Summary
Laccases are oxidases that contain several copper atoms, and catalyse single‐electron oxidations of phenolic compounds with concomitant reduction of oxygen to water. The enzymes are particularly widespread in ligninolytic basidiomycetes, but also occur in certain prokaryotes, insects and plants. Depending on the species, laccases are involved in various biosynthetic processes contributing to carbon recycling in land ecosystems and the morphogenesis of biomatrices, wherein low‐molecular‐weight naturally occurring phenols serve as key enzyme substrates. Studies of these in vivo synthetic pathways have afforded new insights into fungal laccase applicability in green synthetic chemistry. Thus, we here review fungal laccase‐catalysed oxidations of naturally occurring phenols that are particularly relevant to the synthesis of fine organic chemicals, and we discuss how the discovered synthetic strategies mimic laccase‐involved in vivo pathways, thus enhancing the green nature of such reactions. Laccase‐catalysed in vivo processes yield several types of biopolymers, including those of cuticles, lignin, polyflavonoids, humus and the melanin pigments, using natural mono‐ or poly‐phenols as building blocks. The in vivo synthetic pathways involve either phenoxyl radical‐mediated coupling or cross‐linking reactions, and can be adapted to the design of in vitro oxidative processes involving fungal laccases in organic synthesis; the laccase substrates and the synthetic mechanisms reflect in vivo processes. Notably, such in vitro synthetic pathways can also reproduce physicochemical properties (e.g. those of chromophores, and radical‐scavenging, hydration and antimicrobial activities) found in natural biomaterials. Careful study of laccase‐associated in vivo metabolic pathways has been rewarded by the discovery of novel green applications for fungal laccases. This review comprehensively summarizes the available data on laccase‐catalysed biosynthetic pathways and associated applications in fine chemical syntheses.
Laccases in nature and in biotechnology
Laccases are copper‐containing oxidoreductases (EC 1.10.3.2) that catalyse the monoelectronic oxidation of various substrates (e.g. phenols, and aromatic or aliphatic amines) to the corresponding radicals, using molecular oxygen as the final electron acceptor. The enzymes are particularly widespread in ligninolytic basidiomycetes, but also occur in certain prokaryotes, insects and plants, indicating that the laccase redox process is ubiquitous in nature (Claus, 2003; Baldrian, 2006). Laccases play important roles in several biometabolic steps including those involved in fungal pigmentation, plant lignification, lignin biodegradation, humus turnover and cuticle sclerotization, wherein naturally occurring low‐molecular‐weight phenolic compounds and natural fibre polymers are utilized as substrates (O'Malley et al., 1993; Mayer and Staples, 2002; Ruiz‐Duenas and Martinez, 2009).
Studies of laccase action on natural organic substrates in vivo suggested that the enzymes would be useful in biotechnology. Of the laccases available from various species (e.g. bacteria, insects, fungi and plants), fungal laccases are of particular commercial interest because such enzymes have relatively high redox potentials; the enzymes are thus more suitable for use in oxidative processes than are other forms of laccases. Moreover, fungal laccases are secreted extracellularly and enzyme purification is thus very simple, particular advantages for biotechnological applications (Baldrian, 2006). Indeed, fungal laccases have proven to be useful in several areas of biotechnology, including organic syntheses, pulp/textile bleaching, bioremediation, chemical grafting and polymer surface modification (Kunamneni et al., 2008; Mikolasch and Schauer, 2009; Witayakran and Ragauskas, 2009). Laccase‐based oxidation is an alternative to traditional inorganic chemical oxidation. Compared with the latter type of oxidation, laccase‐catalysed reactions show enhanced specificity, a higher oxidation capacity and reduced formation of by‐products, and can be performed under mild reaction conditions. Thus, no hazardous organic solvents are required and it is not necessary to run reactions at extremely high or low pH values. Similarly, neither an increased temperature nor high pressure is required. However, these advantages are also in play when other oxidoreductases, such as peroxidases and catalases, are used (Zamocky and Koller, 1999; Ruiz‐Duenas and Martinez, 2009). The particularly valuable attributes of laccases, not afforded by other enzymes, are a broad substrate specificity and use of molecular oxygen as the final electron acceptor. Laccases are active on a wide range of natural substrates (including phenols, polyphenols, anilines, aryl diamines, methoxy‐substituted phenols, hydroxyindoles, benzenethiols and inorganic/organic metal compounds), making such green enzymes useful in the conduct of several types of oxidative reactions. The use of molecular oxygen as the final acceptor means that oxidation is cost‐effective owing to the abundance of oxygen in nature (Giardina et al., 2010).
The ‘Green chemistry’ approaches aim at the elimination of hazardous substances from chemical processes. The use of enzymes has been highlighted as one of the most fundamental tools leading to green chemistry goals because the catalysts are non‐hazardous itself and also minimize the production of toxic waste (Ran et al., 2008). Towards this end, enzymatic oxidations performed using fungal laccases have been extensively investigated in terms of the relevant mechanisms and to develop green synthetic applications (Riva, 2006; Kunamneni et al., 2008; Mikolasch and Schauer, 2009; Witayakran and Ragauskas, 2009). In particular, the use of naturally occurring phenol substrates is of great interest in terms of novel synthetic applications because such reactions fulfil the basic requirement of green chemistry, in that little toxic waste is produced (the natural substrates are eco‐friendly and an enzyme is a green catalyst). Natural phenols also serve as key substrates for laccase‐catalysed in vivo biosynthetic processes giving rise to natural organics showing various kinds of physicochemical functionalities. Such fact indicates that the biotechnological design of in vitro laccase oxidations involving small phenolics should be guided by the study of natural biometabolic steps. Although several reviews on synthetic applications of fungal laccases have recently appeared (Riva, 2006; Kunamneni et al., 2008; Mikolasch and Schauer, 2009; Witayakran and Ragauskas, 2009), neither the use of natural phenols as substrates nor the fundamental connection between novel synthetic applications and laccase‐related in vivo metabolism has been discussed. Thus, the principal objective of the present review is to summarize current research on fungal laccase‐catalysed oxidation of naturally occurring phenols, in processes that are highly applicable to synthetic chemistry. We discuss how such synthetic strategies can mimic laccase‐associated in vivo biometabolism, making industrial applications ever more ‘green’, and extending enzyme versatility.
Catalytic features of laccases and the implications for fine chemical synthesis
Laccases catalyse four‐electron substrate oxidations, resulting in reductive cleavage of a dioxygen bond; Cu metal atoms within the enzymes play key roles in the reduction of O2 to H2O. The Cu atoms of laccases include one copper of type 1 (Cu1), one of type 2 (Cu2) and two of type 3 (Cu3). Cu1 is the primary electron acceptor in laccase‐catalysed oxidation. The electrons are next transferred via a highly conserved His–Cys–His tripeptide to a trinuclear cluster (TNC) that includes the Cu2 and Cu3 atoms. The electrons finally reduce O2 to H2O (Solomon et al., 2008). The Cu1‐containing cavity on the enzyme surface is fairly wide, thus accommodating a large range of substrates. Cu1 is analogous to a door governing substrate electron entry to the catalytic site, and controls the catalytic rate; reduction of Cu1 is rate‐limiting in the overall catalytic process (Giardina et al., 2010). The redox potentials of Cu1 vary from 420 to 790 mV versus the normal hydrogen electrode, and such relatively low values limit laccase action to phenolic moieties, the redox potentials of which are sufficiently low to allow electron abstraction by Cu1 (Shleev et al., 2004; Canas and Camarero, 2010). The three Cu2/Cu3 ions, arranged in a triangular manner, facilitate dioxygen binding, leading to reduction of molecular oxygen using the four electrons transferred from Cu1 (Ducros et al., 1998; Giardina et al., 2010).
Laccase activity is present in various organisms including fungi, plants, bacteria and insects; the enzymes play key roles in either synthetic or mineralization processes. Laccases can be categorized into two groups of low or high redox potential. Low‐redox‐potential laccases occur in bacteria, plants and insects, whereas high‐redox‐potential enzymes are widely distributed in fungi. The latter enzyme forms are thus particularly useful for the conduct of oxidative processes required in various biotechnological applications (Cherkashin et al., 2007; Mikolasch and Schauer, 2009). White‐rot fungi are the main laccase producers, secreting several isozymes depending on the stage of the fungal life cycle. Most laccases are monomeric glycoproteins of average molecular mass 60–70 kDa, with a carbohydrate content of 10–20% (w/w), which may enhance enzyme stability (Baldrian, 2006; Giardina et al., 2010). Many factors, including nutrient levels, culture conditions and developmental stage, influence laccase synthesis and secretion. Some heavy metals (e.g. Cu2+, Ag+ and Mn2+) (Soden and Dobson, 2001) and lignin‐derived aromatic compounds (e.g. ferulic acid, xylidine and veratric acid) enhance laccase production. The enzymes are also thought to serve in the defence of organisms against oxidative stress caused by reactive oxygen species (ROS) derived from the actions of laccase inducers (Collins and Dobson, 1997). Phenol groups are typical laccase substrates because such enzymes often have low redox potentials. Phenols are oxidized to phenoxyl free radicals by the direct action of laccases, and next engage in either coupling‐based polymerization or radical rearrangement per se, yielding dead‐end products. However, depending on phenoxyl radical stability, redox reversibility featuring oxidation of target compounds may be observed; such reactions use the phenolic substrates as laccase mediators (Canas and Camarero, 2010). Radical‐based coupling or redox recycling of phenolic substrates improves the versatility of laccase catalytic action because non‐laccase substrates can serve as oxidation targets. Phenoxyl radicals are coupled with non‐laccase substrates, thus allowing for formation of new heteromolecular dimers. Otherwise, the phenoxyl radicals feature restored PhO–H bonds, resulting from cleavage of the benzylic C–H bonds of oxidation targets. Such hydrogen atom transfer oxidation, involving phenolic compounds, gives non‐laccase substrates the properties of reactive radicals, finally leading to various types of enzymatic biotransformation. The non‐specific nature of oxidations performed by laccases, combined with the use of enzyme immobilization technology, has encouraged use of the enzymes in green organic syntheses (Riva, 2006; Kunamneni et al., 2008; Mikolasch and Schauer, 2009; Witayakran and Ragauskas, 2009).
