Summary
The occurrence of a range of recalcitrant organic micropollutants in our aquatic environment has led to the development of various tertiary wastewater treatment methods. In this study, biogenic manganese oxides (Bio‐MnOx), biogenic silver nanoparticles (Bio‐Ag0) and ionic silver were used for the oxidative removal of the frequently encountered drug diclofenac and its dechlorinated form, 2‐anilinophenylacetate (APA). Diclofenac was rapidly degraded during ongoing manganese oxidation by Pseudomonas putida MnB6. Furthermore, whereas preoxidized Bio‐MnOx, Bio‐Ag0 and Ag+ separately did not show any removal capacity for diclofenac, an enhanced removal occurred when Bio‐MnOx and silver species were combined. Similar results were obtained for APA. Finally, a slow removal of diclofenac but more rapid APA degradation was observed when silver was added to manganese‐free P. putida biomass. Combining these results, three mechanisms of diclofenac and APA removal could be distinguished: (i) a co‐metabolic removal during active Mn2+ oxidation by P. putida; (ii) a synergistic interaction between preoxidized Bio‐MnOx and silver species; and (iii) a (bio)chemical process by biomass enriched with silver catalysts. This paper demonstrates the use of P. putida for water treatment purposes and is the first report of the application of silver combined with biogenic manganese for the removal of organic water contaminants.
Introduction
Since the discovery of a range of pharmaceuticals and personal care products in surface water, groundwater and even drinking water (Ternes, 1998; Heberer, 2002; Mompelat et al., 2009), tertiary treatment methods were developed to remove micropollutants after conventional wastewater treatment. Physicochemical techniques for the removal of micropollutants are mostly based upon filtration and oxidative processes (Ikehata et al., 2006) but may have specific disadvantages, such as high costs (Johnson and Sumpter, 2001), the formation of toxic by‐products (Guzzella et al., 2002; Richardson et al., 2007) and inefficiency against certain compounds (Ternes et al., 2003). Biogenic metals have shown to be useful catalysts for the removal of recalcitrant organic compounds. Biogenic palladium nanoparticles are efficient catalysts for the reductive transformation of halogenated organics, such as diatrizoate (Hennebel et al., 2010; De Gusseme et al., 2011a) and diclofenac (De Gusseme et al., 2012). However, these reductive treatments are generally limited to a dehalogenation step, upon which other treatments are required to remove the formed reaction products. As such, the end‐product of a reduction of diclofenac with biogenic palladium is its dechlorinated form 2‐anilinophenylacetate (APA) (De Corte et al., 2011; De Gusseme et al., 2012). Conversely, oxidative biological techniques of micropollutant removal are under investigation. For example, biogenic manganese(III,IV) oxides (Bio‐MnOx) can be successfully used for the removal of a range of pharmaceuticals (Forrez et al., 2010; 2011). The oxidative power of manganese oxide compares with that of oxygen gas (Thauer et al., 1977). In addition, the efficiency of micropollutant removal is further improved by the presence of manganese‐oxidizing bacterial biomass: a continuous cyclic reoxidation of manganese can theoretically result in a sustained removal of micropollutants (de Rudder et al., 2004; De Schamphelaire et al., 2007) and oxidation products might be further degraded by the bacterial metabolism, increasing the driving force of the initial reaction. Finally, evidence exists that during the oxidation of Mn2+ to Mn4+ by bacteria such as Pseudomonas putida and Bacillus sp. SG‐1, ligand‐bound Mn3+ intermediates are produced that might sensitively increase the oxidative power of a manganese‐bacteria mixture (Kostka et al., 1995; Parker et al., 2004; Murray and Tebo, 2006).
In water treatment, the disinfecting activity of both chemically and biologically produced silver nanoparticles (Bio‐Ag0) has been shown valuable for the inactivation of bacteria and viruses (Jain and Pradeep, 2005; De Gusseme et al., 2011b). Additionally, silver nanoparticles can be used as catalysts in a range of organic redox reactions (Jiang et al., 2005; Xu et al., 2006). Whereas silver compounds are generally weak redox catalysts, studies have demonstrated a sensitive increase in activity for composite metal catalysts. For example, Haruta and Sano (1983) demonstrated a higher activity of Ag–Co and Ag–Mn composite oxides, in comparison with the single‐component oxides, for the oxidation of hydrogen gas to H2O and carbon monoxide to CO2. Comparable results were obtained for the catalytic oxidation of volatile organic compounds, such as acetone and pyridine (Luo et al., 1998).
This study has explored the applicability of Bio‐MnOx and silver species for the oxidative removal of micropollutants during water treatment, using the chlorinated pharmaceutical compound diclofenac as a model pollutant. The main objectives were (i) to evaluate the influence of the manganese‐oxidizing metabolism of P. putida on the removal of diclofenac with Bio‐MnOx; (ii) to determine the effects of an enrichment of manganese oxides with silver species on the degradation of diclofenac; and (iii) to assess the degradation of 2‐anilinophenylacetate, the dechlorinated product of diclofenac.
