Abstract
Purpose:
Robotic-assisted laparoscopic prostatectomy (RALP) offers reportedly comparable oncologic outcomes for localized disease compared with open radical retropubic prostatectomy (ORRP). However, the oncologic efficacy of RALP in locally-advanced prostate cancer (PCa) is less clear. We report and compare our experience with RALP and ORRP in men with locally advanced PCa.
Methods:
Patients with locally advanced PCa (stage T3 or greater) were identified in both robotic and open cohorts. Clinicopathologic features including age, clinical stage, prostate-specific antigen, surgical margins, and Gleason score were reviewed. We further examined the incidence of positive surgical margins, the effect of the surgical learning curve on margins, and the need for adjuvant therapy.
Results:
From 1997 to 2010, 1,011 patients underwent RALP and 415 patients were identified who underwent radical retropubic prostatectomy (RRP) across four institutions. 140 patients in the RALP group and 95 in the RRP group had locally advanced PCa on final pathology. The overall robotic positive margin rate 47.1% compared with 51.4% in the RRP group. A trend towards a lower positive margin rate was seen after 300 cases in the RALP group, with 66.7% positive margin rate in the first 300 cases compared with 41.8% in the latter 700 cases. In addition, a lower incidence of biochemical recurrence was also noted in the latter cases (30.6% vs. 9.5%).
Conclusions:
Up to 2 out of 3 men undergoing RALP for locally-advanced PCa had positive margins during our initial experience. However, with increasing surgeon experience the overall positive margin rate decreased significantly and was comparable to the positive margin rate for patients with locally advanced disease undergoing ORRP over four academic institutions. We also noted a lower incidence of biochemical recurrence with increasing RALP experience, suggesting better oncologic outcomes with higher volume. Given this data, RALP has comparable oncologic outcomes compared to ORRP, especially with higher volume surgeons.
Keywords: Prostate neoplasms, Oncologic outcomes, Prostatectomy
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed solid malignancy in men and the second leading cause of male cancer related death in the United States [1]. The majority PCa is now diagnosed as clinically localized disease due to widespread prostate-specific antigen (PSA) screening and resulting stage migration, and commonly accepted treatment options for localized disease include active surveillance, radical prostatectomy (RP), and radiotherapy. In cases of locally advanced disease, the role of RP as primary treatment remains uncertain, although a recent series of men with clinical T3 PCa revealed comparable cancer specific survival rates vs. radiation or hormonal treatment [2].
Over the last decade, minimally invasive surgical approaches to PCa have surged in popularity, with an estimated 80% of RPs performed with robotic assistance [3,4]. While several studies have shown comparable oncologic outcomes of robotic-assisted laparoscopic prostatectomy (RALP) and open radical retropubic prostatectomy (RRP) for localized PCa, the oncologic efficacy of RALP vs. RRP for locally advanced PCa is less clear [5–7]. We report on our experience with RALP for pT3 PCa and assess oncologic outcomes. Additionally, we assess the effect of surgeon learning curve on oncologic outcomes and compare our results to men undergoing RRP for pathologic stage (pT3).
MATERIALS AND METHODS
1. Study cohort
Between January 1997 and November 2008, 1,011 men underwent RALP and 415 men underwent RRP across three institutions and patient information was collected to limit selection bias. Our RALP cohort was populated with patients from Yale University Hospital and Washington Hospital Center where 99% of prostatectomies were performed with the robotic approach. Our RRP cohort was populated with patients from Georgetown University Hospital where no surgical robot was available during the study period. Initial evaluation for all men included PSA level, digital rectal exam, and a biopsy-determined Gleason score. Two hundred and thirty-five men (140 RALP vs. 95 RRP) had ≥pT3 PCa on final pathology and were included for analysis. All RALP data was obtained from our Institutional Review Board-approved prospectively collected database while RRP data was obtained from retrospective data collection.
