Abstract
The 41 novel acylthiourea derivatives with hydantoin were synthesized in moderate to excellent yields by using 5-(4-aminophenyl)- and 5-(4-aminobenzyl)- hydantoin or 5-(4-aminobenzyl)-thiohydantoin as raw materials and characterized by IR, 1H NMR spectra and elementary analysis. The preliminary bioassay showed that these compounds exhibit certain selectively herbicidal activities with the 91%, 94% and 87% inhibition rates of 7l, 8o and 8p against B. campestris, 100%, 100% and 95% efficacy against B. campestris in a greenhouse test, respectively. 7a, 7b, 7c and 7d exhibited 74%, 79%, 79% and 71% inhibition rates against F. oxysporum, respectively.
Keywords: acylthiourea, hydantoin, thiohydantoin, herbicidal activity, fungicidal activity
1. Introduction
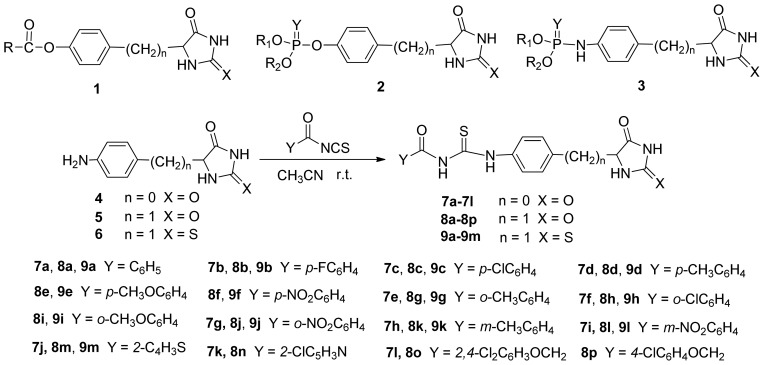
Hydantoin and thiohydantion are important core moiety in the design and synthesis of active molecules as well as natural products; these derivatives have not only been used in medicinal chemistry as anti-HSV, antidiabetic, HDL-cholesterol modulators, but also used as fungicides and herbicides in agrochemical research [1–6]. In addition, these derivatives are very useful building blocks for the synthesis of various heterocycles such as 5-arylidene derivatives of imidazoline-4-one, imidazothiazine, diazinone, and diazepinone [7–9]. In our laboratory, several types of hydantoin and thiohydantioin derivatives (1, 2, 3) were synthesized, and we showed that some of them exhibit good herbicidal, fungicidal and insecticidal activities [10–13]. Acylthioureas are the key structural motifs of numerous compounds that displayed a wide range of biological activity such as antimicrobial, antipathogenic, insecticidal, fungicidal, antitumor activities and influenza virus inhibitors [14–21]. Acylthioureas are widely used as the building blocks for the synthesis of various heterocycles such as thiazolidene-2-imine, imidazole-2-thione and 2-thioxo-4-imidazolidinone [22–33]. We could not find acylthiourea derivatives containing hydantoin and thiohydantion heterocycles in references. Continuing effort of our ongoing project aimed at looking for novel biologically active hydantoin and thiohydantion heterocyclic compounds [10–13], herein, we report a new type of acylthiourea derivatives containing hydantoin and thiohydantion heterocycles (Scheme 1) and their biological activities.
Scheme 1.
The synthetic route of new acylthiourea derivatives.
2. Results and Discussion
In our laboratory, we synthesized 5-aryl derivatives of hydantoin and thiohydantoin to develop the novel inhibitor of Adenylosuccinate Synthetase (AdSS) [10–13], which plays a key role in the two-step conversion of IMP to AMP in the de novo pathway of purine biosynthesis [34–36]. Some of the esters 1 were tested and showed strong herbicidal activities against Zea mays, Triticum aestivum and Arabidopsis thaliana, the further greenhouse test showed that compounds have 60%, 50% and 50% efficacy against Stellaria media, Echinochloa crus-galli and Setaria viridis at the dosage of 1000 g/ha when used as a pre-emergence treatment, respectively [13]. After that, the thiophosphates 2 and phosphoramidates were found to show weak herbicidal activities against Brasica campestris and Echinochloa crus-galli, while one of them exhibited excellent insecticidal activities against Myzus Persicae [11,12]. Then, phosphoramides 3 were further tested and showed increasing herbicidal activities against Brasica campestris as well as insecticidal activities against Myzus Persicae [37]. These results indicated that 5-(4-aminophenyl)-hydantoin, 5-(4-aminobenzyl)-hydantoin and 5-(4-aminobenzyl)-thiohydantoin were important moiety for these active compounds. On the other hand, acylthiourea derivatives have been widely used and synthesized in medicinal chemistry and agrochemical research [14–21] in recent years. Based on these characters, we combined the containing-hydantoin amino derivatives and acylthioureas into a molecule and designed the novel acylthiourea derivatives containing hydantoin and thiohydantoin heterocycles (7, 8, 9). The synthesis was carried out by the reaction of 5-(4-aminophenyl)-hydantoin, 5-(4-aminobenzyl)-hydantoin and 5-(4-aminobenzyl)-thiohydantoin with the freshly prepared acyl isothiocyanates in moderate to excellent yields.
The data in Table 1 showed that some of these compounds, such as 7l, 8o and 8p exhibit 91%, 94% and 87% inhibition rates against B. campestris, respectively, while they only have less than 25% inhibition rates against E. crus-galli at the concentration of 100 μg/mL. Further tests in a greenhouse were performed and the results in Table 2 showed that 7l, 8o and 8p inhibit the growth of B. campestris with 100%, 100% and 95% efficacy after the post-emergence treatments, and only 23%, 46% and 31% efficacy after the pre-emergence treatments at the dosage of 1000 g/ha. However they exhibited less than 15% efficacy against E. crus-galli after the post-emergence or pre-emergence treatments. These results indicated that these compounds exhibit certain selectively herbicidal activities.
Table 1.
The herbicidal activities (inhibition rate, %) of compounds 7, 8 and 9.
| Compd. | B. campestris | E. crus-galli | Compd. | B. campestris | E. crus-galli | Compd. | B. campestris | E. crus-galli |
|---|---|---|---|---|---|---|---|---|
| 7a | 20 | 10 | 8a | 2 | 15 | 9a | 3 | 10 |
| 7b | 38 | 0 | 8b | 12 | 15 | 9b | 44 | 10 |
| 7c | 0 | 15 | 8c | 16 | 20 | 9c | 40 | 5 |
| 7d | 39 | 0 | 8d | 14 | 5 | 9d | 33 | 15 |
| 7e | 29 | 10 | 8e | 0 | 5 | 9e | 0 | 15 |
| 7f | 0 | 5 | 8f | 25 | 0 | 9f | 0 | 10 |
| 7g | 49 | 10 | 8g | 4 | 10 | 9g | 6 | 25 |
| 7h | 23 | 15 | 8h | 10 | 10 | 9h | 29 | 25 |
| 7i | 0 | 0 | 8i | 11 | 15 | 9i | 18 | 25 |
| 7j | 14 | 5 | 8j | 25 | 0 | 9j | 6 | 10 |
| 7k | 6 | 20 | 8k | 0 | 10 | 9k | 0 | 5 |
| 7l | 91 | 0 | 8l | 0 | 0 | 9l | 0 | 10 |
| 8m | 0 | 0 | 9m | 33 | 5 | |||
| 8n | 7 | 10 | ||||||
| 8o | 94 | 10 | ||||||
| 8p | 87 | 10 |
Table 2.