Results
Influence of manganese‐oxidizing metabolism on diclofenac removal
Diclofenac (3 mg l−1) and manganese chloride (3.28 mg Mn2+ l−1) were added simultaneously to a P. putida culture. In a parallel setup, diclofenac was added 113 h after the addition of Mn2+, to allow a complete oxidation of manganese by the bacteria prior to the addition of diclofenac. Diclofenac was readily removed in batches where manganese was simultaneously oxidized, with a decrease of 96 ± 2% within 44 h (first‐order rate constant k = 0.065 h−1), while a lower decrease of 13 ± 6% after 75 h (k = 0.0023 h−1) was observed in batches where manganese was oxidized prior to diclofenac addition (Fig. 1A). Manganese‐free control experiments, in which an equal amount of bacteria was used, showed a diclofenac removal of 14 ± 2% after 142 h.
Figure 1.
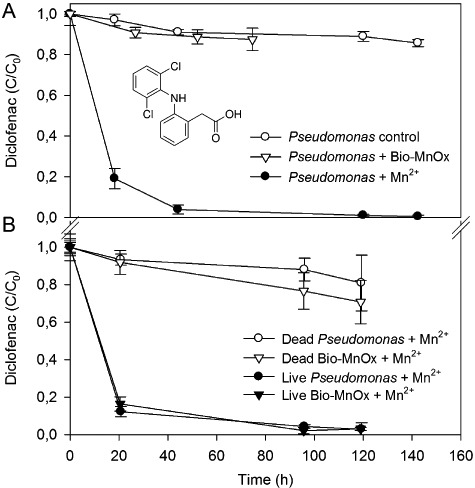
Removal of diclofenac by preformed Bio‐MnOx and by active manganese‐oxidizing metabolism of P. putida. A. During oxidation of 3.28 mg Mn2+ l−1 (‘Pseudomonas + Mn2+’), after complete oxidation of Mn2+ (‘Pseudomonas + Bio‐MnOx’) and in manganese‐free controls (‘Pseudomonas control’). B. In heat‐inactivated P. putida cultures supplied with 3.28 mg Mn2+ l−1 (‘dead Pseudomonas + Mn2+’), in heat‐inactivated Bio‐MnOx (3.28 mg Mn l−1) supplied with another 1.64 mg Mn2+ l−1 added (‘dead Bio‐MnOx + Mn2+’), and in living controls. C0 = 3 mg l−1 diclofenac. Error bars indicate standard deviations of three replicates; sometimes smaller than symbols.
To address the importance of bacterial metabolism during manganese oxidation and thus diclofenac degradation, control experiments using heat inactivated cultures of P. putida were set up (Fig. 1B). Upon addition of 3.28 mg Mn2+ l−1 and 3 mg l−1 diclofenac to non‐viable biomass, no formation of biogenic manganese oxides could be observed and a slight removal of 19 ± 15% diclofenac was seen after 119 h of incubation. In contrast, after the addition of equal amounts of manganese and diclofenac to a viable P. putida culture, repeating the active manganese oxidation of the previous experiment shown in Fig. 1A, a faster removal of diclofenac was observed (97 ± 3% after 119 h, Fig. 1B).
Subsequently, it was tested whether preformed Bio‐MnOx could facilitate the reoxidation of Mn2+ in an autocatalytic manner, rendering the bacterial manganese‐oxidizing metabolism unnecessary for the ensuing removal of diclofenac. To this extent, heat inactivation of preformed Bio‐MnOx (3.28 mg Mn l−1) was performed, after which an additional amount of 1.64 mg Mn2+ l−1 and 3 mg l−1 diclofenac were added. The diclofenac concentration decreased only to a limited extent (29 ± 12% after 119 h), which was in contrast to the diclofenac removal after addition of equal amounts of Mn2+ and diclofenac to viable Bio‐MnOx. Indeed, the latter amounted to 97% ± 1 after 119 h (Fig. 1B), which was again comparable to the diclofenac removal rate during active manganese oxidation obtained in the previous experiments.
Improved diclofenac removal in the presence of silver
To further improve the reactivity of manganese oxides, the influence of silver species on the degradation of diclofenac by Bio‐MnOx was assessed. When diclofenac was added to a mixture of preformed Bio‐MnOx (3.28 mg Mn l−1) and either ionic silver or biogenic nanoparticles (Bio‐Ag0) (5 mg Ag l−1), removal percentages of 92 ± 3% and 91 ± 5%, respectively, were obtained after 159 h (first‐order rate constants k = 0.017 and 0.016 h−1). Control experiments containing Bio‐Ag0, Ag+ (5 mg Ag l−1) or Bio‐MnOx (3.28 mg Mn l−1) separately showed little to no diclofenac removal (Fig. 2A) (k = 0 h−1, 0.0003 h−1 and 0.0023 h−1 respectively).
Figure 2.
