, Ostend, Belgium) was used to demonstrate the best cutoff point for each diagnosis test as well as to calculate its respective PPVs, negative predictive values (NPVs), sensitivities and specificities to predict PCa. The receiver operating characteristics (ROC) curve was employed to graphically demonstrate the sensitivities and specificities of the different diagnostic tests. All statistical analysis was performed considering P<0.05 statistically significant and with a 95% confidence interval (CI).
RESULTS
Four hundred and four patients were finally included in the study. All were included according to data availability from the medical records. A summary of patient characteristics is presented in Table 1. Mean age was 64.06±7.5 years (range, 34 to 84 years). Mean PSA was 45.59 ng/mL (range, 0.4 to 1,400 ng/mL). Mean prostate volume and PSAD were 43.03 and 1.15 respectively. Of the 404, 131 cases (32.4%) were confirmed to be urinary retention and were catheterized. PSA for retention case were examined at least 5 days after catheterization. Positive urine culture was found in 182 cases (45%). Hypoechoic lesion during TRUS was found in 88 cases (22%). Regarding baseline PSA, 2.5% of patients presented with PSA<2.5 ng/mL, 1.7% between 2.5–4 ng/mL, 26.2% between 4.1–10 ng/mL and 69.6% with PSA more than 10 ng/mL. Overall detection of PCa was 11.1% (45 cases). The remaining 359 cases had benign lesions comprising BPH. Twenty nine out of these 45 PCa had nodule from DRE.
Table 1.
Characteristics of 404 patients underwent prostate biopsy
| Variable | No. | Value |
|---|---|---|
| Mean age (yr) | 385 | 64.06 |
| Prostate specific antigen | 397 | 45.59 (0.4–1,400) |
| Mean PSAD | 392 | 1.15 |
| Mean prostate volume | 398 | 43.03 |
| Retention | 404 | |
| No | 273 (67.6) | |
| Yes | 131 (32.4) | |
| Digital rectal examination | 404 | |
| No nodule | 375 (92.3) | |
| Nodule | 29 (7.7) | |
| Hypoechoic area | 400 | |
| No | 312 (78) | |
| Yes | 88 (22) | |
| Bladder stone | 404 | |
| No | 393 (97.3) | |
| Yes | 11 (2.7) | |
| Pathology | 404 | |
| Nodular hyperplasia | 359 (88.9) | |
| Adenocarcinoma | 45 (11.1) | |
| Urine culture | 404 | |
| Negative | 222 (55) | |
| Positive | 182 (45) | |
| Gleason score (sum) | ||
| 0 | 359 (89.1) | |
| 3 | 1 (0.2) | |
| 4 | 2 (0.5) | |
| 5 | 12 (3) | |
| 6 | 6 (1.5) | |
| 7 | 16 (4) | |
| 9 | 7 (1.7) |
Values are presented as mean (range) or number (%). PSAD, prostate-specific antigen density.
The discriminating power to detect PCa as estimated by the ROC curve was 0.778 for PSA and 0.734 for PSAD. Estimates for sensitivity, specificity and predictive values for different PSA and PSAD cutoff points are shown in Fig. 1. The operation characteristics of both tests at maximum discrimination cutoffs were computed. This was 6.95 ng/mL for PSA and 0.7072 for PSAD. For PSA cutoff point of 6.95 ng/mL, the sensitivity was 97.8%, specificity 19.6%, PPV 13.5%, and NPV 98.6%. While for PSAD cutoff point of 0.7072, the sensitivity was 62%, specificity 78.7% and PPV 27.5% (Table 2). There was a significant correlation between cutoff PSA and adenocarcinoma (P =0.004) as well as between cutoff PSAD and pathology result (P=0.000) as shown in Table 3.
Fig. 1.