Representative laccase‐based oxidations of phenolic substrates are summarized in Fig. 1. When the phenolic substrates are natural, the enzymatic reactions are much greener because both substrates (phenols) and catalysts (laccases) are natural products and the in vitro changes mimic those of normal metabolism. Indeed, recent developments in fungal laccase applications in the field of synthetic chemistry have been driven by the results of studies of natural anabolism, involving phenols, in several species; the novel insights thus obtained suggested new enzyme applications. It is thus logical to review representative in vivo anabolic processes in which laccases and natural phenols play roles.
Figure 1.
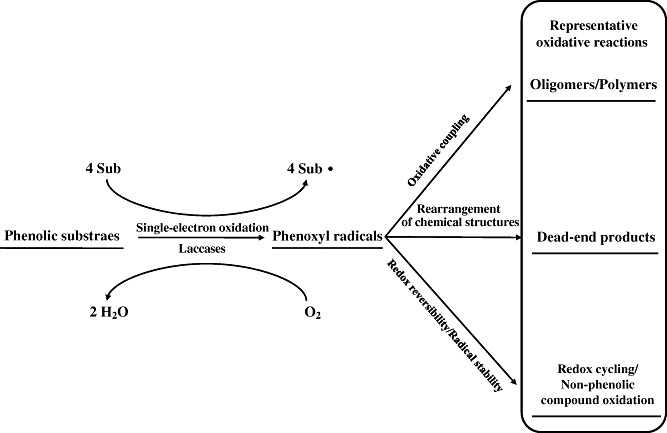
Representative oxidative reactions of phenolic substrates catalysed by laccase enzymes. Sub and Sub· indicate phenolics and phenoxyl radicals respectively. Four electrons obtained from laccase‐catalysed monoelectronic oxidations of four substrates are used for reduction of molecular oxygen to water molecule.
Laccase‐catalysed in vivo anabolic processes
Anabolism versus catabolism
Laccase‐catalysed reactions in fungi, insects and plants play key roles in processes important both on the organismal scale (e.g. in morphogenesis or expression of defensive systems) and in terms of ecosystem interaction (e.g. transformation of polyphenolics or carbon recycling). Representative processes include biodegradation of lignin (Eggert et al., 1997; Wei et al., 2010) and humus (Theuerl and Buscot, 2010); both materials are major components of carbon biomass in land ecosystems. Thus, laccase catabolic action contributes to carbon recycling. However, laccases also perform anabolic polymerizations or cross‐coupling reactions with preformed polymers, leading to synthesis of several biomatrices [e.g. in lignification (O'Malley et al., 1993) and humification (Chefetz et al., 1998)]. Laccase‐catalysed anabolism is also important for polymeric pigment synthesis (Kim et al., 2005) and cuticle sclerotization (Dittmer and Kanost, 2010), depending on the plant/insect species.
As mentioned above, laccases catalyse both anabolic and catabolic reactions. The main role of laccases involved in biometabolic processes is to transform substrates into the corresponding free radicals; the fates of such radicals are thus key in the characterization of reaction mechanisms. In general, laccase‐involved catabolism seems to require the presence of radicals that oxidize target compounds, owing to the low redox potential of laccases per se. Such reactions involve two different mechanisms. Methoxyhydroquinone, which can be produced via fungal metabolic actions and also transformed into a radical form via laccase action, initiates Fenton chemistry, in conjunction with O2 and Fe3+, finally leading to efficient production of ROS (Guillen et al., 2000; Wei et al., 2010). The other pathway boosting laccase oxidation power, and thus stimulating catabolic action, is redox mediation involving low‐molecular‐weight organic compounds (Canas and Camarero, 2010). These mediators are believed to be metabolites derived from oxidation targets, and potentiate in vivo laccase catabolism in a positive feedback manner. Such ROS‐ or mediator‐based oxidations occur principally in basidiomycetes that are able to achieve efficient lignocellulose biodegradation. This strongly indicates that specific metabolic features of basidiomycetes enable the laccases to catalyse powerful oxidative reactions. Some biochemical clues support the notion that fungal secretory organics (e.g. methoxyhydroquinone) (Wei et al., 2010) and other types of extracellular enzymes (e.g. peroxidases) (Schlosser and Hofer, 2002) act synergistically with laccase to destroy natural macromolecules.
In terms of anabolic processes, laccases readily catalyse oxidative coupling reactions, yielding either polymers or cross‐coupled organic materials. Such synthetic processes are ubiquitously found in several species and are involved in morphogenesis and soil humidification. In contrast to the inability of laccases to directly perform catabolic reactions, the redox potentials of the enzymes make coupling reactions thermodynamically possible without requiring additional materials (Mikolasch and Schauer, 2009; Witayakran and Ragauskas, 2009). Coupling reactions are generally conducted using low‐molecular‐weight natural phenols as laccase substrates, and yield several types of polymeric biomatrices. Such in vivo synthetic reactions can be readily reproduced in vitro, indicating that laccase reaction mechanisms and the properties of organic materials synthesized by laccase can provide new insights into green synthetic applications of such enzymes. The following sections examine the biochemical mechanisms and physicochemical properties of representative in vivo biosyntheses involving laccases.
Lignin and polyflavonoid synthesis in plants
Lignin and polyflavonoids are major building blocks of the plant fibre matrices of stems and seeds. Indeed, the role played by laccase in plant fibre synthesis was unclear for many years owing to difficulties associated with enzyme purification. However, technical progress in genetic manipulation and protein purification has provided critical evidence for laccase involvement in plant fibre synthesis (O'Malley et al., 1993). Laccases are ubiquitous enzymes in plant kingdom and seems to be expressed depending on plant development stages (Harakava, 2005; Caparros‐Ruiz et al., 2006; Dardick et al., 2010). Laccases are principally confined to plant cell walls, where lignin and polyflavonoid synthesis occurs. The localization of laccases to cell walls seems to be due to electrostatic interaction between the enzymes and plant fibres. For example, laccases that are strongly positively charged are found in pine xylem, attached to negatively charged fibre components such as pectins. Purified laccases from plant tissues are able to utilize monolignols and flavonoids as substrates (Driouich et al., 1992; Bao et al., 1993), supporting the hypothesis that these materials are enzymatically transformed into free radical intermediates, followed by coupling reactions that yield lignin and polyflavonoids.
Transgenic studies have provided valuable evidence for the involvement of laccases in plant fibre synthesis. The extent of oxidation of phenolic compounds in plant tissues is linked to the laccase expression level, and laccase downregulation impairs synthesis of the natural fibre matrices of xylem and seed coats (Ranocha et al., 2002; Pourcel et al., 2005). Also, addition of copper enhances laccase activity, followed by an increase in lignin synthesis, during the early developmental stages of plant tissues (Lin et al., 2005). These previous physiological studies strongly suggested that laccases were involved in phenolic polymer anabolism, resulting in plant fibre morphogenesis. One important question is: At what stage of lignin and polyflavonoid biosynthesis is laccase action necessary? A detailed analysis of the phenolic compounds in plant tissues clearly revealed that laccase‐mediated polymerization of low‐molecular‐weight natural phenols mainly occurs in apoplasts (Liu et al., 1994; Pourcel et al., 2005). Polymerization seems to be initiated by cell death‐driven bursting of vacuoles during periods of cell wall morphogenesis, allowing for transport of phenolics (e.g. monolignols, epicatechin and proanthocyanidins) into apoplasts, wherein laccases are abundant. Therefore, preformed natural phenolics synthesized via the monolignol or flavonoid pathways are first secreted into apoplasts and then polymerized by laccases. Oligomeric products derived from such reactions are non‐specifically covalently coupled to plant fibre components of cell walls.