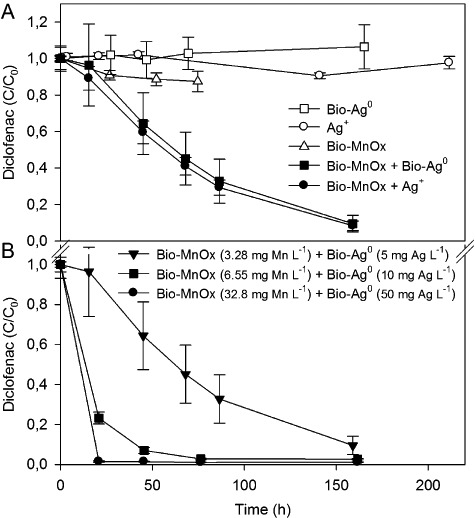
Removal of diclofenac by combined manganese and silver species. A. By Bio‐MnOx (3.28 mg Mn l−1) combined with Bio‐Ag0 or Ag+ (5 mg Ag l−1), and by Bio‐Ag0, Ag+ and Bio‐MnOx as separate controls. B. Diclofenac removal at elevated metal concentrations: 3.28 mg Mn l−1 as Bio‐ MnOx with 5 mg l−1 silver as Bio‐Ag0, and twofold and 10‐fold increases. C0 = 3 mg l−1 diclofenac. Error bars indicate standard deviations of three replicates; sometimes smaller than symbols.
At increased biomass and metal concentrations, diclofenac removal rates were sensitively enhanced (Fig. 2B). When increasing the concentrations of biomass, manganese and silver by a factor 2 and 10, a better removal of 97 ± 0.4% after 76 h and 99 ± 0.4% after 21 h, respectively, could be obtained. This corresponded with a factor 3.6 and 5.7 increase in the diclofenac removal rate constants (k = 0.058 h−1 and 0.091 h−1 respectively).
To distinguish degradation processes from sorption, a respiking experiment was performed for diclofenac removal by Bio‐MnOx (6.55 mg Mn l−1) and Bio‐Ag0 (10 mg Ag l−1) (Fig. 3). Diclofenac was removed by 94 ± 1% after 144 h during a first incubation period. After 173 h, the batches were respiked to the initial concentration of 3 mg l−1 diclofenac. During a second incubation period, diclofenac was removed at a comparable rate, with a decrease of 98 ± 0.5% after 141 h. A parallel control experiment, containing manganese‐free P. putida biomass with Bio‐Ag0, showed a diclofenac removal by 41 ± 9% after 144 h during the first incubation and 42 ± 7% after 141 h during the second incubation period.
Figure 3.
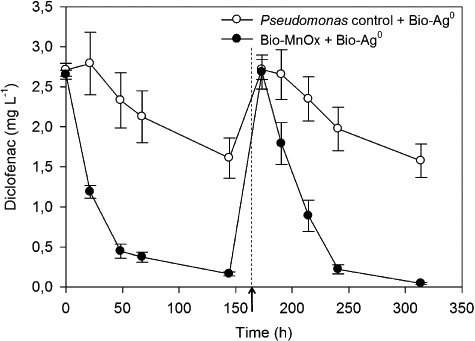
Removal of diclofenac by Bio‐Ag0 (10 mg Ag l−1) combined with P. putida biomass and with preoxidized Bio‐MnOx (6.55 mg Mn l−1). C0 = 3 mg l−1 diclofenac. The arrow marks a respiking of diclofenac to 3 mg l−1 at time t = 173 h. Error bars indicate standard deviations of three replicates; sometimes smaller than symbols.
Removal of APA
To examine the structural importance of the chlorine moieties in the diclofenac molecule and their contribution to its degradability by manganese and silver species, degradation experiments were performed using 2‐anilinophenylacetate (APA) (Fig. 4). When 3.28 mg Mn2+ l−1 and 3 mg APA l−1 were added to a viable P. putida culture, APA was removed by 85 ± 2% after 45 h during active manganese oxidation, after a lag phase of 23 h in which no visible formation of Bio‐MnOx or APA removal was observed. In contrast, control experiments using manganese‐free biomass showed no APA removal. In the presence of preoxidized Bio‐MnOx (3.28 mg Mn l−1) and Bio‐Ag0 (5 mg Ag l−1), APA was removed by 82 ± 8% after 44 h. Surprisingly, a control experiment containing a mixture of Bio‐Ag0 (5 mg Ag l−1) and a manganese‐free P. putida culture showed an even faster removal, with a decrease of APA concentration by 90 ± 2% after 19 h.
Figure 4.
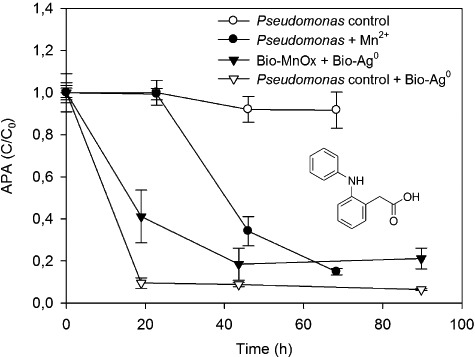
Removal of APA by P. putida during active oxidation of Mn2+ (3.28 mg l−1); by preoxidized Bio‐MnOx (3.28 mg Mn l−1) combined with Bio‐Ag0 (5 mg Ag l−1); and by manganese‐free controls. C0 = 3 mg APA l−1. Error bars indicate standard deviations of three replicates; sometimes smaller than symbols.