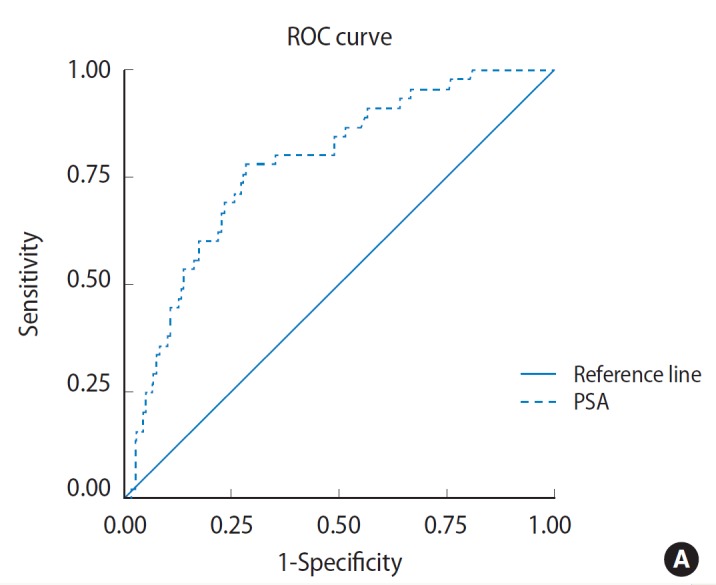
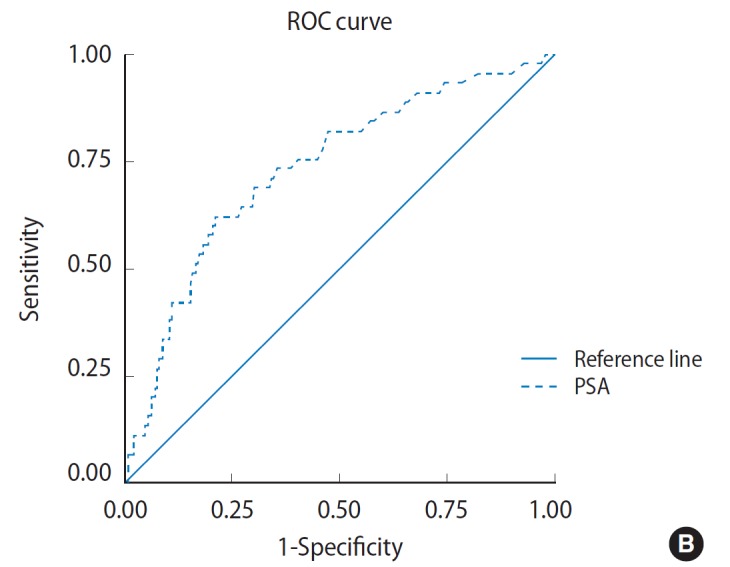
Receiver operating characteristics (ROC) curve depicting diagnostic accuracy of prostate-specific antigen (PSA) (A) and diagnostic accuracy of PSA density (B). The ROC curve shows perpendicular height above the diagonal and wide area under the curve indicating that it is an accurate test.
Table 2.
Performance of both cutoff levels
| PSA (%) | PSAD (%) | |
|---|---|---|
| Cutoff | 6.95 | 0.71 |
| Sensitivity | 97.8 | 62.2 |
| Specificity | 19.6 | 78.7 |
| Positive predictive value | 13.5 | 27.5 |
| Negative predictive value | 98.6 | 94.1 |
PSA, prostate-specific antigen; PSAD, PSA density.
Table 3.
Cross tabulation of PSA and PSAD diagnostic cutoff points for prostate cancer detection
| Category | Pathology
|
Total | |
|---|---|---|---|
| Adeno Ca | No | ||
| ≥Cutoff | |||
| PSA | 44 (13.5) | 283 (86.5) | 327 (100) |
| PSAD | 28 (27.5) | 74 (72.5) | 102 (100) |
| ≤Cutoff | |||
| PSA | 1 (1.4) | 69 (98.6) | 70 (100) |
| PSAD | 17 (5.9) | 273 (94.1) | 290 (100) |
| Total | |||
| PSA | 45 (11.3) | 347 (88.5) | 397 (100) |
| PSAD | 45 (11.5) | 347 (88.5) | 392 (100) |
Values are presented as number (%).
PSA, prostate-specific antigen; PSAD, PSA density.
