Abstract
Transforming growth factor-β (TGF-β) represents a large family of growth and differentiation factors that mobilize complex signaling networks to regulate cellular differentiation, proliferation, motility, adhesion, and apoptosis. TGF-β signaling is tightly regulated by multiple complex mechanisms, and its deregulation plays a key role in the progression of many forms of cancer. Upon ligand binding, TGF-β signals are transduced by Smad proteins, which in turn are tightly dependent on modulation by adaptor proteins such as embryonic liver fodrin, Smad anchor for receptor activation, filamin, and crkl. A further layer of regulation is imposed by ubiquitin-mediated targeting and proteasomal degradation of specific components of the TGF-β signaling pathway. This review focuses on the ubiquitinators that regulate TGF-β signaling and the association of these ubiquitin ligases with various forms of cancer. Delineating the role of ubiquitinators in the TGF-β signaling pathway could yield powerful novel therapeutic targets for designing new cancer treatments.
Introduction
Transforming growth factor-β (TGF-β) signaling
TGF-β represents a large family of growth and differentiation factors that include activin/inhibins and bone morphogenetic proteins (BMPs; Moses & Serra 1996, Massague 1998, Sporn 1999, Hu et al. 2001, Derynck & Zhang 2003, Lange et al. 2004). These proteins mobilize complex signaling networks to regulate cellular differentiation, proliferation, motility, adhesion, and apoptosis. TGF-β signals are conveyed through types I and II serine/threonine kinase receptors to specific intracellular mediators known as Smad proteins (Kimchi et al. 1988, Chen et al. 1997b, Feng & Derynck 1997, Sporn 1999). Activation of the Smad signaling pathway results in nuclear translocation and activation of gene expression (Massague et al. 2000). Vertebrates possess at least nine Smad proteins (Moses & Serra 1996, Heldin et al. 1997, Sporn 1999, Massague et al. 2000, Derynck & Zhang 2003, Tang et al. 2003a) falling into three functional classes: i) receptor-activated Smads (R-Smads): Smad1, Smad2, Smad3, Smad5, and Smad8; ii) co-mediator Smads: Smad4 and Smad 10; and iii) inhibitory Smads (I-Smads): Smad6 and Smad7. Activation of the TGF-β receptor complex by ligand binding results in the phosphorylation of type I receptors by the serine/threonine kinase activity of the type II receptor. R-Smads, such as Smad2 and Smad3, act as direct substrates of specific activated type I receptors, and are phosphorylated on the last two serines at the carboxyl terminus within a highly conserved SSXS motif (Moses & Serra 1996, Heldin et al. 1997, Sporn 1999, Massague et al. 2000, Hu et al. 2001, Weinstein et al. 2001, Derynck & Zhang 2003, Tang et al. 2003b, Lange et al. 2004). Regulation of R-Smads by the receptor kinase confers specificity in this system. Thus, Smad2 and Smad3 are substrates for activin and TGF-β receptors and mediate signaling by these ligands (Kimchi et al. 1988, Chen et al. 1993, Wrana et al. 1994, Feng & Derynck 1997), whereas Smad1, 5, and 8 are targets of BMP receptors and propagate BMP signals (Moses & Serra 1996, Heldin et al. 1997, Kretzschmar et al. 1997). Once phosphorylated, R-Smads associate with the co-mediator Smad, Smad4, and the heteromeric complex then translocates into the nucleus (Abdollah et al. 1997, Heldin et al. 1997). In the nucleus, Smad complexes activate specific genes such as integrins and E-cadherin through cooperative interactions with other DNA-binding and coactivator (or co-repressor) proteins.
Smad proteins consist of two globular Mad homology (MH) domains separated by a flexible linker region. In the basal state, MH1 and MH2 domains are responsible for intrinsic, reciprocal inhibition. MH1 domain inhibition of Smad function is relieved by agonist-induced phosphorylation of the SSXS motif (Massague 1998). The MH2 domains are responsible for homomeric and heteromeric interactions between Smads (Janknecht et al. 1998). Additionally, the MH2 domain is involved in protein–protein interactions such as those between Smad2 and the winged-helix transcription factor fork-head activin signal transducer-1 (FAST-1) (Labbe et al. 1998, Germain et al. 2000). Similar interactions occur between Smad3 and the transcriptional co-activator CREB binding protein (CBP)/p300. TGF-β-mediated transactivation of the cyclin-dependent kinase (cdk) inhibitors p21 (Moustakas & Kardassis 1998) and p15INK4b (Li et al. 1995) involves the Sp1 transcriptional regulator (SP1), whereas TGF-β induction of the plasminogen activator inhibitor (PAI-1) and human collagenase promoters involves transcriptional regulators such as activator protein 1 (AP1) (Zhang et al. 1998). In Xenopus laevis, FAST-1 binds a hexanucleotide repeat motif within the activin response element (ARE) of the activin-inducible Mix.2 promoter (Chen et al. 1997a). In response to activin stimulation, FAST-1 becomes part of a larger ARE-bound complex known as the activin response factor (ARF) that also contains Smad2 and Smad4. Smad2 interacts directly with FAST-1 recruiting Smad4 to the complex. Similarly, in both X. laevis and zebrafish, the activin-inducible promoter of the LIM-homeodomain transcriptional regulatory gene, lim-1, is activated by an ARF that contains FAST-1, Smad2, and Smad4 (Watanabe et al. 2002). This interaction is significant because FAST-1 is involved in primary DNA-binding activity and Smad4 stabilizes DNA binding through its MH1 domain and activates transcription via its MH2 domain (Zhou et al. 1998). A functional interaction between SP1 and a Smad3–Smad4 complex has also been illustrated in TGF-β-mediated activation of the p21 promoter (Koutsodontis et al. 2002).
Direct DNA binding by the MH1 domain has also been demonstrated in Drosophila melanogaster, where the MH1 domain of Mothers of Decapentaplegic (Dpp; Mad), the Smad ortholog, is necessary and sufficient for binding to the vestigial (vg) ‘quadrant’ enhancer (Kim et al. 1997). Similarly, Mad binds to the Dpp response element in the Ultrabithorax (ubx) promoter via its MH1 domain. Additionally, in mammals, direct interaction of Smad3 and Smad4 with three CAGA-box repeats in the TGF-β responsive PAI-1 promoter requires the MH1 domains, as well as agonist stimulation or MH2 domain deletion. Termination of Smad-mediated transactivation by oncoproteins, such as Ski (Smad3) and SnoN (Smad4), as well as Ras, results in negative feedback regulation of TGF-β signaling (Kretzschmar et al. 1999, Stroschein et al. 1999, Sun et al. 1999).
Extrinsic regulatory pathways such as the Erk, c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) pathways contribute to Smad regulation (Denhardt 1996, Kretzschmar et al. 1997, Massague et al. 2000, Saha et al. 2001). In addition, there is a Ca2+-dependent interaction between calmodulin (CaM) and several Smad family members. CaM binds to the N-terminal half of Smad2 with subsequent phosphorylation inhibiting TGF-β-induced nuclear import and transcriptional activity of Smad2 (Zimmerman et al. 1998). Therefore, CaM influences Smad protein function in response to agents that regulate intracellular Ca2+ flux. Other TGF-β-induced signaling pathways involve activation of Rho-like GTPases, including RhoA, Rac, and cdc42 through RhoA-specific guanine exchange factor or Ras activation (Shen et al. 2001, Derynck & Zhang 2003).
TGF-β signaling pathway and malignancy
Growth inhibition by TGF-β, associated with inhibition of c-myc, cdks, reduction in cyclin D1 levels, and inhibition of cdk-4-associated Rb kinase activity as well as induction of cdk inhibitors p15 and p27, has been noted in intestinal epithelial cells (Kurokowa et al. 1987, Ko et al. 1995, 1998). Loss of responsiveness to growth inhibition from TGF-β occurs in many cell types including breast (Arteaga et al. 1988), colorectal carcinoma cells, and pancreatic carcinoma (Beauchamp et al. 1990). Mutational inactivation of TβRII represents one mechanism for the loss of TGF-β-mediated growth inhibition, which in many cases leads to the development of gastrointestinal cancer (Arteaga et al. 1988, 1993, Kimchi et al. 1988, Sun et al. 1994). Thirteen percent of colorectal carcinomas are associated with a replication error (RER) or microsattelite instability phenotype with inactivation of TβRII (Markowitz et al. 1995) and restoration of TβRII by stable transfection results in decreased tumorigenicity (Wang et al. 1995). Accordingly, mutations with loss of function of Smad2 and Smad4 have been noted in 40–50% of pancreatic cancers and 30%of human colorectal cancers (Eppert et al. 1996, Takagi et al. 1996, Thiagalingam et al. 1996). Heterozygous germline mutations in Smad4 are also responsible for a subset of familial juvenile polyposis, a disorder characterized by predisposition to hamartomatous polyps, and gastrointestinal cancer (Howe et al. 1998).