Discussion
Traditional wastewater treatment is focused on the removal of the organic load (chemical oxygen demand, COD) and nutrient concentrations before discharge in natural aquatic systems. However, with continuously increasing global water demands, the need to develop techniques for wastewater reuse arises. Of specific concern for the reuse of water is the presence of persistent organic compounds, such as certain pharmaceuticals, pesticides and industrial chemicals. This research has elaborated on the use of biogenic manganese oxides as novel agents for the removal of micropollutants, in continuation of the research of Sabirova and colleagues (2008) and Forrez and colleagues (2010; 2011). Moreover, this study used for the first time silver as a catalyst, combined with manganese oxides, for the removal of micropollutants.
Removal of diclofenac during active manganese oxidation
Almost no removal of diclofenac in the presence of preformed Bio‐MnOx was observed. In contrast, during active Mn2+ oxidation by P. putida, diclofenac was readily removed, with almost a 30‐fold increase of the first‐order rate constant (Fig. 1). This suggests a participating role of the manganese‐oxidizing metabolism of the bacteria in the removal of diclofenac, especially since heat‐inactivated cultures showed no considerable diclofenac removal. Although it is known that the autocatalytic oxidation of Mn2+ may occur on the surface of preformed manganese oxides (Murray, 1975), these results show that possible intermediates during the autocatalytic oxidation of MnO2 do not contribute to the degradation of diclofenac, but that, in contrast, the metabolic activity of the biomass is the most significant factor herein. The oxidation of manganese by P. putida likely involves a multicopper enzyme, which oxidizes Mn2+ to a ligand‐bound Mn3+ intermediate (Parker et al., 2004; Tebo et al., 2004; Webb et al., 2005). This highly reactive Mn3+ intermediate is then thought to be further oxidized to Mn(IV) oxide by putative enzymes in the extracellular mucous layer of the bacteria (De Vrind et al., 2003). A three‐step model of the oxidizing activity of Bio‐MnOx can be proposed (Fig. 5): (i) Mn2+ is oxidized to a ligand‐bound Mn3+ intermediate, presumably by a multicopper enzyme of P. putida; (ii) this Mn3+ intermediate can either be further oxidized to Mn(IV) oxide, plausibly by enzymes on the cell surface; or (iii) the reactive Mn3+ intermediate can oxidize other compounds, such as micropollutants, upon which Mn2+ is formed again. As such, the degradation of diclofenac can be considered a co‐metabolic process during the oxidation of manganese. The model presented here is in concordance with Forrez and colleagues (2010), who observed that diclofenac removal by Bio‐MnOx was inhibited when P. putida was treated with sodium azide, a biostatic agent. Further study should focus on the technical challenge of verifying this model by demonstrating the formation of unstable Mn3+ intermediates and correlating their presence with the oxidation of diclofenac. Moreover, research on mutant strains should allow to identify the different P. putida enzymes involved in the oxidation of manganese and clarify the mechanism of diclofenac oxidation during this process.
Figure 5.
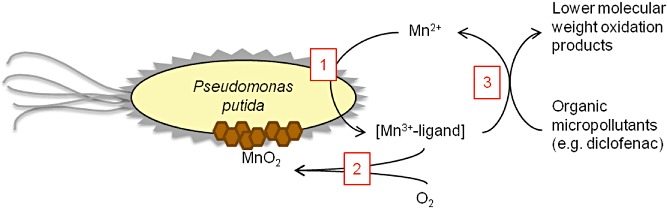
Proposed model of the oxidation of Mn2+ by P. putida and the co‐metabolic degradation of micropollutants such as diclofenac: (1) oxidation of Mn2+ to a ligand‐bound Mn3+ intermediate (after Parker et al., 2004; Tebo et al., 2004; Webb et al., 2005); (2) oxidation of Mn3+ to MnO2 (after De Vrind et al., 2003); (3) reduction of Mn3+ to Mn2+ with concomitant oxidation of the micropollutant.
Removal of diclofenac by the activity of silver species
To increase the overall reactivity, Ag+ and Bio‐Ag0 were added to Bio‐MnOx, upon which diclofenac was rapidly removed. A respiking experiment (Fig. 3) clearly refuted the possibility of sorption as the prime mechanism of diclofenac removal by this silver – Bio‐MnOx mixture, suggesting a catalytic nature of the process. Silver and biogenic manganese oxides cooperate in a synergistic manner, increasing the diclofenac removal rate to a higher level than would be expected from combining the removal rates of both separately: Ag+, Bio‐Ag0 and Bio‐MnOx alone showed little to no diclofenac removal (Fig. 2). Likewise, Imamura and colleagues (1988) observed similar synergistic interactions between manganese and silver oxides for the oxidation of gaseous carbon compounds.
A surprising increase of the diclofenac removal rate was already observed when Bio‐Ag0 was combined with manganese‐free biomass (Fig. 3). One possible explanation could involve the formation of reactive oxygen species (ROS), such as hydroxyl radicals, superoxide and peroxide, since both ionic silver (Matsumura et al., 2003; Park et al., 2009) and silver nanoparticles (Su et al., 2009; Sintubin et al., 2011) have been shown to interact with the cell wall and intracellular respiratory enzymes of bacteria, thereby creating ROS. Given the susceptibility of diclofenac towards oxidation by ROS (Hofmann et al., 2007; Sein et al., 2008), it is considered plausible that the observed removal of diclofenac in the presence of Bio‐Ag0 in a P. putida culture occurs through this reaction.