In urinary retention group, ROC curve analysis revealed two fold PSA cutoff increase (14.55 ng/mL) with discriminating power 0.890 (area under curve [AUC], 0.89; 95% CI, 0.803 to 0.977; P =0.000) (Fig. 2A). Sensitivity and specificity was 90.9% and 50% respectively. While for positive urine culture group, ROC curve analysis revealed almost no PSA cutoff increase (7.065 ng/mL) with discriminating power 0.890 (AUC, 0.783; 95% CI, 0.693 to 0.873; P =0.000) (Fig. 2B). Sensitivity and specificity was 96.9% and 20% respectively.
Fig. 2.
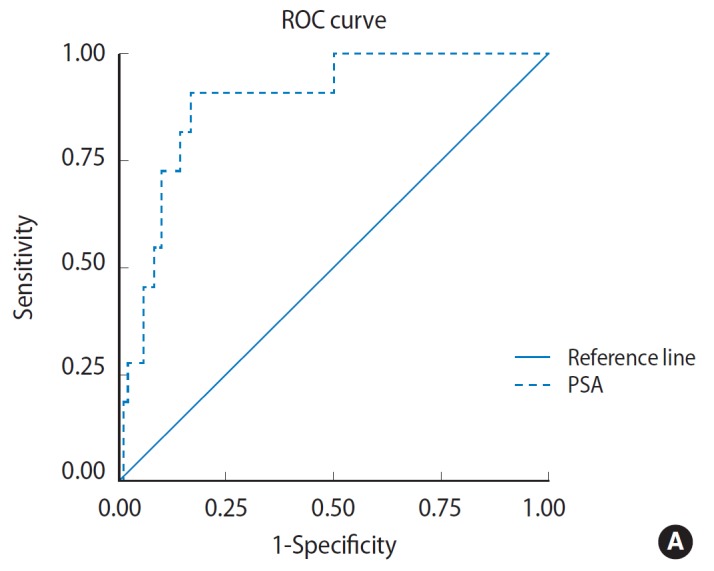
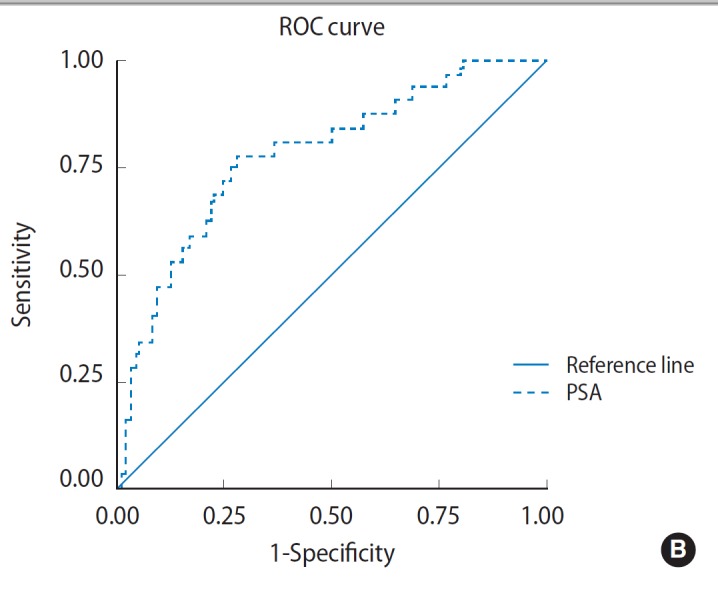
Receiver operating characteristics (ROC) curve shows area under curve for prostate-specific antigen (PSA) cutoff point in urinary retention group (A) and positive urine culture group (B).
Using cross tabulation, there was a significant correlation between hypoechoic area and adenocarcinoma (P =0.000) as shown in Table 4. Statistical calculation revealed higher PCa incidence in hypoechoic area group (22.7%) compared to only 8% in without lesion group. PPV and NPV were 22.7% and 92% respectively.
Table 4.
Cross tabulation of hypoechoic lesion for prostate cancer detection
| Hypoechoic lesion | Pathology
|
Total | |
|---|---|---|---|
| Adeno Ca | No | ||
| Yes | 20 (22.7) | 68 (77.3) | 88 (100) |
| No | 25 (8.0) | 287 (92.0) | 312 (100) |
| Total | 45 (11.3) | 355 (88.8) | 400 (100) |
Values are presented as number (%).