Transgenic mice that are null for Smad3 develop invasive and metastatic colorectal cancers at an early age (Zhu et al. 1998). Additionally, Smad3 is needed to establish the gastrointestinal mucosal immune response to TGF-β signals (Tang et al. 2003b). Hence, Smad3-deficient mice frequently develop gut abscesses and die between 1 and 10 months because of impaired mucosal immunity (Yang et al. 1999). Smad4 is required for gut endoderm lineage, and Smad4+/− mice develop gastric tumors after 12 months (Xu et al. 2000). Furthermore, anaphase-promoting complex (APCΔ716) mice, a model for human familial adenomatous polyposis, intercrossed with Smad4+/− mice develop larger and more invasive colorectal tumors (Takaku et al. 1998).
Adaptor and receptor interacting proteins
Adaptor proteins are important regulators of the TGF-β signaling pathway. For example, Smad2/3 and Smad4 are thought to be distributed along the microtubule (MT) network, and MT stability may be involved in Smad inactivation (Dong et al. 2000, Wrana 2000). Thus, MTs represent a large group of TGF-β signaling adaptor proteins. Additional adaptor proteins include Smad anchor for receptor activation (SARA; Tsukazaki et al. 1998), embryonic liver fodrin (ELF; Tang et al. 2003b), filamin (Sasaki et al. 2001), CrkL (Wurdak et al. 2005), FK506-binding protein 12 (FK506BP12; FK506-binding protein 12 Wang et al. 1996, Chen et al. 1997b), Jab1/CSN5 (Wan et al. 2002, Kim et al. 2004), disabled-2 (Dab2; Hocevar et al. 2001, 2005, Itoh et al. 2003), Drosophila inhibitor of apoptosis-1 and -2, TβRI-associated protein-1 and -2, TβRII binding WD-domain protein TGFβ receptor-interacting protein 1 (TRIP-1; Chen et al. 1995), X-linked inhibitor of apoptosis (Yamaguchi et al. 1999, Birkey Reffey et al. 2001, Harlin et al. 2001), Death domain-associated protein (Daxx; Yang et al. 1997, Perlman et al. 2001), associated molecule with the SH3 domain of STAM-2 (AMSH-2; Ibarrola et al. 2004), and CD2-associated protein (CD2AP; Schiffer et al. 2004).
SARA is a scaffolding protein that regulates the subcellular localization of unactivated R-Smads, potentially scaffolding the TGF-β receptor kinase to the Smad2 substrate (Tsukazaki et al. 1998, Wu et al. 2000). Filamins are a family of actin polymerization proteins that also form scaffolds for a range of signaling proteins including SAP kinases such as MKK-4, small GTPases Rho and Ras, as well as Smad2 and Smad5 (Sasaki et al. 2001).
ELF, a β-Spectrin, is a component of TGF-β signaling that functions to recruit Smads to the receptor by controlling the subcellular localization of Smad3 and Smad4 (Mishra et al. 1998, 1999). ELF plays a critical role in Smad4 localization upon TGF-β stimulation, suggesting that these molecules interact and translocate to the cell nucleus. ELF does not appear to interact with SARA or filamin, and in elf mutants, SARA and filamin distribution is the same as in wild-type mice (Tang et al. 2003b). Thus, TGF-β signaling through R-Smad/ELF interactions may work by way of a different mechanism than that of SARA and filamin.
Adaptors and malignancy
The role of adaptor proteins may prove to be critical for tumor development. However, ELF is one of the few adaptor proteins known to serve as a tumor suppressor. In elf−/− homozygotes, liver development is defective with loss of gastrointestinal epithelial cell shape and polarity. This phenotype is similar to that of Smad2+/−/Smad3+/− double heterozygous mice (Weinstein et al. 2001). Interestingly, there is a dramatically high prevalence of hepatocellular carcinoma in elf+/− heterozygotes. Smad4+/− mice were found to develop hyperplasia of the fundus and antrum, and intercrosses with elf mutants exacerbated the phenotype. Interestingly, elf+/−/Smad4+/− mutants also develop colonic adenomas (Tang et al. 2005). None of the other mutants in the TGF-β family, including Smad3 or Smad4 mutants, develop HCCs in the absence of a carcinogen, establishing that ELF has sufficient anti-oncogenic activity on its own.
Loss of ELF in human gastric cancer is a significant aspect of gastric tumor suppression by TGF-β. Little or no ELF is expressed in three out of six well-defined human gastric cancer cell lines. Importantly, ELF is inactivated in one (NCI-N87) cancer cell line in which the rest of the TGF-β pathway is intact. In contrast, Smad4 expression is decreased in only one cell line.
Control of TGF-β signaling by ubiquitinators
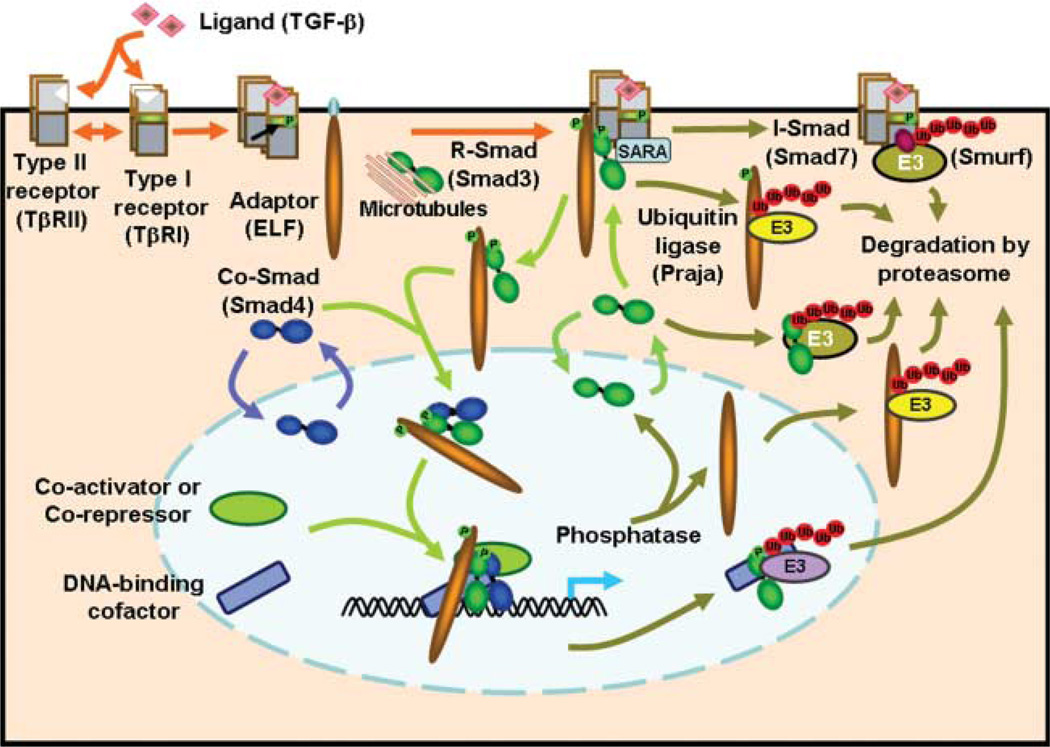
Interactions involving ubiquitination are an integral part of the TGF-β/Smad signaling pathway (Fig. 1). The ubiquitin–proteasome system is composed of two discrete steps. First, multiple ubiquitin molecules are attached to the target protein. Second, the polyubiquitinated protein is degraded by the 26S proteasome complex. Ubiquitination is mediated by at least three enzymes: 1) ubiquitin-activating enzyme (E1); 2) ubiquitin-conjugating enzyme (E2); and 3) ubiquitin ligase (E3). E3 ubiquitin ligases are primarily responsible for the recognition of specific target proteins and deregulation of E3 ubiquitin ligases is linked to the development of cancer (Nakayama & Nakayama 2006). E3 ubiquitin ligases are categorized into four major groups based on specific structural motifs. These are the homologous to the E6-AP carboxyl terminus (HECT)-type, really interesting new gene (RING)-finger type, U-box type, and plant homeo domain (PHD)-finger type proteins. HECT-type ligases that are involved in TGF-β signaling include Smurf1 and Smurf2, Neural precursor cell expressed, developmentally down-regulated 4-2 (Nedd4-2), WW domain containing protein 1 (WWP1)/Tiul1, Itch, Cbl-b, and Arkadia. A single U-box type ligase, carboxyl terminus of hsc70-interacting protein (CHIP), is additionally implicated in this pathway. RING-finger type, the largest group of E3 ligases, includes Skp1/Culin/F-box protein (SCF)–Skp2, SCF–β-TrCP1, Roc1–SCF–β-TrCP1, anaphase promoting complex (APC)–CDH1, Ectodermin, and PRAJA. The role of these ubiqintiators and their association with development of cancer is presented below and in Tables 1 and 2.
