Abstract
AIM
To determine serum osteocalcin levels in South Chinese males with non-alcoholic fatty liver disease (NAFLD) and to examine the relation between serum osteocalcin and NAFLD.
METHODS
Data were collected from 1683 men attending the Fangchenggang Area Male Healthy and Examination Survey (FAMHES) from September 2009 to December 2009. Serum osteocalcin was measured with electrochemiluminescence immunoassay. An abdominal ultrasonographic examination for all individuals was performed by two experienced ultrasonographers. The associations of serum osteocalcin with NAFLD were evaluated.
RESULTS
The levels of serum osteocalcin were lower in 364 NAFLD participants than in 1319 non-NAFLD participants (24.51 ± 1.38 ng/mL vs. 20.81 ± 1.33 ng/mL, p < 0.001). Serum osteocalin level was associated with the scale of NAFLD (r = −0.150, p < 0.01). Serum osteocalin level tended to decrease with the scale of NAFLD. Binary logistic regression analysis showed that decreased ORs for NAFLD were observed from the first to the fourth osteocalcin quartiles.
CONCLUSIONS
Our findings suggest that a lower serum osteocalcin level is associated with the presence of NAFLD.
Keywords: osteocalin, Insulin resistance, non-alcoholic fatty liver disease, ultrasonography
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a type of fatty liver disease when fat is deposited in the liver but not due to excessive alcohol use [1]. Some epidemiological surveys have showed that NAFLD has become a serious public health problem in China and even Asia [2–5]. To date, the mechanism of NAFLD is not fully understood. However, NAFLD is now considered to be a manifestation of metabolic syndrome (MetS) and it has been reported to relate to insulin resistance (IR) [6,7]. IR is almost a universal finding in NAFLD [8].
Osteocalcin, a hormone secreted by osteoblasts, can stimulate insulin expression in β-cells and protect animals from obesity and glucose intolerance [9]. Recent studies suggest that serum osteocalcin plays a key role in the pathogenesis of IR and energy expenditure [10,11]. Moreover, decreased serum osteocalcin levels have been reported in patients with metabolic syndrome [12]. Fernández-Real et al. thought that osteocalcin may play a role in the development of insulin resistance-associated fatty liver disease [13]. However, there are few studies that have explored the association between osteocalcin and NAFLD [14–16], which reported that in East Chinese men and Turkish patients, NAFLD patients were seen with a decreased serum osteocalcin level than in the controls. This prompted us to investigate the role of osteocalcin in the development of NAFLD.
Therefore, we conducted a cross-sectional study to investigate serum osteocalcin levels in South Chinese males with abdominal ultrasonography proven NAFLD, and to examine the relation of serum osteocalin and NAFLD.
2. Results and Discussion
Among 1683 individuals enrolled in this study, the prevalence of NAFLD was 21.74%. The characteristics of the participants were divided into non-NAFLD (n = 1319) and NAFLD (n = 364) groups shown in Table 1. Compared with participants in non-NAFLD group, those in NAFLD group had lower serum osteocalcin levels (p < 0.001) (Table 1). In non-NAFLD group, the mean of serum osteocalcin was 24.51 ng/mL with a SD of 1.38 ng/mL while in NAFLD group the mean of serum osteocalcin was 20.81 ng/mL with a SD of 1.33 ng/mL. All variables had statistical differences between these two groups (Table 1).
Table 1.
Baseline characteristics of the subjects in non-NAFLD and non-alcoholic fatty liver disease (NAFLD) groups.
| Characteristic | Non-NAFLD (n = 1319) | NAFLD (n = 364) | p value |
|---|---|---|---|
| Age (years) | 36.69 ± 11.37 | 40.52 ± 10.18 | <0.001 |
| Osteocalcin (ng/mL) | 24.51 ± 1.38 | 20.81 ± 1.33 | <0.001 |
| WC (cm) | 77.74 ± 7.68 | 89.29 ± 6.84 | <0.001 |
| BMI (kg/m2) | 22.25 ± 2.67 | 26.25 ± 2.74 | <0.001 |
| ALT (U/L) | 36.90 ± 12.65 | 48.82 ± 15.25 | <0.001 |
| Glucose (mmol/L) | 5.20 ± 0.82 | 5.57 ± 1.30 | <0.001 |
| TC (mmol/L) | 5.60 ± 1.01 | 6.05 ± 1.06 | <0.001 |
| TG (mmol/L) | 1.24 ± 0.92 | 2.44 ± 1.40 | <0.001 |
| HDL-C (mmol/L) | 1.43 ± 0.29 | 1.30 ± 0.40 | <0.001 |
| LDL-C (mmol/L) | 2.88 ± 0.79 | 3.24 ± 0.78 | <0.001 |
| Physical activity (n, %) | |||
| Low * | 848(64.3) | 208(57.1) | |
| Moderate * | 368(27.9) | 131(36.0) | 0.011 |
| High * | 103(7.8) | 25(6.9) |
Note: The physical activity level was classified as low, moderate, or high according to Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) (available at: http://www.ipaq.ki.se/scoring.pdf). High: equivalent to approximately at least one hour per day or more, of at least moderate-intensity activity above the basal level of physical activity; Moderate: equivalent to half an hour of at least moderate-intensity physical activity on most days; Low: not meeting any of the criteria for either of the previous categories [17].