The Gleason score (GS) group for PSA greater than 6.95 ng/mL was 81.4% for moderate and 16.3% for high grade. All patients with PSA less than 6.95 ng/mL shows moderate GS. Cross tabulation shows no significant correlation between PSA cutoff point and GS (P=0.893). Among those with PSAD >0.7072, 88.9% were categorized as moderate GS and 7.4% as high-grade GS. Meanwhile, among pastients with PSAD <0.7072, moderate and high grade GS was 70.6% and 29.4%, respectively. Statistical calculation using cross tabulation shows no significant correlation between PSAD cutoff point and GS (P=0.121) as shown in Table 5.
Table 5.
Cross tabulation of PSA and PSAD diagnostic cutoff points with Gleason score
| Category | Gleason score
|
Total | ||
|---|---|---|---|---|
| Mild | Moderate | High | ||
| ≤Cutoff | ||||
| PSA | 0 (0) | 1 (100) | 0 (0) | 1 (100) |
| PSAD | 0 (0) | 12 (70.6) | 5 (29.4) | 17 (100) |
| ≥Cutoff | ||||
| PSA | 1 (2.3) | 35 (81.4) | 7 (16.3) | 43 (100) |
| PSAD | 1 (3.7) | 24 (88.9) | 2 (7.4) | 27 (100) |
| Total | ||||
| PSA | 1 (2.3) | 36 (81.8) | 7 (15.9) | 44 (100) |
| PSAD | 1 (2.3) | 36 (81.8) | 7 (15.9) | 44 (100) |
Values are presented as number (%).
PSA, prostate-specific antigen; PSAD, PSA density.
DISCUSSION
In United States, PCa has been the common noncutaneous malignancy in men since 1984, now accounting for one quarter of all such cancers. The estimated lifetime risk of disease is 16.72%, with a lifetime risk of death at 2.57%. Its incidence varies widely between countries and ethnic populations, with disease rates differing by more than 100-fold. Although the specific causes of PCa initiation and progression are not yet known, considerable evidence suggests that both genetic and environment play a role in the origin and evolution of this disease [11].
Almost all cases of PCa are adenocarcinoma. About 4% of PCa cases exhibit transitional cell morphology and are believed to have developed from the urogenital lining of the prostatic urethra [12]. Today, PSA-based screening of a symptomatic men has led to the adaptation of TRUS biopsy as the standard of care for routine prostate biopsy. Currently, most clinicians recommend biopsy once a patient’s serum PSA rises above 4.0 ng/mL, although significant research efforts are ongoing to identify the optimal PSA threshold to recommend prostate biopsy in the asymptomatic patient. Evidence to lower the PSA threshold from work by Catalona’s group showed higher rates of organ-confined disease at the time of radical retropubic prostatectomy in samples from PSAs in the 2.6–4.0 ng/mL range. These findings have led many urologists to now recommend prostate biopsy to men younger than 60 years of age once their PSA level rises above 2.5 ng/mL. Regardless of initial PSA value, a PSAV greater than 0.75 ng/mL/yr is frequently associated with PCa and warrants biopsy [11].
Although numerous grading systems exist for the evaluation of prostatic adenocarcinoma, the GS system is the most widely accepted. The GS is based on the glandular pattern of the tumor as identified at relatively low magnification, both the primary (predominant) and the secondary (second most prevalent) architectural patterns [7]. One point needs to be clarified is that the primary Gleason grade is perhaps the most important with respect to placing patients in prognostic groups [13].
Serum PSA above 4 ng/mL with or without an abnormal DRE is generally accepted as an indication for needle biopsy of the prostate [14]. Disruption of the normal prostatic architecture, such as by prostatic disease, inflammation, or trauma, allows greater amounts of PSA to enter the general circulation [2]. PSA was initially introduced for postoperative follow-up and was gradually used as a screening test, which brought about tremendous change in the morbidity and mortality of PCa [3].
