Abstract
We recently described a novel, non-host-derived, phage-mediated immunity active at mucosal surfaces, the main site of pathogen entry in metazoans. In that work, we showed that phage T4 adheres to mucus glycoproteins via immunoglobulin-like domains displayed on its capsid. This adherence positions the phage in mucus surfaces where they are more likely to encounter and kill bacteria, thereby benefiting both the phage and its metazoan host. We presented this phage-metazoan symbiosis based on an exclusively lytic model of phage infection. Here we extend our bacteriophage adherence to mucus (BAM) model to consider the undoubtedly more complex dynamics in vivo. We hypothesize how mucus-adherent phages, both lytic and temperate, might impact the commensal microbiota as well as protect the metazoan epithelium from bacterial invasion. We suggest that BAM may provide both an innate and an acquired antimicrobial immunity.
Keywords: phage, bacteriophage, immune system, mucus, lysogen, lytic
Background
While the essential role of microbial-metazoan symbioses has become increasingly apparent and accepted, investigations of metazoan microbiomes continue to overlook or ignore a key player: the phages. In other ecosystems, these ubiquitous microbial predators have been shown to impel bacterial diversification and shape the composition of microbial communities. Nonetheless, our knowledge of both the range of their ecological functions and the cascade of consequent effects remains grossly incomplete. Undoubtedly phages are significant participants in microbiomes. We further posit that phages, like their bacterial associates, engage in mutually beneficial symbioses with their metazoan hosts.
Investigating this reciprocity has been hampered by various factors, not the least of which is the dynamic complexity of these communities. Early phage research was mostly confined to laboratory studies using one phage/one host model systems. More recent research efforts have begun to investigate phage-host interactions within the context of complex natural environments. One environment of considerable import is that of metazoan mucosal surfaces, the main points of entry for pathogenic microorganisms. Although little is yet known of phage dynamics within this ecological niche, numerous studies have jumped ahead to test the feasibility of novel modes of phage therapy to defend these sites from infection. Initial investigations assessed the immediate effects of delivering exogenous phage to the mucosal surfaces lining the gut and the lung, as well as to the external surface of coral polyps.1-3 Their preliminary results have been propitious. The worldwide rise of pathogens with resistance to multiple—sometimes all available—antibiotics has spurred a renewed and urgent interest in phage therapy. Historically, phage therapy has been plagued by numerous failings and misconceptions. According to Brüssow,4 however, the current lack of successful applications is primarily a consequence of the reductionist approaches that have dominated phage research. The fact remains that, despite decades of investigation, we simply do not understand the fundamental biology governing phage-host interactions within naturally occurring systems—not even that of the model organisms T4 and E. coli in the human gut.
Mucus as an Environment
What are the structural and spatial dynamics that phages encounter upon approaching a mucosal surface? Mucus presents a complex, stratified, and transient environment with defined turnover dynamics.5 The innermost zone is firmly anchored to the membrane of the underlying epithelial cells and is typically devoid of bacteria. Residing above that is a 10–700 μm thick stratified zone of secreted gel-like mucus that supports microbial growth.6,7 The underlying epithelium constantly secretes mucin glycoproteins, the primary macromolecular constituents of mucus, thus driving the continual upward migration of the mucus. As it moves upwards, the mucus becomes less dense and is eventually sloughed from the surface.5 Mucus layers typically harbor large and genetically diverse populations of microbes that range from transient drop-ins to host-specific commensals, from potential pathogens to symbionts. Past studies have found that some symbionts provide their metazoan hosts with services such as access to otherwise unavailable nutrient sources8 and protection from infection or other environmental insults,9 but much remains to be investigated.