In this study, no definite conclusions could be made regarding the mechanism of the observed Bio‐MnOx – silver interaction during the degradation of diclofenac. Yet, the results hitherto suggest a twofold mode of action: (i) silver species (Ag+ and Bio‐Ag0) interact with P. putida biomass in such a way that diclofenac is slowly removed; and (ii) silver species enhance the reactivity of Bio‐MnOx, in such a way that diclofenac is more rapidly removed. It is suggested that further research should attempt to demonstrate an increased amount of ROS when the bacterial cells are triggered by silver and the subsequent effects on micropollutants such as diclofenac. Likewise, the mechanism of the observed catalytic manganese – silver synergy should be addressed. Furthermore, given the antibacterial activity of silver (Morones et al., 2005; Bjarnsholt et al., 2007; Yoon et al., 2007), careful study should determine whether silver may be added to active P. putida biomass at concentrations sufficiently low not to inhibit the oxidation of manganese and the associated diclofenac removal, while still allowing the catalytic silver – Bio‐MnOx interaction to further enhance diclofenac removal rates.
Differences in reactivity between diclofenac and APA
The end‐product of a reductive treatment of diclofenac with, for example, palladium or palladium – gold catalysts is 2‐anilinophenylacetate (APA) (De Corte et al., 2011; De Gusseme et al., 2012). In order to explore the use of manganese and silver species to complement such reductive treatments, additional removal experiments were conducted with APA. During active bacterial oxidation of manganese, APA was rapidly removed after a 23‐hour lag phase, in which no manganese oxide formation and APA removal occurred (Fig. 4). After this delayed onset of manganese oxidation, the APA removal rate was comparable to that of diclofenac under equal circumstances. Remarkably, when silver was added to a manganese‐free P. putida culture, a faster APA removal was observed than when combined with Bio‐MnOx. Thus, it is concluded that silver acts as an efficient catalyst for the removal of APA in the presence of P. putida biomass and that manganese is not required in this reaction.
A comparison of the degradation kinetics of diclofenac with those of APA might provide more insight in the structural importance of the chlorine moieties of diclofenac and in the mechanism by which it is transformed. The main bacterial degradation pathway of diclofenac is an oxidation to 5‐hydroxydiclofenac, followed by (aut)oxidation to diclofenac‐2,5‐iminoquinone (Gröning et al., 2007). Likewise, these two compounds were the only metabolites detected for diclofenac degradation by Bio‐MnOx (Forrez et al., 2010), although they only accounted for 5–10% of the total diclofenac removal. In this study, both diclofenac and APA were removed at comparable rates during the ongoing oxidation of manganese by P. putida. Therefore, it is concluded that the chlorine moieties of diclofenac do not affect its principal degradation pathway, which is likely to involve a hydroxylation of the ring structure. When added to a combination of Bio‐MnOx and Bio‐Ag0, APA was removed at a lower rate than in a manganese‐free bacterial culture containing only Bio‐Ag0 (Fig. 4), suggesting that manganese oxides are not required for the degradation of APA by Bio‐Ag0 and even interfere with it. On the contrary, an enhanced removal rate was observed for diclofenac in the presence of both manganese oxides and silver, compared with silver in a manganese‐free bacterial culture (Fig. 3). It is therefore concluded that the principal degradation mechanism of diclofenac by manganese and silver might differ from that of APA and that the chlorine moieties of diclofenac counter its degradability by silver in manganese‐free biomass. Possibly, APA is, more than diclofenac, sensitive to degradation by the ROS that are produced extra‐ and intracellularly in the presence of silver ions. This might explain the higher APA removal rate in the absence of Bio‐MnOx, since deposits of manganese oxides on the bacterial cell wall are expected to obstruct cross‐membrane transport. Diclofenac, on the other hand, is primarily degraded as the result of a hitherto unspecified synergistic interaction between manganese oxides and silver.
Conclusive remarks
This research has shown that biogenic manganese and silver species can be used as novel agents for the oxidative and catalytic removal of micropollutants. Further research should focus on determining the range of different micropollutants that can be removed by these agents and optimizing the removal conditions, as well as unravelling the reaction mechanisms and identifying the formed metabolites. Additionally, careful study should allow assessing and improving the economic feasibility of water treatment with Bio‐MnOx and Bio‐Ag0, in order to transform these promising, innovative materials into an effective solution to the problem of recalcitrant organic water pollution.
Experimental procedures
Bacterial growth conditions
Pure cultures of P. putida MnB6 (BCCM/LMG 2322) were grown on a platform shaker (111 r.p.m.) at 28°C under aerobic conditions, in 100 ml or 250 ml Erlenmeyer flasks. The growth medium was prepared according to Boogerd and de Vrind (1987). The cultures were incubated until an optical density (610 nm) of 1.0 ± 0.3 was reached, measured after syringe‐and‐needle homogenization.