Age-adjusted Spearman correlation coefficient between osteocalcin and variables was shown in Table 2. There was a statistical negative correlation between osteocalcin and WC, BMI, TC, LDL-C, glucose, TG and ALT after adjustment for age (all p < 0.001). However, there was no statistically significant correlation between osteocalcin and HDL-C (p = 0.437). The strongest correlation was observed between osteocalcin and BMI (r = −0.246). The important finding in our study was that serum osteocalcin levels were statistically associated with the scale of NAFLD after adjustment of age and physical activity. Osteocalcin showed a decreased trend with the scale (r = −0.150, p < 0.001).
Table 2.
Age-adjusted Spearman correlations between osteocalcin and variables.
| Variable | Correlation coefficients | p value |
|---|---|---|
| WC | −0.223 | <0.001 |
| BMI | −0.246 | <0.001 |
| ALT | −0.151 | <0.001 |
| TC | −0.105 | <0.001 |
| HDL | 0.019 | 0.437 |
| LDL | −0.083 | <0.001 |
| Glucose | −0.094 | <0.001 |
| TG | −0.142 | <0.001 |
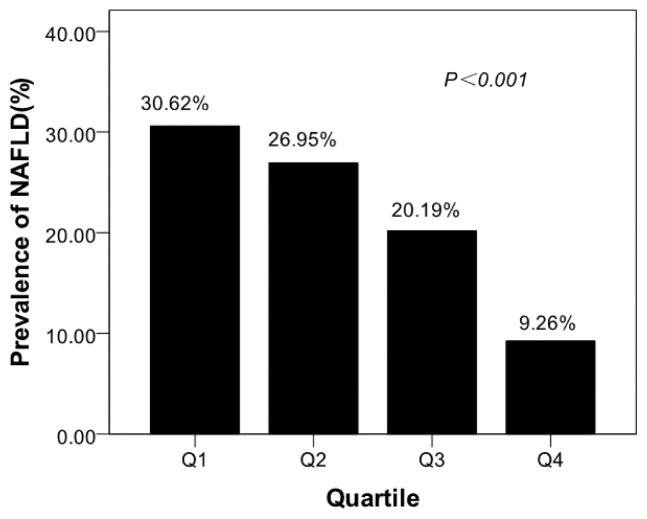
Participants with lower serum osteocalcin levels might be suffer from NAFLD. The prevalence of NAFLD of different quartiles was shown in Figure 1. The prevalence of NAFLD from the lowest to highest osteocalcin quartile were 30.62%, 26.95%, 20.19%, 9.26%, respectively (p < 0.001). The binary logistic regression analysis results were shown in Table 3. Decreased ORs for NAFLD were observed from the first to the fourth osteocalcin quartiles. Compared with the subjects in the fourth osteocalcin quartile, those in the first quartile had an OR of 4.32 (95% confidence interval (CI), 2.93–6.38); those in the second quartile had an OR of 3.54 (CI, 2.39–5.25) and those in the third quartile had an OR of 2.47 (CI, 1.65–3.71).
Figure 1.
Prevalence of non-alcoholic fatty liver disease (NAFLD) in different quartiles of osteocalcin. The prevalence of NAFLD from the lowest to highest osteocalcin quartile were 30.62%, 26.95%, 20.19%, 9.26%, respectively (p < 0.001).
Table 3.