2. Variables
Preoperative demographic and pathologic data including age, clinical stage, surgical margin status, and biopsy Gleason score were recorded. All RALP clinical staging was confirmed with intraoperative examination under anesthesia prior to surgery. Postoperative pathologic data including margin status, and PSA data were also recorded. Postoperative PSA values were obtained during regular clinic or telephone follow-up intervals based on surgeon preference. Biochemical recurrence was defined as two consecutive detectable PSA levels ≥0.2 ng/mL.
3. Statistical analysis
The student’s t-test and multivariate analysis of variation test were used for comparison of preoperative and postoperative variables with P<0.05 were considered statistically significant. Logistic regression and Kaplan-Meier curve analysis was performed for biochemical recurrence free survival. Statistical analyses were performed using the SPSS ver. 12 (SPSS Inc., Chicago, IL, USA).
RESULTS
Table 1 illustrates the baseline preoperative characteristics as well as postoperative pathologic and oncologic outcomes both RALP and RRP cohorts. There were no differences in age or preoperative PSA data. While 46.4% (65/140) of men undergoing RALP had clinically palpable disease, this data was not available for the RRP cohort. There were no significant differences in postoperative Gleason score. The overall positive margin rate in men with advance disease undergoing RALP was 47.1% (66/140) compared with 58.9% (56/95) in the RRP group (P=0.08). At a follow-up of 3 years, 18.9% patients (18/95) in the RRP group had biochemical recurrence compared to 18.5% of patients (26/140) in the RALP group. Kaplan-Meier analysis was performed and showed a similar biochemical recurrence-free survival advantage between our RRP and RALP patients (Fig. 1). Median length of biochemical recurrence-free survival was twelve months for both groups (P =0.742; 95% confidence interval [CI], 0.51 to 1.69; hazard ratio [HR], 0.93).
Table 1.
Preoperative and postoperative clinicopathologic data
| Variable | RRP (n=95) | RALP (n=140) | P-value |
|---|---|---|---|
| Preoperative patient data | |||
| Mean follow-up (yr) | 9.4 | 4.5 | |
| Mean age (yr) | 60.3 | 62.1 | 0.185 |
| Preoperative Gleason score | 6.7 | 6.6 | 0.642 |
| <6 | 52.6 (50) | 50 (70) | 0.845 |
| 7 | 26.3 (25) | 25.0 (35) | 0.765 |
| >8 | 21.1 (20) | 25.0 (35) | 0.345 |
| Mean preoperative PSA | 9.1 | 8.3 | 0.502 |
| <10 | 81.1 (77) | 80.0 (112) | 0.876 |
| 10–20 | 11.6 (11) | 14.3 (20) | 0.324 |
| >20 | 7.3 (7) | 5.7 (8) | 0.234 |
| Clinical stage | |||
| T1 | 55.8 (53) | 53.6 (75) | 0.756 |
| T2 | 44.2 (42) | 46.4 (65) | 0.245 |
| >T3 | 0 (0) | 0 (0) | |
| Postoperative pathologic data | |||
| Final Gleason score | |||
| Mean | 7.2 | 7.2 | 0.893 |
| <6 | 22.1 (21) | 17.1 (24) | 0.564 |
| 7 | 46.3 (44) | 55.7 (78) | 0.345 |
| >8 | 31.6 (30) | 27.2 (38) | 0.456 |
| Pathologic stage | |||
| T3a | 49.5 (47) | 47.1 (66) | 0.456 |
| T3b | 42.1 (40) | 45.7 (64) | 0.754 |
| T4 | 8.4 (8) | 7.2 (10) | 0.652 |
| Positive margin | 58.9 (56) | 47.1 (66) | 0.083 |
| Biochemical recurrence | 18.9 (18) | 18.5 (26) | 0.943 |
| Median biochemical recurrence free survival (mo) | 12.0 | 12.0 | 0.740 |
Values are presented as number (%).
RRP, radical retropubic prostatectomy; RALP, robotic-assisted laparoscopic prostatectomy; PSA, prostate specific antigen.
Fig. 1.