The physicochemical properties of phenolic polymers derived from plant tissues have been also characterized in mutant plants. Laccase‐deficient plants showed delayed browning of seed coats, concomitant with an increase in the level of monomeric phenolics, indicating that phenol‐coupling reactions catalysed by laccases cause chromophore formation via repeated double‐bond conjugation; such reactions are essential for plant pigmentation (Pourcel et al., 2005). In addition, the polymeric products seem to be involved in pathogen defence systems. Two major pathways towards biosynthesis of antibacterial compounds have been suggested, based on the catalytic properties of laccase and the structural characteristics of phenolic oligomers (Dizhbite et al., 2004; Pourcel et al., 2005). These are, first, formation of reactive quinones from phenolic moieties via laccase action, followed by oxidative attack on pathogenic microbes; and, second, inhibition of essential microbial oxidative processes via induction of radical‐scavenging activities attributable to phenolic moieties or the several double bonds of phenolic oligomers. Such oligomers also have roles as building blocks, used to construct physical barriers that strengthen cell wall rigidity (Ranocha et al., 2002), thus protecting plant tissues from environmental stresses such as ultraviolet light and drought.
Insect cuticle sclerotization
Cuticle sclerotization is essential for stabilization of the insect exoskeleton (Hopkins and Kramer, 1992). Biochemical and mutagenic studies have revealed that laccase action is involved in cross‐coupling of phenolic materials to polymeric matrices consisting of proteins and chitin. Such processes induce morphogenetic diversity within the cuticle, finally creating highly hydrophobic, dense polymeric matrices. As with the plant pectin–laccase interactions described above, insect laccases are bound to cuticles, thus localizing the enzymes to a specific region of the cell. This means that the coupling reactions occur inside the cuticle matrix. By means of such reactions, empty spaces within cuticles are efficiently filled and the matrices are functionalized (Sugumaran et al., 1992). Catechol, N‐acetyldopamine and N‐β‐alanyldopamine serve as laccase substrates during sclerotization, although the sources of these low‐molecular‐weight materials remain to be determined (Kramer et al., 2001). The laccase reaction mechanisms involved in insect cuticle sclerotization are similar to those involved in plant fibre synthesis, in that phenolic compounds are transformed into quinones, followed by radical coupling with cuticle polymers. However, cross‐coupling to preformed polymeric materials seems to be more common during cuticle sclerotization than is self‐polymerization of phenolic monomers. The coupling reactions result in formation of carbon–nitrogen and carbon–oxygen covalent linkages between low‐molecular‐weight phenolics and histidyl residues of cuticular proteins.
The laccase‐dependent processes in cuticle synthesis are associated with morphogenesis wound healing, and immune system development in insects. Beetles in which laccase action was downregulated via RNA interference were paler in colour and more soft‐bodied than were wild‐type insects (Arakane et al., 2005). Such morphogenetic defects indicate that cross‐coupling of low‐molecular‐weight phenolics to protein‐based matrices is essential for cuticle pigmentation and hardening. The cross‐linking reactions increase the level of double‐bond conjugations in cuticle matrices, which thus become brown in colour. In addition, the positive charges of histidine imidazole groups, which act as hydrophilic sites, are easily blocked by cross‐coupling. Thus, the cuticles become water‐resistant, and serve as a barrier protecting against environmental stresses such as drought and pathogen invasion. Physical wounding and immune system challenge also induce laccase‐catalysed oxidation of low‐molecular‐weight phenolic compounds in insects. Polymeric products derived from enzymatic oxidation act as building blocks to fill wound sites. Such reactions can also encapsulate foreign invaders, inhibiting their proliferation in insects (Dittmer and Kanost, 2010).
Fungal melanogenesis
Fungal melanins produced by oxidative polymerization of low‐molecular‐weight phenolics by laccases are important secondary polymeric metabolites, contributing to several physiological responses of fungi. Melanins are known to be involved in fungal virulence, survival, pigmentation and longevity of propagules. Melanin synthesis catalysed by fungal laccases can use both endogenous and exogenous substrates (Eisenman et al., 2007; Frases et al., 2007). A well‐characterized dihydroxynaphthalene (DHN)‐melanin pathway involves the actions of several enzymes including polyketide synthases and laccases, and initiates DHN synthesis from acetyl‐CoA or malonyl‐CoA, followed by laccase‐catalysed transformation of preformed DHN into DHN‐melanin (Bell and Wheeler, 1986; Tsai et al., 1999). Otherwise, laccase substrates seem to arise from exogenous sources. Extracellularly provided homogentisic acid or dihydroxyphenylalanine significantly enhances melanin pigment synthesis in fungi. Such phenolic compounds are directly transformed into polymeric pigments by laccase (Eisenman et al., 2007; Frases et al., 2007). The role of laccases in melanogenesis involves induction of oxidative couplings of either exogenously or endogenously produced phenolic compounds. As is also the case in lignin and polyflavonoid biosynthesis and cuticle sclerotization, such coupling reactions yield phenolic macromolecules concomitant with development of pigmentation. Laccase anabolic action is also important for polyketide pigment synthesis. Laccase‐deficient fungus accumulates unidentified intermediate compounds during polyketide synthetic pathways (Kim et al., 2005), indicating that laccases catalyse biotransformation of the intermediates to polymeric pigments, as did in DHN‐melanin pathways.
The physiological roles of melanins in fungi reflect the physicochemical properties of the materials. Melanin synthesis causes fungi to become resistant to host immune systems, and such synthesis is thus correlated with fungal virulence. An interesting hypothesis is that melanin may scavenge ROS produced by phagocytic cells. Indeed, melanin is rich in unpaired electrons owing to the presence of many quinone moieties produced by laccase‐catalysed single‐electron oxidations of phenol moieties. Experimental evidence for the ability of melanin to scavenge radicals such as OH•, SO4•− and CCl3O2• has been reported (Rózanowska et al., 1999). The protective role of melanogenesis in terms of fungal virulence is enhanced by the adhesive properties of such materials. Melanins are negatively charged and strongly hydrophobic; these characteristics are attributable to the phenolic monomers present in the compounds. Hydroxyphenyl groups are easily deprotonated, resulting in the appearance of negative charges. In addition, the conjugated benzene rings of melanins are very hydrophobic. Owing to such adhesive properties, melanins are structural components required for correct assembly of fungal cell walls. These assembly patterns affect the adherence of fungi to host tissue, thereby affecting fungal virulence (Pihet et al., 2009).
Insights into synthetic applications
Representative laccase‐catalysed in vivo synthetic processes clearly show that laccase action in various species is important in the synthesis of functionalized building blocks used in morphogenesis. Naturally occurring phenols used in synthetic anabolic reactions are regarded as key laccase substrates, and the synthetic routes seem to be limited to oxidative coupling of natural phenols per se or cross‐coupling thereof into preformed biomacromolecules (Fig. 2). Representative data on laccase‐associated in vivo synthetic pathways are summarized in Table 1. The synthetic products basically serve as physical barriers, but also show varied physicochemical properties; the materials may have antioxidant or antibiotic functions, can be involved in chromophore formation, or may mediate electrostatic adherence. Such characteristics are mainly attributable to the hydroquinone, hydroxyphenyl or double‐bond moieties of the polymeric products. Purified laccases and plant‐derived phenols are excellent starting materials for synthetic reactions, yielding several oligomeric, polymeric and/or cross‐coupled organics. In principle, the conduct of such reactions in vitro can yield products with several valuable physicochemical characteristics ubiquitously found in biomatrices. Moreover, the properties of such synthesized materials may be further engineered, depending on the types of phenolic monomers used for oligomer and polymer syntheses. Recent developments in fungal laccase‐based synthetic applications have shown that mimicking of laccase‐dependent in vivo phenolic anabolism can offer new opportunities for the development of eco‐friendly applications. Towards this end, we next describe recent advances in our understanding of fungal laccase‐catalysed oxidations of naturally occurring phenols that are particularly applicable to in vitro organic syntheses, and discuss how studies of laccase‐associated in vivo anabolism have guided the development of useful biotechnological applications.
Figure 2.
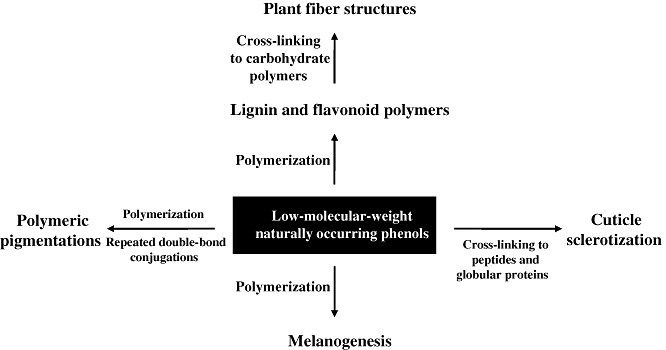
Laccase‐catalysed in vivo anabolic pathways show the key roles played by naturally occurring phenols of low molecular weight as laccase substrates.
Table 1.