The herbicidal activities (efficacy, %) of compounds 7l, 8o and 8p in greenhouse test.
| Compd. | B. campestris | E. crus-galli | ||
|---|---|---|---|---|
| Pre-emergence | Post-emergence | Pre-emergence | Post-emergence | |
| 7l | 23 | 100 | 0 | 0 |
| 8o | 46 | 100 | 0 | 10 |
| 8p | 31 | 95 | 10 | 15 |
The data in Table 3 showed that only 7a, 7b, 7c, 7d and 7h exhibit more than 70% inhibition rates against Fusarium oxysporum, and the others including 8 and 9 (data not shown in Table 3) have less than 55% inhibition rates against Alternaria solani, Botryospuaeria berengeriana, Cercospora arachidcola and Fusahum graminearum at the concentration of 100 μg/mL. The inhibition rates of 7a, 7b, 7c, 7d and 7h against F. oxysporum were 74%, 79%, 79%, 71% and 71%, respectively. They are weaker when compared to carbendazin (the positive control) against F. oxysporum and need further structure modification to increase the fungicidal activity.
Table 3.
The fungicidal activities (inhibition rate, %) of compounds 7 against several plant fungi.
| Compd. | F. oxysporum | A. Solani | B. berengeriana | C. arachidcola | F. graminearum |
|---|---|---|---|---|---|
| 7a | 74 | 20 | 32 | 0 | 25 |
| 7b | 79 | 24 | 23 | 0 | 54 |
| 7c | 79 | 24 | 23 | 0 | 40 |
| 7d | 71 | 21 | 7 | 6 | 44 |
| 7e | 59 | 17 | 30 | 6 | 25 |
| 7f | 24 | 27 | 28 | 6 | 10 |
| 7g | 24 | 27 | 37 | 6 | 18 |
| 7h | 71 | 3 | 20 | 0 | 40 |
| 7i | 24 | 13 | 25 | 6 | 16 |
| 7j | 24 | 27 | 37 | 6 | 18 |
| 7k | 41 | 20 | 23 | 0 | 40 |
| 7l | 15 | 17 | 40 | 0 | 12 |
| Carbendazin | 100 | 44 | 97 | 8 | 100 |
3. Experimental Section
3.1. General Information
All reactions were performed under room temperature with magnetic stirring. Unless otherwise stated, all reagents were purchased from commercial suppliers and used without further purification. Organic solutions were concentrated under reduced pressure using a rotary evaporator or oil pump. Melting points were measured on a Yanagimoto apparatus (Yanagimoto MFG Co., Kyoto, Japan) and uncorrected. Infrared spectra were recorded using a Shimadzu IR-435 instrument with KBr plates. 1H NMR spectra were obtained on Bruker DPX 300 spectrometer (Bruker Biospin Co., Stuttgart, Germany) with DMSO-d6 as a solvent and TMS as an internal standard. Elemental analysis was performed on a Vario EL instrument (Elementar Vario Micro Cube, Hanau, Germany).
3.2. Synthesis
3.2.1. Synthesis of 5-(4-Aminobenzyl)-, 5-(4-Aminophenyl)-Hydantoin (4 and 5), 5-(4-Aminobenzyl)-Thiohydantoin (6) and Acyl Isothiocyanate Derivatives
The synthesis of the intermediates 4, 5 and 6 were carried out according to the protocols in our previous paper and their spectral data were identical with that in the reference [37]. To a suspension of benzoic acid derivative (20 mmol) in 25 mL of CH2Cl2 in a 50 mL three-necked flask, 8 mL SOCl2 and a drop of N,N-dimethylformamide (DMF) were added. After stirring at room temperature for 3 h, the solution was evaporated. The resulting acyl chloride was dissolved in 15 mL of anhydrous acetonitrile and added to a solution of 20 mmol potassium thiocyanate in 25 mL of acetonitrile with two drops of polyethylene glycol-400 (PEG-400). After stirring at room temperature for 2 h, the mixture was filtered to give the acyl isothiocyanate derivatives, which were used without further purification [14,15].
3.2.2. General Procedure for the Synthesis of Compounds 7, 8 and 9
To a stirred solution of 20 mmol 5-(4-aminophenyl)-hydantoin (4), or 5-(4-aminobenzyl)-hydantoin (5) or 5-(4-aminobenzyl)-2-thiohydantoin (6) in 20 mL of anhydrous acetonitrile, the acyl isothiocyanate solution in acetonitrile freshly prepared were added dropwise at ambient temperature. The reaction was monitored by TLC. After leaving it overnight, the reaction was stopped and the product was filtered. The products were further purified by recrystallization using DMF-EtOH-H2O to afford the compounds 7, 8 and 9.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)benzamide 7a, white solid, yield 82%, m.p. 140–142 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.60 (s, 1H, NH), 11.58 (s, 1H, NH), 10.82 (s, 1H, NH), 8.43 (s, 1H, NH), 7.97 (d, J = 8.4 Hz, 2H, ArH), 7.73–7.52 (m, 5H, ArH), 7.38 (d, J = 8.4 Hz, 2H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3160, 3053, 1787, 1725, 1672, 1597 cm−1. Anal calcd. for C17H14N4O3S: C 57.62, H 3.98, N 15.81; Found: C 57.59, H 3.90, N 15.78.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-4-fluorobenzamide 7b, white solid, yield 70%, m.p. 146–148 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.55 (s, 1H, NH), 11.64 (s, 1H, NH), 10.83 (s, 1H, NH), 8.44 (s, 1H, NH), 8.09–8.03 (m, 2H, ArH), 7.70 (d, 2H, J = 8.4 Hz, ArH), 7.42–7.32 (m, 4H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3125, 3041, 1784, 1729, 1666, 1601 cm−1. Anal calcd. for C17H13FN4O3S: C 54.83, H 3.52, N 15.05; Found: C 54.80, H 3.50, N 15.01.