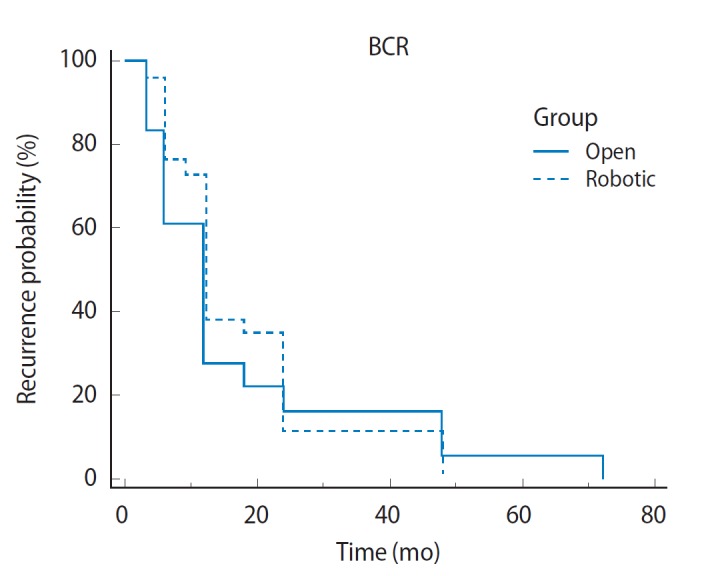
Biochemical recurrence (BCR) free survival in open vs. robotic-assisted laparoscopic prostatectomy T3 patients.
Table 2 compares oncologic outcomes of our initial 300 RALP vs. the latter 700. A trend towards a lower positive margin rate was seen after 300 cases in the RALP cohort with 66.7% (24/36) positive margin rate in the first 300 cases compared with 39.4% (41/104) in the latter 700. Additionally, there was a lower incidence of biochemical recurrence in the latter cases (30.6 [11/36] vs. 9.3% [13/104]). Kaplan-Meier analysis was performed between these two cohorts and showed a significant biochemical recurrence-free survival advantage of our latter cases at 24 months of follow-up (Fig. 2). Median length of biochemical recurrence-free survival was three months in our initial cohort with an increase to 24 months with more experience (P=0.009; 95% CI, 0.19 to 1.13; HR, 0.46).
Table 2.
Learning curve effect on RALP oncologic outcome for pT3 prostate cancer
| First 300 RALP (n=36) | Latter 700 RALP (n=104) | P-value | |
|---|---|---|---|
| Mean follow-up (yr) | 6.1 | 3.5 | |
| Mean age (yr) | 59.2 | 60.3 | 0.507 |
| Mean preoperative PSA (ng/dL) | 7.3 | 8.26 | 0.469 |
| Preoperative PSA | |||
| <10 | 75.0 (27) | 81.7 (85) | 0.543 |
| 10–20 | 16.7 (6) | 13.5 (14) | 0.734 |
| >20 | 8.3 (3) | 4.8 (5) | 0.385 |
| Clinical stage | |||
| T1 | 44.4 (16) | 56.7 (59) | 0.425 |
| T2 | 55.6 (20) | 43.3 (45) | 0.244 |
| >T3 | 0 (0) | 0 (0) | |
| Mean preoperative Gleason | 6.8 | 7.1 | 0.135 |
| Preoperative Gleason | |||
| <6 | 55.6 (20) | 48.1 (50) | 0.367 |
| 7 | 11.1 (4) | 29.8 (31) | 0.654 |
| >8 | 33.3 (12) | 22.1 (23) | 0.478 |
| Final Gleason | |||
| Mean | 7.1 | 7.5 | 0.597 |
| <6 | 33.3 (12) | 11.5 (12) | |
| 7 | 36.1 (13) | 62.5 (65) | |
| >8 | 30.6 (11) | 26.0 (27) | |
| Positive margin | 66.7 (24) | 39.4 (41) | 0.004 |
| Pathologic stage | |||
| T3a | 52.8 (19) | 45.2 (47) | 0.105 |
| T3b | 44.4 (16) | 46.2 (48) | 0.156 |
| T4 | 2.8 (1) | 8.6 (9) | 0.236 |
| Biochemical recurrence | 30.6 (11) | 12.5 (13) | 0.002 |
| Median biochemical recurrence free survival (mo) | 3.0 | 24.0 | 0.009 |
Values are presented as percentage (number).
