SUMMARY
Perturbations of cell surface synapse organizing proteins, particularly α-neurexins, contribute to neurodevelopmental and psychiatric disorders. From an unbiased screen, we identify calsyntenin-3 (alcadein-β) as a novel synapse organizing protein unique in binding and recruiting α-neurexins but not β-neurexins. Calsyntenin-3 is present in many pyramidal neurons throughout cortex and hippocampus but is most highly expressed in interneurons. The transmembrane form of calsyntenin-3 can trigger excitatory and inhibitory presynapse differentiation in contacting axons. However, calsyntenin-3 shed ectodomain, which represents about half the calsyntenin-3 pool in brain, suppresses the ability of multiple α-neurexin partners including neuroligin 2 and LRRTM2 to induce presynapse differentiation. Clstn3 −/− mice show reductions in excitatory and inhibitory synapse density by confocal and electron microscopy and corresponding deficits in synaptic transmission. These results identify calsyntenin-3 as an α-neurexin-specific binding partner required for normal functional GABAergic and glutamatergic synapse development.
INTRODUCTION
Synapse organizing complexes mediate local differentiation of presynaptic and postsynaptic specializations by recruiting molecular components and organelles involved in neurotransmitter release and reception. Emerging evidence indicates that multiple synapse organizing complexes act in concert to regulate the density, composition, and function of synapses on each neuron (Missler et al., 2012; Shen and Scheiffele, 2010; Siddiqui and Craig, 2011).
The presynaptic neurexins constitute one of the best-characterized families of synapse organizing proteins (Krueger et al., 2012; Sudhof, 2008). Each of the three neurexin genes in humans and rodents produces long α isoforms from an upstream promoter and shorter β isoforms from a downstream promoter. Alternative splicing creates additional diversity resulting in >3000 potential neurexin isoforms (Tabuchi and Sudhof, 2002). Importantly, α-neurexins perform essential functions that are not shared with β-neurexins. Mice that lack α-neurexins but express normal β-neurexin levels die at birth due to major deficits in synaptic function (Missler et al., 2003), and synaptic transmission can only be rescued by transgenic expression of neurexin-1α but not of neurexin-1β (Zhang et al., 2005). Neurexins have acquired particular notoriety because alterations in NRXN genes were found to contribute to non-syndromic autism spectrum disorders and schizophrenia (Betancur et al., 2009; Sudhof, 2008; Szatmari et al., 2007). Interestingly, the majority of disease-associated NRXN variations selectively affect α-neurexins and not β-neurexins (Ching et al., 2010; Gauthier et al., 2011; Schaaf et al., 2012; Vaags et al., 2012). Despite the obvious functional importance of α-neurexins, and the identification of multiple extracellular partners for neurexin isoforms, the only identified α-neurexin-specific interacting proteins are the small secreted neuropeptide-like neurexophilins (Missler and Sudhof, 1998).
Neurexins control excitatory and inhibitory synapse development through isoform-selective interactions with different sets of postsynaptic partners. Neuroligins were the first postsynaptic neurexin interactors to be identified. As with neurexins, mutations in NLGN genes are linked to neurodevelopmental disorders (Jamain et al., 2003; Krueger et al., 2012; Sudhof, 2008), and animal models support a causative role (e.g. (Jamain et al., 2008). The main neuroligin at glutamatergic synapses, neuroligin 1 (with the B insert), binds only β-neurexins, while the other neuroligins, including neuroligin 2 which is the main neuroligin of GABAergic synapses, bind all neurexins (Boucard et al., 2005). Acting cooperatively with neuroligins at subsets of glutamatergic synapses, postsynaptic LRRTM1 and LRRTM2 bind α and β neurexins lacking an insert at splice site 4 while cerebellin1 bridges postsynaptic GluRδ2 to neurexins containing an insert at splice site 4 (de Wit et al., 2009; Ko et al., 2009; Linhoff et al., 2009; Siddiqui et al., 2010; Uemura et al., 2010). Neurexins and these diverse postsynaptic binding partners control multiple aspects of synapse development including stabilization and morphological and functional maturation (Ito-Ishida et al., 2012; Kwon et al., 2012; Soler-Llavina et al., 2011; Uemura et al., 2010; Varoqueaux et al., 2006). Modulators of synapse organizing complexes also regulate synapse development. For example, MDGA1 reduces inhibitory synapse density by blocking the interaction of neuroligin 2 with neurexins (Pettem et al., 2013).
In the present study, we employed an unbiased screen for synaptogenic proteins to identify calsyntenin-3 as a novel synapse organizing protein. Calsyntenin-3, also known as alcadein-β (Alzheimer-related cadherin-like protein β), is a brain-specific transmembrane protein with extracellular cadherin and LNS domains and subject to ectodomain shedding (Araki et al., 2004; Araki et al., 2003; Hintsch et al., 2002). We present evidence here indicating that calsyntenin-3 is an α-neurexin-specific binding partner essential for proper excitatory and inhibitory synapse development.