The PSAD parameter was developed to undermine the influence of the prostate volume on the serum level of PSA [6]. Meanwhile, PSAD greater than 0.15 is associated with 25% incidence of cancer, and a PSAD less than 0.10 with 5% incidence of cancer [7]. Two studies performed to determine the reference values of serum PSA for Iranian men revealed that the PSA values were significantly lower than those for white and black Western men, and slightly lower than those for Japanese men [7]. Black men are at 60% increased risk for PCa and at 150% increased risk for PCa mortality. In contrast, Asian/Pacific Islander American men are at an overall 40% age adjusted lower risk for PCa and at 55% lower risk for PCa mortality than white men [15].
Catalona et al. [16] reported that a PSA level of 4 ng/mL or higher was appropriate as the PSA cutoff value for the screening of PCa. Since then, this value has been most commonly used clinically; whereas its sensitivity is 67.5% to 80% and the specificity is only 20% to 30%. When total PSA cutoff level is considered high, some clinically important cancers may be overlooked. In contrast, when it is considered low, unnecessary biopsies would increase morbidity and cost. However, approximately 20% of PCa patients have serum total PSA levels below 4 ng/mL. In some studies, PCa detection rate was between 2% to 28% in patients who had PSA less than 4.0 ng/mL. Thus, patient selection, core number, core localization, and the suitable rebiopsy time are still controversial issues [1].
Colberg et al. [17] reported that PCa detection rate was 7.2% in 121 volunteers with suspicious DRE and/or TRUS and serum total PSA level between 2.9–4.0 ng/mL. In a study by Babaian et al, [18] 151 volunteers had serum total PSA value between 2.5–4.0 ng/mL, and PCa was found in 24.5% cases. Another study evaluated 883 patients with serum total PSA level between 2.0–3.9 ng/mL, and PCa rate was 14.3%. Clinically important cancers, 20% of which were beyond prostate, were reported in 89% of the patients with serum total PSA level less than 4.0 ng/mL [1]. Wu and Huang [9] in 2004 found that 162 biopsies (13.1%) might be avoided for Chinese population if the PSA cutoff was 6.0 ng/mL but 4 cancers (1.6%) would have been overlooked.
A cross-sectional study involving 805 patients from 40 to 95 years by Mochtar et al. [19] in Jakarta-Indonesia found 105 and 303 unnecessary prostate biopsies for intermediate and high PSA level, subsequently. Among 805 patients, only 35 patients had histologically confirmed PCa, i.e., 3 of 108 patients with PSA 4–10 ng/mL and PSAD >0.15, and 32 of 335 patients with PSA >10 ng/mL. With a PSA cutoff level of ≥8 ng/mL, they found 100% sensitivity to PCa. PSAD ≥0.20 within a PSA level of 8–30 ng/mL gave 100% sensitivity to prostate cancer. Using these new cutoffs there would be 148 biopsies (33.4%) saved. Unfortunately, therefore, they concluded that the commonly accepted values of serum PSA level and PSAD resulted in many unnecessary biopsies in Indonesian patients [10]. Unfortunately, they did not discuss urinary retention and the possibility of urinary tract infection that may alter this cutoff value as almost half of Indonesian BPH patients came to seek medical helps after urinary retention.
In our study, we defined PSA 6.95 ng/mL as the best cutoff point to detect PCa (sensitivity 97.8%, specificity 42.2%, PPV 13.5%). We also found that this cutoff has a significant correlation with pathology result (P =0.004). Compared with several prior studies using cutoff PSA 4 ng/mL; the sensitivity, specificity and rate of misdiagnostic are almost the same. Since Indonesia has low rate of PCa and previous study shows only 2.8% PCa detection rate in intermediate PSA range (4–10 ng/mL) with PSAD >0.15, this lead to presumption that for PSA less than 4 ng/mL the incidense rate must be much lower. Consequently, biopsy for PSA less than 4 ng/mL is not mandatory and recommended only for specific cases. This is intended to reduce morbidity and avoid unnecessary biopsies.