RRP, radical retropubic prostatectomy; RALP, robotic-assisted laparoscopic prostatectomy; PSA, prostate specific antigen.
Fig. 2.
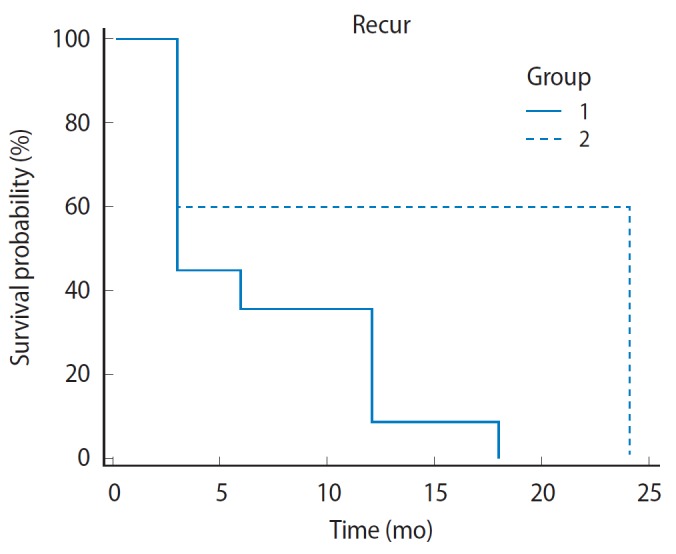
Biochemical recurrence free survival between initial (group 1) and latter (group 2) robotic experience.
DISCUSSION
Historically, surgery has not been the preferred approach in the treatment locally advanced PCa due to the risk of subclinical metastatic disease and elevated rates of positive surgical margins. Additionally, no randomized data comparing surgery to other treatment modalities are yet available, making it difficult to justify the morbidity of surgery [8]. However, in recent years, several series have reported oncologic outcomes with primary surgical treatment that are similar to reported series of patients treated with radiotherapy in the setting of locally advanced disease.
Ward et al. [9] first reported their series of 842 patients with locally advanced disease treated with RRP in 2005 with a 5-year disease specific survival rate of 95%. In 2007, Freed-land et al. [2] and Hsu et al. [10] reported on 200 and 58 patient cohorts respectively with identical 5 and 10 year PCa specific survival of 98% and 91%. More recently in 2009, Xylinas et al. [11,12] published a similar series of 100 patients with a 90% 5 year disease specific survival rate. These survival rates favor comparatively to published rates for other treatment modalities, namely radiotherapy with and without androgen deprivation. Bolla et al. [13] published EORTC (European Organisation for Research and Treatment of Cancer) long term results in 2002 of patients who underwent immediate androgen deprivation and primary external beam radiotherapy. Of their 412 patients with a median follow up of 66 months, the reported 5 year cancer specific survival rates were 40% and 74% for patients receiving radiotherapy alone and combined treatment respectively [13].
A study published in Cancer comparing outcomes of surgery and radiotherapy with and without androgen deprivation further validates the role of surgery as a treatment option for locally advanced disease. Boorjian et al. [14] retrospectively examined 2 large databases of patients with high-risk PCa treated with either radiation therapy or RRP. In total, 1,238 patients underwent RRP, and 609 patients received with external beam radiotherapy (EBRT; 344 received EBRT plus androgen deprivation therapy [ADT], and 265 received EBRT alone), between 1988 and 2004. The median follow-up was 10.2 years, 6.0 years, and 7.2 years after RRP, EBRT plus ADT, and EBRT alone, respectively. The 10-year cancer-specific survival rate was 92%, 92%, and 88% after RRP, EBRT plus ADT, and EBRT alone, respectively (P =0.06). The risk of all-cause mortality, however, was greater after EBRT plus ADT than after RRP (HR, 1.60; 95% CI, 1.25 to 2.05; P=0.0002) [14,15].