Diclofenac and APA removal during active manganese oxidation
Pseudomonas putida cultures with optical density (610 nm) of 1.0 ± 0.3 were enriched with manganese chloride (3.28 mg Mn2+ l−1) from a stock solution of 500 mg MnCl2 l−1 (218 mg Mn2+ l−1) that was previously filter sterilized (0.22 µm, Millipore) and stored at 4°C. Diclofenac was added at the desired time from a cool‐stored stock solution in distilled water. Likewise, APA was added from a cool‐stored stock solution in 70% ethanol. Experiments were incubated in 100 ml or 250 ml Erlenmeyer flasks, leaving a headspace volume equal to or greater than the volume of the culture liquid, to ensure aerobic conditions. For heat‐inactivation experiments, Erlenmeyer flasks containing P. putida biomass or preoxidized Bio‐MnOx were placed in hot water for 10 min at 60°C. All experiments were performed in triplicate. Between sampling points, batches were stored on a platform shaker (111 r.p.m.) at 28°C. Manganese‐free P. putida controls were treated identically.
Diclofenac and APA removal by preformed Bio‐MnOx and silver species
Preoxidized Bio‐MnOx was produced according to Forrez and colleagues (2010). In brief, P. putida cultures were grown to an optical density (610 nm) of 1.0 ± 0.3 and enriched with manganese chloride (3.28 mg Mn2+ l−1) as described above, and stored on a platform shaker at 28°C during 24 h for a complete oxidation of the Mn2+. The Bio‐MnOx were then centrifuged (10 min at 7500 g) and washed twice (10 min at 10 000 g) with phosphate buffer (10 mM KH2PO4/K2HPO4, pH 7–8). The pellets were resuspended at the desired concentration in a test solution containing 0.6 g NaCl l−1 and 10 mM phosphate buffer (pH 7). Manganese‐free control cultures were treated identically.
The production of Bio‐Ag0 was performed following the protocol of Sintubin and colleagues (2009): Lactobacillus fermentum G2/10 was grown in Man‐Rogosa‐Sharpe (MRS) broth (Oxoid) at 28°C on a platform shaker (111 r.p.m.) for 48 h. The biomass was harvested and washed twice with Milli‐Q water by means of centrifugation (5 min at 10 000 g). After determination of the cell dry weight (CDW ≈ cell wet weight × 0.23), the biomass was resuspended in Milli‐Q water to a concentration of 4.6 g CDW l−1 and alkalified with 0.025 M NaOH. Silver (1 g l−1) was added in the diamine‐complexed form, from a stock solution of silver nitrate that was dosed with ammonium hydroxide until all formed silver precipitates were redissolved. After 24h of incubation (28°C, 111 r.p.m.), the biomass and the formed Bio‐Ag0 were washed thrice (15 min, 10 000 g) with Milli‐Q water. The concentration of silver, present on the biomass as Bio‐Ag0, was determined by atomic absorption spectroscopy (AA‐6300 atomic absorption spectroscope, Shimadzu) after addition of 2 ml HNO3 (65%) and 2 ml H2O2 (30%) to a 2 ml Bio‐Ag0 sample and subsequent heating until the solution was clear.
According to the experimental setup, Bio‐Ag0 and Ag+, from a freshly made stock solution of AgNO3 in Milli‐Q water, were added to the Bio‐MnOx solution or to Milli‐Q water in 100 ml Erlenmeyer flasks. After addition of diclofenac or APA (3 mg l−1), batches were stored on a platform shaker (111 r.p.m.) at 28°C between sampling points. All experiments were performed in triplicate.
Analytical procedures
Samples for diclofenac and APA measurements were filtered with regenerated cellulose filters (RC, 0.20 µm, Phenex) or polyvinylidene fluoride filters (PVDF, 0.22 µm, Millipore), which both tested negative for sorption of diclofenac and APA (data not shown). Prior to analysis, the samples were stored in glass vials at 4°C. Measurements were made using a Dionex P580 HPLC system with a TCC‐100 column oven and a diode array detector (UVD340U) and processing was done using Chromeleon 6.80 software. A Genesis C18 column (150 × 4.6 mm, 4 µm, Alltech) with a guard column was used for separation. The injection volume was 50 µl. The separation method was modified from Forrez and colleagues (2010): at a temperature of 40°C and a flow rate of 1 ml min−1, the elution was isocratic with 80% HPLC grade methanol and 20% of a solution of 0.1% formic acid in Milli‐Q water. Diclofenac was detected by absorbance at a wavelength of 203 nm, at a retention time of 4 min. APA was analysed by HPLC as described for diclofenac. The absorbance was measured at a wavelength of 275 nm and APA appeared at a retention time of 3 min. Standards ranging from 0 to 5 mg l−1 were prepared for calibration. The limit of detection (LOD), i.e. the lowest non‐zero point in the calibration series, was 100 µg l−1.
Acknowledgments
T. Hennebel is supported by Ghent University Multidisciplinary Research Partnership (MRP) – Biotechnology for a sustainable economy (01 MRA 510W). This work was part of research projects obtained from the EU Commission within the Program of the Seventh Framework FP7‐KBBE‐2010‐4: EU ULIXES project (266473) and FP7‐KBBE‐2010.3.5.01: EU BIOTREAT project (266039). We gratefully thank Bart De Gusseme, Pieter Verhagen, Sam Van Nevel and Liesje Sintubin for their assistance and helpful suggestions and Jessica Benner and Simon De Corte for carefully reviewing the manuscript.