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-4-chlorobenzamide 7c, white solid, yield 55%, m.p.230–232 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.50 (s, 1H, NH), 11.69 (s, 1H, NH), 10.81 (s, 1H, NH), 8.43 (s, 1H, NH), 7.99 (d, J = 7.4 Hz, 2H, ArH), 7.70 (d, J = 8.4 Hz, 2H, ArH), 7.62 (d, J = 7.4 Hz, 2H, ArH), 7.37 (d, J = 8.4 Hz, 2H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3145, 3035, 1783, 1728, 1670, 1593 cm−1; Anal calcd. for C17H13ClN4O3S: C 52.51, H 3.37, N 14.41; Found: C 52.53, H 3.41, N 14.36.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-4-methylbenzamide 7d, white solid, yield 53%, m.p.220–222 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.65 (s, 1H, NH), 11.50 (s, 1H, NH), 10.82 (s, 1H, NH), 8.44 (s, 1H, NH), 7.91 (d, J = 7.5 Hz, 2H, ArH), 7.71 (d, J = 8.4 Hz, 2H, ArH), 7.39–7.34 (m, 4H, ArH), 5.20 (s, 1H, CH), 2.40 (s, 3H, CH3); IR (KBr) ν: 3150, 3035, 1786, 1726, 1664, 1598 cm−1; Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.63, H 4.40, N 15.11.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-2-methylbenzamide 7e, white solid, yield 54%, m.p.138–140 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.62 (s, 1H, NH), 11.51 (s, 1H, NH), 10.82 (s, 1H, NH), 8.43 (s, 1H, NH), 7.84–7.70 (m, 4H, ArH), 7.47–7.37 (m, 4H, ArH), 5.20 (s, 1H, CH), 2.40 (s, 3H, CH3); IR (KBr) ν: 3170, 3045, 1789, 1715, 1677, 1598 cm−1; Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.62, H 4.55, N 15.35.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-2-chlorobenzamide 7f, white solid, yield 55%, m.p.222–224 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.34 (s, 1H, NH), 12.03 (s, 1H, NH), 10.83 (s, 1H, NH), 8.44 (s, 1H, NH), 7.72 (d, J = 7.4 Hz, 2H, ArH), 7.65–7.43 (m, 4H, ArH), 7.38 (d, J = 7.4 Hz, 2H, ArH), 5.21 (s, 1H, CH); IR (KBr) ν: 3301, 3056, 1783, 1715, 1694, 1593 cm−1; Anal calcd. for C17H13ClN4O3S: C 52.51, H 3.37, N 14.41; Found: C 52.60, H 3.41, N 14.44.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-2-nitrobenzamide 7g, white solid, yield 63%, m.p.234–236 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.25 (s, 1H, NH), 12.17 (s, 1H, NH), 10.84 (s, 1H, NH), 8.46 (s, 1H, NH), 8.23 (d, J = 8.1 Hz, 1H, ArH), 7.94–7.71 (m, 5H, ArH), 7.39 (d, J = 7.4 Hz, 2H, ArH), 5.22 (s, 1H, CH); IR (KBr) ν: 3146, 3034, 1764, 1716, 1690, 1593 cm−1; Anal calcd. for C17H13N5O5S: C 51.12, H 3.28, N 17.54; Found: C 51.14, H 3.21, N 17.25.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-3-methylbenzamide 7h, white solid, yield 54%, m.p.158–160 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.62 (s, 1H, NH), 11.54 (s, 1H, NH), 10.83 (s, 1H, NH), 8.45 (s, 1H, NH), 7.83–7.70 (m, 4H, ArH), 7.49–7.36 (m, 4H, ArH), 5.21 (s, 1H, CH), 2.39 (s, 3H, CH3); IR (KBr) ν: 3210, 3047, 1769, 1726, 1668, 1600 cm−1; Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.64, H 4.40, N 15.16.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-3-nitrobenzamide 7i, white solid, yield 52%, m.p.162–164 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.42 (s, 1H, NH), 12.04 (s, 1H, NH), 10.83 (s, 1H, NH), 8.78 (d, J = 2.1 Hz, 1H, ArH), 8.51–8.47 (m, 1H, ArH), 8.45 (s, 1H, NH), 8.37 (dd, J = 7.8, 2.4Hz, 1H, ArH), 7.84 (t, J = 7.8 Hz, 1H, ArH), 7.72 (d, J = 8.4 Hz, 2H, ArH), 7.39 (d, J = 8.4 Hz, 2H, ArH), 5.21 (s, 1H, CH); IR (KBr) ν: 3229, 3047, 1776, 1723, 1677, 1600 cm−1; Anal calcd. for C17H13N5O5S: C 51.12, H 3.28, N 17.54; Found: C 51.02, H 3.30, N 17.54.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)thiophene-2-carboxamide 7j, white solid, yield 80%, m.p. 226–228 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.44 (s, 1H, NH), 11.64 (s, 1H, NH), 10.81 (s, 1H, NH), 8.43 (s, 1H, NH), 8.38 (d, J = 3.8 Hz, 1H, ThH), 8.05 (d, J = 5.0 Hz, 1H, ThH), 7.70 (d, J = 7.4 Hz, 2H, ArH), 7.36 (d, J = 7.4 Hz, 2H, ArH), 7.26 (dd, J = 3.8, 5.0 Hz, 1H, ThH), 5.20 (s, 1H, CH); IR (KBr) ν: 3138, 3043, 1778, 1722, 1657, 1592 cm−1. Anal calcd. for C15H12N4O3S2: C 49.99, H 3.36, N 15.55; Found: C 49.97, H 3.39, N 15.56.
2-Chloro-N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)isonicotinamide 7k, white solid, yield 73%, m.p. 212–214 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.25 (s, 1H, NH), 11.96 (s, 1H, NH), 10.81 (s, 1H, NH), 8.62 (d, J = 5.4 Hz, 1H, PyH), 8.43 (s, 1H, NH), 7.99 (s, 1H, PyH), 7.84 (dd, J = 1.2, 5.4 Hz, 1H, PyH), 7.70 (d, J = 7.4 Hz, 2H, ArH), 7.39 (d, J = 7.4 Hz, 2H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3150, 3042, 1765, 1719, 1666, 1593 cm−1. Anal calcd. for C16H12ClN5O3S: C 49.30, H 3.10, N 17.97; Found: C 49.26, H 3.08, N 17.90.