Figure 1.
The TGF-β signaling pathway is regulated at multiple points by ubiquitin-mediated targeting and proteasomal degradation. Receptors, adaptor proteins, Smads, co-activator/co-repressor and DNA binding co-factors are targeted by E3 ubiquitin ligases for ubiquitination and degredation by the proteasome. These protein degredation pathways impart precise and specific control over the cellular response to TGF-β signaling.
Table 1.
E3 ubiquitin ligases implicated in transforming growth factor-β (TGF-β) signaling
Table 2.
E3 ligases associated with transforming growth factor-β (TGF-β) signaling and cancer
| E3 ligase | Cancer cell lines | Altered expression in cancer |
|---|---|---|
| Smurf1 | RhoA – tumor cell migration, Boyer et al. (2006) and Sahai et al. (2007) | Amplified – pancreatic adenocarcinomas, Loukopoulos et al. (2007); amplified – pancreatic cancer, Bashyam et al. (2005) |
| Smurf2 | High expression correlates with poor prognosis of esophageal squamous cell carcinoma, Joazeiro et al. (1999) and Fukuchi et al. (2002) | |
| Smurf2 | p14ARF mutant melanoma, Packer et al. (2007) | |
| Nedd4-2 | Lung, colon, gastric, breast, ovary, and melanoma, Kuratomi et al. (2005) | |
| WWP1 | Breast, Chen et al. (2007) | Breast cancer, Chen et al. (2007) |
| PRAJA | Gastrointestinal, Saha et al. (2006) | |
| SCF–β-TrCP1 | Pancreatic, Wan et al. (2005) | Pancreatic ductal adenocarcinomas, Wan et al. (2005) |
| Ecto | Colorectal and breast, Dupont et al. (2005) |
Smurf1
Smurf1 modulates BMP signaling through multiple mechanisms thereby influencing a variety of BMP-induced cellular behaviors. Smurf1 ubiquitinates BMP-regulated R-Smads. In Xenopus, Smurf1 inhibits BMP signaling through Smad1, Smad5, and Smad8, while potentiating activin signaling through Smad2 and Smad3 (Zhu et al. 1999). In mouse lung, Smurf1 over-expression reduces levels of Smad1 and Smad5, but not Smad8. In addition, Smurf1 inhibits lung epithelial branching, which is rescued by over-expression of Smad1 or BMP4 (Shi et al. 2004). In bone, LIM mineralization protein-1 (LMP-1) directly interacts with the Smurf1 WW2 domain and can effectively compete with Smad1 and Smad5 for binding, thus potentiating BMP signaling (Sangadala et al. 2006). In C2C12 cells, Smurf1 promotes myogenic differentiation, while blocking BMP-induced osteogenic conversion, yet Smurf1 has no effect on TGF-β-induced differentiation arrest. In these cells, elevated Smurf1 reduces the level of endogenous Smad5, but not Smad2, Smad3, or Smad7. Adding back Smad5 to the Smurf1 over-expressing C2C12 cells restores BMP-mediated osteoblast conversion. Conversely, depletion of the endogenous Smurf1 through RNA interference blocks myogenic differentiation and promotes BMP-induced osteogenic conversion (Ying et al. 2003).
Smurf1 negatively regulates BMP signaling together with the I-Smads, Smad6/7. Smurf1 interacts with nuclear Smad7 and induces Smad7 ubiquitination and translocation into the cytoplasm. Additionally, Smurf1 associates with BMP type I receptors (TβRI) via Smad7, with subsequent enhancement of degradation of both the receptor and Smad7. Thus, Smad7 functions to induce the degradation of TβRI by recruiting the E3 ubiquitin ligase, Smurf1, to the receptor (Ebisawa et al. 2001). Further, Smurf1 induces ubiquitination and degradation of Smad1/5. Moreover, Smurf1 can associate with Smad1/5 indirectly through I-Smads and induce their ubiquitination and degradation. Thus, Smurf1 controls BMP signaling with and without I-Smads through multiple mechanisms (Murakami et al. 2003).
In activin signaling, the cytoplasmic immunophilin FKBP12, a 12 kDa FK506-binding protein, interacts with Smad7 in an activin-dependent manner and forms a complex with Smad7 on the type I receptor. The interaction of FKBP12 and Smad7 enhances the ubiquitination of the type I receptor by Smurf1. Thus, FKBP12 acts as an adaptor molecule for the Smad7–Smurf1 complex to regulate the duration of the activin signal (Yamaguchi et al. 2006).
Smurf1, which otherwise cannot directly bind to Smad4, mediates ubiquitination of Smad4 in the presence of Smad6 or Smad7. Smad2 can also act as an adaptor for Smurf1 binding and ubiquitination of Smad4. Ternary complexes of Smad4, Smad7, and Smurf1 primarily co-localize in the cytoplasm and in peripheral cell protrusions. Smad2 or Smad7 mutants, defective in Smad4 interaction, fail to induce Smurf1-mediated down-regulation of Smad4. Likewise, Smad4 mutants defective in Smad2 or Smad7 interaction are not effectively down-regulated by Smurf1 (Moren et al. 2005).
Smurf1 also modulates transcription by inducing the ubiquitination and degradation of Runx2. Runx2 is a bone-specific transcription factor that plays a critical role in bone development, postnatal bone formation, and chondrocyte maturation. Smad6 enhances Smurf1-induced Runx2 degradation in an ubiquitin–proteasome-dependent manner. Thus, Smurf1 induces Runx2 degradation in a Smad6-dependent manner and serves as a negative regulatory mechanism for the BMP–Smad–Runx2 signaling pathway (Shen et al. 2006).
Smurf2
Smurf2 interacts with R-Smads, including Smad1, Smad2, and Smad3 but not Smad4. Smurf2 over-expression is sufficient to reduce the steady-state levels of Smad1 and Smad2 but not Smad3 or Smad4. Significantly, Smurf2 exhibits higher-binding affinity to activated Smad2 and displays a preference for Smad2 as its target for degradation (Lin et al. 2000). Upon TGF-β activation, phosphorylated Smad2 translocates to the nucleus, where its accumulation results in Smad2 ubiquitination and degradation, thereby terminating TGF-β signaling (Lo & Massague 1999). On the other hand, Zhang et al. report that Smurf2 preferentially targets Smad1 for ubiquitination- and proteasome-mediated degradation. At higher Smurf2 expression levels, Smad2, but not Smad3, also decreases. In Xenopus embryos, ectopic Smurf2 specifically inhibits Smad1 responses and thereby affects embryonic patterning by BMP signals (Zhang et al. 2001). As seen with Smurf1, Smurf2 can also mediate ubiquitination and degradation of Smad4 in the presence of Smad7 or Smad2 (Moren et al. 2005).