Over the last five years, robotic surgery has become increasingly utilized for the treatment of PCa, however, primarily for those patients with localized disease. Although long term oncologic data for RALP is not yet available, excellent visualization, enhanced instrument familiarity and surgical precision has lead to the popularity of the robotic approach. Additionally, aggressive marketing and the advantages of minimally invasive surgery, including shorter hospital stay, less postoperative pain and blood loss has led to an increase in the demand of this modality for primary treatment. The goal of this study was to evaluate whether robotic surgery can be safely offered to those patients with locally advanced diseases by closely examining oncologic outcomes.
In 2009, Ham et al. [3] published a series of 121 patients with locally advanced PCa evaluating the feasibility of robotic surgery for primary treatment. In their study, 48.8% of patients had positive margins on final pathology. Jayram et al. [16] more recently reported oncologic outcomes for a group of high risk PCa patients (PSA ≥20 ng/mL, clinical stage ≥T2c, or preoperative Gleason grade ≥8) who underwent robotic assisted prostatectomy. The total rate of positive surgical margins was 20.9% and final pathology demonstrated extracapsular disease in 54.1% of their patients. At 2 years of follow-up, 21.3% of patients had experienced biochemical recurrence or had persistent disease after treatment [16].
In our experience, results were similar with 47.1% of patients exhibiting positive margins on final pathology. With a mean follow up 4.5 years, 18.5% of the RALP had biochemical recurrence. When compared to our historical cohort who underwent RRP for T3 disease, there was trend towards a lower positive margin rate (58.9% vs. 47.1%), however, this did not meet statistical significance (P =0.08). Additionally, there were no significant differences in the rate of biochemical recurrence (18.9% vs. 18.5%, P=0.94) between the two cohorts. Median biochemical free recurrence was 12 months for both groups and there were no statistically significant differences on logistic regression or Kaplan-Meier analyses.
Furthermore, we have found that with increasing surgical experience, oncologic outcomes improved. When comparing our latter 700 RALP cases with our first 300, the positive margin rate was significantly lower after a higher volume of cases (66.7% vs. 39.4%, P =0.004). Kaplan-Meier analysis of the cohorts shows a significant biochemical recurrence-free survival advantage of our latter cases at 24 months of follow-up (Fig. 1). Median length of biochemical recurrence-free survival was three months in our initial cohort with an increase to 24 months with more experience (95% CI, 0.19 to 1.13; HR, 0.46; P=0.009) (Table 2).
This data mimics several other studies that have shown that after the learning curve for robotic surgery has been reached, surgical outcomes improve. Notably, a study from Tulane University in 2006 evaluated positive surgical margin rates with increasing surgical experience for those undergoing RALP. Atug et al. [17] found that after 100 consecutive cases, the positive margin rate of their last third was significantly better than the first third, concluding that oncologic outcome is affected by the experience of the robotic surgeon. Additional studies from high volume robotic surgery centers also share the same experience as the authors experienced greater oncologic outcomes with more experience [18–20].
While the existing data on robotically treated locally advanced PCa remains limited, we present the first comparison to our knowledge of oncologic outcomes for those undergoing open and robotic prostatectomy for pT3 disease. Our results have shown that robotic surgical treatment gives comparable oncologic outcomes to open surgery. This study is not without its limitations. It’s retrospective nature, no central pathologic review, and variable follow-up among patients may produce bias in our results. Additionally, pathologic nodal status was not able to be obtained on all our patients and was left out of our review. Despite this, there appears to be an emerging role for robotic prostatectomy in patients with locally advanced disease, however, additional studies are needed to validate the results shown from our experience.