RESULTS
An Unbiased Expression Screen Identifies Calsyntenin-3 as a Novel Synaptogenic Protein
We performed an unbiased mammalian expression screen that we developed to identify novel proteins involved in synapse development (Linhoff et al., 2009). The assay is based on co-cultures of non-neuronal cells with neurons following a strategy first used to demonstrate the synaptogenic activity of neuroligins (Scheiffele et al., 2000). Pools of a custom cDNA expression library from developing rat brain were expressed in COS cells which were then co-cultured with rat hippocampal neurons and assessed for their ability to induce presynapse differentiation in contacting axons (Figure S1A and B). A positive cDNA library pool, PC151, was identified that induced hemi-synapse formation between a COS cell and neurons, as shown by the clustering of synapsin in the absence of excitatory or inhibitory postsynaptic scaffolds PSD-95 family proteins or gephyrin (to exclude endogenous axon-dendrite synapses). This positive pool was further screened by PCR and found to lack cDNAs encoding known synaptogenic factors, including neuroligins and LRRTMs (not shown). Hence, we subdivided PC151 into successively smaller pools which we tested for synaptogenic activity and ultimately identified the single active clone to encode calsyntenin-3. Calsyntenin-3 (Hintsch et al., 2002), also known as alcadein-β (Araki et al., 2003), is a single pass transmembrane protein that is highly enriched in brain but functionally poorly understood.
Calsyntenin-3 Induces Excitatory and Inhibitory Presynapse Differentiation
To better assess synaptogenic function of calsyntenin-3, we added a Myc-tag at its mature N-terminus and a CFP tag at its C-terminus. These tags did not alter the activity of calsyntenin-3 to induce clustering of synapsin in the co-culture assay (Figure S1C and S1D). Myc-calsyntenin-3-CFP also induced clustering of the synaptic vesicle protein synaptophysin and the active zone protein bassoon (Figure S1E). Moreover, Myc-calsyntenin-3-CFP induced uptake of an antibody against the luminal domain of synaptotagmin which becomes accessible on the neuronal surface only during active recycling of vesicles (Figure 1A). Thus, calsyntenin-3 induces the differentiation of functional presynaptic terminals. Similar to CFP-neuroligin-2 (Figure 1C), Myc-calsyntenin-3-CFP induced a 24.6-fold increase in synapsin clustering in isolated axons contacting transfected COS cells, as compared to control protein membrane-associated CFP (mCFP). Myc-calsyntenin-3-CFP induced a 2.4-fold increase relative to mCFP in tau-positive axon contact area with transfected COS cells (Figure S1F), indicating that calsyntenin-3 promotes adhesion to axons. However, even after normalizing per axon-COS cell contact area, Myc-calsyntenin-3-CFP induced a 10.1 fold increase in synapsin clustering as compared to mCFP (Figure 1D), indicating that calsyntenin-3 directly and potently induces presynapse differentiation in contacting axons independently of promoting adhesion.
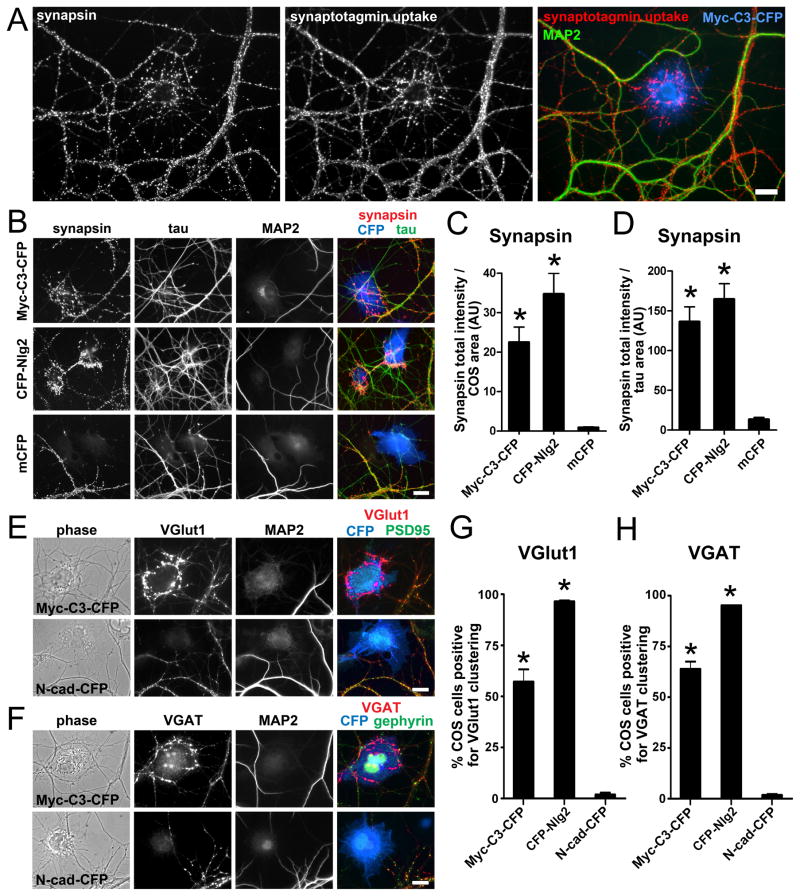
Figure 1. Calsyntenin-3 Induces Excitatory and Inhibitory Presynapse Differentiation.