2-(2,4-dichlorophenoxy)-N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)acetamide 7l, white solid, yield 59%, m.p. 258–260 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 11.85 (s, 1H, NH), 10.77 (s, 1H, NH), 10.24 (s, 1H, NH), 8.37 (s, 1H, NH), 7.63–7.60 (m, 3H, ArH), 7.37 (dd, J = 2.4, 8.4 Hz, 1H, ArH), 7.27 (d, J = 7.5 Hz, 2H, ArH), 7.09 (d, J = 8.4 Hz, 1H, ArH), 5.11 (s, 1H, CH), 4.86 (s, 2H, CH2); IR (KBr) ν: 3288, 3060, 1780, 1740, 1682, 1600 cm−1. Anal calcd. for C18H14Cl2N4O4S: C 47.69, H 3.11, N 12.36; Found: C 47.58, H 3.12, N 12.34.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)benzamide 8a, white solid, yield 93%, m.p. 240–242 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.61 (s, 1H, NH), 11.53 (s, 1H, NH), 10.48 (s, 1H, NH), 7.99–7.96 (m, 3H, ArH+NH), 7.70–7.64 (m, 3H, ArH), 7.57–7.52 (m, 2H, ArH),7.23 (d, J = 7.8 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.99–2.89 (m, 2H, CH2); IR (KBr) ν: 3172, 3064, 1766, 1726, 1668, 1597 cm−1. Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.98, H 4.30, N 15.25.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-flurobenzamide 8b, white solid, yield 83%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.56 (s, 1H, NH), 11.58 (s, 1H, NH), 10.48(s, 1H, NH), 8.09–8.03(m, 2H, ArH), 7.96 (s, 1H, NH), 7.64 (d, J = 8.0 Hz, 2H, ArH), 7.40–7.35 (m, 2H, ArH), 7.23 (d, J = 8.0 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH,), 2.97–2.94 (m, 2H, CH2); IR (KBr) ν: 3178, 3060, 1762, 1727, 1669, 1600 cm−1. Anal calcd for C18H15FN4O3S: C 55.95, H 3.91, N 14.50; Found: C 55.86, H 4.01, N 14.51.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-chlorobenzamide 8c, white solid, yield 63%, m.p. 232–234 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.51 (s, 1H, NH), 11.64 (s, 1H, NH), 10.49 (s, 1H, NH), 7.98–7.93 (m, 3H, ArH + NH), 7.66–7.56 (m, 4H, ArH), 7.23 (d, 2H, J = 8.4 Hz, ArH), 4.37–4.33 (m, 1H, CH), 2.95–2.93 (m, 2H, CH2); IR (KBr) ν: 3155, 3034, 1758, 1726, 1666, 1594 cm−1. Anal calcd. for C18H15ClN4O3S: C 53.67, H 3.75, N 13.91; Found: C 53.78, H 3.79, N 13.90.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methylbenzamide 8d, white solid, yield 66%, m.p. 228–230 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.67 (s, 1H, NH), 11.43 (s, 1H, NH), 10.48 (s, 1H, NH), 7.96 (s, 1H, NH), 7.91 (d, J = 8.1 Hz, 2H, ArH), 7.65 (d, J = 8.4 Hz, 2H, ArH), 7.35 (d, J = 8.1 Hz, 2H, ArH), 7.23 (d, J = 8.4 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.96–2.93 (m, 2H, CH2), 2.37 (s, 3H, CH3); IR (KBr) ν: 3171, 3067, 1767, 1730, 1668, 1598 cm−1. Anal calcd. for C19H18N4O3S: C 59.67, H 4.74, N 14.65; Found: C 59.58, H 4.67, N 14.48.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methoxybenzamide 8e, white solid, yield 69%, m.p. 224–226 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.74 (s, 1H, NH), 11.39 (s, 1H, NH), 10.50 (s, 1H, NH), 8.02 (d, J = 8.7 Hz, 2H, ArH,), 7.99 (s, 1H, NH),7.64 (d, J = 8.4 Hz, 2H, ArH), 7.22 (d, J = 8.4 Hz, 2H, ArH), 7.07 (d, J = 8.7 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 3.86 (s, 3H, OCH3), 2.95–2.93 (m, 2H, CH2); IR (KBr) ν: 3176, 3051, 1766, 1726, 1667, 1596 cm−1. Anal calcd. for C19H18N4O4S: C 57.27, H 4.55, N 14.16; Found: C 57.22, H 4.55, N 14.03.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-nitrobenzamide 8f, yellow solid, yield 82%, m.p. 226–228 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.75 (s, 1H, NH), 11.47(s, 1H, NH), 10.44 (s, 1H, NH), 8.38–7.93 (m, 5H, ArH + NH), 7.35–7.20 (m, 4H, ArH), 4.38–4.33 (m, 1H, CH), 2.97–2.92 (m, 2H, CH2); IR (KBr) ν: 3112, 3047, 1768, 1728, 1667, 1592 cm−1. Anal calcd. for C18H15N5O5S: C 52.30, H 3.66, N 16.94; Found: C 52.23, H 3.68, N 16.85.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methylbenzamide 8g, white solid, yield 56%, m.p. 212–214 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.56 (s, 1H, NH), 11.68 (s, 1H, NH), 10.48 (s, 1H, NH), 7.96 (s, 1H, NH), 7.67 (d, J = 8.4 Hz, 2H, ArH), 7.51~7.22 (m, 6H, ArH), 4.37–4.34 (m, 1H, CH), 2.96–2.91 (m, 2H, CH2), 2.42 (s, 3H, CH3); IR (KBr) ν: 3181, 3066, 1767, 1722, 1673, 1595 cm−1. Anal calcd. for C19H18N4O3S: C 59.67, H 4.74, N 14.65; Found: C 59.69, H 4.75, N 14.74.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-chlorobenzamide 8h, white solid, yield 60%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.36 (s, 1H, NH), 11.98 (s, 1H, NH), 10.49 (s, 1H, NH), 7.97 (s, 1H, NH), 7.67–7.43 (m, 6H, ArH), 7.23 (d, J = 8.5 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.99–2.93 (m, 2H, CH2); IR (KBr) ν: 3194, 3060, 1766, 1717, 1679, 1592, 1537 cm−1. Anal calcd. for C18H15ClN4O3S: C 53.67, H 3.75, N 13.91; Found: C 53.60, H 3.75, N 13.86.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methoxybenzamide 8i, white solid, yield 60%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.57 (s 1H, NH), 12.21 (s, 1H, NH), 10.49 (s, 1H, NH), 7.97 (s, 1H, NH), 7.94–7.91 (m, 1H, ArH), 7.70–7.64 (m, 3H, ArH), 7.31–7.14 (m, 4H, ArH), 4.37–4.34 (m, 1H, CH), 4.01 (s, 3H, OCH3), 2.96–2.94 (m, 2H, CH2); IR (KBr) ν: 3219, 3036, 1769, 1716, 1665, 1595 cm−1. Anal calcd. for C19H18N4O4S: C 57.27, H 4.55, N 14.16; Found: C 57.51, H 4.53, N 14.14.