Smad7 associates constitutively with Smurf2. Smurf2 is nuclear, but binding to Smad7 induces export and recruitment to activated TβRI, where it causes degradation of receptors and Smad7 via proteasomal and lysosomal pathways. IFNγ, which stimulates expression of Smad7, induces Smad7–Smurf2 complex formation and increases TGF-β receptor turnover. TGF-β receptor turnover is stabilized by blocking Smad7 or Smurf2 expression. Furthermore, Smad7 mutants that interfere with recruitment of Smurf2 to the receptors are compromised in their inhibitory activity. Thus, Smad7 acts as an adaptor in an E3 ubiquitin ligase complex that targets activated TβRI for degradation (Kavsak et al. 2000).
In the presence of TGF-β signaling, Smad2 interacts through its proline-rich PPXY motif with the tryptophan-rich WW domains of Smurf2. Activated Smad2 mediates association of Smurf2 with the transcriptional co-repressor SnoN (Ski-related novel protein N). This allows the HECT domain of Smurf2 to target SnoN for ubiquitin-mediated degradation by the proteasome, thus relieving SnoN-mediated repression of transcription (Bonni et al. 2001).
NEDD4-2
NEDD4-2 is a direct-binding partner of Smad7. NEDD4-2 associates with TGF-β type I receptors via Smad7 and induces its ubiquitin-dependent degradation. Additionally, NEDD4-2 binds to activated Smad2 and Smad3, and induces degradation of Smad2, but not Smad3 (Kuratomi et al. 2005). Furthermore, NEDD4-2, in the presence of Smad7, can mediate ubiquitination of Smad4 (Moren et al. 2005). In contrast to Smurf2, NEDD4-2 fails to induce ubiquitination of SnoN, although NEDD4-2 binds to SnoN via Smad2 more strongly than Smurf2. Over-expression of NEDD4-2 prevents transcriptional activity induced by TGF-β and BMP, whereas silencing of NEDD4-2 by siRNA enhances the responsiveness to TGF-β superfamily cytokines (Kuratomi et al. 2005). Thus, NEDD4-2 is a negative regulator of TGF-β signaling.
WWP1/Tiul1
WW domain-containing protein 1, WWP1, also known as TGIF-interacting ubiquitin ligase 1, Tiul1, is structurally related to Smurf E3 ubiquitin ligases. WWP1/Tiul1 associates with Smad7, induces Smad7 nuclear export, and enhances the binding of Smad7 to activated TβRI resulting in their ubiquitination and degradation, without affecting the expression levels of Smad7. WWP1/Tiul 1 can also mediate ubiquitination of Smad4 in the presence of Smad7 (Moren et al. 2005). Over-expression of WWP1/Tiul1 suppresses TGF-β-induced growth arrest and transcriptional responses, and inhibits TGF-β stimulated phosphorylation of Smad2 (Seo et al. 2004). Thus, WWP1/Tiul1 over-expression, in cooperation with Smad7, negatively regulates TGF-β signaling. However, unlike Smurfs, WWP1/Tiul1 fails to ubiquitinate R-Smads and SnoN. Importantly, WWP1/Tiul1 and Smurfs are expressed in distinct patterns in human tissues and carcinoma cell lines, suggesting unique pathophysiological roles of WWP1/Tiul1 and Smurfs (Komuro et al. 2004).
Upon activation of TGF-β signaling, WWP1/Tiul1 interacts with Smad2 and the nuclear co-repressor TGIF. Steady-state levels of TGIF are not affected by WWP1/Tiul1, but the interaction of WWP1/Tiul1 and Smad2 with TGIF, targets Smad2 for degradation. Silencing of WWP1/Tiul1 or TGIF by siRNA suppresses TGF-β-dependent degradation of Smad2 and enhances TGF-β-mediated gene expression. Thus, WWP1/Tiul1 and TGIF act as negative regulators of TGF-β signaling (Seo et al. 2004).
Itch
Itch, also know as atrophin-1-interacting protein 4 (AIP4), is an E3 ligase for Notch and JunB (Qiu et al. 2000, Gao et al. 2004). Itch also regulates TGF-β signaling, where it facilitates complex formation between TβRI and Smad2 and enhances TGF-β-induced transcription. In addition, Itch promotes ubiquitination of Smad2. Moreover, Itch augments Smad2 phosphorylation, which requires intact ligase activity. In mouse embryonic fibroblasts (MEFs), loss of Itch reduced susceptibility to TGF-β-induced cell growth arrest and decreased phosphorylation of Smad2, without altering protein levels for Smad2, Smad4, and Smad7. Thus, Itch positively regulates TGF-β signaling via proteolysis-independent ubiquitination (Bai et al. 2004).
HEF1, human enhancer of filamentation 1, functions as a multidomain docking protein implicated in signaling pathways such as those mediated by integrin, T-cell receptor, and B-cell receptor. HEF1 is also involved in TGF-β signaling pathways by interacting with Smad3. The interaction of Smad3 with HEF1 induces HEF1 proteasomal degradation, which is further enhanced upon TGF-β stimulation. Itch functions as an ubiquitin E3 ligase for HEF1, forming a complex with both Smad3 and HEF1 through its WW domains in a TGF-β-independent manner. This complex regulates HEF1 ubiquitination and degradation, and can be enhanced by TGF-β stimulation. Smad3-regulated proteasomal degradation of HEF1 by Itch therefore serves to broaden the network of cross-talk between TGF-β signaling and those pathways involving HEF1 and Itch (Feng et al. 2004).
Arkadia
Arkadia was originally identified as a protein that enhances signaling activity of Nodal and induces mammalian nodes during early embryogenesis. Arkadia is widely expressed in mammalian tissues, and it enhances both TGF-β and BMP signaling. Arkadia physically interacts with Smad7 and induces its ubiquitination and degradation. In contrast to Smurfs, however, Arkadia does not associate with TβRI and does not induce degradation of TGF-β receptors. TGF-β down-regulates Arkadia, while it up-regulates Smad7. Silencing of Arkadia by siRNA results in repression of the transcriptional activities induced by TGF-β and BMP, and in the accumulation of Smad7 protein. Arkadia therefore, may play a role as an amplifier of TGF-β signaling by reducing levels of inhibitory Smad7 (Koinuma et al. 2003).
Axin, a pivotal player in Wnt signaling that is required for the constitutive degradation of β-catenin, also coordinates TGF-β signaling by forming a multimeric complex consisting of Smad7 and Arkadia. Specific knockdown of Axin or Arkadia demonstrates that Axin and Arkadia cooperate with each other in promoting Smad7 ubiquitination. Coexpression of Wnt-1, which downregulates Axin levels, reduces Smad7 ubiquitination by Arkadia. Thus, Axin acts as an intrinsic regulator in TGF-β as well as in Wnt signaling, and therefore may participate in cross-talk between these signaling pathways (Liu et al. 2006).
Cbl-b
In T cells, loss of Cbl-b results in defective TGF-β-mediated Smad2 phosphorylation. Cbl-b loss also prevents TGF-β-mediated induction of Foxp3+ functional regulatory T cells. Cbl-b−/− mice show significantly enhanced responses to a tumor that is strictly TGF-β regulated. Thus, the E3 ubiquitin ligase Cbl-b plays an integral role in T-cell TGF-β signaling, and its absence results in multifunctional TGF-β-related defects that have important implications in cancer.
CHIP
CHIP is a U-box-dependent E3 ubiquitin ligase that directly binds and interacts with Smad1 (Li et al. 2005). Over-expression of CHIP results in ubiquitin-mediated degradation of Smad1 and Smad4. Conversely, reduction of CHIP levels with RNAi results in enhanced BMP signaling (Li et al. 2004).
CHIP directly mediates ubiquitination and degradation of Smad3 independently of TGF-β signaling, thereby regulating the basal level of Smad3. In cell culture luciferase assays, over-expression of CHIP inhibits TGF-β signaling, whereas silencing CHIP by siRNA increases sensitivity to TGF-β signaling. In cell lines with stably over-expressed CHIP, Smad3 is greatly decreased and TGF-β signaling is abolished, based on cell proliferation assays and JunB expression. Thus, CHIP can modulate the sensitivity of the TGF-β signaling by controlling the basal level of Smad3 through ubiquitin-mediated degradation (Xin et al. 2005).