List of laccases in in vivo anabolism associated with biological functions.
| In vivo laccase sources | Proposed anabolic functions of laccases | References |
|---|---|---|
| Zinnia elegans | Laccase anabolic actions correlated with lignin biosynthesis in Zinnia stems | Liu et al. (1994) |
| Populus trichocarpa | Xylem fibre synthesis contributing to cell wall structure | Ranocha et al. (2002) |
| Eucalyptus | Lignin biosynthesis through polymerization of monolignols without H2O2 | Harakava (2005) |
| Glycine max | Copper‐induced activation of laccases followed by enhancement of lignin synthesis | Lin et al. (2005) |
| Arabidopsis thaliana | Seed coat formation via oxidative polymerization of flavonoids | Pourcel et al. (2005) |
| Zea mays | Lignin polymerization for cell wall structures | Caparros‐Ruiz et al. (2006) |
| Prunus persica | Seed stone formation via lignin biosynthesis | Dardick et al. (2010) |
| Manduca sexta | Cuticle sclerotization via protein cross‐linking reactions | Kramer et al. (2001) |
| Tribolium castaneum | Cuticle sclerotization leading to tanning process | Arakane et al. (2005) |
| Cryptococcus neoformans | Melanin pigment synthesis through polymerization of various melanin precursors including homogentisic acid and dihydroxyphenylalanine | Williamson et al. (1998); Eisenman et al. (2007); Frases et al. (2007) |
| Aspergillus fumigatus | Melanogenesis through polymerization of dihydroxynaphthalene, a well‐known melanin precursor | Tsai et al. (1999) |
| Gibberella zeae | Polyketide pigment synthesis | Kim et al. (2005) |
| Chaetomium thermophilium | Humification during composting with precursors of phenolic compounds | Chefetz et al. (1998) |
Fungal laccase‐dependent synthetic applications derived from an understanding of in vivo anabolism
Lessons from laccase‐mediator system refinement
The first efforts to mimic natural metabolism and extend laccase applications used laccase‐mediator system (LMS) refinement. With this approach, studies of laccase‐catalysed catabolic steps yielded novel insights useful for the development of LMS. The non‐phenolic organics that form the major proportion of lignin cannot be directly oxidized by laccases owing to the low redox potentials of such enzymes. Indeed, it has been shown that laccases alone do not depolymerize lignin, but rather polymerize the material, generating minor lignin surface modifications (Bajpai, 1999). However, the finding that white‐rot fungi, which produce laccases as the principal secretory oxidases, can extensively mineralize lignin (Eggert et al., 1996; 1997), strongly suggested that small organic molecules acting as mediators of laccase activity should be present, making it possible for laccases to oxidize the recalcitrant polymer. Although genetic clues supporting potential role of other peroxidases in lignin mineralization have been identified (Morgenstern et al., 2010), this laccase mediator hypothesis has been shown to be correct; various laccase mediators have been discovered. The first compound identified was ABTS (2,2′‐azino‐bis[3‐ethylbenzothiazoline‐6‐sulfonic acid]) (Bourbonnais and Paice, 1990). In the time since the discovery of ABTS, a great deal of work has focused on identification of new mediators, and 1‐hydroxybenzotriazole (HBT), violuric acid and promazine have been described (Bourbonnais et al., 1997; Li et al., 1999).
Other than synthetic mediators, natural laccase substrates either secreted by fungi or that are common plant metabolites have been exploited as technologically suitable options owing to their low cost and environmental compatibility (Johannes and Majcherczyk, 2000; Camarero et al., 2005; Canas and Camarero, 2010). In particular, the experimental confirmation that naturally occurring phenols related in structure to lignin monomers act as laccase mediators suggested that novel LMS could be developed by closely mimicking the natural environment of lignin biodegradation mediated by fungi, which involves radical‐creating reactions catalysed by laccase. Lignin‐related phenols, including syringaldehyde, vanillin, acetosyringone and p‐coumaric acid, are derived from lignin biodegradation. Thus, it is not unreasonable to suggest that natural phenols may act as laccase mediators during in vivo destruction of lignin. Although the presence of natural laccase mediators has not been proved during in vivo wood decay with fungi, some lignin‐related phenols have mediation capacities similar to those of widely used synthetic mediators (i.e. ABTS and HBT) when used in processes designed to remove several types of organic pollutants, including dyes (Camarero et al., 2005; Murugesan et al., 2009), PAHs (Canas et al., 2007) and pesticides (Torres‐Duarte et al., 2009). Thus, laccase‐dependent natural mediation systems could be valuable for soil bioremediation.
As shown by the shift from use of synthetic mediators to natural compounds, studies of in vivo catabolic steps catalysed by laccases present in wood‐rotting fungi can offer new insights into LMS development. Similarly, study of in vivo anabolism has also guided developments in the synthetic applications of laccases. Here, we discuss how studies of synthetic applications and in vivo anabolism are mutually informative.
Materials and synthetic routes
As described above, naturally occurring phenols are key substrates in laccase‐catalysed anabolic actions. One important question is: Where can key materials involved in laccase‐catalysed anabolic actions be obtained for synthetic applications? Natural phenolics used as raw materials in laccase‐catalysed biotransformations are widely distributed in the plant kingdom and are easily extracted by simple mechanical processes. In general, homogenized plant materials, including fruits, vegetables, herbs and trees, are extracted using hydrophilic solvents (often water). The extracts are then lyophilized, yielding mixtures of natural phenolics in the solid phase; further extractions remove residual sugars and carbohydrates and thus increase the purity of natural phenolics. Several studies have shown that such simple extraction methods effectively yield natural phenolics from biomass (Jeon et al., 2009; Dai and Mumper, 2010). The tested biomasses include both edible and waste plant materials, indicating that phenolics can be obtained cheaply and safely. Laccases are readily purified from fermentations performed by white‐rot fungi because the enzymes are secreted extracellularly. Several laccases from edible white‐rot fungi that are safe to handle have been obtained in quantity (Baldrian, 2006). Remarkably, crude laccases directly harvested from liquid or solid cultures, thus not subjected to elaborate purification steps, have proven to be effective in synthetic applications (Mikolasch and Schauer, 2009; Witayakran and Ragauskas, 2009). Thus, the green catalysts are cheap to prepare and use. In conclusion, both laccases and naturally occurring phenols, key components of laccase‐catalysed in vivo anabolism in various species, are safely and cheaply available for use in synthetic chemistry.
As shown for the in vivo anabolic steps catalysed by laccases, the reactions of natural phenols in vitro are limited to oxidative coupling of natural phenols per se or cross‐coupling to preformed macromolecules. In vitro coupling reactions involving phenolic substrates are initiated by the formation of radical cations from hydroxyphenyl groups, followed by intermolecular attack, producing homo‐ or heterodimers (Mikolasch and Schauer, 2009; Jeon et al., 2010). Structural changes in dimers occur upon electron delocalization, leading to the formation of new covalent bonds. Through such coupling reactions, carbon–carbon, carbon–oxygen and carbon–nitrogen conjugations between phenoxyl radicals and coupled organics are preferentially formed. Oligomers and polymers can also be synthesized from such dimers, but this requires much longer incubation times. The oxidative coupling reactions can also be extended to achieve fibre surface functionalization via cross‐coupling to preformed macro‐matrices; phenoxyl radicals formed by laccase action seem to be involved in this process in two different ways. First, a LMS with an oxidation capacity much greater than that of laccase alone results in fibre bleaching (Camarero et al., 2007). Under the LMS‐based bleaching, phenoxyl radicals derived from laccase action on natural phenolics take hydrogen radicals from the surfaces of fibres (i.e. redox cycling of natural phenols between laccases and fibres). The oxidized surfaces of fibres further perform electron delocalization, finally leading to oxidative bond cleavage of fibres (i.e. bleaching process via LMS). Second, oxidative coupling between monomeric radicals and polymer surfaces enzymatically grafts phenolic monomers onto fibres, thus giving rise to the physicochemical characteristics of the monomers on the fibre surface (Aracri et al., 2010). Unlike the LMS‐based bleaching, in this coupling process, phenoxyl radicals are nucleophilically attacked to surfaces of fibres, resulting in the formation of new covalent bonds between natural phenols and the surfaces of fibres (i.e. grafting via cross‐coupling). Both mechanisms seem to be simultaneously active; bleaching and grafting are thus competitive in nature. However, several studies have shown that laccase‐catalysed oxidations of natural phenols effectively modify the surfaces of natural and artificial polymeric materials via cross‐coupling.
The products of laccase‐catalysed oxidations of natural phenols are diverse, ranging from dimers to macromolecules. For example, dimeric products can be synthesized from totarol (Ncanana et al., 2007) and 17β‐oestradiol (Nicotra et al., 2004b) via oxidative coupling‐induced carbon–carbon or carbon–oxygen bond formation. Additionally, intramolecular coupling is apparent when natural phenolics are used as substrates. As shown by the biotransformation of procyanidin B‐2 into procyanidin A‐2 by laccases (Osman and Wong, 2007), a radical site formed by electron abstraction catalysed by laccase precedes nucleophilic attack by a hydroxyl group located at another site, finally leading to formation of a new covalent intramolecular bond. This intra‐coupling process seems to require the proximity of electron‐rich functional groups that readily mount nucleophilic attacks on laccase‐reactive hydroxyphenyl moieties. Furthermore, enzymatic synthesis of much higher‐molecular‐weight phenolic polymers is also feasible. The molecular weights and yields of polymers can be manipulated by varying the reaction time, the buffer composition and the initial monomer concentration; laccase‐catalysed polymerization of methoxyphenols (Tanaka et al., 2010) and syringic acid (Ikeda et al., 1996) resulted in synthesis of polymers of more than 18 000 Da when reaction time and buffer composition were appropriately adjusted. The use of mediators such as HBT and polyoxometalates during natural phenol polymerization can also effectively increase the molecular weights of the final products (Prasetyo et al., 2010; Kim et al., 2011). The polymerization reactions described above are based on bulk supply of monomers. However, end‐wise polymerization conducted with gradual supply of monomers seems to occur preferentially during plant fibre synthesis such as lignifications (Morreel et al., 2004). This end‐wise coupling process gives rise to different coupling bond formation and increased molecular weight of polymers (Grabber et al., 2003). Together with the use of organic solvents and redox mediators in the laccase‐catalysed polymerizations, the fine control of monomer supply might lead to much greater heterogeneity of polymerization products.