(A) COS cells expressing Myc-calsyntenin-3-CFP (Myc-C3-CFP) induce the formation of presynaptic specializations in contacting axons of co-cultured hippocampal neurons. Induced presynaptic sites show synapsin clustering and uptake of a luminal domain antibody against synaptotagmin indicating functional actively recycling terminals. Induced presynaptic sites are differentiated from endogenous axon-dendrite synapses by lack of apposing MAP2-positive dendrites.
(B) Myc-calsyntenin-3-CFP (Myc-C3-CFP) expressed in COS cells induces clustering of synapsin at contact sites with tau-positive axons lacking associated MAP2-positive dendrites. CFP-neuroligin-2 (CFP-Nlg2) has similar synaptogenic activity whereas co-cultures with membrane-associated CFP (mCFP) show only endogenous axon-dendrite synapses.
(C and D) Quantitation of the total integrated intensity of synapsin immunofluorescence associated with COS cells expressing the indicated CFP fusion protein and not associated with MAP2 divided by the COS cell area (C) or divided by the tau-positive axon-COS cell contact area (D). ANOVA, p < 0.0001, n = 30 cells from 3 independent experiments; *p < 0.001 compared to mCFP by post-hoc Bonferroni multiple comparison test, p > 0.05 Myc-calsyntenin-3-CFP compared with CFP-neuroligin-2.
(E) Myc-calsyntenin-3-CFP (Myc-C3-CFP) expressed in COS cells induces glutamatergic excitatory marker VGlut1 clustering in contacting axons. Absence of associated PSD-95 family proteins was used to exclude inter-neuronal synapses. N-cadherin-CFP (N-cad-CFP) lacks similar synaptogenic activity.
(F) Myc-calsyntenin-3-CFP (Myc-C3-CFP) expressed in COS cells induces GABAergic inhibitory marker VGAT clustering in contacting axons. Absence of associated gephyrin was used to exclude inter-neuronal synapses. N-cadherin-CFP (N-cad-CFP) lacks similar synaptogenic activity.
(G and H) Quantitation of the percentage of COS cells expressing the indicated CFP fusion that induced clusters of VGlut1 lacking PSD-95 (G) or of VGAT lacking gephyrin (H). ANOVA, p < 0.0001, n≥3 experiments counting≥100 cells per experiment, *p < 0.001 compared to negative control N-cadherin-CFP by post-hoc Bonferroni multiple comparison test.
Scale bars: 20 μm. Data are presented as mean ± SEM. See also Figure S1.
We next tested whether calsyntenin-3 specifically induces glutamatergic or GABAergic presynapse differentiation. Myc-calsyntenin-3-CFP in COS cells induced clustering of vesicular glutamate transporter VGlut1 and of vesicular GABA transporter VGAT in contacting axons in hippocampal neuron co-culture at sites lacking apposed PSD-95 or gephyrin, respectively (Figure 1E–H). Thus, calsyntenin-3 induces differentiation of glutamatergic and GABAergic presynaptic specializations.
The calsyntenin family contains two other members which share 51–53% amino acid sequence identity with calsyntenin-3 (Hintsch et al., 2002). We thus tested calsyntenin-1 and calsyntenin-2 for synaptogenic activity in the co-culture assay, both in untagged form and with Myc and CFP tags to confirm expression as for calsyntenin-3. However, calsyntenin-1 and calsyntenin-2 were unable to induce synapsin clustering in contacting hippocampal axons (Figure S1G) or cortical axons (not shown).
Calsyntenin-3 is a Selective α-Neurexin Binding Partner
Calsyntenin-3 induces presynapse differentiation in co-culture assays presumably by binding to a protein on axons. Two main families of axonal proteins have been found to mediate presynapse differentiation, neurexins and type II protein tyrosine phosphatases (PTPs), which each bind multiple postsynaptic partners (Siddiqui and Craig, 2011; Takahashi and Craig, 2013). Thus, we tested for interactions of calsyntenin-3 with representatives of the neurexin and PTP families. Purified soluble fusion protein of the calsyntenin-3 ectodomain with human immunoglobulin constant region, calsyntenin-3-Fc, bound to COS cells expressing neurexin-1α but not to cells expressing PTPσ or PTPδ (Figure 2A). Saturable binding was observed, and Scatchard analysis revealed an apparent dissociation constant of 43.7 nM for the interaction of calsyntenin-3-Fc with cell-expressed neurexin-1α (Figure 2B), suggesting a physiologically significant interaction. Interaction of neurexins with many partners is calcium-dependent. Indeed, binding of calsyntenin-3-Fc to neurexin-1α was abolished in nominally calcium-free buffer containing 50 mM EGTA (Figure 2C) indicating a calcium-dependent interaction.