Binary logistic regression model examining associations of serum osteocalcin level and the presence of non-alcoholic fatty liver disease (NAFLD) Age-adjusted Spearman correlations between osteocalcin and variables.
| Quartile of osteocalcin (ng/mL) | Odds ratio (95%CI) | p value | Adjusted 1 Odds ratio (95%CI) | p value | Adjusted 2 Odds ratio (95%CI) | p value |
|---|---|---|---|---|---|---|
| Q4 (>29.21) | 1 | 1 | 1 | |||
| Q3 (23.41–29.21) | 2.47 (1.65–3.71) | <0.001 | 2.30 (1.53–3.46) | <0.001 | 1.83 (1.14–2.93) | =0.012 |
| Q2 (18.98–23.41) | 3.54 (2.39–5.25) | <0.001 | 3.10 (2.08–4.63) | <0.001 | 2.28 (1.44–3.63) | <0.001 |
| Q1 (<18.98) | 4.32 (2.93–6.38) | <0.001 | 3.60 (2.41–5.39) | <0.001 | 2.25 (1.42–3.55) | <0.001 |
After the adjustment of age, BMI and physical activity, the ORs decreased obviously. Compared with the subjects in the fourth osteocalcin quartile, participants in the first quartile had an OR of 2.25 (CI, 1.42–3.55), those in the second quartile had an OR of 2.28 (CI, 1.44–3.63), and those in the third quartile had an OR of 1.83 (CI, 1.14–2.93).
In original studies on osteocalcin and insulin resistance, Lee et al. found that mice were lacking osteocalcin displayed decreased β-cell proliferation, glucose intolerance, and insulin resistance [9]. A great deal of interest followed this report and some studies tried to evaluate the relation between serum osteocalcin levels and metabolic syndrome [14,18–20], which showed that osteocalcin, plasma CK-18, and SREBF-1c polymorphism are associated with liver fibrosis and NAFLD. The recently reported association of osteocalcin with insulin resistant and metabolic syndrome prompts us to evaluate serum osteocalcin levels in patients with NAFLD.
Our investigation demonstrated that the level of serum osteocalcin in NAFLD group was lower than in non-NAFLD group (20.81 ± 1.33 ng/mL vs. 24.51 ± 1.38ng/mL; p < 0.001). One novel finding in our study was that serum osteocalcin levels were statistically associated with the scale of NAFLD after adjustment of age and physical activity. Osteocalcin showed a decreased trend with the scale of NAFLD (r = −0.150, p < 0.001). Moreover, after adjustment of age, physical activity and BMI, the binary logistic regression analysis results showed decreased ORs for NAFLD were observed from the first to the fourth osteocalcin quartiles. It is uncertain which mechanisms lead to lower osteocalcin levels in patients with NAFLD [21]. The relationship between serum osteocalcin levels and NAFLD could be hypothetically explained by the fact that osteocalcin increases insulin and adiponectin expression, decreases fat mass, improves insulin sensitivity and may thereby protect people against the development of NAFLD.
Some findings indicated that serum osteocalcin level was one of the predictors of insulin sensitivity and metabolic syndrome. [11,22–24] This study also kept consistent with prior reports. The glucose level in NAFLD group was higher than non-NAFLD group (5.57 ± 1.30 mmol/L vs. 5.20 ± 0.82 mmol/L; p < 0.001). Other variables in Table 1 had statistical differences between these two groups. We further found an inverse association between serum osteocalcin and fasting plasma glucose, BMI and waist circumference (Table 2).
Fatty liver disease is the most common cause of mild to moderate elevation of liver enzymes. Fernández-Real et al. found that circulating osteocalcin concentration was associated with parameters of liver fat infiltration (serum ALT and gamma glutamyl transferase levels) [13]. Consistent with their previous reports, we found serum osteocalcin levels to be inversely related to ALT. Compared with participants in non-NAFLD group, those with NAFLD had higher serum ALT levels (p < 0.001) (Table 1). In non-NAFLD group, the mean of serum osteocalcin was 36.90 U/L with a SD of 12.65 U/L while in NAFLD group the mean of serum ALT was 48.82 U/L with a SD of 15.25 U/L. In addition, serum ALT levels were linked to serum osteocalcin levels (r = −0.144, p < 0.001). The results implied that osteocalcin could be a potential novel marker to assess the progression of NAFLD.
There has been some research on the association of osteocalcin with NAFLD. To the best of our knowledge, there only one study has showed an association of decreased osteocalcin levels with the presence of NAFLD in a relatively larger population in East China [14]. However, two limitations in our study should be addressed. Because this study was performed in relatively healthy Chinese males, it had selection bias. However, such a large population-based sample limited the selection bias to some extent. It is well known that liver biopsy remains the best way to diagnose NAFLD and establish the presence of fibrosis, but it cannot be performed easily in a large population because of its invasiveness [12]. In fact, abdominal ultrasonography is more accessible than liver biopsy [25–27]. It is the optimal choice for NAFLD epidemiological surveys [28].