SCF–Skp2
The SCF is a multi-protein E3 ubiquitin ligase complex, containing Skp, Cullin, and an F-box protein. SCF–Skp2 mediates the metabolic instability of cancer-derived mutant Smad4. Skp2, the F-box component of SCF–Skp2, physically interacts with Smad4. Several cancer-derived unstable Smad4 mutants exhibit significantly increased binding to Skp2, which leads to increased ubiquitination and proteolysis (Liang et al. 2004).
SCF–βTrCP1
SCF–βTrCP1 is a critical determinant for Smad4 degradation. F-box protein βTrCP1 in this E3 ligase interacts with Smad4, but has no interaction with Smad2 and has weak interaction with Smad3. The βTrCP1/Smad3 interaction is abolished by Smad4 gene silencing, indicating that the interaction is indirect and is through Smad4. Ectopic expression of SCF–βTrCP1 induces the ubiquitination and degradation of Smad4, while suppression of βTrCP1 by siRNA increases expression of Smad4. Consistent with these results, cells that over-express SCF–βTrCP1 have reduced TGFβ-dependent transcriptional activity and an impaired cell cycle arrest function. Thus, SCF–βTrCP1 abrogates TGF-β function in vivo by decreasing Smad4 stability (Wan et al. 2004).
SCF–βTrCP1 inhibits TGF-β biological activity in pancreatic cancer cells by decreasing Smad4 stability. In human pancreatic ductal adenocarcinoma cells, Smad4 levels are very low as determined by immunohistochemistry. In pancreatic tumor-derived Smad4 mutants, most point-mutated Smad4 proteins, except those within or very close to a mutation cluster region, exhibit higher interaction affinity with β-TrCP1 and significantly elevated ubiquitination. Two cancer cell lines harboring Smad4 point mutations, AsPC-1 and Caco-2, exhibit rapid SCF–βTrCP1-mediated Smad4 degradation. Conversely, reduction of SCF–βTrCP1 expression by siRNA in pancreatic cancer cells results in elevated levels of Smad4 and TGF-β signaling. Therefore, inhibition of Smad4-specific E3 ligase might be a target for therapeutic intervention in pancreatic cancer (Wan et al. 2005).
ROC1–SCF–β-TrCP1
Activated Smad3 is degraded by the ubiquitin–proteasome pathway through an interaction with the C-terminal MH2 domain of ROC1, a RING finger protein. An E3 ubiquitin ligase complex regulator of cullins 1 (ROC1)–SCF–β-TrCP1 consisting of ROC1, Skp1, Cullin1, and β-TrCP1 (also known as Fbw1a) induces ubiquitination of Smad3. Recruitment of p300, a transcriptional coactivator, to nuclear Smad3 facilitates the interaction with ROC1–SCF–β-TrCP1 and triggers export from the nucleus to the cytoplasm for proteasomal degradation (Fukuchi et al. 2001).
APC
The APC is a multi-subunit ubiquitin E3 ligase that mediates Smad2- or Smad3-induced degradation of SnoN. SnoN is an important negative regulator of TGF-β signaling, which functions to maintain the repressed state of TGF-β target genes in the absence of ligand. Upon TGF-β stimulation, Smad3 and Smad2 translocate into the nucleus and induce a rapid degradation of SnoN, allowing activation of TGF-β target genes. Smad3 and to a lesser extent, Smad2, interact with both the APC and SnoN, resulting in the recruitment of the APC to SnoN and subsequent ubiquitination of SnoN in a destruction box (D box)-dependent manner. In addition to the D box, efficient ubiquitination and degradation of SnoN requires the Smad3-binding site in SnoN as well as key lysine residues necessary for ubiquitin attachment. Mutation of either the Smad3-binding site or the lysine residues results in stabilization of SnoN and in enhanced antagonism of TGF-β signaling (Stroschein et al. 2001, Wan et al. 2001).
The APC activator CDH1 forms a quaternary complex with SnoN, Smad3, and APC, in which CDH1 and Smad3 synergistically regulate SnoN degradation (Wan et al. 2001). CDH1 also regulates HEF1 levels. HEF1, CDH1, Smad3, and APC10, a component APC, physically interact. Distinct subdomains within the MH2 domain of Smad3 bind to APC10 and HEF1, suggesting the formation of a complex of HEF1, Smad3, APC10, and CDH1. In addition, over-expression of APC10 and CDH1 leads to a reduction in HEF1 protein levels. Thus, Smad3 may recruit the APC complex via a direct interaction with APC10 to regulate the ubiquitination and degradation of HEF1 by the CDH1 subunit of the APC complex (Nourry et al. 2004). Over-expression of Smad3 triggers the proteasomal degradation of HEF1. In addition, TGF-β stimulation induces rapid degradation of endogenous HEF1 in different TGF-β-responsive cell lines. Interestingly, in TGF-β-treated epithelial cells, the degradation of HEF1 is followed closely by an increase in HEF1 mRNA, resulting in a time-dependent increase in HEF1 protein. Elevated HEF1 protein levels inhibit TGF-β-induced gene responses. Thus, the TGF-β signaling pathway is regulated by HEF1 via a negative feedback mechanism (Liu et al. 2000).
Ecto
Ecto, Ectodermin, is a RING-type E3 ubiquitin ligase for Smad4. In Xenopus embryos, Ecto is essential for the specification of ectoderm and acts by restricting mesoderm-inducing activity of TGF-β signals to the mesoderm thus favoring neural induction. Depletion of Ecto in human cells enforces TGF-β-induced cytostasis. Ecto additionally plays a causal role in limiting the antimitogenic effects of Smad4 in tumor cells. Thus, Ecto may act as a switch in the control of TGF-β gene responses during early embryonic development and cell proliferation (Dupont et al. 2005).
PRAJA
PRAJA, a RING-H2 protein, interacts with ELF in a TGF-β-dependent manner. PRAJA manifests substantial E3-dependent ubiquitination of ELF and Smad3, but not Smad4. Treatment with potent proteasomal inhibitors MG132 results in the accumulation of ELF, indicating that PRAJA ubiquitinates ELF and that its degradation is mediated via the proteasomal pathway. Deletion of the RING finger domain at the C terminus of PRAJA, Δ-PRAJA, abolishes ubiquitination of ELF. In a cell line that stably over-expresses PRAJA, ELF expression is low compared with normal controls. In contrast, in a Δ-PRAJA stable cell line, ELF expression is high compared with normal controls. Moreover, in PRAJA-transfected hepatocytes, the degradation rate of ELF is substantially higher in TGF-β stimulated cells compared with unstimulated cells. After 30 min of TGF-β induction, ELF associates with PRAJA, which is approximately when the ELF–Smad heteromeric complex translocates into the nucleus. This raises possibilities that PRAJA interaction and degradation of ELF could result in the disruption of Smad4 signaling, with a potential role in development of cancer.
There is a fivefold increase of PRAJA expression and a subsequent decrease in ELF and Smad4 expression in gastrointestinal cancer cell lines. Therefore, alteration of ELF and/or Smad4 expression in the TGF-β signaling pathway may be induced by enhancement of ELF degradation, which is mediated by a high-level expression of PRAJA in gastrointestinal cancers (Saha et al. 2006).
Overall, these studies suggest a unique mechanism by showing that the pathway and growth of cells that are dependent on the TGF-β adaptor protein, ELF, are inactivated by the RING E3 ubiquitin ligase, PRAJA. Also, multiple other cancers derived from meso-endodermally derived epithelium are associated with the TGF-β/BMP pathway inactivation, where it may regulate progenitor cell fate (Souchelnytskyi et al. 2002, Siegel & Massague 2003, Tang et al. 2003b). Indeed, the functions of TGF-β are more complex than simply inhibiting cell growth, as TGF-β can induce the growth of mesenchymal cells and alter synthesis of extracellular matrix components as well as metalloproteases involved in cell invasion (Sporn & Roberts 1990, Heldin et al. 1997, Derynck et al. 1998, Souchelnytskyi et al. 2002, Itoh et al. 2003, Tang et al. 2003a). TGF-β signals also modulate the immune response to tumors and are thought to play a role in tumor angiogenesis (Wieser 2001). The development of gut tumors in elf+/− and elf+/− Smad4+/− mutants points to the critical role played by ELF as an essential adaptor protein for the proper transmission of signals generated by the TGF-β pathway. Furthermore, modification of the TGF-β signaling pathway by loss of expression of ELF through PRAJA could play a significant role in the development of gastrointestinal tumors. Further research into the role of the ubiquitin–proteasome system in modulating TGF-β signaling could be the key to unlocking the mechanisms behind many cancers, and lead to unique therapeutic approaches to treating these diseases.