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-nitrobenzamide 8j, yellow solid, yield 50%, m.p. 222–224 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.27 (s, 1H, NH), 12.12 (s, 1H, NH), 10.49 (s, 1H, NH), 8.24–8.21 (m, 1H, ArH), 7.96 (s, 1H, NH), 7.94–7.77 (m, 3H, ArH), 7.66 (d, 2H, J = 8.4 Hz, ArH), 7.24 (d, 2H, J = 8.4 Hz, ArH), 4.38–4.34 (m, 1H, CH), 2.97–2.95 (m, 2H, CH2); IR (KBr) ν: 3219, 3036, 1769, 1716, 1665, 1595 cm−1. Anal calcd. for C18H15N5O5S: C 52.30, H 3.66, N 16.94; Found: C 52.25, H 3.76, N 16.84.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-methylbenzamide 8k, white solid, yield 64%, m.p. 220–221 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.64 (s, 1H, NH), 11.48 (s, 1H, NH), 10.49 (s, 1H, NH), 7.97 (s, 1H, NH), 7.83–7.63 (m, 4H, ArH), 7.49–7.40 (m, 2H, ArH), 7.23 (d, J = 8.4 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.97–2.92 (m, 2H, CH2), 2.36 (s, 3H, CH3); IR (KBr) ν: 3169, 3056, 1767, 1726, 1668, 1596 cm−1. Anal calcd. for C19H18N4O3S: C 59.67, H 4.74, N 14.65; Found: C 59.62, H 4.75, N 14.54.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-nitrobenzamide 8l, little yellow solid, yield 79%, m.p. 168–170 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.43 (s, 1H, NH), 12.01 (s, 1H, NH), 10.49 (s, 1H, NH), 8.78 (s, 1H, ArH), 8.51–8.36 (m, 2H, ArH), 7.99 (s, 1H, NH), 7.86–7.68 (m, 3H, ArH), 7.24 (d, J = 8.4 Hz, 2H, ArH), 4.38–4.34 (m, 1H, CH), 2.97–2.91 (m, 2H, CH2); IR (KBr) ν: 3180, 3056, 1770, 1719, 1669, 1602 cm−1. Anal calcd. for C18H15N5O5S: C 52.30, H 3.66, N 16.94; Found: C 52.32, H 3.60, N 16.84.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)thiophene-2-carboxamide 8m, white solid, yield 82%, m.p. 244–246 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.46 (s, 1H, NH), 11.58 (s, 1H, NH), 10.48 (s, 1H, NH), 8.39–8.37 (m, 1H, ThH), 8.06–8.04 (m, 1H, ThH), 7.96 (s, 1H, NH), 7.63 (d, J = 8.5 Hz, 2H, ArH), 7.27–7.21 (m, 3H, ArH + ThH), 4.37–4.32 (m, 1H, CH), 2.96–2.91 (m, 2H, CH2); IR (KBr) ν: 3171, 3061, 1762, 1716, 1654, 1596 cm−1. Anal calcd. for C16H14N4O3S2: C 51.32, H 3.77, N 14.96; Found: C 51.60, H 3.81, N 14.96.
2-Chloro-N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)isonicotinamide 8n, white solid, yield 80%, m.p. 202–204 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.25 (s, 1H, NH), 11.92 (s, 1H, NH), 10.48 (s, 1H, NH), 8.61 (d, J = 5.1 Hz, 1H, PyH), 7.99–7.82 (m, 3H, PyH + NH), 7.63 (d, J = 8.3 Hz, 2H, ArH), 7.24 (d, J = 8.3 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.99–2.89 (m, 2H, CH2); IR (KBr) ν: 3150, 3042, 1765, 1719, 1666, 1593 cm−1. Anal calcd. for C17H14ClN5O3S: C 50.56, H 3.49, N 17.34; Found: C 50.70, H 3.57, N 17.27.
2-(2,4-dichlorophenoxy)-N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)acetamide 8o, white solid, yield 71%, m.p. 262–264 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 11.95 (s, 1H, NH), 10.41 (s, 1H, NH), 10.14 (s, 1H, NH), 7.91 (s, 1H, NH), 7.50 (d, J = 8.5 Hz, 2H, ArH), 7.40~7.08 (m, 4H, ArH), 4.83 (s, 2H, CH2), 4.36–4.30 (m, 1H, CH), 2.90–2.81 (m, 2H, CH2); IR (KBr) ν: 3159, 3048, 1762, 1732, 1669, 1597 cm−1. Anal calcd. for C19H16Cl2N4O4S: C 48.83, H 3.45, N 11.99; Found: C 48.72, H 3.55, N 11.92.
2-(4-dichlorophenoxy)-N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)acetamide 8p, white solid, yield 70%, m.p. 210–212 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 11.94 (s, 1H, NH), 10.42 (s, 1H, NH), 10.06 (s, 1H, NH), 7.92 (s, 1H, NH), 7.52 (d, J = 8.5 Hz, 2H, ArH), 7.39~7.33 (m, 2H, ArH), 7.14~7.10 (m, 2H, ArH), 7.04~6.99 (m, 2H, ArH), 4.69 (s, 2H, CH2), 4.34–4.28 (m, 1H, CH), 2.93–2.85 (m, 2H, CH2); IR (KBr) ν: 3116, 3043, 1769, 1709, 1687, 1598 cm−1. Anal calcd. for C19H17ClN4O4S: C 52.72, H 3.96, N 12.94; Found: C 52.62, H 3.86, N 12.90.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)benzamide 9a little yellow solid, yield 75%, m.p. 240–242 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.62 (s, 1H, NH), 11.51 (s, 2H, NH), 10.09 (s, 1H, NH), 7.99~7.96 (m, 2H, ArH), 7.69~7.52 (m, 5H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.96 (m, 2H, CH2); IR (KBr) ν: 3185, 3081, 1772, 1742, 1655, 1597 cm−1. Anal calcd. for C18H16N4O2S2: C 56.23, H 4.19, N 14.57; Found: C 56.16, H 4.21, N 14.55.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-flurobenzamide 9b little yellow solid, yield 56%, m.p. 238–240 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.57 (s, 1H, NH), 11.59 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 8.09~8.04 (m, 2H, ArH), 7.67~7.64 (m, 2H, ArH), 7.41~7.35 (m, 2H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.95 (m, 2H, CH2); IR (KBr) ν: 3309, 3047, 1775, 1754, 1658, 1593 cm−1. Anal calcd. for C18H15FN4O2S2: C 53.72, H 3.76, N 13.92; Found: C 53.53, H 3.88, N 13.91.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-chlorobenzamide 9c little yellow solid, yield 58%, m.p. 240–242 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.52 (s, 1H, NH), 11.64 (s, 1H, NH), 11.50 (s, 1H, NH), 10.10 (s, 1H, NH), 8.00~7.97 (m, 2H, ArH), 7.67~7.60 (m, 4H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.96 (m, 2H, CH2); IR (KBr) ν: 3158, 3077, 1776, 1732, 1617, 1597 cm−1. Anal calcd. for C18H15ClN4O2S2: C 51.61, H 3.61, N 13.37; Found: C 51.46, H 3.72, N 13.47.