3. Experimental Section
3.1. Subjects
The Fangchenggang Area Male Health and Examination Survey was a population–based study conducted among non–institutionalized Chinese males aging from 17 to 88 years old in Guangxi. This study was designed to investigate the effects of environmental and genetic factors and their interaction with the development of age-related chronic diseases. A comprehensive demographic and health survey was conducted among 4303 men, who participated in a large-scale physical examination in the Medical Centre of Fangchenggang First People’s Hospital from September to December in 2009. Each method and potential risks were explained in detail to the participants, who gave written informed consent before the survey. In addition, the study received approval from ethics committee of Guangxi medical university.
Participants were included in this study based on the following criteria: (1) aged above 17 years old Fangchenggang area males; (2) having a stable and settled lifestyle.
Participants were excluded from this study based on the following criteria: (1) currently diagnosed with diabetes mellitus, coronary heart disease, stroke, hyperthyroidism, rheumatoid arthritis, and cancer; (2) currently diagnosed with hepatitis B virus (determined by the presence of hepatitis B surface antigen); with other hepatitis history and impaired hepatic function (alanine transaminase >2.0 times upper limit of normal); with liver cirrhosis or hepatic carcinoma; (3) taking any kind of medication (such as dehydrocortisone, methotrexate and so on) known to cause fatty liver’s images (images of brightechoes in liver parenchyma) during the previous year; (4) with excessive alcohol consumption (≥20 g/d, according to the published report [21]); (5) with incompleted follow-up clinical or laboratory examination, or ultrasound examination.
3.2. Epidemiological Survey
Data on demographic characteristics (age, education, occupation, etc.), lifestyle characteristics (alcohol consumption and physical activity), health status, and medical history were collected by using a standardized questionnaire (details were described by Tan et al. [12]). Body measurements including body weight and standing height were performed by a trained examiner using a standardized protocol. Body mass index (BMI) was calculated as weight (kilograms) divided by squared height (meters). The amount of alcohol intake was obtained from a series of questions including quantity of alcohol intake each time, times of alcohol intake each day, months of alcohol intake each year, years of alcohol intake, type and alcoholicity of beverage, drinking and dietary habits. From these data, we calculated the average daily and total alcohol assumption based on previous published method [3,29].
3.3. Ultrasonography
An abdominal ultrasonographic examination for all individuals was performed by two experienced ultrasonographer using a portable ultrasound device (GE, LOGIQ e, 5.0-MHz transducer, Fairfield, CT, USA). The liver of each participant was assessed for size, contour, echogenicity, structure and posterior beam attenuation.
The ultrasonographic criteria used to diagnose fatty liver disease included the presence of increased liver echogenicity (bright), stronger echoes in the hepatic parenchyma than in the renal parenchyma, vessel blurring, and narrowing of the lumen of the hepatic veins [23,29–31]. The degree of fatty liver disease on ultrasonography was divided into three scales (mild, moderate or severe) according to the criteria described by Saadeh et al. [23].
3.4. Laboratory Assessments
Overnight fasting venous blood specimens were drawn. Serum total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and glucose levels were measured enzymatically on Dimension-RxL Chemistry Analyzer (Dade Behring, Newark, DE, USA) in the Department of Clinical Laboratory at the Fangchenggang First People’s Hospital. Serum osteocalcin was measured with electrochemiluminescence immunoassay on COBAS 6000 system E601 (Elecsys module) immunoassay analyzer (Roche Diagnostics, GmbH, Mannheim, Germany) with the same batch of reagents. The interassay coefficient of variation was 4.5%.
3.5. Quality Control
All researchers were given systematic training before the investigation. For further quality control, 5% of the questionnaires and blood sample results were randomly chosen for the re-examination; kappa analysis of these samples showed good consistency in the results of the diagnostic tests (data not shown). Abdominal ultrasonic examinations were performed by two experienced ultrasonographers using the same portable ultrasound device.