Laccase oxidative action induces structural variability during polymerization of natural phenols; hydroxyl groups of polymers are modified with methoxy or hydrogen groups, or monomer composition in polymers is variable. Laccases catalyse non‐specific radical reactions using natural phenols as substrates. The hydroxyl groups of natural phenols are the principal electron‐donating moieties, and radical sites are readily formed at these locations via laccase oxidative action using molecular oxygen. The radical sites move to different places via electron delocalization, resulting in the appearance of various radical intermediates from a single phenoxyl radical. Such formation of non‐specific intermediates contributes to the structural heterogeneity of polymeric products, including non‐specific substitution of hydroxyl sites with methoxy or hydrogen groups (Marjasvaara et al., 2006). Consistent with what has been found when homopolymers are synthesized from single monomers, heteropolymers, composed of more than two types of monomers, can be readily synthesized by the action of laccases on natural phenol blends. From such phenolic mixtures, several types of phenoxyl radicals can be simultaneously or sequentially formed, depending on the substrate specificities of the laccase for the natural phenols, thus facilitating coupling of different monomers (i.e. heteropolymer synthesis). Analyses of the products of laccase‐catalysed oxidations of dual‐natural phenol blends indicate that both the enzyme substrate specificities and the tendency of laccase‐generated radicals to couple with other monomers interact in a complex manner to determine monomer composition of the heteropolymer (Jeon et al., 2010). Two interesting features distinguish heteropolymer from homopolymer synthesis with respect to biotechnological development. First, compared with homopolymer synthesis from single monomers, enzymatic heteropolymer synthesis using natural phenol blends yields polymers that are diverse in function owing to structural diversity caused by variation in monomer composition. Second, desirable qualities of polymers enzymatically synthesized for biotechnological applications are easily engineered via an appropriate choice of monomer (Mikolasch and Schauer, 2009; Jeon et al., 2010).
Green materials such as laccases and natural phenolics can be cheaply and safely obtained from fungal cultures and biomasses respectively. Several studies on organic syntheses in vitro have shown that coupling reactions proceed efficiently, yielding polymeric products formed via coupling of natural phenols per se, or surface modifications of macromolecules via cross‐coupling with natural phenolics. Overall, the key synthetic routes of laccase‐catalysed in vivo anabolism are readily reproduced in vitro, thus allowing green reactions to be used in biotechnology.
Physicochemical features of polymers useful in biotechnology
Apart from the development of new synthetic routes, and characterization of the products of laccase‐catalysed oxidations of natural phenols, novel functional properties imparted by special physical or chemical features of the synthesized materials have been of great interest because such functionalities are important in biotechnological applications. We describe the physicochemical properties of organic materials synthesized via laccase action on natural phenols, and discuss how such properties are shared with those of polymers synthesized by in vivo anabolic reactions.
The physicochemical properties of coupling or grafting products oxidatively synthesized from natural phenols and laccases can largely be predicted from the properties of the monomeric natural phenols (Uyama, 2007; Jeon et al., 2009). Natural phenols are versatile in function and have antioxidant, anti‐inflammatory, antibiotic, antimutagenic and immunoregulatory activities. One important question is whether laccase‐mediated oligomerization and polymerization of natural phenols might enhance the functional properties of such materials. Several studies have shown that laccase‐catalysed polymerization of natural phenols, including rutin (Kurisawa et al., 2003), epigallocatechin gallate (Kurisawa et al., 2004) and catechin (Chung et al., 2003), yields antioxidant materials that are more powerful than the natural monomeric phenols. The fact that such in vitro synthetic processes enhance antioxidant capacity is consistent with the fact that high‐molecular‐weight natural polyphenols are much stronger antioxidants than are low‐molecular‐weight polyphenols of plants (Ihara et al., 2004). Although exact mechanisms regarding enhanced antioxidant effects of high‐molecular‐weight phenolics and coupling products of phenolics remain to be determined, it seems to be clear that the proximity of several aromatic rings and hydroxyl groups are more important for free radical scavenging than specific functional groups of natural phenols (Hagerman et al., 1998). Notably, natural phenol‐derived products were also strong inhibitors of certain enzymes involved in disease (i.e. xanthine oxidase, collagenase and tyrosinase); this was attributed to the development of non‐covalent interactions between the synthesized green materials and the enzymes (Ihara et al., 2004; Kurisawa et al., 2004; Uyama, 2007).
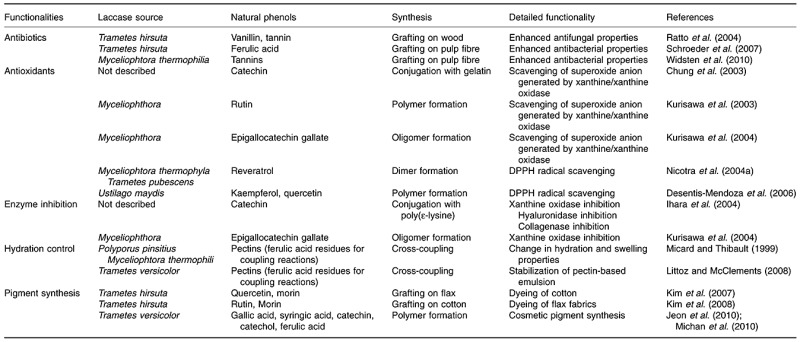
Another functional feature of coupled materials is their antibiotic properties. Natural phenols can be coupled to well‐known antibiotic compounds, to synthesize new drugs, or cross‐coupled with macro‐matrices to functionalize surfaces. Use of methylcatechol as a laccase substrate, in reactions with antibiotics such as ampicillin and cefadroxil, resulted in heterodimer coupled products via nuclear amination, but the antibiotic potencies of the resulting derivatives were similar to those of the original antibiotics (Mikolasch et al., 2008). However, natural phenol grafting mediated by laccase action yielded fibres with enhanced antibiotic properties. For example, tannins with antibiotic properties (the ability to achieve bacterial cell wall penetration and to chelate essential iron) were covalently immobilized onto lignocellulosic materials by laccase oxidative action. The modified fibres showed antibacterial effects on both Gram‐positive and ‐negative bacteria (Widsten et al., 2010). It has also been shown that grafting with vanillin imparted antifungal properties to wood fibres, indicating that laccase‐catalysed grafting of natural phenols can be used to make wood preservatives that inhibit colonization by wood‐decaying fungi (Ratto et al., 2004).
Gelation or hydration activities of biomacromolecules such as pectin can be manipulated using laccase‐catalysed coupling reactions. Phenolic moieties present in such biopolymers act as laccase‐reactive sites for coupling reactions; in the case of pectins, ferulic acid residues serve as the principal coupling sites. Globular protein‐coated oil‐in‐water emulsions can be rendered more stable via laccase‐catalysed coupling of pectins. The coupling of pectin to protein seems to increase protein ionic strength, thus inhibiting lipid droplet aggregation (Littoz and McClements, 2008). In addition, laccase‐catalysed oxidative coupling of pectins per se controls the extent of hydration of pectin powders. Such behaviour is associated with the compact structures of pectin‐based biopolymers, achieved via oxidative coupling (Micard and Thibault, 1999). The extent of oxidative coupling in pectins is proportional to the compactness of pectin aggregates, and enhanced coupling renders pectin less sensitive to variation in ionic strength in the presence of water. Such eco‐friendly modifications of biopolymers are particularly useful in the food processing industries (the biopolymers can be used as stabilizing or thickening agents) and in medical applications (the polymers are useful for drug encapsulation or to form biocontainers for controlled release of active molecules).
Chromophore formation by oxidative coupling of natural phenols is also of particular interest owing to the need for green processes in dye synthesis. During coupling reactions resulting in oligomer or polymer synthesis, chromophore formation is achieved via repetitive double‐bond conjugation; colourful materials are thus synthesized (Kim et al., 2007; 2008; Jeon et al., 2010). To date, the textile industry is the main area in which eco‐coloration has been valuable. Dyeing of cotton and flax can be achieved via laccase‐catalysed flavonoid‐mediated coloration followed by grafting of polymeric dyes into fabrics. Such polymerized flavonoids are generally dark brown in colour, and the extent of coupling to fabric surfaces can be manipulated by varying pH, temperature and the extent of mechanical agitation (Kim et al., 2007; 2008). Apart from applications in textile dyeing, enzymatically controlled coloration is relevant in the fields of cosmetic production and food processing, owing to the eco‐friendly features of the synthesized dyes (Jeon et al., 2010). The use of phenolics from plant fibres, and blends thereof, in laccase‐catalysed polymerization, yields diverse colours in the visible spectrum, thus permitting desirable colours to be precisely formulated. For example, a deep black colour required for cosmetic hair applications in Asia was synthesized using a combination of catechin and catechol. In addition, the statistical associations between compositions of the phenol blends and colour values of the synthesized polymers (i.e. the Hunter L*, a* and b* values) are significant, indicating that organic synthesis of desirable colours can be predicted by mathematical modelling of phenolic blends (Jeon et al., 2010).