Of particular interest is the possible role of the metazoan host in shaping the mucus community. A recently proposed model posits that metazoan epithelial cells select for commensal bacteria in the mucus region nearest the epithelium by secretion of antimicrobials and nutrients.10 Both top-down and bottom-up selection applied in this zone proximal to the epithelium would have disproportionately strong effects throughout the mucus layer due to the constant outward flux of the mucus. In contrast, environmentally-derived nutrients or antimicrobials would primarily affect the outer mucus surface and its associated bacteria that are positioned for prompt sloughing and removal from the mucus layer. Further, it was reasoned that secretion of antimicrobials could, in principle, select for commensal microbes faster than nutrient secretions—provided such agents preferentially killed the non-beneficial strains within the mucus layer. Most antimicrobial secretions, however, lack such specificity.11
Phages in Mucus
Phages, on the other hand, are ‘antimicrobials’ that can be highly specific for individual bacterial strains. This feature, in conjunction with their ubiquity and abundance, suggests that phages might be an effective mechanism for selective microbial killing within mucus environments. Such action, however, entails more than the ability of phages to lyse bacteria. One obvious requirement is that the phages must be present in the mucus in sufficient numbers. Earlier incidental observations had suggested that this condition might be met. In our recent research, we confirmed and extended those reports by demonstrating the enrichment of phages on varied mucosal surfaces.12 For this we collected mucus and adjacent environmental samples from nine diverse metazoans. Our results quantified a greater abundance of phages and an elevated ratio of phages-to-bacteria within all of the mucus environments sampled. Also required is that relevant phages possess a mechanism facilitating their adherence to mucus. In our model system using phage T4, this adherence was mediated by capsid proteins with immunoglobulin-like (Ig-like) folds that interacted with the glycan residues decorating mucin glycoproteins.12 Such Ig-like proteins had been previously found in structural proteins of ~25% of the sequenced tailed phages (Caudovirales) where they were postulated to facilitate adherence to bacterial cell surfaces during infection.13 Their role in the adherence of phage T4 to mucus is the first experimentally determined function for capsid-displayed Ig-like phage proteins.12
Innate Immunity
To serve as effective antimicrobials for the host, adherent phages must reduce bacterial colonization of the mucus. Our in vitro studies demonstrated that mucus-adherent lytic phage reduced such colonization and in so doing protected the underlying epithelium from the otherwise resultant cell death. Based on these findings, we consider that lytic phages provide a previously unrecognized, non-host-derived immunity that actively protects the mucosal surfaces of their metazoan host. We referred to this novel mechanism of immunity as the Bacteriophage Adherence to Mucus (BAM) model.12
Classically the immune system has been divided into two branches: the innate immune response that defends the host from infection in a non-specific manner, and the acquired or adaptive immune response that reacts to specific antigens and possesses a memory that expedites subsequent responses to the same agent. The innate immune system is considered the metazoan host’s first line of defense against infection. Contributing to this protection are the physical barriers presented by the epithelia, phagocytic cells, and plasma proteins, among others. The mucus layer itself is considered to be part of the innate immune system by virtue of its protective mucin glycoproteins14,15 and broad-spectrum antimicrobial compounds.11,16 Likewise, the BAM model as originally presented considers the accumulation of lytic phage on mucosal surfaces to actively contribute to innate immunity.12
Lytic Phage and BAM Innate Immunity
Both the adherence and the accumulation of phages are subject to mucus dynamics. In our model the ability of any particular phage to adhere depends on the specific glycans present. Different mucin glycoproteins with distinct glycosylation patterns are expressed at the various mucosal surfaces of each metazoan, and the mucin glycosylation at any location can be altered by the host in response to mucosal infection and inflammation.14 The accumulation of adherent phage is opposed by the perpetual outward flux of the mucus that is driven by epithelial secretion and that culminates in the sloughing of the outer mucus. This suggests that lytic phages adhering to the outer mucus are inevitably destined for prompt removal. However, other factors might postpone their eviction. It is possible that the rate of diffusion of even adherent phages through the viscous mucus may be sufficient to counter the mucus conveyor belt, thus allowing the phages to penetrate to deeper regions. If a phage encounters a susceptible bacterial host deeper within the mucus and replicates, the progeny virions released would then diffuse in all directions, thereby reseeding the surrounding mucus. Thus a balance must be struck between the strength of phage adherence and the rate of mucus turnover. Perhaps the weak interactions we observed between T4 phage and glycans reflect this balancing act. Stronger adherence would slow phage diffusion, perhaps to the point where the mucus flux would carry the phages outward faster than the phages can diffuse inward.
We previously demonstrated through the use of replication negative phage mutants that phage replication within the mucus was required for BAM protection of epithelial cells against bacterial infection.12 This conclusion was based on studies of a one phage/one host in vitro system. Undoubtedly the great diversity of phage and bacteria within mucus influences BAM innate immunity in vivo. Typically lytic phages efficiently infect only a narrow range of closely related bacterial species or strains.17 In our initial model we assumed that every bacterium entering the mucus layer is accompanied by and supports it’s own lytic phage population. The populations of phage predators and bacterial prey would cycle within the mucus in accordance with the classic kill-the-winner dynamic.18 Notably, BAM innate immunity is both transient and perpetual: transient in that loss of the bacterial host from the mucus occasions loss of its predator phage, as well; perpetual in that incoming bacteria are always accompanied by lytic phages preying upon them (Fig. 1).