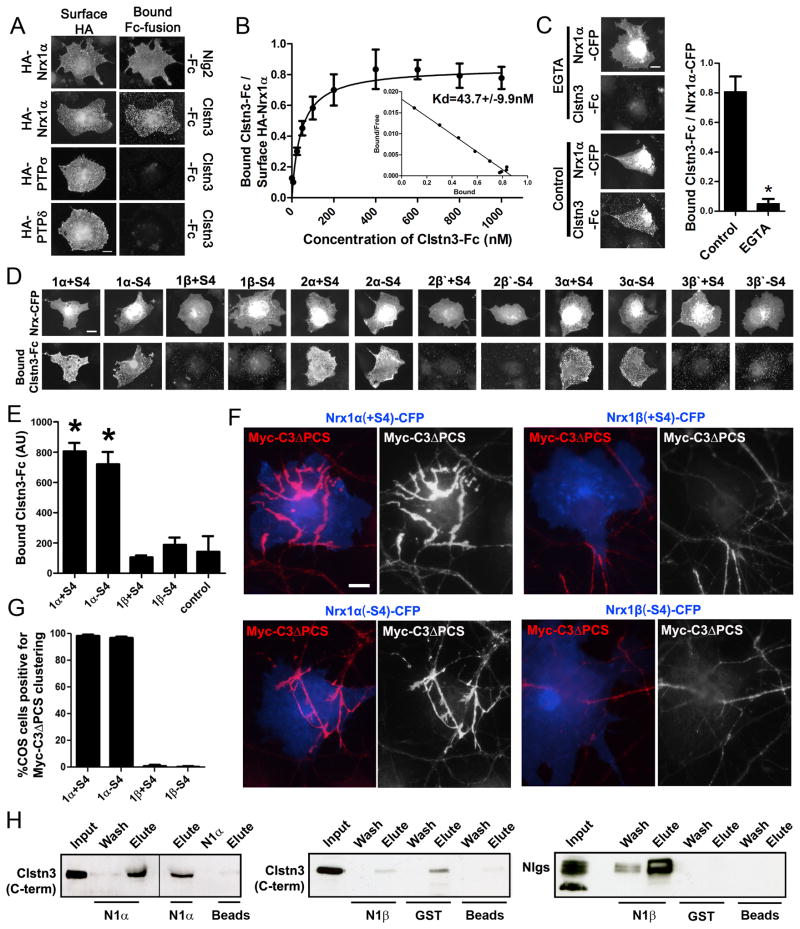
Figure 2. Calsyntenin-3 Binds and is Recruited by α-Neurexins but Not β-Neurexins.
(A) Like neuroligin-2-Fc (Nlg2-Fc), calsyntenin-3 fusion protein Clstn3-Fc bound to COS cells expressing HA-neurexin1α (HA-Nrx1α) but did not bind to COS cells expressing HA-PTPσ or HA-PTPδ. The neurexin-1α variant lacking the insert at splice site 4 was used for panels A-C.
(B) By Scatchard analysis, affinity of binding of Clstn3-Fc to HA-Nrx1α expressed on COS cells was estimated at 43.7 nM (n ≥ 15 cells each).
(C) Binding of Clstn3-Fc to neurexin Nrx1α-CFP was abolished in calcium-free media containing 50 mM EGTA. *p < 0.0001, Student’s t-test, n = 24 cells each.
(D) Clstn3-Fc bound to COS cells expressing all α-neurexin isoforms tested, α-neurexins 1, 2 and 3 containing (+S4) or lacking (−S4) the insert at splice site 4. Clstn3-Fc did not bind to COS cells expressing any β-neurexin isoform tested; 2β′ and 3β ′ contain the LNS domain from neurexin 2 or 3, respectively, fused to neurexin 1β flanking sequences.
(E) Quantitation of Clstn3-Fc bound to COS cells expressing neurexin 1α or 1β containing (+S4) or lacking (−S4) the insert at splice site 4 or to non-transfected control cells. ANOVA p < 0.0001, n ≥ 20 cells each, * p < 0.001compared to control by post-hoc Bonferroni multiple comparison test.
(F) In a recruitment assay, neurons expressing Myc-calsyntenin-3ΔPCS (Myc-C3ΔPCS) were co-cultured with COS cells expressing the indicated neurexin isoforms tagged with CFP. The α-neurexins but not β-neurexins in COS cells recruited Myc-C3ΔPCS to contact sites.