Representative applications of the use of laccases and natural phenols in organic syntheses are summarized in Table 2. In general, the use of natural phenolic compounds in such syntheses has facilitated the development of new multifunctional oligomeric/polymeric products with antibiotic, hydration and antioxidant properties that inhibit disease‐associated enzymes and absorb visible light. Based on such useful physicochemical properties, applications of in situ laccase‐catalysed polymerization, or the resulting polymeric products, in the food, cosmetic and medical fields are becoming commonplace. Interestingly, laccase‐catalysed synthetic reactions in various species can produce such valuable products. As described above, the antioxidant activities of phenolic oligomers/polymers are used in plants and fungi as immune defences. Radical scavenging by phenolic products is an antibiotic property, inhibiting key metabolic steps of invaders. Dehydration is essential to ensure cell wall solidification in plants and insects. Complex laccase‐catalysed coupling of monolignols to polysaccharides such as pectins or the coupling of small phenols to peptides contributes greatly to dehydration. Detailed reaction routes for the in vitro dehydration are also shared with in vivo laccase synthetic reactions. For example, as shown for in vitro coupling reactions of pectins, which yield products valuable in the food‐processing industry, lignin matrices become stiffened and dehydrated during cell wall development via formation of diferulic linkages between polysaccharide‐bound lignins and polysaccharides. Chromophore formation, yielding organic dyes, is also associated with several biological processes including plant fibre synthesis (Pourcel et al., 2005; Dardick et al., 2010) and mushroom coloration (Frases et al., 2007), both of which involve laccase action on natural phenols. The synthesis of coloured polymers in vivo is generally accompanied by natural fibre synthesis that yields flavonoid polymers (Pourcel et al., 2005) and lignins (Ranocha et al., 2002; Dardick et al., 2010), both of which are ubiquitous constituents of many biomatrices.
Table 2.
Laccase‐catalysed oxidation of naturally occurring phenols for organic synthesis.
The in vivo synthetic steps involving laccase action in the creation of notable biological phenomena are readily reproduced in vitro, indicating that the development of novel synthetic applications of fungal laccases should be guided by studies of in vivo anabolic pathways involving the enzymes. For example, the diversity of colour shadings of biomatrices derived during natural fibre synthesis indicate that the colour range of organic dyes synthesized in vitro should be much broader than is currently the case. Colour diversification, thus maximizing colour choice, is essential for extension of organic dye applicability in situations in which the esthetic features of dyes are important. The main factor influencing colour diversity is inclusion of various types of natural phenols in polymerization reactions. This notion is supported by the observation that diverse phenolic compounds are contained in black liquors derived from wood fibres (Ibarra et al., 2006; Gutierrez et al., 2007). Notably, this insight encouraged the conduct of experiments showing that variations in natural phenolic substrate compositions used for laccase‐catalysed organic dye synthesis yielded products with different shades of colour (Jeon et al., 2010). Close mimicking of the natural actions of laccases has led to the discovery of even more versatile properties, thus extending the applications of laccase‐catalysed organic syntheses.
What further work on laccase‐catalysed syntheses is required? The synthesis of organic composites composed of natural phenols, followed by studies on the adsorptive properties, remain important owing to the great demand for organic adsorbents. Previous in vivo studies on laccase‐catalysed plant fibre morphogenesis and the adsorptive properties of the fibres thus synthesized provided valuable clues for the development of in vitro applications. In fact, the present authors have observed that the adsorptive properties of polymers synthesized by laccase from natural phenols are similar to those of plant biomasses such as lignins (unpublished data). The conjugation of natural phenolics to protein, mediated by laccase, may allow the in vitro synthesis of insect cuticle‐like materials with high wet strength. Such a synthetic approach to development of a cuticle‐like material has been shown to be feasible, although tyrosinase was also used (Miessner et al., 2001).
Conclusions
Laccase‐catalysed oxidations are biochemically versatile, and the enzymes accept a wide range of substrates; laccases are thus very useful in organic syntheses. The innate green nature of synthetic applications involving laccase can be further enhanced by using low‐molecular‐weight natural phenols as substrates. Such phenols are transformed by laccase into phenoxyl radicals that subsequently participate in either oxidative coupling of natural phenols per se, or cross‐coupling to preformed macromolecules, yielding several types of oligomers/polymers with diverse physicochemical properties useful in the food, medical and cosmetic industries. Interestingly, such coupling reactions are prevalent in nature because natural phenols are key in vivo substrates of laccases in fungal, plant and insect systems. Several properties (i.e. antibiotic and antioxidant activities, dehydration ability and visible light absorbance) that appear during the progress of in vivo synthetic reactions of plant fibre morphogenesis, fungal melanogenesis and insect cuticle hardening can be reproduced in vitro, indicating that in vivo features of laccase action can guide laccase applications in biotechnology. As research on organic syntheses has shown, future novel green applications of fungal laccases will be optimized if close attention is paid to laccase‐associated in vivo metabolic processes.
Acknowledgments
This work was supported by a Korean Science and Engineering Foundation (KOSEF) grant from the Korean government (MEST) (No. R01‐2008‐000‐20244‐0) and Brain Korea 21 project. P. Baldrian was supported by the Institutional Research Concept of the Institute of Microbiology ASCR (AV0Z50200510).
References
- Aracri E., Fillat A., Colom J.F., Gutierrez A., del Rio J.C., Martinez A.T., Vidal T. Enzymatic grafting of simple phenols on flax and sisal pulp fibres using laccases. Bioresour Technol. 2010;101:8211–8216. doi: 10.1016/j.biortech.2010.05.080. [DOI] [PubMed] [Google Scholar]
- Arakane Y., Muthukrishnan S., Beeman R.W., Kanost M.R., Kramer K.J. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci USA. 2005;102:11337–11342. doi: 10.1073/pnas.0504982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai P. Application of enzymes in the pulp and paper industry. Biotechnol Prog. 1999;15:147–157. doi: 10.1021/bp990013k. [DOI] [PubMed] [Google Scholar]
- Baldrian P. Fungal laccases – occurrences and properties. FEMS Microbial Rev. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- Bao W., O'Malley D.M., Whetten R., Sederoff R.R. A laccase associated with lignification in loblolly pine xylem. Science. 1993;260:672–674. doi: 10.1126/science.260.5108.672. [DOI] [PubMed] [Google Scholar]
- Bell A.A., Wheeler M.H. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol. 1986;24:411–451. [Google Scholar]
- Bourbonnais R., Paice M.G. Oxidation of non‐phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M.G., Freiermuth B., Bodie E., Borneman S. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol. 1997;63:4627–4632. doi: 10.1128/aem.63.12.4627-4632.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero S., Ibarra D., Martinez M.J., Martinez A.T. Lignin‐derived compounds as efficient laccase mediators for decolorization of different type of recalcitrant dyes. Appl Environ Microbiol. 2005;71:1775–1784. doi: 10.1128/AEM.71.4.1775-1784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero S., Ibarra D., Martinez A.T., Romero J., Gutierrez A., del Rio J.C. Paper pulp delignification using laccase and natural mediators. Enzyme Microb Technol. 2007;40:1264–1271. [Google Scholar]
- Canas A.I., Camarero S. Laccases and their natural mediators: biotechnological tools for sustainable eco‐friendly processes. Biotechnol Adv. 2010;28:694–705. doi: 10.1016/j.biotechadv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Canas A., Alcalde M., Plou F.J., Matinez M.J., Martinez A.T., Camarero S. Transformation of polycyclic aromatic hydrocarbons by laccase is strongly enhanced by phenolic compounds present in soil. Environ Sci Technol. 2007;41:2964–2971. doi: 10.1021/es062328j. [DOI] [PubMed] [Google Scholar]
- Caparros‐Ruiz D., Fornale S., Civardi L., Puigdomenech P., Rigau J. Isolation and characterization of a family of laccases in maize. Plant Sci. 2006;171:217–225. [Google Scholar]