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methylbenzamide 9d little yellow solid, yield 70%, m.p. 237–238 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.68 (s, 1H, NH), 11.51 (s, 1H, NH), 11.43 (s, 1H, NH), 10.10 (s, 1H, NH), 7.92~7.89 (m, 2H, ArH), 7.67~7.64 (m, 2H, ArH), 7.36~7.33 (m, 2H, ArH), 7.24~7.21 (m, 2H, ArH), 4.59–4.56 (m, 1H, CH), 3.01–2.95 (m, 2H, CH2), 2.40 (s, 3H, CH3); IR (KBr) ν: 3284, 3047, 1773, 1752, 1655, 1596 cm−1. Anal calcd. for C19H18N4O2S2: C 57.27, H 4.55, N 14.06; Found: C 57.20, H 4.65, N 14.11.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methoxybenzamide 9e little yellow solid, yield 46%, m.p. 232–234 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.74 (s, 1H, NH), 11.50 (s, 1H, NH), 11.35 (s, 1H, NH), 10.10 (s, 1H, NH), 8.03~8.00 (m, 2H, ArH), 7.67~7.64 (m, 2H, ArH), 7.23~7.20 (m, 2H, ArH), 7.08~7.05 (m, 2H, ArH), 4.59–4.56 (m, 1H, CH), 3.86 (s, 3H, OCH3), 3.01–2.96 (m, 2H, CH2); IR (KBr) ν: 3306, 3051, 1774, 1752, 1668, 1596 cm−1. Anal calcd. for C19H18N4O3S2: C 55.05, H 4.38, N 13.52; Found: C 54.82, H 4.45, N 13.49.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-nitrobenzamide 9f little yellow solid, yield 85%, m.p. 244–245 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.40 (s, 1H, NH), 11.91 (s, 1H, NH), 11.50 (s, 1H, NH), 10.10 (s, 1H, NH), 8.36~8.33 (m, 2H, ArH), 8.18~8.15 (m, 2H, ArH), 7.68~7.64 (m, 2H, ArH), 7.25~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.05–2.97 (m, 2H, CH2); IR (KBr) ν: 3202, 3060, 1775, 1746, 1672, 1594 cm−1. Anal calcd. for C18H15N5O4S2: C 50.34, H 3.52, N 16.31; Found: C 50.18, H 3.67, N 16.24.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methylbenzamide 9g little yellow solid, yield 63%, m.p. 220–222 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.58 (s, 1H, NH), 11.68 (s, 1H, NH), 11.50 (s, 1H, NH), 10.10 (s, 1H, NH), 7.70~7.66 (m, 2H, ArH), 7.51~7.41 (m, 2H, ArH), 7.32~7.27 (m, 2H, ArH), 7.24~7.20 (m, 2H, ArH), 4.59–4.56 (m, 1H, CH), 3.02–2.95 (m, 2H, CH2), 2.42 (s, 3H, CH3); IR (KBr) ν: 3187, 3094, 1774, 1745, 1676, 1587 cm−1. Anal calcd. for C19H18N4O2S2: C 57.27, H 4.55, N 14.06; Found: C 57.14, H 4.62, N 14.35.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-chlorobenzamide 9h little yellow solid, yield 53%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.38 (s, 1H, NH), 11.98 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 7.68~7.43 (m, 6H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.02–2.96 (m, 2H, CH2); IR (KBr) ν: 3224, 3062, 1776, 1745, 1685, 1593 cm−1. Anal calcd. for C18H15ClN4O2S2: C 51.61, H 3.61, N 13.37; Found: C 51.44, H 3.70, N 13.36.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methoxybenzamide 9i little yellow solid, yield 69%, m.p. 244–246 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.58 (s, 1H, NH), 11.50 (s, 1H, NH), 11.20 (s, 1H, NH), 10.09 (s, 1H, NH), 7.94~7.91 (m, 1H, ArH), 7.70~7.64 (m, 3H, ArH), 7.31~7.15 (m, 4H, ArH), 4.59–4.56 (m, 1H, CH), 4.01 (s, 3H, OCH3), 3.02–2.94 (m, 2H, CH2); IR (KBr) ν: 3159, 3084, 1775, 1752, 1651, 1595 cm−1. Anal calcd. for C19H18N4O3S2: C 55.05, H 4.38, N 13.52; Found: C 54.92, H 4.47, N 13.63.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-nitrobenzamide 9j little yellow solid, yield 54%, m.p. 202–204 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.29 (s, 1H, NH), 12.12 (s, 1H, NH), 11.51 (s, 1H, NH), 10.11 (s, 1H, NH), 8.24~8.21 (m, 1H, ArH), 7.94~7.88 (m, 1H, ArH), 7.81~7.66 (m, 4H, ArH), 7.25~7.22 (m, 2H, ArH), 4.61–4.56 (m, 1H, CH), 3.02–2.96 (m, 2H, CH2); IR (KBr) ν: 3166, 3030, 1775, 1740, 1693, 1598 cm−1. Anal calcd. for C18H15N5O4S2·1/3H2O: C 49.64, H 3.63, N 16.08; Found: C 49.67, H 3.68, N 16.19.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-methylbenzamide 9k little yellow solid, yield 88%, m.p. 234–236 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.65 (s, 1H, NH), 11.50 (s, 1H, NH), 11.47 (s, 1H, NH), 10.10 (s, 1H, NH), 7.83~7.65 (m, 4H, ArH), 7.49~7.40 (m, 2H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.94 (m, 2H, CH2), 2.39 (s, 3H, CH3); IR (KBr) ν: 3176, 3051, 1776, 1751, 1657, 1597 cm−1. Anal calcd. for C19H18N4O2S2: C 56.42, H 4.65, N 13.85; Found: C 56.50, H 4.63, N 14.11.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-nitrobenzamide 9l little yellow solid, yield 73%, m.p. 242–243 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.43 (s, 1H, NH), 11.97 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 8.78~8.76 (m, 1H, ArH), 8.50~8.47 (m, 1H, ArH), 8.38~8.35 (m, 1H, ArH), 7.86~7.81 (m, 1H, ArH), 7.68~7.65 (m, 2H, ArH), 7.25~7.22 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.02–2.96 (m, 2H, CH2); IR (KBr) ν: 3172, 3090, 1774, 1738, 1692, 1604 cm−1. Anal calcd. for C18H15N5O4S2: C 50.34, H 3.52, N 16.31; Found: C 49.94, H 3.68, N 16.26.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)thiophene-2-carboxamide 9m little yellow solid, yield 68%, m.p. 242–244 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.48 (s, 1H, NH), 11.58 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 8.39–8.37 (m, 1H, ThH), 8.06–8.04 (m, 1H, ThH), 7.66~7.63 (m, 2H, ArH), 7.27~7.20 (m, 3H, ArH + ThH), 4.60–4.55 (m, 1H, CH), 3.01–2.95 (m, 2H, CH2); IR (KBr) ν: 3176, 3107, 1776, 1737, 1668, 1599 cm−1. Anal calcd. for C16H14N4O2S3: C 49.21, H 3.61, N 14.35; Found: C 49.16, H 3.72, N 14.52.