In patients with serum PSA more than 10 ng/mL, applying PSAD of 0.15 will increase the specificity and PPV of the test without significantly compromising the test sensitivity. In addition, it seems reasonable that all men with PSA greater than 4 ng/mL should have a TRUS biopsy if their PSAD is greater than 0.15 [7]. In general, the detection rates of PCa based on the PSA level are 10–20% at 2.5–4.0 ng/mL; 25% at 4.1–10.0 ng/mL; and 50–60% at 10 ng/mL or higher [3]. Sheikh et al. [14] found PSAD cutoff point 0.32 in Kuwaiti people has a specificity of 80%, suggesting that it can be used as a confirmatory but not an exclusionary test. A retrospective study of 132 uncatheterized BPH and PCa cases in Jakarta-Indonesia by Mochtar et al. [19] revealed an optimum PSAD cutoff level of 0.19. At this level, the measured sensitivity was 100% with a specificity of 79%. From statistical analysis using ROC, we also found 0.7072 as the best PSAD cutoff in Indonesian men (relatively in this series). Sensitivity, specificity, and PPV was 62%, 78.7% and, 27.5%, respectively. There was also a significant correlation between cutoff PSAD and pathology result (P =0.000). This high cutoff is equivalent with high mean PSAD (1.15). In contrast to literature study, this Indonesian men (Asian) shows high PSA and PSAD.
Total PSA can be reduced by certain treatments such as 5-α reductase inhibitors and may be increased in acute urine retention, during the 48 hours after ejaculation, in prostate biopsies or placement of a urinary catheter, transurethral resection of the prostate, BPH and prostatitis [20]. In our study, positive urine culture alters almost no PSA increase (from 6.95 to 7.065) and it is contrary to previous studies. This slight increase phenomenon may occur because we did not separate symptomatic and asymptomatic group. Therefore, positive urine culture could not describe the urinary tract infection or prostate inflammatory status. Consequently, we recommend the same PSA cutoff (6.95 ng/mL) as the best level to detect PCa.
Elevation of PSA can also be found in urinary retention. In 2003, Chawla et al. [21] reported significant PSA elevation in patients urinary retention. Among the 74 BPH patients with urinary retention, 42 (56.8%) has elevated PSA; 8 (10.8%) of these patients even showed PSA concentrations above 25 ng/mL. While in no-retention subgroup, only 11 out of 38 (28.9%) had above normal PSA concentrations and none of these had PSA more than 25 ng/mL. The effect of urinary retention in PSA elevations was further confirmed by significantly high odds ratio of 3.22 (95% CI, 1.29 to 8.15). Erdogan et al. [20] randomly divided 35 BPH patients into 2 groups, urethral catheterization and suprapubic cystostomy. He found that PSA level significantly increased 2 hours, 12 hours and 7 days after catheterization (P <0.05) but not in cyctostomy group. Lipsky et al. [22] studied 1,492 consecutive BPH patients and reported that overall PSA in catheterized patients was twice as high compared to patients without catheter. In contrast to positive urine culture group, urinary retention made a big impact on PSA cutoff level. We found that urinary retention could double PSA cutoff to 14.55 ng/mL with sensitifity 90.9% and specificity 50%.
Despite the higher prevalence of cancers discovered in prostates with hypoechoic areas, Onur et al. [23] found that hypoechoic lesion itself was not associated with increased cancer prevalence compared with biopsy cores from isoechoic areas in a contemporary series of almost 4,000 patients. While Gosselaar et al. [24] found PCa detected from directed hypoechoic lesion biopsy was limited to only 3.5% of the 1,840 men. In this series, we found significant correlation between hypoechoic lesion and PCa (PPV 22.7% and NPV 92%). Lotfi et al. [7] in 2009 found 80% of the cancers diagnosed in 102 patients were in the mid or high-grade range of GS. From multivariate analysis, they also failed to prove correlation among PSA, PSAD and GS [7]. While Hofner et al. [25] in 2010 found only minor relationship between PSAD and final GS (R2 =0.33, P =0.001). In this series, we found no significant correlation between new PSA cutoff value and GS, as well as new PSAD cutoff value with GS.