- Chefetz B., Chen Y., Hadar Y. Purification and characterization of laccase from Chaetomium thermophilium and its role in humification. Appl Environ Microbiol. 1998;64:3175–3179. doi: 10.1128/aem.64.9.3175-3179.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkashin E.A., Stepanova E.V., Landesman E.O., Koroleva O.V., Tishkov V.I. Comparative analysis of gene sequences of three high‐redox‐potential laccases from basidiomycetes. Dokl Biochem Biophys. 2007;417:348–351. doi: 10.1134/s1607672907060178. [DOI] [PubMed] [Google Scholar]
- Chung J.E., Kurisawa M., Uyama H., Kobayashi S. Enzymatic synthesis and antioxidant property of gelatin‐catechin conjugates. Biotechnol Lett. 2003;25:1993–1997. doi: 10.1023/b:bile.0000004391.27564.8e. [DOI] [PubMed] [Google Scholar]
- Claus H. Laccases and their occurrence in prokaryotes. Arch Microbiol. 2003;179:145–150. doi: 10.1007/s00203-002-0510-7. [DOI] [PubMed] [Google Scholar]
- Collins P.J., Dobson A. Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol. 1997;63:3444–3450. doi: 10.1128/aem.63.9.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: extraction, analysis, and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardick C.D., Callahan A.M., Chiozzotto R., Schaffer R.J., Piagnani M.C. Stone formation in peach fruit exhibits spatial coordination of the lignin and flavonoid pathways and similarity to Arabidopsis dehiscence. BMC Biol. 2010;8:13–29. doi: 10.1186/1741-7007-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desentis‐Mendoza R.M., Hernandez‐Sanchez H., Moreno A., Rojas del c E., Chel‐Guerrero L.C., Tamariz J., Jaramillo‐Flores M.E. Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis. Biomacromolecules. 2006;7:1845–1854. doi: 10.1021/bm060159p. [DOI] [PubMed] [Google Scholar]
- Dittmer N.T., Kanost M.R. Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem Mol Biol. 2010;40:179–188. doi: 10.1016/j.ibmb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Dizhbite T., Telysheva G., Jurkjane V., Viesturs U. Characterization of the radical scavenging activity of lignins – natural antioxidants. Bioresour Technol. 2004;95:309–317. doi: 10.1016/j.biortech.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Driouich A., Laine A.‐C., Vian B., Faye L. Characterization and localization of laccase forms in stem and cell cultures of sycamore. Plant J. 1992;2:13–24. [Google Scholar]
- Ducros V., Brzozowski A.M., Wilson K.S., Brown S.H., Ostergaard P., Schneider P. Crystal structure of the type‐2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol. 1998;5:310–316. doi: 10.1038/nsb0498-310. et al. [DOI] [PubMed] [Google Scholar]
- Eggert C., Temp U., Eriksson K.E.L. The ligninolytic system of the white‐rot fungus Pycnoporus cinnabarinnus: purification and characterization of the laccase. Appl Environ Microbiol. 1996;62:1511–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert C., Temp U., Eriksson K.E.L. Laccase is essential for lignin degradation by the white‐rot fungus Pycnoporus cinnabarinnus. FEBS Lett. 1997;407:89–92. doi: 10.1016/s0014-5793(97)00301-3. [DOI] [PubMed] [Google Scholar]
- Eisenman H.C., Mues M., Weber S.E., Frases S., Chaskes S., Gerfen G., Casadevall A. Cryptococcus neoformans laccase catalyses melanin synthesis from both D‐ and L‐DOPA. Microbiology-SGM. 2007;153:3954–3962. doi: 10.1099/mic.0.2007/011049-0. [DOI] [PubMed] [Google Scholar]
- Frases S., Salazar A., Dadachova E., Casadevall A. Cryptococcus neoformas can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl Environ Microbiol. 2007;73:615–621. doi: 10.1128/AEM.01947-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina P., Faraco V., Pezzella C., Piscitelli A., Vanhulle S., Sannia G. Laccases: a never‐ending story. Cell Mol Life Sci. 2010;67:369–385. doi: 10.1007/s00018-009-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabber J.H., Hatfield R.D., Ralph J. Apoplastic pH and monolignol addition rate effects on lignin formation and cell wall degradability in Maize. J Agric Food Chem. 2003;51:4984–4989. doi: 10.1021/jf030027c. [DOI] [PubMed] [Google Scholar]
- Guillen F., Gomez‐Toribio V., Martinez M.J., Martinez A.T. Production of hydroxyl radical by the synergistic action of fungal laccase and aryl alcohol oxidase. Arch Biochem Biophys. 2000;383:142–147. doi: 10.1006/abbi.2000.2053. [DOI] [PubMed] [Google Scholar]
- Gutierrez A., Rencoret J., Ibarra D., Molina S., Camarero S., Romero J. Removal of lipophilic extractives from paper pulp by laccase and lignin‐derived phenols as natural mediators. Environ Sci Technol. 2007;41:4124–4129. doi: 10.1021/es062723+. et al. [DOI] [PubMed] [Google Scholar]
- Hagerman A.E., Riedl K.M., Jones A., Sovik K.N., Ritchard N.T., Hartzfeld P.W., Riechel T.L. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Harakava R. Genes encoding enzymes of the lignin biosynthesis pathway in Eucalyptus. Genet Mol Biol. 2005;28:601–607. [Google Scholar]
- Hopkins T.L., Kramer K.J. Insect cuticle sclerotization. Annu Rev Entomol. 1992;37:273–302. [Google Scholar]
- Ibarra D., Romero J., Martinez M.J., Martinez A.T., Camarero S. Exploring the enzymatic parameters for optimal delignification of eucalypt pulp by laccase‐mediator. Enzyme Microb Technol. 2006;39:1319–1327. [Google Scholar]
- Ihara N., Schmitz S., Kurisawa M., Chung J.E., Uyama H., Kobayashi S. Amplification of inhibitory activity of catechin against disease‐related enzymes by conjugation on poly(ε‐lysine) Biomacromolecules. 2004;5:1633–1636. doi: 10.1021/bm049823x. [DOI] [PubMed] [Google Scholar]
- Ikeda R., Uyama H., Kobayashi S. Novel synthetic pathway to a poly(phenylene oxide): laccase‐catalyzed oxidative polymerization of syringic acid. Macromolecules. 1996;29:3053–3054. [Google Scholar]
- Jeon J.R., Kim E.J., Kim Y.M., Murugesan K., Kim J.H., Chang Y.S. Use of grape seed and its natural polyphenol extracts as a natural organic coagulant for removal of cationic dyes. Chemosphere. 2009;77:1090–1098. doi: 10.1016/j.chemosphere.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Jeon J.R., Kim E.J., Murugesan K., Park H.K., Kim Y.M., Kwon J.H. Laccase‐catalyzed polymeric dye synthesis from plant‐derived phenols for potential application in hair dyeing: enzymatic colorations driven by homo‐ or hetero‐polymer synthesis. Microb Biotechnol. 2010;3:324–335. doi: 10.1111/j.1751-7915.2009.00153.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes C., Majcherczyk A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol. 2000;66:524–528. doi: 10.1128/aem.66.2.524-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.‐E., Han K.‐H., Jin J., Kim J.‐C., Yun S.‐H., Lee Y.‐W. Putative polyketide synthase and laccase genes for biosynthesis of aurofusarin in Gibberella zeae. Appl Environ Microbiol. 2005;71:1701–1708. doi: 10.1128/AEM.71.4.1701-1708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Moldes D., Cavaco‐Paulo A. Laccase for enzymatic coloration of unbleached cotton. Enzyme Microb Technol. 2007;40:1788–1793. [Google Scholar]
- Kim S., Lopez C., Guebitz G., Cavaco‐Paulo A. Biological coloration of flax fabrics with flavonoids using laccase from Trametes hirsuta. Eng Life Sci. 2008;8:324–330. [Google Scholar]
- Kim S., Silva C., Evtuguin D.V., Gamelas J.A.F., Cavaco‐Paulo A. Polymetalate/laccase‐mediated oxidative polymerization of catechol for textile dyeing. Appl Microbiol Biotechnol. 2011;89:981–987. doi: 10.1007/s00253-010-2932-5. [DOI] [PubMed] [Google Scholar]
- Kramer K.J., Kanost M.R., Hopkins T.L., Jiang H., Zhu Y.C., Xu R. Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron. 2001;57:385–392. et al. [Google Scholar]
- Kunamneni A., Camarero S., Garcia‐Burgos C., Plou F.J., Ballesteros A., Alcalde M. Engineering and applications of fungal laccases for organic synthesis. Microb Cell Fact. 2008;7:32–48. doi: 10.1186/1475-2859-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisawa M., Chung J.E., Uyama H., Kobayashi S. Enzymatic synthesis and antioxidant properties of poly(rutin) Biomacromolecules. 2003;4:1394–1399. doi: 10.1021/bm034136b. [DOI] [PubMed] [Google Scholar]
- Kurisawa M., Chung J.E., Uyama H., Kobayashi S. Oxidative coupling of epigallocatechin gallate amplifies antioxidant activity and inhibits xanthine oxidase activity. Chem Commun (Camb) 2004;294:294–295. doi: 10.1039/b312311a. [DOI] [PubMed] [Google Scholar]
- Li K., Xu F., Eriksson K.‐E. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compounds. Appl Environ Microbiol. 1999;65:2654–2660. doi: 10.1128/aem.65.6.2654-2660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.C., Chen L.M., Liu Z.H. Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci. 2005;168:855–861. [Google Scholar]
- Littoz F., McClements D.J. Bio‐mimetic approach to improving emulsion stability: cross‐linking adsorbed beet pectin using laccase. Food Hydrocolloids. 2008;22:1203–1211. [Google Scholar]