Figure 1. BAM Innate Immunity is mediated by lytic phage replication and kill-the-winner dynamics. Continual mucus secretion removes lytic phages unless they are actively replenished by ongoing productive phage infection.
Increased understanding of the dynamic role of lytic phages at mucosal surfaces will enable development of new tactics for their manipulation to treat and prevent disease. One potential application already under investigation is the use of lytic phages for prophylactic treatments to deter pathogenic colonization of at-risk mucosal epithelia. While the effectiveness of such treatments would likely be short-lived, protection for up to four days has been recently reported when the phages were applied before their bacterial host had established in the mucus.19-21 Clearly extensive investigation of phage dynamics in vivo is a prerequisite for the development of therapeutic protocols.
Acquired Immunity
An invading pathogen that penetrates innate immune defenses will be accosted by agents of the acquired (or adaptive) immune system. This complex, multi-component system is characterized by its ability to respond to novel antigens with a highly specific riposte and to react to subsequent encounters with greater speed and efficiency. The human adaptive immune system entails the collaborative functioning of B cells, T cells, antibodies, and numerous other components. That phages make use of an immunoglobulin-like (Ig-like) protein fold to adhere to mucus struck us as an interesting twist, given the key role of immunoglobulin proteins in our acquired immunity. Our immune system uses junctional diversity to generate antigen-binding sites with a great variety of binding affinities without disrupting the underlying protein scaffolding. These binding sites are formed by the loop structures connecting the β-sheets of the immunoglobulin fold. In contrast, some phages utilize a mechanism of targeted mutagenesis during reverse transcription to generate diversity that is restricted to an exposed surface of the β-sheet within the Ig-like fold.13,22 Notably, the diversity generated in both of these situations supports specific binding interactions, albeit serving quite different purposes. In the canonical acquired immune system, this diversity facilitates binding of infecting pathogens even on first exposure, such binding being essential for a killing response. In the BAM model, we postulate that the diversified Ig-like folds, exposed on the surface of the phage capsids, facilitate phage adherence to mucus despite the frequent shifting of mucin glycosylation patterns by the metazoan host.12
Temperate Phages and BAM Acquired Immunity
As discussed above, mucus-adherent lytic phage are likely transient within mucus layers as their maintenance requires ongoing productive infections. Thus their persistence is contingent not only on the presence of a sufficient population of sensitive hosts, but also on other factors such as an adequate growth rate of their hosts and chance encounters. In contrast, those problematic factors have little effect on the maintenance of temperate phages residing as prophages within their hosts. Temperate phages are known to be present on mucosal surfaces,22-24 but their impact on community dynamics in general, and BAM-mediated immunity in particular, has not been investigated. Here we suggest some intriguing possibilities.
Nutrient secretions by mucosal epithelia have been credited with aiding the selection of gut commensal bacteria.10 We assert that these tactics would, by proxy, also select for temperate phages that migrate with their commensal bacterial hosts throughout the mucus layer. In the mucus environment, these epithelially-derived secretions encourage commensals to take up residence in the stratified mucus layer closest to the epithelium. Virions released by induction of prophages on board would act as antimicrobials throughout the mucus layer while they are conveyed outward by the continual flux of the mucus. Further, it has been suggested that commensal bacteria establish in the gut by deploying their temperate phages as biological weapons.24,25 As prophages, they protect their host from infection by the same or closely related phages; as virions, they kill closely related non-lysogens that might compete with their host. Temperate phages are widely known to confer beneficial phenotypic traits on their bacterial hosts, but these initial studies suggest they may also benefit their metazoan hosts by contributing to the selection of the commensal mucosal microbiota.