(G) Quantitation of the percentage of COS cells expressing the indcated neurexin that induced clustering of Myc-C3ΔPCS in contacting neuronal processes. ANOVA, p < 0.0001, n = 3 experiments.
(H) Pull-down of calsyntenin-3 from cow brain synaptosomes by neurexin1α but not neurexin1β. Left blot, Calsyntenin-3 was pulled down by neurexin1α ectodomain attached to beads in two independent experiments (lane 3 & 4) but not beads alone (lane 6). Neurexin1α ectodomain bait which is similar in size to calsyntenin-3 does not cross-react with the calsyntenin 3 antibody (lane 5). Middle blot, calsyntenin-3 could not be pulled down by neurexin1β-GST (compare with GST or beads alone). Right blot, under the same conditions, neuroligins (Nlgs) were pulled down by neurexin1β-GST but not GST or beads alone.
Scale bars: 10 μm. Data are presented as mean ± SEM. See also Figure S2.
As discussed above, neurexin promoter usage and alternative splicing at site 4 control its extracellular interactions. Thus, we tested multiple neurexin variants for their interaction with calsyntenin-3. When expressed in COS cells, all tested α-neurexins (1, 2, and 3 with or without an insert at splice site 4) bound calsyntenin-3-Fc, whereas none of the six β-neurexin variants tested bound calsyntenin-3-Fc (Figure 2D). Quantification of neurexin-1 binding showed that only neurexin-1α but not neurexin-1β mediated significant calsyntenin-3 binding as compared with nontransfected cells, and the presence or absence of an insert at splice site 4 in neurexin-1α did not affect the apparent affinity for calsyntenin-3-Fc (Figure 2E). We further tested the specificity of interactions in a cell-based recruitment assay. COS cells expressing all α but not β isoforms of neurexins 1, 2 and 3 tested were able to recruit Myc-calsytenin-3ΔPCS expressed in neurons to contact sites (Figures 2F, 2G and S2; ΔPCS indicates a small deletion to improve neuronal surface expression as described further below). Thus, in this modified co-culture assay, the ectodomains of calsyntenin-3 on the neuron surface and α-neurexins but not β-neurexins on the COS cell surface bound in trans at cell-cell interaction sites.
To confirm the interaction of native calsyntenin-3 with α-neurexin but not β-neurexin, we did pull-down experiments from brain. Recombinant ectodomain proteins for neurexin-1α or neurexin-1β were produced, attached to beads, and used for affinity chromatography with cow brain synaptosomes. Neurexin-1α ectodomain attached to beads, but not neurexin-1β-GST or GST or beads alone, pulled down calsyntenin-3 from synaptosomes (Figure 2H). As a positive control for neurexin-1β-GST, we found that this neurexin-1β ectodomain efficiently pulled down neuroligins from synaptosomes as expected from previous studies (Boucard et al., 2005). Altogether, these data identify α-neurexins as a selective binding partner for calsyntenin-3.
Full-length but Not Cleaved Calsyntenin-3 Induces Presynapse Differentiation
In a manner similar to amyloid β-precursor protein, calsyntenin-3 is subject to primary membrane-proximal proteolytic cleavage resulting in ectodomain shedding followed by secondary intramembranous cleavage by a presenilin-dependent γ-secretase (Araki et al., 2004; Hata et al., 2009). To determine whether the full-length and/or cleaved ectodomain forms are present in brain, we generated an antibody that detects an extracellular epitope present in calsyntenin-3 but not the related calsyntenin-1 or -2 (Figure 3A; validated against tissue lacking calsyntenin-3 in Figure 7B). In mouse brain homogenate, this antibody detected the full-length and large cleaved extracellular fragment of calsyntenin-3 in roughly equal amounts (Figure 3A). Thus, about half of the calsyntenin-3 in brain is present in the cleaved form. The degree of calsyntenin-3 cleavage may even be higher as some of the cleavage products may end up in the cerebrospinal fluid (Hata et al., 2011; Vogt et al., 2001).