3.6. Statistical Analysis
Statistical analyses were performed using SPSS 18.0 software (IBM, Chicago, IL, USA). Descriptive results of continuous variables were expressed as either mean (±SD) or median and quartiles, and categorical data were expressed as percentage. The distribution of variables was analyzed with Kolmogorov-Smirnov test. Natural logarithmic transformations were performed so that osteocalcin values changed from a markedly skewed distribution to approximate normality. Basic characteristics of different groups were compared using Student’s t test or chi-square test when it is appropriate. The chi-square test was also used to compare the prevalence of NAFLD between different osteocalcin quartiles. Spearman correlation coefficients (adjusted for age) were calculated between serum osteocalcin and the following variables: waist circumference (WC), Glucose, BMI, ALT, TC, TG, HDL, and LDL. The association between serum osteocalcin levels and the scale of NAFLD was also evaluated. The binary logistic regression model was used to assess whether serum osteocalcin levels were associated with the presence of NAFLD. The odds ratio (OR) was calculated for the presence of NAFLD in quartiles of osteocalcin, with the participants in the highest quartile of osteocalcin considered as the reference group. Levels of statistical significance were set at p < 0.05.
4. Conclusions
Our findings suggest that lower serum osteocalcin levels are associated with measures of insulin resistance (fasting plasma glucose) and the presence of NAFLD. In the future, further studies are needed to establish the role of serum osteocalcin in patients with NAFLD.
Acknowledgments
The work described in this paper was supported by grants from the National Natural Science Foundation of China (Grant No. 30945204) and the Provincial departments of Finance and Education, Guangxi Zhuang Autonomous Region, China (Grant No. 2009GJCJ150). We express our sincere thanks to the local research teams from Fangchenggang First People’s Hospital, Fangchenggang, China, for their contribution to the survey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Adams L.A., Angulo P., Lindor K.D. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou X.H., Zhu Y.X., Lu H.J., Chen H.F., Li Q., Jiang S., Xiang K.S., Jia W.P. Non-alcoholic fatty liver disease’s prevalence and impact on alanine aminotransferase associated with metabolic syndrome in the Chinese. J. Gastroenterol. Hepatol. 2011;26:722–730. doi: 10.1111/j.1440-1746.2010.06509.x. [DOI] [PubMed] [Google Scholar]
- 3.Fan J.G., Saibara T., Chitturi S., Kim B.I., Sung J.J., Chutaputti A Asia-Pacific Working Party for NAFLD. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific. J. Gastroenterol. Hepatol. 2007;22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 4.Amarapurkar D.N., Hashimoto E., Lesmana L.A., Sollano J.D., Chen P.J., Goh K.L. Asia-Pacific Working Party on NAFLD. How common is non–alcoholic fatty liver disease in the Asia–Pacific region and are there local differences. J. Gastroenterol. Hepatol. 2007;22:788–793. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- 5.Fan J.G., Farrell G.C. Epidemiology of non–alcoholic fatty liver disease in China. J. Hepatol. 2009;50:204–210. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 6.McCullough A.J. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2006;40:S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 7.Utzschneider K.M., Kahn S.E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 8.Jornayvaz F.R., Samuel V.T., Shulman G.I. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu. Rev. Nutr. 2010;30:273–290. doi: 10.1146/annurev.nutr.012809.104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee N.K., Sowa H., Hinoi E., Ferron M., Ahn J.D., Confavreux C., Dacquin R., Mee P.J., McKee M.D., Jung D.Y., et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducy P. The role of osteocalcin in the endocrine cross–talk between bone remodelling and energy metabolism. Diabetologia. 2011;54:1291–1297. doi: 10.1007/s00125-011-2155-z. [DOI] [PubMed] [Google Scholar]
- 11.Pittas A.G., Harris S.S., Eliades M., Stark P., Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J. Clin. Endocrinol. Metab. 2009;94:827–832. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan A.H., Gao Y., Yang X.B., Zhang H.Y., Qin X., Mo L.J., Peng T., Xia N., Mo Z.N. Low serum osteocalcin level is a potential marker for metabolic syndrome: Results from a Chinese male population survey. Metabolism. 2011;60:1186–1192. doi: 10.1016/j.metabol.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Real J.M., Ortega F., Gómez-Ambrosi J., Salvador J., Frühbeck G., Ricart W. Circulating osteocalcin concentrations are associated with parameters of liver fat infiltration and increase in parallel to decreased liver enzymes after weight loss. Osteoporos. Int. 2010;21:2101–2107. doi: 10.1007/s00198-010-1174-9. [DOI] [PubMed] [Google Scholar]