- Liu L., Dean J.F.D., Friedman W.E., Eriksson K.E.L. A laccase‐like phenoloxidase is correlated with lignin biosynthesis in Zinnia elegans stem tissues. Plant J. 1994;6:213–224. [Google Scholar]
- Marjasvaara A., Torvinen M., Kinnunen H., Vainiotalo P. Laccase‐catalyzed polymerization of two phenolic compounds studied by matrix‐assisted laser desorption/ionization time‐of‐flight and electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry with collision‐induced dissociation experiments. Biomacromolecules. 2006;7:1604–1609. doi: 10.1021/bm060038p. [DOI] [PubMed] [Google Scholar]
- Mayer A.M., Staples R.C. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- Micard V., Thibault J.‐F. Oxidative gelation of sugar‐beet pectins: use of laccases and hydration properties of the cross‐linked pectins. Carbohydr Res. 1999;39:265–273. [Google Scholar]
- Michan C., Daniels C., Fernandez M., Solano J., Campa A.M., Ramos J.L. Sugar (ribose), spice (peroxidase) and all thing nice (laccase hair‐dyes) Microb Biotechnol. 2010;3:131–133. doi: 10.1111/j.1751-7915.2010.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miessner M., Peter M.G., Vincent J.F.V. Preparation of insect‐cuticle‐like biomimetic materials. Biomacromolecules. 2001;2:369–372. doi: 10.1021/bm005652u. [DOI] [PubMed] [Google Scholar]
- Mikolasch A., Schauer F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol. 2009;82:605–624. doi: 10.1007/s00253-009-1869-z. [DOI] [PubMed] [Google Scholar]
- Mikolasch A., Wurster M., Lalk M., Witt S., Seefeldt S., Hammer E. Novel β‐lactam antibiotics synthesized by amination of catechols using fungal laccase. Chem Pharm Bull. 2008;56:902–907. doi: 10.1248/cpb.56.902. et al. [DOI] [PubMed] [Google Scholar]
- Morgenstern I., Robertson D.L., Hibbett D.S. Characterization of three mnp genes of Fomitiporia mediterranea and report of additional class II peroxidases in the order Hymenochaetales. Appl Environ Microbiol. 2010;76:6431–6440. doi: 10.1128/AEM.00547-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreel K., Ralph J., Kim H., Lu F., Goeminne G., Ralph S. Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol. 2004;136:3537–3549. doi: 10.1104/pp.104.049304. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan K., Yang I.H., Kim Y.M., Jeon J.R., Chang Y.S. Enhanced transformation of malachite green by laccase of Ganoderma lucidum in the presence of natural phenolic compounds. Appl Microbiol Biotechnol. 2009;82:341–350. doi: 10.1007/s00253-008-1819-1. [DOI] [PubMed] [Google Scholar]
- Ncanana S., Baratto L., Roncaglia L., Riva S., Burton S.G. Laccase‐mediated oxidation of totarol. Adv Synth Catal. 2007;349:1507–1513. [Google Scholar]
- Nicotra S., Cramarossa M.R., Mucci A., Pagnoni U.M., Riva S., Forti L. Biotransformation of reveratrol: synthesis of trans‐dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens. Tetrahedron. 2004a;60:595–600. [Google Scholar]
- Nicotra S., Intra A., Ottolina G., Riva S., Danieli B. Laccase‐mediated oxidation of the steroid hormone 17β‐estradiol in organic synthesis. Tetrahedron Asymmetry. 2004b;15:2927–2931. [Google Scholar]
- O'Malley D.M., Whetten R., Bao W., Chen C.‐L., Sederoff R.R. The role of laccase in lignification. Plant J. 1993;4:751–757. [Google Scholar]
- Osman A.M., Wong K.K.Y. Laccase (EC 1.10.3.2) catalyses the conversion of procyanidin B‐2 (epicatechin dimer) to type A‐2. Tetrahedron Lett. 2007;48:1163–1167. [Google Scholar]
- Pihet M., Vandeputte P., Tronchin G., Renier G., Saulniet P., Georgeault S. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigates conidia. BMC Microbiol. 2009;9:177–187. doi: 10.1186/1471-2180-9-177. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L., Routaboul J.‐M., Kerhoas L., Caboche M., Lepiniec L. TRANSPARENT TESTA10 encodes a laccase‐like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant cell. 2005;17:2966–2980. doi: 10.1105/tpc.105.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasetyo E.N., Kudanga T., Ostergaard L., Rencoret J., Gutierrez A., Rio J.C.D. Polymerization of lignosulfonates by the laccase‐HBT (1‐hydroxybenzotriazole) system improves dispersibility. Bioresour Technol. 2010;101:5054–5062. doi: 10.1016/j.biortech.2010.01.048. et al. [DOI] [PubMed] [Google Scholar]
- Ran N., Zhao L., Chen Z., Tao J. Recent applications of biocatalysis in developing green chemistry for chemical synthesis at the industrial scale. Green Chem. 2008;10:361–372. [Google Scholar]
- Ranocha P., Chabannes M., Chamayou S., Danoun S., Janueau A., Boudet A.‐M., Goffner D. Laccase down‐regulation causes alternations in phenolic metabolism and cell wall structure in Poplar. Plant Physiol. 2002;129:145–155. doi: 10.1104/pp.010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto M., Ritschkoff A.‐C., Viikari L. Enzymatically polymerized phenolic compounds as wood preservatives. Holzforschung. 2004;58:440–445. [Google Scholar]
- Riva S. Laccases: blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–226. doi: 10.1016/j.tibtech.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Rózanowska M., Sarna T., Land E.J., Truscott T.G. Free radical scavenging properties of melanin interaction of eu‐ and pheo‐melanin models with reducing and oxidizing radicals. Free Radic Biol Med. 1999;26:518–525. doi: 10.1016/s0891-5849(98)00234-2. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Duenas F.J., Martinez A.T. Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb Biotechnol. 2009;2:164–177. doi: 10.1111/j.1751-7915.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser D., Hofer C. Laccase‐catalyzed oxidation of Mn2+ in the presence of natural Mn3+ chelators as a novel source of extracellular H2O2 production and its impact on manganese peroxidase. Appl Environ Microbiol. 2002;68:3514–3521. doi: 10.1128/AEM.68.7.3514-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M., Aichernig N., Guebitz G.M., Kokol V. Enzymatic coating of lignocellulosic surfaces with polyphenols. Biotechnol J. 2007;2:334–341. doi: 10.1002/biot.200600209. [DOI] [PubMed] [Google Scholar]
- Shleev S.V., Morozova O., Nikitina O., Gorshina E.S., Rusinova T., Serezhenkov V.A. Comparison of physic‐chemical characteristics of four laccases from different basidiomycetes. Biochimie. 2004;86:693–703. doi: 10.1016/j.biochi.2004.08.005. et al. [DOI] [PubMed] [Google Scholar]
- Soden D.M., Dobson A.D. Differential regulation of laccase gene expression in Pleurotus sajor‐caju. Microbiology. 2001;147:1755–1763. doi: 10.1099/00221287-147-7-1755. [DOI] [PubMed] [Google Scholar]
- Solomon E.I., Augustine A.J., Yoon J. O2 reduction to H2O by the multicopper oxidases. Dalton Trans. 2008;30:3921–3932. doi: 10.1039/b800799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumaran M., Giglio L., Kundzicz H., Saul S., Semepsi V. Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch Insect Biochem Physiol. 1992;19:271–283. doi: 10.1002/arch.940190406. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Takahashi M., Hagino H., Nudejima S.‐I., Usui H., Fujii T., Taniguchi M. Enzymatic oxidative polymerization of methoxyphenols. Chem Eng Sci. 2010;65:569–573. [Google Scholar]
- Theuerl S., Buscot F. Laccases: toward disentangling their diversity and functions in relation to soil organic matter cycling. Biol Fertil Soils. 2010;46:215–225. [Google Scholar]
- Torres‐Duarte C., Roman R., Tinoco R., Vazquez‐Duhalt R. Halogenated pesticide transformation by a laccase‐mediator system. Chemosphere. 2009;77:687–692. doi: 10.1016/j.chemosphere.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Tsai H.‐F., Wheeler M.H., Chang Y.C., Kwon‐Chung K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigates. Appl Environ Microbiol. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama H. Artificial polymeric flavonoids: synthesis and applications. Macromol Biosci. 2007;7:410–422. doi: 10.1002/mabi.200700005. [DOI] [PubMed] [Google Scholar]
- Wei D., Houtman C.J., Kapich A.N., Hunt C.G., Cullen D., Hammel K.E. Laccase and its role in production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycetes Postia placenta. Appl Environ Microbiol. 2010;76:2091–2097. doi: 10.1128/AEM.02929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widsten P., Healthcote C., Kandelbauer A., Guebitz G., Nyanhongo G.S., Prasetyo E.N., Kudanga T. Enzymatic surface functionalization of lignocellulosic materials with tannins for enhancing antibacterial properties. Process Biochem. 2010;45:1072–1081. [Google Scholar]
- Williamson P.R., Wakamatsu K., Ito S. Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol. 1998;180:1570–1572. doi: 10.1128/jb.180.6.1570-1572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witayakran S., Ragauskas A.J. Synthetic applications of laccase in green chemistry. Adv Synth Catal. 2009;351:1187–1209. [Google Scholar]
- Zamocky M., Koller F. Understanding the structure and function of catalases: clues from molecular evolution and in vivo mutagenesis. Prog Biophys Mol Biol. 1999;72:19–66. doi: 10.1016/s0079-6107(98)00058-3. [DOI] [PubMed] [Google Scholar]