References
- Bjarnsholt T., Kirketerp‐Møller K., Kristiansen S., Phipps R., Nielsen A.K., Jensen P.Ø. Silver against Pseudomonas aeruginosa biofilms. Acta Path Micro Im. 2007;115:921–928. doi: 10.1111/j.1600-0463.2007.apm_646.x. et al. [DOI] [PubMed] [Google Scholar]
- Boogerd F.C., de Vrind J.P. Manganese oxidation by Leptothrix discophora. J Bacteriol. 1987;169:489–494. doi: 10.1128/jb.169.2.489-494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Corte S., Hennebel T., Fitts J.P., Sabbe T., Bliznuk V., Verschuere S. Bio‐supported bimetallic Pd‐Au nanocatalysts for dechlorination of environmental contaminants. Environ Sci Technol. 2011;45:8506–8513. doi: 10.1021/es2019324. et al. [DOI] [PubMed] [Google Scholar]
- De Gusseme B., Hennebel T., Vanhaecke L., Soetaert M., Desloover J., Wille K. Biogenic palladium enhances diatrizoate removal from hospital wastewater in a microbial electrolysis cell. Environ Sci Technol. 2011a;45:5737–5745. doi: 10.1021/es200702m. et al. [DOI] [PubMed] [Google Scholar]
- De Gusseme B., Hennebel T., Christiaens E., Saveyn H., Verbeken K., Fitts J.P. Virus disinfection in water by biogenic silver immobilized in polyvinylidene fluoride membranes. Water Res. 2011b;45:1856–1864. doi: 10.1016/j.watres.2010.11.046. et al. [DOI] [PubMed] [Google Scholar]
- De Gusseme B., Soetaert M., Hennebel T., Vanhaecke L., Boon N., Verstraete W. Catalytic dechlorination of diclofenac by biogenic palladium in a microbial electrolysis cell. Microbial biotech. 2012 doi: 10.1111/j.1751-7915.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schamphelaire L., Rabaey K., Boon N., Verstraete W., Boeckx P. Minireview: the potential of enhanced manganese redox cycling for sediment oxidation. Geomicrobiol J. 2007;24:547–558. [Google Scholar]
- De Vrind J., De Groot A., Brouwers G.J., Tommassen J., De Vrind‐de Jong E. Identification of a novel Gsp‐related pathway required for secretion of the manganese‐oxidizing factor of Pseudomonas putida strain GB‐1. Mol Microbiol. 2003;47:993–1006. doi: 10.1046/j.1365-2958.2003.03339.x. [DOI] [PubMed] [Google Scholar]
- Forrez I., Carballa M., Verbeken K., Vanhaecke L., Schlüsener M., Ternes T. Diclofenac oxidation by biogenic manganese oxides. Environ Sci Technol. 2010;44:3449–3454. doi: 10.1021/es9027327. et al. [DOI] [PubMed] [Google Scholar]
- Forrez I., Carballa M., Fink G., Wick A., Hennebel T., Vanhaecke L. Biogenic metals for the oxidative and reductive removal of pharmaceuticals, biocides and iodinated contrast media in a polishing membrane bioreactor. Water Res. 2011;45:1763–1773. doi: 10.1016/j.watres.2010.11.031. et al. [DOI] [PubMed] [Google Scholar]
- Gröning J., Held C., Garten C., Claußnitzer U., Kaschabek S.R., Schlömann M. Transformation of diclofenac by the indigenous microflora of river sediments and identification of a major intermediate. Chemosphere. 2007;69:509–516. doi: 10.1016/j.chemosphere.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Guzzella L., Feretti D., Monarca S. Advanced oxidation and adsorption technologies for organic micropollutant removal from lake water used as drinking‐water supply. Water Res. 2002;36:4307–4318. doi: 10.1016/s0043-1354(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Haruta M., Sano H. Preparation of highly active composite oxides of silver for hydrogen and carbon monoxide oxidation. Stud Surf Sci Catal. 1983;16:225–236. [Google Scholar]
- Heberer T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J Hydrol. 2002;266:175–189. [PubMed] [Google Scholar]
- Hennebel T., De Corte S., Vanhaecke L., Vanherck K., Forrez I., De Gusseme B. Removal of diatrizoate with catalytically active membranes incorporating microbially produced palladium nanoparticles. Water Res. 2010;44:1498–1506. doi: 10.1016/j.watres.2009.10.041. et al. [DOI] [PubMed] [Google Scholar]
- Hofmann J., Freier U., Wecks M., Hohmann S. Degradation of diclofenac in water by heterogeneous catalytic oxidation with H2O2. Appl Catal B Environ. 2007;70:447–451. [Google Scholar]
- Ikehata K., Naghashkar N., Ei‐Din M. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone Sci Eng. 2006;28:353–414. [Google Scholar]
- Imamura S., Sawada H., Uemura K., Ishida S. Oxidation of carbon monoxide catalyzed by manganese‐silver composite oxides. J Catal. 1988;109:198–205. [Google Scholar]
- Jain P., Pradeep T. Potential of silver nanoparticle‐coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng. 2005;90:59–63. doi: 10.1002/bit.20368. [DOI] [PubMed] [Google Scholar]
- Jiang Z‐J., Liu C‐Y., Sun L‐W. Catalytic properties of silver nanoparticles supported on silica spheres. J Phys Chem B. 2005;109:1730–1735. doi: 10.1021/jp046032g. [DOI] [PubMed] [Google Scholar]