3.3. Bioassay of Herbicidal and Fungicidal Activity
The preliminary herbicidal activities of compounds 7–9 against B. campestris and E. crus-galli were assayed using the protocols in the references [11–13]. The preliminary fungicidal activities of compounds 7–9 against F. oxysporurm, A. solani, B. berengeriana, C. arachidcola and F. graminearum were evaluated using methods in the references [7,10] by the mycelium growth rate test [38]. The culture was incubated at 25 ± 0.5 ºC. Three replicates were performed and the mean measurements were calculated from the three replicates.
The greenhouse test was performed using the procedures in reference [39] according to the pre-emergence and post-emergence applications. The formulations were sprayed before the seedlings were planted in a pot or the formulations were sprayed during one to two-leaf appeared after the seedlings were planted in a pot. Then they were kept in the greenhouse to observe the root and stem growth of the plants in three weeks, and the inhibition rates of compounds were obtained comparison the fresh plant weights with the blank control. Three replicates were performed and the mean measurements were calculated from the three replicates.
4. Conclusions
The novel acylthiourea derivatives with hydantoin or thiohydantoin were synthesized in moderate to excellent yields using 5-(4-aminophenyl)- and 5-(4-aminobenzyl)-hydantoin or 5-(4-aminobenzyl)- thiohydantoin as raw materials and characterized by IR, 1H NMR spectra and elementary analysis. The preliminary bioassay showed that these compounds exhibit some herbicidal selectivity with the 91%, 94% and 87% inhibition rates of 7l, 8o and 8p against B. campestris, and 100%, 100% and 95% efficacy against B. campestris in a greenhouse test, respectively. Compounds 7a, 7b, 7c and 7d exhibited 74%, 79%, 79% and 71% inhibition rates against F. oxysporum, respectively.
Acknowledgments
This project was founded by National Natural Science Foundation of China (Nos. 21172254 and 20772150), the 12th Five-year National Key Technologies R & D Program of China (No. 2011BAE06B03) and National Key Laboratory of Elemento-Organic Chemistry in Nankai University (201003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Li K., Shi D.Q. Synthesis and herbicidal activity of 3-aryl-1-[2-(aryloxy)propanoyl]imidazolidine- 2,4-diones. J. Heterocycl. Chem. 2009;46:544–547. [Google Scholar]
- 2.Thenmozhiyal J.C., Wong P.T.-H., Chui W.K. Anticonvulsant activity of phenylmethylene-hydantoins: A structure-activity relationship study. J. Med. Chem. 2004;47:1527–1535. doi: 10.1021/jm030450c. [DOI] [PubMed] [Google Scholar]
- 3.Elokdah H., Abou-Gharbia M., Hennan J.K., Mcfarlane G., Mugford C.P., Krishnamurthy G., Crandall D.L. Tiplaxtinin, a novel orally efficacious inhibitor of plasminogen activator inhibitor-1: Design, synthesis, and preclinical characterization. J. Med. Chem. 2004;47:3491–3494. doi: 10.1021/jm049766q. [DOI] [PubMed] [Google Scholar]
- 4.Brady S.F., Bauer J.D., Clarke-Pearson M.F., Daniels R. Natural products from isnA-containing biosynthetic gene clusters recovered from the genomes of cultured and uncultured bacteria. J. Am. Chem. Soc. 2007;129:12102–12103. doi: 10.1021/ja075492v. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima M., Itoi K., Takamatsu Y., Kinoshita T., Okazaki T., Kawakubo K., Shindo M., Honma T., Tohjigamori M., Haneishi T. Hydantocidin: A new compound with herbicidal activity from Streptomyes hygroscopicus. J. Antibiot. 1991;44:293–300. doi: 10.7164/antibiotics.44.293. [DOI] [PubMed] [Google Scholar]
- 6.Zhao B.G., Du H.F., Shi Y.A. A Cu(I)-catalyzed C-H α-Amination of esters. Direct synthesis of hydantoins. J. Am. Chem. Soc. 2008;130:7220–7221. doi: 10.1021/ja802242h. [DOI] [PubMed] [Google Scholar]
- 7.Lei J.P., Han J.T., Xu Z.H., Dong H.B., Wang M.A. Synthesis and fungicidal activity of 5-cyclohexylidene-2-aminoimidazolin-4-one derivatives. Chin. J. Org. Chem. 2012;32:1993–1998. [Google Scholar]
- 8.Kiec-Kononowicz K., Szymanska E. Antimycobacterial activity of 5-arylidene derivatives of hydantoin. Farmaco. 2002;57:909–916. doi: 10.1016/s0014-827x(02)01292-2. [DOI] [PubMed] [Google Scholar]
- 9.Kiec-Kononowicz K., Karolak-Wojicechowska J., Muller C.E., Schumacher B., Pekela E., Szymanska E. Imidazo-thiazine, -diazinone and -diazepinone derivatives. Synthesis, structure and benzodiazepine receptor binding. Eur. J. Med. Chem. 2001;36:407–419. doi: 10.1016/s0223-5234(01)01239-9. [DOI] [PubMed] [Google Scholar]
- 10.Han J.T., Dong H.B., Xu Z.H., Lei J.P., Wang M.A. Facile synthesis of 5-arylidene thiohydantoin by sequential sulfonylation/desulfination reaction. Int. J. Mol. Sci. 2013;14:12484–12495. doi: 10.3390/ijms140612484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J.T., Wang J.M., Dong H.B., Xu Z.H., Liu B., Wang M.A. Synthesis and biological activity of novel phosphoramidate with hydantoin. Chin. J. Org. Chem. 2013;33:596–601. [Google Scholar]
- 12.Xu Z.H., Wang J.M., Han J.T., Liu B., Wang M.A. Synthesis and biological activity of novel (thio)phosphates with hydantoin. Chin. J. Org. Chem. 2012;32:2134–2140. [Google Scholar]
- 13.Han J.T., Dong H.B., Wang J.M., Lei J.P., Wang M.A., Fang J.X. Synthesis and herbicidal activity of 5-(4-hydroxybenzyl)-2-thioxoimidazolidin-4-one esters. Molecules. 2011;16:2833–2845. doi: 10.3390/molecules16042833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J.F., Liu C., Ma Y., Wang B.L., Xiong L.X., Yu S.J., Li Z.M. Synthesis of novel 3-chloropyridin-2-yl-pyrazole derivatives and their insecticidal, fungicidal activities and QSAR Study. Lett. Drug Des. Discov. 2013;10:497–506. [Google Scholar]