Acknowledgements
The authors wish to thank Tiffany Blake for critical review of the manuscript. Grant Support: NIH RO1 CA106614A (L M), NIH RO1 DK56111 (L M), NIH RO1 CA4285718A (L M), V A Merit Award (L M), R Robert and Sally D Funderburg Research Scholar (L M).The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2–Smad4 complex formation and signaling. Journal of Biological Chemistry. 1997;272:27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- Arteaga CL, Tandon AK, Von Hoff DD, Osborne CK. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Research. 1988;48:3898–3904. [PubMed] [Google Scholar]
- Arteaga CL, Carty-Dugger T, Moses HL, Hurd SD, Pietenpol JA. Transforming growth factor beta 1 can induce estrogen-independent tumorigenicity of human breast cancer cells in athymic mice. Cell Growth and Differentiation. 1993;4:193–201. [PubMed] [Google Scholar]
- Bai Y, Yang C, Hu K, Elly C, Liu YC. Itch E3 ligase-mediated regulation of TGF-beta signaling by modulating smad2 phosphorylation. Molecular Cell. 2004;15:825–831. doi: 10.1016/j.molcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Bashyam MD, Bair R, Kim YH, Wang P, Hernandez-Boussard T, Karikari CA, Tibshirani R, Maitra A, Pollack JR. Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia. 2005;7:556–562. doi: 10.1593/neo.04586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp RD, Lyons RM, Yang EY, Coffey RJ, Moses HL. Expression of and response to growth regulatory peptides by two human pancreatic carcinoma cell lines. Pancreas. 1990;5:369–380. doi: 10.1097/00006676-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Birkey Reffey S, Wurthner JU, Parks WT, Roberts AB, Duckett CS. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-beta signaling. Journal of Biological Chemistry. 2001;276:26542–26549. doi: 10.1074/jbc.M100331200. [DOI] [PubMed] [Google Scholar]
- Bonni S, Wang HR, Causing CG, Kavsak P, Stroschein SL, Luo K, Wrana JL. TGF-beta induces assembly of a Smad2–Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nature Cell Biology. 2001;3:587–595. doi: 10.1038/35078562. [DOI] [PubMed] [Google Scholar]
- Boyer L, Turchi L, Desnues B, Doye A, Ponzio G, Mege JL, Yamashita M, Zhang YE, Bertoglio J, Flatau G, et al. CNF1-induced ubiquitylation and proteasome destruction of activated RhoA is impaired in Smurf1−/− cells. Molecular and Cellular Biology. 2006;17:2489–2497. doi: 10.1091/mbc.E05-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Ebner R, Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-β activities. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- Chen RH, Miettinen PJ, Maruoka EM, Choy L, Derynck R. A WD-domain protein that is associated with and phosphorylated by the type II TGF-β receptor. Nature. 1995;377:548–552. doi: 10.1038/377548a0. [DOI] [PubMed] [Google Scholar]
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997a;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- Chen YG, Liu F, Massague J. Mechanism of TGFbeta receptor inhibition by FKBP12. EMBO Journal. 1997b;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhou Z, Ross JS, Zhou W, Dong JT. The amplified WWP1 gene is a potential molecular target in breast cancer. International Journal of Cancer. 2007;121:2834–2841. doi: 10.1002/ijc.22653. [DOI] [PubMed] [Google Scholar]
- Denhardt DT. Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochemical Journal. 1996;318:729–747. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng X-H. Transcriptional activators of TGF-β responses: Smads. Cell. 1998;95:737. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. Microtubule binding to Smads may regulate TGF-β activity. Molecular Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, Rugge M, Piccolo S. Germ-layer specification and control of cell growth by ectodermin, a Smad4 ubiquitin ligase. Cell. 2005;121:87–99. doi: 10.1016/j.cell.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. Journal of Biological Chemistry. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, et al. MADR2 maps to 18q21 and encodes a TGF-β-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. A kinase subdomain of transforming growth factor-beta (TGF-β) type I receptor determines the TGF-beta intracellular signaling specificity. EMBO Journal. 1997;16:3912–3923. doi: 10.1093/emboj/16.13.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Guedes S, Wang T. Atrophin-1-interacting protein 4/human Itch is a ubiquitin E3 ligase for human enhancer of filamentation 1 in transforming growth factor-beta signaling pathways. Journal of Biological Chemistry. 2004;279:29681–29690. doi: 10.1074/jbc.M403221200. [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Molecular Biology of the Cell. 2001;12:1431–1443. doi: 10.1091/mbc.12.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Fukai Y, Masuda N, Miyazaki T, Nakajima M, Sohda M, Manda R, Tsukada K, Kato H, Kuwano H. High-level expression of the Smad ubiquitin ligase Smurf2 correlates with poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Research. 2002;62:7162–7165. [PubMed] [Google Scholar]
- Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- Germain S, Howell M, Esslemont GM, Hill CS. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes and Development. 2000;14:435–451. [PMC free article] [PubMed] [Google Scholar]
- Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Molecular and Cellular Biology. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO Journal. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor {beta} (TGFβ)-stimulated fibronectin synthesis through TGFβ-activated kinase 1 and activation of the JNK pathway. Journal of Biological Chemistry. 2005;280:25920–25927. doi: 10.1074/jbc.M501150200. [DOI] [PubMed] [Google Scholar]
- Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang X, Zhong Q, Fisher AB, Bryington M, Zuckerman KS. Differential effects of transforming growth factor on cell cycle regulatory molecules in human myeloid leukemia cells. Oncogene. 2001;20:6840–6850. doi: 10.1038/sj.onc.1204790. [DOI] [PubMed] [Google Scholar]
- Ibarrola N, Kratchmarova I, Nakajima D, Schiemann WP, Moustakas A, Pandey A, Mann M. Cloning of a novel signaling molecule, AMSH-2, that potentiates transforming growth factor beta signaling. BMC Cell Biology. 2004;5:2. doi: 10.1186/1471-2121-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Thorikay M, Kowanetz M, Moustakas A, Itoh F, Heldin C-H, ten Dijke P. Elucidation of Smad requirement in transforming growth factor-beta type I receptor-induced responses. Journal of Biological Chemistry. 2003;278:3751–3761. doi: 10.1074/jbc.M208258200. [DOI] [PubMed] [Google Scholar]
- Janknecht R, Wells NJ, Hunter T. TGF-β-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes and Development. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CA, Wing SS, Huang H-K, Leverson JD, Hunter T, Liu Y-C. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Molecular Cell. 2000;6:1365. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Kim J, Johnson K, Hul Ju C, Carroll S, Laughon A. Drosophila mad binds to DNA and directly mediates activation of vestigial by decapentaplegic. Nature. 1997;388:304. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- Kim BC, Lee HJ, Park SH, Lee SR, Karpova TS, McNally JG, Felici A, Lee DK, Kim SJ. Jab1/CSN5, a component of the COP9 signalosome, regulates transforming growth factor beta signaling by binding to Smad7 and promoting its degradation. Molecular and Cellular Biology. 2004;24:2251–2262. doi: 10.1128/MCB.24.6.2251-2262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A, Wang XF, Weinberg RA, Cheifetz S, Massague J. Absence of TGF-β receptors and growth inhibitory responses in retinoblastoma cells. Science. 1988;240:196–199. doi: 10.1126/science.2895499. [DOI] [PubMed] [Google Scholar]
- Ko TC, Sheng HM, Reisman D, Thompson EA, Beauchamp RD. Transforming growth factor-beta 1 inhibits cyclin D1 expression in intestinal epithelial cells. Oncogene. 1995;10:177–184. [PubMed] [Google Scholar]
- Ko TC, Yu W, Sakai T, Sheng H, Shao J, Beauchamp RD, Thompson EA. TGF-β1 effects on proliferation of rat intestinal epithelial cells are due to inhibition of cyclin D1 expression. Oncogene. 1998;16:3445–3454. doi: 10.1038/sj.onc.1201902. [DOI] [PubMed] [Google Scholar]