An important limitation of our study is the retrospective design. Another drawback is the calculation of PSAD based on transrectal ultrasound. Though TRUS volume measurements correlate with pathological prostate volumes, they do not represent the exact values, but underestimate true prostate volumes in most cases. Nevertheless, interobserver reliability of transrectal volume measurements is high. Bias could also be introduced by different TRUS biopsy operator. Since PSA <4 ng/mL and or intermediate PSA with PSAD <0.15 were not included in biopsy criteria, this can lead to differences in statistical assessment. Acute urinary retention and positive urine culture could also make a bias value, therefore PSA test was examined at least 5 days after acute urinary retention and catheterization, and repeat PSA test in cases with a high results. Obviously, it will be essential to prove our concept of new PSA and PSAD cutoff value in larger series; thereby, the exact lower cutoff value could be refined.
In conclusion, PSA and PSAD cutoff point for Indonesian men in this series is relatively different from international consensus. The difference might be caused by racial variation of either prostate volume. Furthermore, these data show that PSA and PSAD cutoff point must be adjusted to racial variation to discriminate between malignant and benign disease. Urinary retention is a significant factor for PSA cutoff increase.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Aslan Y, Tekdogan, Tuncel A, Uzun MB, Karabulut E, Atan A. Serum dehydroepiandrosterone sulfate usage for early detection of prostate cancer in men with serum prostate specific antigen level between 2.5 and 4.0 ng/mL: a pilot study. Turk J Med Sci. 2008;38:399–404. [Google Scholar]
- 2.Carrol P, Albertsen PC, Greene K, Babaian RJ, Carter HB, Gann PH, et al., editors. Prostate-specific antigen best practice statement: 2009 update. Linthicum: American Urological Association Education and Research Inc.; 2009. [Google Scholar]
- 3.Kim HW, Ko YH, Kang SH, Lee JG. Predictive factors for prostate cancer in biopsy of patients with prostate-specific antigen levels equal to or less than 4 ng/ml. Korean J Urol. 2011;52:166–71. doi: 10.4111/kju.2011.52.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis LO, Zani EL, Alonso JC, Simoes FA, Rejowski RF, Ferreira U. Does the criterion for prostate biopsy indication impact its accuracy? A prospective population-based outpatient clinical setting study. Actas Urol Esp. 2011;35:10–4. doi: 10.1016/j.acuro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Gregorio EP, Grando JP, Saqueti EE, Almeida SH, Moreira HA, Rodrigues MA. Comparison between PSA density, free PSA percentage and PSA density in the transition zone in the detection of prostate cancer in patients with serum PSA between 4 and 10 ng/mL. Int Braz J Urol. 2007;33:151–60. doi: 10.1590/s1677-55382007000200004. [DOI] [PubMed] [Google Scholar]
- 6.Martins ACP, Borelli- Bovo TJ, Reis RB, Paschoalin EL, Cologna AJ, Suaid HJ. Performance Of PSA and of PSA density in the diagnosis of prostate carcinoma. Acta Cir Bras. 2002;17:7–11. [Google Scholar]
- 7.Lotfi M, Assadsangabi R, Shirazi M, Jali R, Assadsangabi A, Nabavizadeh SA. Diagnostic value of prostate specific antigen and its density in Iranian men with prostate cancer. Iran Red Crescent Med J. 2009;11:170–5. [Google Scholar]
- 8.Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology. 2005;65:549–53. doi: 10.1016/j.urology.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 9.Wu TT, Huang JK. The clinical usefulness of prostate-specific antigen (PSA) level and age-specific PSA reference ranges for detecting prostate cancer in Chinese. Urol Int. 2004;72:208–11. doi: 10.1159/000077116. [DOI] [PubMed] [Google Scholar]