- 15.Zhang J.F., Xu J.Y., Wang B.L., Li Y.X., Xiong L.X., Li Y.Q., Ma Y., Li Z.M. Synthesis and insecticidal activities of novel anthranilic diamides containing acylthiourea and acylurea. J. Agric. Food Chem. 2012;60:7565–7572. doi: 10.1021/jf302446c. [DOI] [PubMed] [Google Scholar]
- 16.Limban C., Marutescu L., Chifiriuc M.C. Synthesis, spectroscopic properties and antipatho-genic activity of new thiourea derivatives. Molecules. 2011;16:7593–7607. doi: 10.3390/molecules16097593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limban C., Missir A.V., Chirita I.C., Nitulescu G.M., Caproiu M.T., Chifiriuc M.C., Israil A.M. Synthesis and antimicrobial properties of new 2-((4-ethylphenoxy)methyl)benzoylthioureas. Chem. Pap. 2011;65:60–69. [Google Scholar]
- 18.Rao X.P., Wu Y., Song Z.Q., Shang S.B., Wang Z.D. Synthesis and antitumor activities of unsymmetrically disubstitutedacylthioureas fused with hydrophenanthrene structure. Med. Chem. Res. 2011;20:333–338. [Google Scholar]
- 19.Sun R.F., Zhang Y.L., Chen L., Li Y.Q., Li Q.S., Song H.B., Huang R.Q., Bi F.C., Wang Q.M. Design, synthesis, bioactivity, and structure−activity relationship (SAR) studies of novel benzoylphenylureas containing oxime ether group. J. Agric. Food Chem. 2008;56:11376–11391. doi: 10.1021/jf801901h. [DOI] [PubMed] [Google Scholar]
- 20.Hallur G., Jimeno A., Dalrymple S., Zhu T., Jung M.K., Hidalgo M., Isaacs J.T., Sukumar S., Hamel E., Khan S.R. Benzoylphenylurea sulfur analogues with potent antitumor activity. J. Med. Chem. 2006;49:2357–2360. doi: 10.1021/jm051261s. [DOI] [PubMed] [Google Scholar]
- 21.Sun C.W., Huang H., Feng M.Q., Shi X.L., Zhang X.D., Zhou P. A novel class of potent influenza virus inhibitors: Polysubstitutedacylthiourea and its fused heterocycle derivatives. Bioorg. Med. Chem. Lett. 2006;16:162–166. doi: 10.1016/j.bmcl.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Junejo J., Ghosh S.K., Shaikh M., Gahtori P., Singh U.P. Facile synthesis, antibacterial activity and molecular properties prediction of some new 1,3-dihydroimidazol-2-thione derivatives. Lett. Drug Des. Discov. 2011;8:763–768. [Google Scholar]
- 23.Aamer S., Mahira B. Synthesis and bioactivity of some new 1-tolyl-3-aryl-4-Methyl imidazole-2-thiones. Med. Chem. Res. 2007;16:143–154. [Google Scholar]
- 24.Wang X.C., Wang F., Quan Z.J., Wang M.G., Li Z. An efficient and clean synthesis of 1-aroyl-3-aryl-4-substituted imidazole-2-thiones in water. J. Chem. Res. 2005;11:689–690. [Google Scholar]
- 25.Zeng R.S., Zou J.P., Zhi S.J., Chen J., Shen Q. Novel synthesis of 1-aroyl-3-aryl-4-substituted imidazole-2-thiones. Org. Lett. 2003;5:1657–659. doi: 10.1021/ol030024l. [DOI] [PubMed] [Google Scholar]
- 26.Majid M.H., Setareh M. An efficient synthesis of thiazol-2-imine derivatives via a one-pot, three-component reaction. Tetrahedron Lett. 2012;53:392–394. [Google Scholar]
- 27.Wang L., Pan X.Q., Rao W.D., Zou J.P. Synthesis of 1,3-disubstituted-2-thioxo-4- imidazolidinones. Chin. J. Org. Chem. 2011;31:1939–1942. [Google Scholar]
- 28.Aamer S., Uzma S., Abdul H., Faiza K. Synthesis and antimicrobial activity of some novel 2-(substituted fluorobenzoylimino)-3-(substituted fluorophenyl)-4-methyl-1,3-thiazolines. J. Fluorine Chem. 2010;131:333–339. [Google Scholar]
- 29.Murru S., Singh C.B., Kavala V., Patel B.K. A convenient one-pot synthesis of thiazol-2- imines: Application in the construction of pifithrin analogues. Tetrahedron. 2008;64:1931–1942. [Google Scholar]
- 30.Aamer S., Sabah Z., Michael B. Synthesis and crystal structure of some novel 2-aroylimino-3- aryl-4-phenyl-1,3-thiazolines. Synth. Commun. 2008;38:2185–2199. [Google Scholar]
- 31.Singh C.B., Siva M., Veerababurao K., Bhisma K.P. 3-Aryl-1-benzoylthioureas with α-bromoketones in water form 2-N-benzoyl-3-arylthiazol-2(3H)-imines, not 3-aryl-1-benzoylimidazoline- 2-thiones. J. Chem. Res. 2007;3:136–137. [Google Scholar]
- 32.Aamer S., Madood P. Synthesis, crystal structure of Some new 2-(4-methylbenzoyl-imino)-3- aryl-4-methyl-1,3-thiazolines. J. Heterocycl. Chem. 2006;43:1027–1030. [Google Scholar]
- 33.Singh C.B., Siva M., Veerababurao K., Bhisma K.P. It is “thiazolidene-2-imine” and not imi-dazole-2-thione as the reaction product of 1-benzoyl-3-phenylthiourea with Br2/enolizable ketone. Org. Lett. 2006;23:5397–5399. doi: 10.1021/ol062371b. [DOI] [PubMed] [Google Scholar]
- 34.Walters E.W., Lee S.F., Niderman T., Bemasconi P., Subramanian M.V., Siehl D.L. Adenylosuccinate synthetase from maize. Purification, properties, and mechanism of inhibition by 5’-phosphohydantocidin. Plant Physiol. 1997;114:549–555. doi: 10.1104/pp.114.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cseke C., Gerwick B.C., Crouse G.D., Murdoch M.G., Green S.B., Heim D.R. 2-Phosphohydantocidin: the in vivo adenylosuccinate synthetase inhibitor responsible for hydantocidin phytotoxicity. Pestic. Biochem. Physiol. 1996;55:210–217. [Google Scholar]
- 36.Fonne-Pfister R., Chemla P., Ward E., Girardet M., Kreuz K.E., Hinzatko R.B., Fromm H., Schar H.P., Grutter M.G., Cowan-Jacob S.W. The mode of action and the structure of a herbicide in complex with its target: Binding of activated hydantocidin to the feedback regulation site of adenylosuccinate synthetase. Proc. Natl. Acad. Sci. USA. 1996;93:9431–9436. doi: 10.1073/pnas.93.18.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J.M., Xu Z.H., Han J.T., Dong H.B., Liu B., Wang M.A. Synthesis and biological activity of novel phosphoramide with hydantoin. Chin. J. Org. Chem. 2013 doi: 10.6023/cjoc201305023.. [DOI] [Google Scholar]
- 38.Chen N.C. The Bioassay Technologies for Pesticides. Beijing Agricultural University Press; Beijing, China: 1991. pp. 161–162. [Google Scholar]
- 39.Chen W.Y. The Research & Development of New Pesticide—Methods & Progress. Chemical Industry Press; Beijing, China: 2007. pp. 132–134. [Google Scholar]