As demonstrated for the canonical temperate phage λ, low multiplicity of infection (MOI) favors entry into the lytic pathway, high MOI favors establishment of lysogeny. In a structured environment such as mucus, virions released by lysis are not immediately dispersed, but rather remain concentrated in the local environment where they attack nearby susceptible bacteria at high MOI. The local population of potential hosts thereby becomes mostly lysogenized, thus immune from infection by any free virions of the same phage. When this occurs for a commensal bacterium, there develops a stable lysogenized population that continually produces a low level of free virions through spontaneous induction.25
Host Range and Lysogeny
Classically, phages were thought to possess very narrow and specific host ranges. However, this conclusion stemmed from cross-infection experiments that were biased because the phages studied were typically isolated by methods favoring specialist lytic phage.17,26 Modeling has suggested that there exists a trade-off between phage replication efficiency and host range: specialist phages infect a smaller host range with greater efficiency, and host range expansion inflicts a fitness cost on the phage.27 Hence the ecological importance of generalist phages has been difficult to experimentally validate. However, a recent study demonstrated that antibiotic stress increased phage host range in the mouse gut, exemplifying the adaptive capacity of phage infection networks and suggesting that phages with broad host range may be a significant factor in vivo.28 Diverse mechanisms are known that enhance phage polyvalency, including recombination, gene inversion cassettes, and reverse transcriptase-mediated tropism switching.29
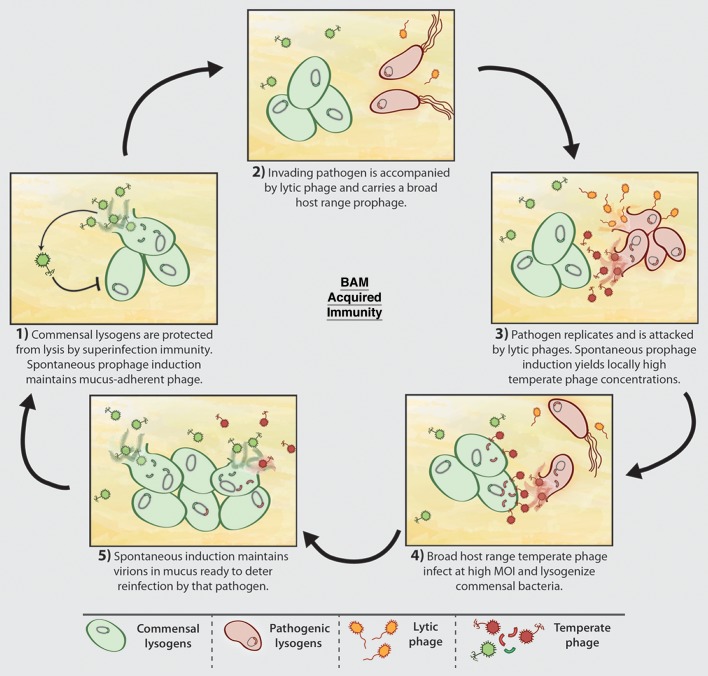
Once we acknowledge that phages occupy a spectrum of host range from specialist to generalist, the door is open to the intriguing possibility of an acquired BAM immunity. Picture an invading bacterial pathogen carrying a generalist prophage that, after induction, is able to infect and lysogenize one of the commensal bacteria (that may, or may not, be a lysogen bearing an unrelated prophage). After the pathogen has been eliminated from the mucus layer, its generalist temperate phage, ensconced as a prophage in a commensal, would remain. Spontaneous induction of commensal strains would maintain a low level of dispersed virions on duty, ready to pounce should that pathogen attempt to reinfect this mucosal epithelium.30 For maximum effectiveness, we would also posit that those virions be mucus-adherent, thus apt to linger longer within the mucus layer. In this fashion, we hypothesize that temperate phages residing in the mucus confer a ‘phage memory’ of previous bacterial insults, thereby providing a layer of phage-mediated acquired immunity for the metazoan host (Fig. 2). This mechanism may further explain the persistence of an individual’s unique virome over time.23
Figure 2. BAM Acquired Immunity is maintained by prophages within commensal bacteria that provide a memory of previous bacterial invasions. Key to this are broad host range temperate phages that not only infect and kill a pathogen, but also lysogenize a commensal.