- 10.Rahardjo D, Kamil ST, Pakasi LS. Rationale for using serum prostate-specific antigen (PSA) level and PSA density (PSAD) to detect prostatic malignancy in a country with low prostate cancer incidence. Gan To Kagaku Ryoho. 2000;27(Suppl 2):563–70. [PubMed] [Google Scholar]
- 11.Loeb S, Carter HB. Early detection, diagnosis, and staging of prostate cancer. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 10th ed. Philadelphia: Elsevier; 2012. pp. 2763–70. [Google Scholar]
- 12.Crawford ED. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology. 2009;73:S4–10. doi: 10.1016/j.urology.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Presti JC, Kane CJ, Shinohara K, Carroll PR. Neoplasm of the prostate gland. In: Tanagho EA, McAninch JW, editors. Smith’s general urology. 17th ed. Columbus: McGraw-Hill Co.; 2008. pp. 348–74. [Google Scholar]
- 14.Sheikh M, Al-Saeed O, Kehinde EO, Sinan T, Anim JT, Ali Y. Utility of volume adjusted prostate specific antigen density in the diagnosis of prostate cancer in Arab men. Int Urol Nephrol. 2005;37:721–6. doi: 10.1007/s11255-005-4683-2. [DOI] [PubMed] [Google Scholar]
- 15.Elliott CS, Shinghal R, Presti JC., Jr Racial variations in the performance of prostate specific antigen and prostate specific antigen density in the era of extended prostate biopsy schemes. J Urol. 2008;180:1318–23. doi: 10.1016/j.juro.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270:948–54. [PubMed] [Google Scholar]
- 17.Colberg JW, Smith DS, Catalona WJ. Prevalence and pathological extent of prostate cancer in men with prostate specific antigen levels of 2.9 to 4.0 ng/ml. J Urol. 1993;149:507–9. doi: 10.1016/s0022-5347(17)36130-x. [DOI] [PubMed] [Google Scholar]
- 18.Babaian RJ, Johnston DA, Naccarato W, Ayala A, Bhadkamkar VA, Fritsche HH., Jr The incidence of prostate cancer in a screening population with a serum prostate specific antigen between 2.5 and 4.0 ng/ml: relation to biopsy strategy. J Urol. 2001;165:757–60. [PubMed] [Google Scholar]
- 19.Mochtar CA, Rahardjo D, Umbas R. A higher PSA-density cut-off level in patients with intermediate PSA values for the early detection of prostate cancer. Gan To Kagaku Ryoho. 2000;27(Suppl 2):514–22. [PubMed] [Google Scholar]
- 20.Erdogan K, Gurdal M, Tekin A, Kirecci S, Sengor F. The effect of urethral catheterisation on serum prostate-specific antigen levels in male patients with acute urinary retention. Yonsei Med J. 2003;44:676–8. doi: 10.3349/ymj.2003.44.4.676. [DOI] [PubMed] [Google Scholar]
- 21.Chawla R, Abraham R, Arora U, Mammen K. Effect of urinary retention on the levels of prostate specific antigen (PSA) and prostatic acid phosphatase (PAP) in prostatic disease. Indian J Urol. 2003;19:120–4. [Google Scholar]
- 22.Lipsky K, Schips L, Pummer K, Rehak P, Zigeuner R. Influence of indwelling transurethral catheters on PSA-levels in patients with benign prostatic hyperplasia [abstract] Eur Urol Suppl. 2005;4(3):106. [Google Scholar]
- 23.Onur R, Littrup PJ, Pontes JE, Bianco FJ., Jr Contemporary impact of transrectal ultrasound lesions for prostate cancer detection. J Urol. 2004;172:512–4. doi: 10.1097/01.ju.0000131621.61732.6b. [DOI] [PubMed] [Google Scholar]
- 24.Gosselaar C, Roobol MJ, Roemeling S, Wolters T, van Leenders GJ, Schroder FH. The value of an additional hypoechoic lesion-directed biopsy core for detecting prostate cancer. BJU Int. 2008;101:685–90. doi: 10.1111/j.1464-410X.2007.07309.x. [DOI] [PubMed] [Google Scholar]
- 25.Hofner T, Pfitzenmaier J, Alrabadi A, Pahernik S, Hadaschik B, Wagener N, et al. PSA density lower cutoff value as a tool to exclude pathologic upstaging in initially diagnosed unilateral prostate cancer: impact on hemiablative focal therapy. World J Urol. 2012;30:91–5. doi: 10.1007/s00345-010-0631-6. [DOI] [PubMed] [Google Scholar]