In conclusion, up to 2 out of 3 men undergoing RALP for locally-advanced PCa had positive margins during our initial experience. With increasing surgeon experience, the overall positive margin rate decreased significantly and was comparable to that of patients undergoing RRP for advanced disease at our institution. With short-term follow-up, there is a comparable rate of biochemical recurrence between the two groups. Given this data, we conclude that RALP and RRP have comparable oncologic outcomes in advanced PCa, especially with higher volume surgeons.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Freedland SJ, Partin AW, Humphreys EB, Mangold LA, Walsh PC. Radical prostatectomy for clinical stage T3a disease. Cancer. 2007;109:1273–8. doi: 10.1002/cncr.22544. [DOI] [PubMed] [Google Scholar]
- 3.Ham WS, Park SY, Rha KH, Kim WT, Choi YD. Robotic radical prostatectomy for patients with locally advanced prostate cancer is feasible: results of a single-institution study. J Laparoendosc Adv Surg Tech A. 2009;19:329–32. doi: 10.1089/lap.2008.0344. [DOI] [PubMed] [Google Scholar]
- 4.The New York Times. Results unproven, robotic surgery wins converts [Internet] New York: The New York Times Co.; c2010. [cited 2012 Oct 17]. Available from: http://www.nytimes.com/2010/02/14/health/14robot.html?pagewanted=1&hp. [Google Scholar]
- 5.Smith JA, Jr, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385–9. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–63. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen ME, Partin AW. The impact of definitions of failure on the interpretation of biochemical recurrence following treatment of clinically localized prostate cancer. Rev Urol. 2007;9:57–62. [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrentschuk N, Trottier G, Kuk C, Zlotta AR. Role of surgery in high-risk localized prostate cancer. Curr Oncol. 2010;17(Suppl 2):S25–32. doi: 10.3747/co.v17i0.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005;95:751–6. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, Joniau S, Oyen R, Roskams T, Van Poppel H. Outcome of surgery for clinical unilateral T3a prostate cancer: a single-institution experience. Eur Urol. 2007;51:121–8. doi: 10.1016/j.eururo.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Xylinas E, Drouin SJ, Comperat E, Vaessen C, Renard-Penna R, Misrai V, et al. Oncological control after radical prostatectomy in men with clinical T3 prostate cancer: a single-centre experience. BJU Int. 2009;103:1173–8. doi: 10.1111/j.1464-410X.2008.08208.x. [DOI] [PubMed] [Google Scholar]
- 12.Xylinas E, Dache A, Roupret M. Is radical prostatectomy a viable therapeutic option in clinically locally advanced (cT3) prostate cancer? BJU Int. 2010;106:1596–600. doi: 10.1111/j.1464-410X.2010.09630.x. [DOI] [PubMed] [Google Scholar]
- 13.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 14.Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, Horwitz EM, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–91. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzbeierlein JM. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2830–2. doi: 10.1002/cncr.25899. [DOI] [PubMed] [Google Scholar]
- 16.Jayram G, Decastro GJ, Large MC, Razmaria A, Zagaja GP, Shalhav AL, et al. Robotic radical prostatectomy in patients with high-risk disease: a review of short-term outcomes from a high-volume center. J Endourol. 2011;25:455–7. doi: 10.1089/end.2010.0349. [DOI] [PubMed] [Google Scholar]
- 17.Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol. 2006;49:866–71. doi: 10.1016/j.eururo.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 18.Hong YM, Sutherland DE, Linder B, Engel JD. “Learning curve” may not be enough: assessing the oncological experience curve for robotic radical prostatectomy. J Endourol. 2010;24:473–7. doi: 10.1089/end.2009.0121. [DOI] [PubMed] [Google Scholar]
- 19.Freire MP, Choi WW, Lei Y, Carvas F, Hu JC. Overcoming the learning curve for robotic-assisted laparoscopic radical prostatectomy. Urol Clin North Am. 2010;37:37–47. doi: 10.1016/j.ucl.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Ou YC, Yang CR, Wang J, Yang CK, Cheng CL, Patel VR, et al. The learning curve for reducing complications of robotic-assisted laparoscopic radical prostatectomy by a single surgeon. BJU Int. 2011;108:420–5. doi: 10.1111/j.1464-410X.2010.09847.x. [DOI] [PubMed] [Google Scholar]