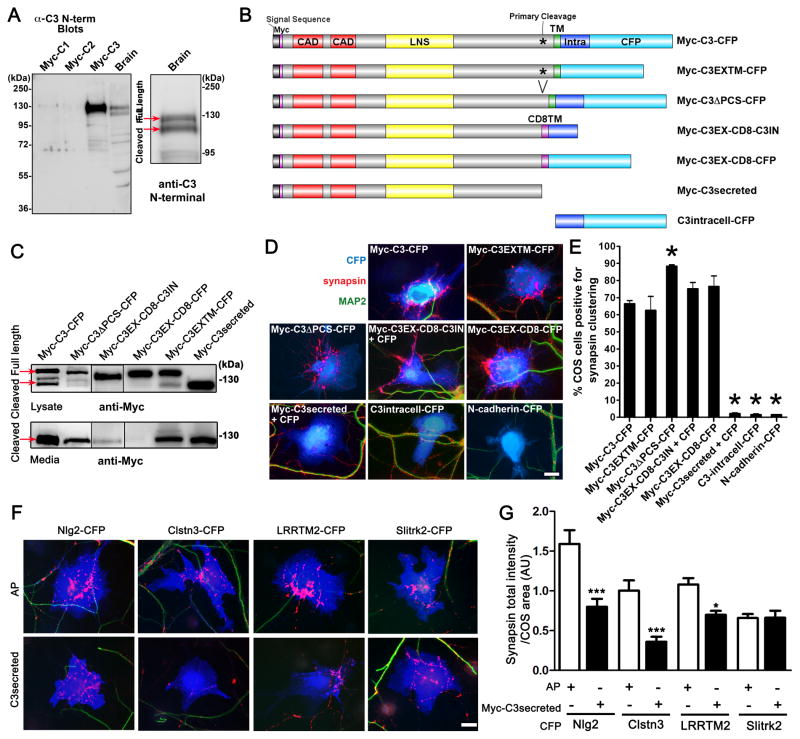
Figure 3. Full-Length Calsyntenin-3 is Synaptogenic but Cleaved Calsyntenin-3 Inhibits the Synaptogenic Activity of α-Neurexin Partners.
(A) An antibody raised against an N-terminal region of calsyntenin-3 recognizes Myc-calsyntenin-3 (Myc-C3) and shows little or no signal for Myc-calsyntenin-1 or -2 (Myc-C1, Myc-C2) expressed in COS cells. In mouse brain homogenate, the two major bands correspond to full-length (F. L.) calsyntenin-3 and the cleaved extracellular fragment. The COS cell lysate analyzed here contains mainly full-length calsyntenin-3; the cleaved extracellular fragment is found mainly in COS cell media.
(B) Domain structure of Myc-calsyntenin-3-CFP and variants: CAD, cadherin domain; LNS, laminin neurexin sex hormone binding protein domain; TM, transmembrane domain; Intra, intracellular domain; CD8TM, the calsyntenin-3 transmembrane domain was replaced with the CD8 lymphocyte protein transmembrane domain.
(C) Western blot with anti-Myc antibody of lysate and media from COS cells expressing the indicated constructs confirms secretion of Myc-C3secreted and indicates that Myc-C3EX-CD8-CFP was not cleaved whereas all other constructs assayed were partially cleaved generating large fragments in the media.
(D) Representative images of calsyntenin-3 variants in the co-culture assay. All membrane-associated forms with the calsyntenin-3 extracellular domain are synaptogenic including the non-cleavable Myc-C3EX-CD8-CFP.
(E) Quantitation of the percentage of COS cells expressing the indicated construct that induced clusters of synapsin lacking MAP2 in the co-culture assay. ANOVA, p < 0.0001, n≥3 experiments counting≥100 cells per experiment, ◆p < 0.001 compared to negative control N-cadherin-CFP by post-hoc Bonferroni multiple comparison test, * p < 0.001 compared to Myc-C3-CFP by post-hoc Bonferroni multiple comparison test.
(F) Representative co-culture images showing suppression of synaptogenic activity of CFP-tagged α-neurexin partners neuroligin 2 (Nlg2), calsyntenin-3 (Clstn3) and LRRTM2 by co-expression of Myc-C3secreted mimicking the shed calsyntenin-3 ectodomain as compared with co-expression of alkaline phosphatase (AP) control. Myc-C3secreted had no effect on Slitrk2 which acts via a non-neurexin presynaptic partner.
(G) Quantification of suppression of synaptogenic activity of α-neurexin partners by Myc-C3secreted. Clusters of synapsin associated with CFP-positive COS cells and lacking MAP2 were measured. ANOVA, p<0.0001, n= >30 cells each, *** p < 0.001 and * p < 0.05 compared to AP control by post-hoc Bonferroni multiple comparison test.
Scale bars: 20 μm. Data are presented as mean ± SEM.