- Koinuma D, Shinozaki M, Komuro A, Goto K, Saitoh M, Hanyu A, Ebina M, Nukiwa T, Miyazawa K, Imamura T, et al. Arkadia amplifies TGF-β superfamily signalling through degradation of Smad7. EMBO Journal. 2003;22:6458–6470. doi: 10.1093/emboj/cdg632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro A, Imamura T, Saitoh M, Yoshida Y, Yamori T, Miyazono K, Miyazawa K. Negative regulation of transforming growth factor-beta (TGF-β) signaling by WW domain-containing protein 1 (WWP1) Oncogene. 2004;23:6914–6923. doi: 10.1038/sj.onc.1207885. [DOI] [PubMed] [Google Scholar]
- Koutsodontis G, Moustakas A, Kardassis D. The role of Sp1 family members, the proximal GC-rich motifs, and the upstream enhancer region in the regulation of the human cell cycle inhibitor p21WAF-1/Cip1 gene promoter. Biochemistry. 2002;41:12771–12784. doi: 10.1021/bi026141q. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGF-β/Smad signaling by oncogenic Ras. Genes and Development. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratomi G, Komuro A, Goto K, Shinozaki M, Miyazawa K, Miyazono K, Imamura T. NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-β (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-β type I receptor. Biochemical Journal. 2005;386:461–470. doi: 10.1042/BJ20040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokowa M, Lynch K, Podolsky DK. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochemical and Biophysical Research Communications. 1987;142:775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- Labbe E, Silvestri C, Hoodless PA, Wrana JL, Attisano L. Smad2 and Smad3 positively and negatively regulate TGF beta-dependent transcription through the forkhead DNA-binding protein FAST2. Molecular Cell. 1998;2:109–120. doi: 10.1016/s1097-2765(00)80119-7. [DOI] [PubMed] [Google Scholar]
- Lange PA, Samson CM, Bird MA, Hayden MA, Behrns KE. Cirrhotic hepatocytes exhibit decreased TGF-β growth inhibition associated with downregulated Smad protein expression. Biochemical and Biophysical Research Communications. 2004;313:546–551. doi: 10.1016/j.bbrc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Li JM, Nichols MA, Chandrasekharan S, Xiong Y, Wang XF. Transforming growth factor beta activates the promoter of cyclin-dependent kinase inhibitor p15INK4B through an Sp1 consensus site. Journal of Biological Chemistry. 1995;270:26750–26753. doi: 10.1074/jbc.270.45.26750. [DOI] [PubMed] [Google Scholar]
- Li L, Xin H, Xu X, Huang M, Zhang X, Chen Y, Zhang S, Fu XY, Chang Z. CHIP mediates degradation of Smad proteins and potentially regulates Smad-induced transcription. Molecular and Cellular Biology. 2004;24:856–864. doi: 10.1128/MCB.24.2.856-864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RF, Zhang F, Lu YJ, Sui SF. Specific interaction between Smad1 and CHIP: a surface plasmon resonance study. Colloids and Surfaces. B, Biointerfaces. 2005;40:133–136. doi: 10.1016/j.colsurfb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Liang M, Liang YY, Wrighton K, Ungermannova D, Wang XP, Brunicardi FC, Liu X, Feng XH, Lin X. Ubiquitination and proteolysis of cancer-derived Smad4 mutants by SCFSkp2. Molecular and Cellular Biology. 2004;24:7524–7537. doi: 10.1128/MCB.24.17.7524-7537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Liang M, Feng X-H. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. Journal of Biological Chemistry. 2000;275:36818–36822. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- Liu X, Elia AE, Law SF, Golemis EA, Farley J, Wang T. A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO Journal. 2000;19:6759–6769. doi: 10.1093/emboj/19.24.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, et al. Axin is a scaffold protein in TGF-β signaling that promotes degradation of Smad7 by Arkadia. EMBO Journal. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo RS, Massague J. Ubiquitin-dependent degradation of TGF-β-activated smad2. Nature Cell Biology. 1999;1:472–478. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- Loukopoulos P, Shibata T, Katoh H, Kokubu A, Sakamoto M, Yamazaki K, Kosuge T, Kanai Y, Hosoda F, Imoto I, et al. Genome-wide array-based comparative genomic hybridization analysis of pancreatic adenocarcinoma: identification of genetic indicators that predict patient outcome. Cancer Science. 2007;98:392–400. doi: 10.1111/j.1349-7006.2007.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, et al. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annual Review of Biochemistry. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Mishra L, Cai T, Levine A, Weng D, Mezey E, Mishra B, Gearhart J. Identification of elf1, a beta-spectrin, in early mouse liver development. International Journal of Developmental Biology. 1998;42:221–224. [PubMed] [Google Scholar]
- Mishra L, Cai T, Yu P, Monga SP, Mishra B. Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene. 1999;18:353–364. doi: 10.1038/sj.onc.1202313. [DOI] [PubMed] [Google Scholar]
- Moren A, Imamura T, Miyazono K, Heldin CH, Moustakas A. Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. Journal of Biological Chemistry. 2005;280:22115–22123. doi: 10.1074/jbc.M414027200. [DOI] [PubMed] [Google Scholar]
- Moses HL, Serra R. Regulation of differentiation by TGF-beta. Current Opinion in Genetics and Development. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Kardassis D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. PNAS. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Molecular and Cellular Biology. 2003;14:2809–2817. doi: 10.1091/mbc.E02-07-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nature Reviews. Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Nourry C, Maksumova L, Pang M, Liu X, Wang T. Direct interaction between Smad3, APC10, CDH1 and HEF1 in proteasomal degradation of HEF1. BMC Cell Biology. 2004;5:20. doi: 10.1186/1471-2121-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer LM, Pavey SJ, Boyle GM, Stark MS, Ayub AL, Rizos H, Hayward NK. Gene expression profiling in melanoma identifies novel downstream effectors of p14ARF. International Journal of Cancer. 2007;121:784–790. doi: 10.1002/ijc.22725. [DOI] [PubMed] [Google Scholar]
- Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-β-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nature Cell Biology. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- Qiu L, Joazeiro C, Fang N, Wang H-Y, Elly C, Altman Y, Fang D, Hunter T, Liu Y-C. Recognition and ubiquitination of Notch by Itch, a Hect-type E3 ubiquitin ligase. Journal of Biological Chemistry. 2000;275:35734–35737. doi: 10.1074/jbc.M007300200. [DOI] [PubMed] [Google Scholar]
- Saha D, Datta PK, Beauchamp RD. Oncogenic ras represses transforming growth factor-beta/Smad signaling by degrading tumor suppressor Smad4. Journal of Biological Chemistry. 2001;276:29531–29537. doi: 10.1074/jbc.M100069200. [DOI] [PubMed] [Google Scholar]
- Saha T, Vardhini D, Tang Y, Katuri V, Jogunoori W, Volpe EA, Haines D, Sidawy A, Zhou X, Gallicano I, et al. RING finger-dependent ubiquitination by PRAJA is dependent on TGF-β and potentially defines the functional status of the tumor suppressor ELF. Oncogene. 2006;25:693–705. doi: 10.1038/sj.onc.1209123. [DOI] [PubMed] [Google Scholar]
- Sahai E, Garcia-Medina R, Pouyssegur J, Vial E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. Journal of Cell Biology. 2007;176:35–42. doi: 10.1083/jcb.200605135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L. LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads. Journal of Biological Chemistry. 2006;281:17212–17219. doi: 10.1074/jbc.M511013200. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Masuda Y, Ohta Y, Ikeda K, Watanabe K. Filamin associates with Smads and regulates transforming growth factor-beta signaling. Journal of Biological Chemistry. 2001;276:17871–17877. doi: 10.1074/jbc.M008422200. [DOI] [PubMed] [Google Scholar]
- Schiffer M, Mundel P, Shaw AS, Bottinger EP. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. Journal of Biological Chemistry. 2004;279:37004–37012. doi: 10.1074/jbc.M403534200. [DOI] [PubMed] [Google Scholar]
- Seo SR, Lallemand F, Ferrand N, Pessah M, L’Hoste S, Camonis J, Atfi A. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO Journal. 2004;23:3780–3792. doi: 10.1038/sj.emboj.7600398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZY, Xu LY, Chen XH, Cai WJ, Shen J, Chen JY, Huang TH, Zeng Y. The genetic events of HPV-immortalized esophageal epithelium cells. International Journal of Molecular Medicine. 2001;8:537–542. doi: 10.3892/ijmm.8.5.537. [DOI] [PubMed] [Google Scholar]