Looking to the future, these phage-mediated defenses could be employed for the development of clinical treatments that assist mucosal epithelia in thwarting invading bacterial pathogens. We envision the use of a novel immunization mechanism for mucosal surfaces that would employ cocktails of mucus-adherent temperate phages or of commensal bacteria carrying appropriate prophages. However, before that can possibly become a reality, much research is needed on multiple levels. What role do temperate phages play within these environments? Through lysogenic conversion they confer specialized metabolic functions on their host bacteria, provide them with virulence factors and resistance genes, and grant them access to vast genetic diversity of mostly unknown function.17,29 When contemplating utilization of temperate phages for clinical treatments, the consequences of introducing such genes into the commensal mucosal bacterial populations must be taken into account.25,28 Many other questions remain. How significant is the contribution of phage-mediated innate and acquired immunity in vivo? What governs the dynamic interplay of lytic and temperate phages within the mucus environment? Are there specialist phages carrying Ig-like proteins adapted to particular mucosal environments? What are the trade-offs in terms of mucosal defense between the longer-term accumulation of phages and the protection afforded by mucus renewal? Do these mechanisms benefit the commensal microbes? A vast unknown territory awaits investigation at the mucosal surface of your choice.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bacteriophage/article/25857
References
- 1.Maura D, Galtier M, Le Bouguénec C, Debarbieux L. Virulent bacteriophages can target O104:H4 enteroaggregative Escherichia coli in the mouse intestine. Antimicrob Agents Chemother. 2012;56:6235–42. doi: 10.1128/AAC.00602-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 3.Cohen Y, Joseph Pollock F, Rosenberg E, Bourne DG. Phage therapy treatment of the coral pathogen Vibrio coralliilyticus. MicrobiologyOpen (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüssow H. What is needed for phage therapy to become a reality in Western medicine? Virology. 2012;434:138–42. doi: 10.1016/j.virol.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Johansson MEV, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4659–65. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–41. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012;15:57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 9.McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A. 2013;110:3229–36. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schluter J, Foster KR. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol. 2012;10:e1001424. doi: 10.1371/journal.pbio.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–8. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A. 2013;110:10771–6. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser JS, Yu Z, Maxwell KL, Davidson AR. Ig-like domains on bacteriophages: a tale of promiscuity and deceit. J Mol Biol. 2006;359:496–507. doi: 10.1016/j.jmb.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–97. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieleg O, Vladescu I, Ribbeck K. Characterization of particle translocation through mucin hydrogels. Biophys J. 2010;98:1782–9. doi: 10.1016/j.bpj.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthésy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–15. doi: 10.1016/S1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 17.Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004;28:127–81. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Thingstad TF, Lignell R. Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat Microb Ecol. 1997;13:19–27. doi: 10.3354/ame013019. [DOI] [Google Scholar]
- 19.Maura D, Morello E, du Merle L, Bomme P, Le Bouguénec C, Debarbieux L. Intestinal colonization by enteroaggregative Escherichia coli supports long-term bacteriophage replication in mice. Environ Microbiol. 2012;14:1844–54. doi: 10.1111/j.1462-2920.2011.02644.x. [DOI] [PubMed] [Google Scholar]
- 20.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 21.Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS One. 2011;6:e16963. doi: 10.1371/journal.pone.0016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minot S, Grunberg S, Wu GD, Lewis JD, Bushman FD. Hypervariable loci in the human gut virome. Proc Natl Acad Sci U S A. 2012;109:3962–6. doi: 10.1073/pnas.1119061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes A, Haynes M, Hanson N, Angly FE, Heath AC, Rohwer F, Gordon JI. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–8. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duerkop BA, Clements CV, Rollins D, Rodrigues JLM, Hooper LV. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc Natl Acad Sci U S A. 2012;109:17621–6. doi: 10.1073/pnas.1206136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gama JA, Reis AM, Domingues I, Mendes-Soares H, Matos AM, Dionisio F. Temperate bacterial viruses as double-edged swords in bacterial warfare. PLoS One. 2013;8:e59043. doi: 10.1371/journal.pone.0059043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen EC, Schrader HS, Rieland B, Thompson TL, Lee KW, Nickerson KW, Kokjohn TA. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl Environ Microbiol. 1998;64:575–80. doi: 10.1128/aem.64.2.575-580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jover LF, Cortez MH, Weitz JS. Mechanisms of multi-strain coexistence in host-phage systems with nested infection networks. J Theor Biol. 2013;332:65–77. doi: 10.1016/j.jtbi.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Modi SR, Lee HH, Spina CS, Collins JJ. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature. 2013;499:219–22. doi: 10.1038/nature12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chibani-Chennoufi S, Bruttin A, Dillmann M-L, Brüssow H. Phage-host interaction: an ecological perspective. J Bacteriol. 2004;186:3677–86. doi: 10.1128/JB.186.12.3677-3686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemire S, Figueroa-Bossi N, Bossi L. Bacteriophage crosstalk: coordination of prophage induction by trans-acting antirepressors. PLoS Genet. 2011;7:e1002149. doi: 10.1371/journal.pgen.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]