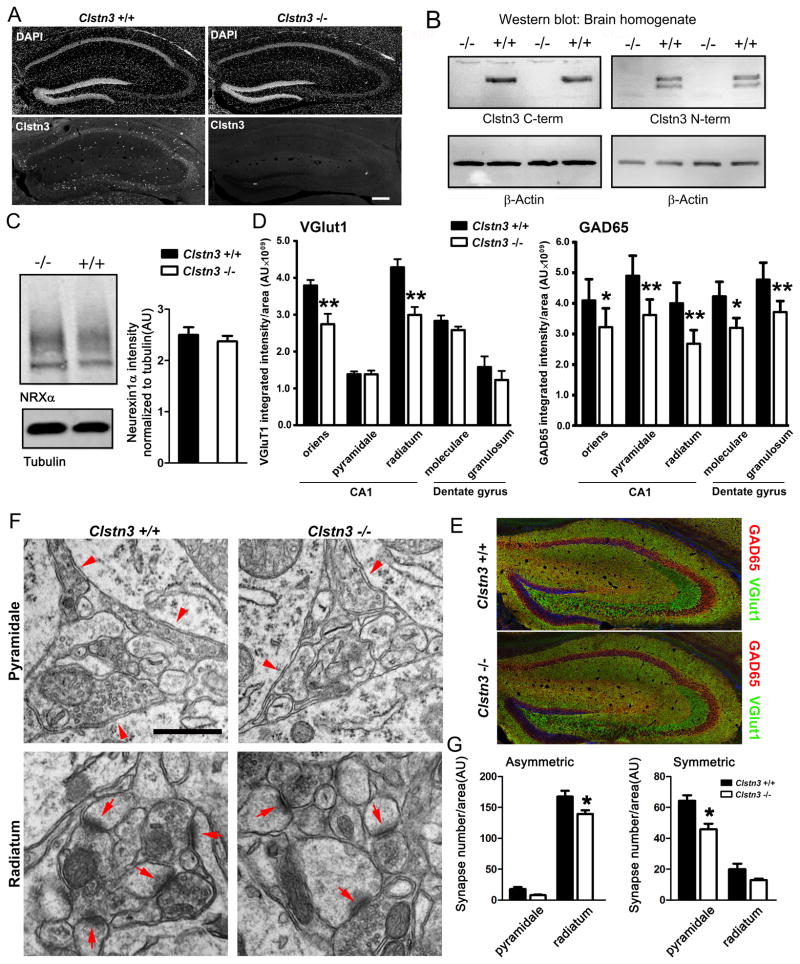
Figure 7. Clstn3 −/− Mice Show Reduced Inhibitory and Excitatory Synaptic Development.
(A) Immunofluorescence confirmation of loss of calsyntenin-3 immunoreactivity (using the C-terminal antibody) in hippocampus of Clstn3 −/− mice. Clstn3 −/− brains exhibited normal morphology and cellular distributions indicated with the DAPI nuclear co-stain.
(B) Western blot confirmation of loss of calsyntenin-3 (Clstn-3) protein detected with both C-terminal and N-terminal antibodies in Clstn3 −/− mouse brain homogenate with β-actin as loading control.
(C) Western blot and quantification showed the level of α-neurexin was indistinguishable in crude synaptosome fractions of Clstn3 −/− and +/+ littermate mice. Student’s t-test, p > 0.05, n = 3.
(D) Quantitation of VGlut1 and GAD65 punctate integrated intensity per tissue area from high resolution images. ANOVA, p < 0.0001, n = 5 mice each after averaging data from 6 sections per mouse, age P30; *p < 0.05, **p < 0.01, ***p < 0.001 by post-hoc Bonferroni multiple comparison test comparing Clstn3 +/+ and −/− for each region. VGlut1 and GAD65 were measured from the same regions of double-labeled sections.(E) Clstn3 +/+ and Clstn3 −/− mice showed no gross difference in hippocampal distribution of immunoreactivity for VGlut1, GAD65, or the nuclear marker DAPI, imaged at low resolution. (F,G) Ultrastructure analysis showed asymmetric and symmetric synapse loss in hippocampal CA1 region of Clstn3 −/− mice. ANOVA, p < 0.0001, n = 4 mice each after averaging data from more than 50 images per mouse per region, age P50–60; * p < 0.01 by post-hoc Bonferroni multiple comparison test comparing Clstn3 +/+ and −/− for each region.
Scale bars: 200 μm in A and E, 500 nm in F. Data are expressed as mean ± SEM. See also Figure S6.
To determine which calsyntenin-3 variants have synaptogenic activity, we generated a series of deletion constructs from Myc-calsyntenin-3-CFP and tested them for cleavage and synaptogenic activity in the co-culture assay (Figure 3B–E). Deleting the intracellular domain did not reduce synaptogenic activity (comparing Myc-C3EXTM-CFP to Myc-calsyntenin-3-CFP), and the intracellular domain alone (C3intracell-CFP) was not active. Deleting 20 amino acids surrounding the primary cleavage site (Myc-C3ΔPCS-CFP) or replacing the region from the primary cleavage site to the end of the transmembrane domain with the transmembrane domain of the unrelated lymphocyte protein CD8 (Myc-C3EX-CD8-C3IN) did not reduce synaptogenic activity, nor did these manipulations block partial cleavage in COS cells. We thus fused only the extracellular region of calsyntenin-3 up to the primary cleavage site to a partial CD8-CFP fusion and found that this construct, Myc-C3EX-CD8-CFP, showed no cleavage in COS cells, yet exhibited a similar synaptogenic activity as Myc-calsyntenin-3-CFP. Thus, calsyntenin-3 cleavage is not essential for synaptogenic activity. Furthermore, when Myc-tagged calsyntenin-3 ectodomain (Myc-C3secreted) was expressed and released into the media, mimicking the cleaved ectodomain, no synaptogenic activity was observed. Thus, the transmembrane form of calsyntenin-3 is synaptogenic and proteolytic release of its ectodomain abolishes the synaptogenic activity of calsyntenin-3.