- Johnson A.C., Sumpter J.P. Removal of endocrine‐disrupting chemicals in activated sludge treatment works. Environ Sci Technol. 2001;35:4697–4703. doi: 10.1021/es010171j. [DOI] [PubMed] [Google Scholar]
- Kostka J.E., Luther G.W., Nealson K.H. Chemical and biological reduction of Mn(III)‐pyrophosphate complexes: potential importance of dissolved Mn(III) as an environmental oxidant. Geochim Cosmochim Acta. 1995;59:885–894. [Google Scholar]
- Luo M‐F., Yuan X‐X., Zheng X‐M. Catalyst characterization and activity of Ag‐Mn, Ag‐Co and Ag‐Ce composite oxides for oxidation of volatile organic compounds. Appl Catal A Gen. 1998;175:121–129. [Google Scholar]
- Matsumura Y., Yoshikata K., Kunisaki S., Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microb. 2003;69:4278–4281. doi: 10.1128/AEM.69.7.4278-4281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mompelat S., Le Bot B., Thomas O. Occurrence and fate of pharmaceutical products and by‐products, from resource to drinking water. Environ Int. 2009;35:803–814. doi: 10.1016/j.envint.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- Murray J.W. The interaction of metal ions at the manganese dioxide‐solution interface. Geochim Cosmochim Acta. 1975;39:505–519. [Google Scholar]
- Murray K.J., Tebo B.M. Cr(III) is indirectly oxidized by the Mn(II)‐oxidizing bacterium Bacillus sp. strain SG‐1. Environ Sci Technol. 2006;41:528–533. doi: 10.1021/es0615167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.‐J., Kim J.Y., Kim J., Lee J.‐H., Hahn J.‐S., Gu M.B., Yoon J. Silver‐ion‐mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009;43:1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Parker D.L., Sposito G., Tebo B.M. Manganese(III) binding to a pyoverdine siderophore produced by a manganese(II)‐oxidizing bacterium. Geochim Cosmochim Acta. 2004;68:4809–4820. [Google Scholar]
- Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., DeMarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by‐products in drinking water: a review and roadmap for research. Mutat Res-Rev Mutat. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- de Rudder J., Van de Wiele T., Dhooge W., Comhaire F., Verstraete W. Advanced water treatment with manganese oxide for the removal of 17[alpha]‐ethynylestradiol (EE2) Water Res. 2004;38:184–192. doi: 10.1016/j.watres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Sabirova J.S., Cloetens L.F.F., Vanhaecke L., Forrez I., Verstraete W., Boon N. Manganese‐oxidizing bacteria mediate the degradation of 17[alpha]‐ethinylestradiol. Microbial Biotech. 2008;1:507–512. doi: 10.1111/j.1751-7915.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sein M.M., Zedda M., Tuerk J., Schmidt T.C., Golloch A., von Sonntag C. Oxidation of diclofenac with ozone in aqueous solution. Environ Sci Technol. 2008;42:6656–6662. doi: 10.1021/es8008612. [DOI] [PubMed] [Google Scholar]
- Sintubin L., De Windt W., Dick J., Mast J., van der Ha D., Verstraete W., Boon N. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biot. 2009;84:741–749. doi: 10.1007/s00253-009-2032-6. [DOI] [PubMed] [Google Scholar]
- Sintubin L., De Gusseme B., Van der Meeren P., Pycke B., Verstraete W., Boon N. The antibacterial activity of biogenic silver and its mode of action. Appl Microbiol Biot. 2011;91:153–162. doi: 10.1007/s00253-011-3225-3. [DOI] [PubMed] [Google Scholar]
- Su H.‐L., Chou C.‐C., Hung D.‐J., Lin S.‐H., Pao I.C., Lin J.‐H. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009;30:5979–5987. doi: 10.1016/j.biomaterials.2009.07.030. et al. [DOI] [PubMed] [Google Scholar]
- Tebo B.M., Bargar J.R., Clement B.G., Dick G.J., Murray K.J., Parker D. Biogenic manganese oxides: properties and mechanisms of formation. Annu Rev Earth Planet Sci. 2004;32:287–328. et al. [Google Scholar]
- Ternes T. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998;32:3245–3260. [Google Scholar]
- Ternes T., Stuber J., Herrmann N., McDowell D., Ried A., Kampmann M. Ozonation: a tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003;37:1976–1982. doi: 10.1016/S0043-1354(02)00570-5. [DOI] [PubMed] [Google Scholar]
- Thauer R.K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S.M., Dick G.J., Bargar J.R., Tebo B.M. Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II) Proc Natl Acad Sci USA. 2005;102:5558–5563. doi: 10.1073/pnas.0409119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Wang D.S., Zhang J.T., Li Y.D. Shape‐dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem Asian J. 2006;1:888–893. doi: 10.1002/asia.200600260. [DOI] [PubMed] [Google Scholar]
- Yoon K.‐Y., Hoon Byeon J., Park J.‐H., Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373:572–575. doi: 10.1016/j.scitotenv.2006.11.007. [DOI] [PubMed] [Google Scholar]