- 14.Dou J., Ma X., Fang Q., Hao Y., Yang R., Wang F., Zhu J., Bao Y., Jia W. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in Chinese men. Clin. Exp. Pharmacol. Physiol. 2013;40:282–288. doi: 10.1111/1440-1681.12063. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz Y., Kurt R., Eren F., Imeryuz N. Serum osteocalcin levels in patients with nonalcoholic fatty liver disease: Association with ballooning degeneration. Scand. J. Clin. Lab. Invest. 2011;71:631–636. doi: 10.3109/00365513.2011.604427. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz Y. Review article: Non-alcoholic fatty liver disease and osteoporosis-clinical and molecular crosstalk. Aliment. Pharmacol. Ther. 2012;36:345–352. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 17.Saadeh S., Younossi Z.M., Remer E.M., Gramlich T., Ong J.P., Hurley M., Mullen K.D., Cooper J.N., Sheridan M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 18.Aller R., Castrillon J.L., de Luis D.A., Conde R., Izaola O., Sagrado M.G., Velasco M.C., Alvarez T., Pacheco D. Relation of osteocalcin with insulin resistance and histopathological changes of non alcoholic fatty liver disease. Ann. Hepatol. 2011;10:50–55. [PubMed] [Google Scholar]
- 19.Cusi K., Chang Z., Harrison S., Lomonaco R., Bril F., Orsak B., Ortiz-Lopez C., Hecht J., Feldstein A.E., Webb A., et al. Limited Value of Plasma Cytokeratin-18 as a Biomarker for NASH and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease (NAFLD) J. Hepatol. 2013 doi: 10.1016/j.jhep.2013.07.042.. [DOI] [PubMed] [Google Scholar]
- 20.Musso G., Bo S., Cassader M., de Michieli F., Gambino R. Impact of sterol regulatory element-binding factor-1c polymorphism on incidence of nonalcoholic fatty liver disease and on the severity of liver disease and of glucose and lipid dysmetabolism. Am. J. Clin. Nutr. 2013;98:895–906. doi: 10.3945/ajcn.113.063792. [DOI] [PubMed] [Google Scholar]
- 21.Adams L.A., Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad. Med. J. 2006;82:315–322. doi: 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeap B.B., Chubb S.A.P., Flicker L., McCaul K.A., Ebeling P.R., Beilby J.P., Norman P.E. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur. J. Endocrinol. 2010;163:265–272. doi: 10.1530/EJE-10-0414. [DOI] [PubMed] [Google Scholar]
- 23.Saleem U., Mosley T.H., Jr., Kullo I.J. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2010;30:1474–1478. doi: 10.1161/ATVBAHA.110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M., Ma X., Li H., Pan X., Tang J., Gao Y., Hou X., Lu H., Bao Y., Jia W. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur. J. Endocrinol. 2009;161:723–729. doi: 10.1530/EJE-09-0585. [DOI] [PubMed] [Google Scholar]
- 25.Adams L.A., Talwalkar J.A. Diagnostic evaluation of nonalcoholic fatty liver disease. J. Clin. Gastroenterol. 2006;40:S34–S38. doi: 10.1097/01.mcg.0000168642.38945.f1. [DOI] [PubMed] [Google Scholar]
- 26.Mottin C.C., Moretto M., Pan A.V., Swarowsky A.M., Toneto M.G., Glock L., Repetto G. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes. Surg. 2004;14:635–637. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 27.Joy D., Thava V.R., Scott B.B. Diagnosis of fatty liver disease: Is biopsy necessary. Eur. J. Gastroenterol. Hepatol. 2003;15:539–543. doi: 10.1097/01.meg.0000059112.41030.2e. [DOI] [PubMed] [Google Scholar]
- 28.Sanyal A.J. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 29.Fan J.G. Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: An updated and revised edition. Chin. J. Hepatol. 2010;18:163–166. [PubMed] [Google Scholar]
- 30.Meziri M., Pereira W.C., Abdelwahab A., Degott C., Laugier P. In vitro chronic hepatic disease characterization with a multiparametric ultrasonic approach. Ultrasonics. 2005;43:305–313. doi: 10.1016/j.ultras.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Pulzi F.B., Cisternas R., Melo M.R., Ribeiro C.M., Malheiros C.A., Salles J.E. New clinical score to diagnose nonalcoholic steatohepatitis in obese patients. Diabetol. Metab. Syndr. 2011 doi: 10.1186/1758-5996-3-3.. [DOI] [PMC free article] [PubMed] [Google Scholar]