- Shen R, Chen M, Wang YJ, Kaneki H, Xing L, O’Keefe RJ, Chen D. Smad6 interacts with Runx2 and mediates Smad ubiquitin regulatory factor 1-induced Runx2 degradation. Journal of Biological Chemistry. 2006;281:3569–3576. doi: 10.1074/jbc.M506761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Chen H, Sun J, Chen C, Zhao J, Wang YL, Anderson KD, Warburton D. Overexpression of Smurf1 negatively regulates mouse embryonic lung branching morphogenesis by specifically reducing Smad1 and Smad5 proteins. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2004;286:L293–L300. doi: 10.1152/ajplung.00228.2003. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nature Reviews. Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S, Moustakas A, Heldin CH. TGF-β signaling from a three-dimensional perspective: insight into selection of partners. Trends in Cell Biology. 2002;12:304–307. doi: 10.1016/s0962-8924(02)02300-0. [DOI] [PubMed] [Google Scholar]
- Sporn MB. TGF-β: 20 years and counting. Microbes and Infection. 1999;1:1251–1253. doi: 10.1016/s1286-4579(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB. The transforming growth factor-betas: past, present, and future. Annals of the New York Academy of Sciences. 1990;593:1–6. doi: 10.1111/j.1749-6632.1990.tb16095.x. [DOI] [PubMed] [Google Scholar]
- Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K. Negative feedback regulation of TGF-β signaling by the SnoN oncoprotein. Science. 1999;286:771–774. doi: 10.1126/science.286.5440.771. [DOI] [PubMed] [Google Scholar]
- Stroschein SL, Bonni S, Wrana JL, Luo K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes and Development. 2001;15:2822–2836. doi: 10.1101/gad.912901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu G, Willson JK, Zborowska E, Yang J, Rajkarunanayake I, Wang J, Gentry LE, Wang XF, Brattain MG. Expression of transforming growth factor beta type II receptor leads to reduced malignancy in human breast cancer MCF-7 cells. Journal of Biological Chemistry. 1994;269:26449–26455. [PubMed] [Google Scholar]
- Sun Y, Liu X, Eaton EN, Lane WS, Lodish HF, Weinberg RA. Interaction of the Ski oncoprotein with Smad3 regulates TGF-β signaling. Molecular Cell. 1999;4:499–509. doi: 10.1016/s1097-2765(00)80201-4. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kohmura H, Futamura M, Kida H, Tanemura H, Shimokawa K, Saji S. Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology. 1996;111:1369–1372. doi: 10.1053/gast.1996.v111.pm8898652. [DOI] [PubMed] [Google Scholar]
- Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, Wakefield LM. a TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. Journal of Clinical Investigation. 2003;112:1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003b;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- Tang Y, Katuri V, Srinivasan R, Fogt F, Redman R, Anand G, Said A, Fishbein T, Zasloff M, Reddy EP, et al. Transforming growth factor-β suppresses nonmetastatic colon cancer through Smad4 and adaptor protein ELF at an early stage of tumorigenesis. Cancer Research. 2005;65:4228–4237. doi: 10.1158/0008-5472.CAN-04-4585. [DOI] [PubMed] [Google Scholar]
- Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, et al. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nature Genetics. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE, domain protein that recruits Smad2 to the TGFβ receptor. Cell. 1998;95:779. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-β signaling by targeting SnoN for destruction. Molecular Cell. 2001;8:1027–1039. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- Wan M, Cao X, Wu Y, Bai S, Wu L, Shi X, Wang N, Cao X. Jab1 antagonizes TGF-β signaling by inducing Smad4 degradation. EMBO Reports. 2002;3:171–176. doi: 10.1093/embo-reports/kvf024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Tang Y, Tytler EM, Lu C, Jin B, Vickers SM, Yang L, Shi X, Cao X. Smad4 protein stability is regulated by ubiquitin ligase SCF beta-TrCP1. Journal of Biological Chemistry. 2004;279:14484–14487. doi: 10.1074/jbc.C400005200. [DOI] [PubMed] [Google Scholar]
- Wan M, Huang J, Jhala NC, Tytler EM, Yang L, Vickers SM, Tang Y, Lu C, Wang N, Cao X. SCFβ-TrCP1 controls Smad4 Protein stability in pancreatic cancer cells. American Journal of Pathology. 2005;166:1379–1392. doi: 10.1016/s0002-9440(10)62356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun L, Myeroff L, Wang X, Gentry LE, Yang J, Liang J, Zborowska E, Markowitz S, Willson JKV, et al. Demonstration that mutation of the type II transforming growth factor β receptor inactivates its tumor suppressor activity in replication error-positive colon carcinoma cells. Journal of Biological Chemistry. 1995;270:22044. doi: 10.1074/jbc.270.37.22044. [DOI] [PubMed] [Google Scholar]
- Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGF-β family type I receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Rebbert ML, Andreazzoli M, Takahashi N, Toyama R, Zimmerman S, Whitman M, Dawid IB. Regulation of the Lim-1 gene is mediated through conserved FAST-1/FoxH1 sites in the first intron. Developmental Dynamics. 2002;225:448–456. doi: 10.1002/dvdy.10176. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Monga SP, Liu Y, Brodie SG, Tang Y, Li C, Mishra L, Deng CX. Smad proteins and hepatocyte growth factor control parallel regulatory pathways that converge on beta1-integrin to promote normal liver development. Molecular and Cellular Biology. 2001;21:5122–5131. doi: 10.1128/MCB.21.15.5122-5131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R. The transforming growth factor-beta signaling pathway in tumorigenesis. Current Opinion in Oncology. 2001;13:70–77. doi: 10.1097/00001622-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clarke RB. Cutting edge: deficiency in the E3 Ubiquitin Ligase Cbl-b results in a multifunctional defect in T cell TGF-β sensitivity in vitro and in vivo. Journal of Immunology. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Wu G, Chen Y-G, Ozdamar B, Gyuricza CA, Chong PA, Wrana JL, Massagué J, Shi Y. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;287:92–97. doi: 10.1126/science.287.5450.92. [DOI] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. Inactivation of TGF-β signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes and Development. 2005;19:530–535. doi: 10.1101/gad.317405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Xu X, Li L, Ning H, Rong Y, Shang Y, Wang Y, Fu X-Y, Chang Z. CHIP controls the sensitivity of transforming growth factor-β signaling by modulating the basal level of Smad3 through ubiquitin-mediated degradation. Journal of Biological Chemistry. 2005;280:20842–20850. doi: 10.1074/jbc.M412275200. [DOI] [PubMed] [Google Scholar]
- Xu X, Brodie SG, Yang X, Im YH, Parks WT, Chen L, Zhou YX, Weinstein M, Kim SJ, Deng CX. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene. 2000;19:1868–1874. doi: 10.1038/sj.onc.1203504. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H, Matsumoto K. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO Journal. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Kurisaki A, Yamakawa N, Minakuchi K, Sugino H. FKBP12 functions as an adaptor of the Smad7-Smurf1 complex on activin type I receptor. Journal of Molecular Endocrinology. 2006;36:569–579. doi: 10.1677/jme.1.01966. [DOI] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO Journal. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SX, Hussain ZJ, Zhang YE. Smurf1 facilitates myogenic differentiation and antagonizes the bone morphogenetic protein-2-induced osteoblast conversion by targeting Smad5 for degradation. Journal of Biological Chemistry. 2003;278:39029–39036. doi: 10.1074/jbc.M301193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. PNAS. 2001;98:974–979. doi: 10.1073/pnas.98.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Zawel L, Lengauer C, Kinzler KW, Vogelstein B. Characterization of human FAST-1, a TGF-β and activin signal transducer. Molecular Cell. 1998;2:121–127. doi: 10.1016/s1097-2765(00)80120-3. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- Zimmerman CM, Kariapper MS, Mathews LS. Smad proteins physically interact with calmodulin. Journal of Biological Chemistry. 1998;273:677–680. doi: 10.1074/jbc.273.2.677. [DOI] [PubMed] [Google Scholar]