Released Calsyntenin-3 Ectodomain Inhibits the Synaptogenic Activity of Multiple α-Neurexin Partners
We wondered further whether the released calsyntenin-3 ectodomain might have anti-synaptogenic activity by competing with full-length calsyntenin-3. The free calsyntenin-3 ectodomain might bind to α-neurexin along axons without triggering its local aggregation, thus blocking binding of full-length calsyntenin-3 and potentially other synaptogenic partners to α-neurexin and counteracting their presynaptic inducing activity. To test this idea, we co-expressed Myc-C3secreted mimicking the shed calsyntenin-3 ectodomain, or the presumably inert secreted protein alkaline phosphatase (AP) as a control, along with calsyntenin-3ΔPCS or other synaptogenic proteins neuroligin-2, LRRTM2, or Slitrk2. Indeed, Myc-C3secreted compared with control AP significantly reduced presynaptic induction by calsyntenin-3ΔPCS, or by neuroligin-2 or LRRTM2 which act through binding neurexins (α-neurexins in part) (Boucard et al., 2005; Siddiqui et al., 2010)), but not by Slitrk2 which acts through binding PTPσ (Yim et al., 2013) (Figure 3F and 3G). Thus, the released calsyntenin-3 ectodomain inhibits the presynaptic inducing activity of full-length calsyntenin-3 and of other α-neurexin partners neuroligin and LRRTM.
Cadherin and LNS Domains of Calsyntenin-3 are Necessary for α-Neurexin Binding and for Synaptogenic Activity
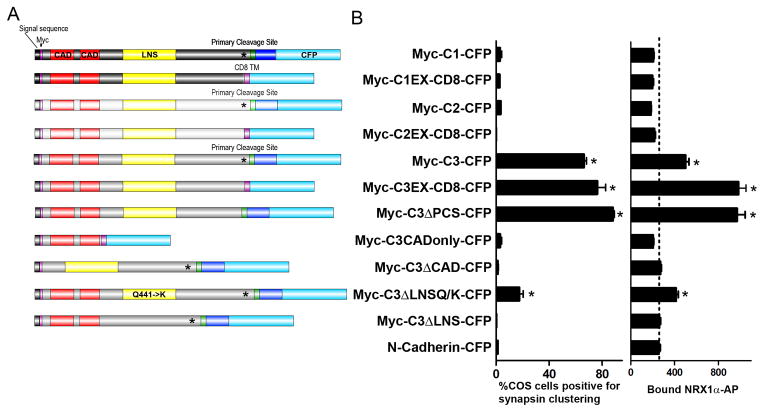
We generated another series of deletion constructs to map the domains of calsyntenin-3 important for neurexin binding and for presynaptic inducing activity (Figure 4A). Calsyntenin-3 expressed on COS cells mediated binding of neurexin1-α ectodomain fused to alkaline-phosphatase (neurexin1α-AP) but not of neurexin1β-AP, further confirming the interaction (Figure S3A). We then assayed Myc-calsyntenin-3-CFP and mutants for binding of neurexin1α-AP and for synaptogenic activity in the co-culture assay (Figure 4B). Deleting the cadherin domains (Myc-C3ΔCAD-CFP) abolished both neurexin binding and synaptogenic activity, indicating that the cadherin domains are essential. However, the cadherin domains alone (Myc-C3CADonly-CFP) expressed on the COS cell surface were unable to bind neurexin1α-AP or induce presynapse differentiation in co-culture. Furthermore, deleting the LNS domain abolished both neurexin binding and synaptogenic activity. All mutants tested reached the COS cell surface with efficiencies similar to or better than Myc-calsyntenin-3-CFP (Figure S3B); thus, deficits in binding or synaptogenic activity were not due to trafficking alterations. We also tested a point mutation in the LNS domain Q441→K mimicking a mutation E→K in the C. elegans single calsyntenin ortholog CASY-1 which results in defects in learning (Ikeda et al., 2008). This LNS point mutation significantly reduced but did not abolish both α-neurexin binding and synaptogenic activity. These data indicate that the cadherin and LNS domains of calsyntenin-3 are essential for α-neurexin binding and for synaptogenic activity.
Figure 4. The Cadherin and LNS Domains are Required for Calsyntenin-3 Synaptogenic Activity and α-Neurexin Binding.
(A) Domain structure of calsyntenin variants. The darker and lighter shading for calsyntenin-1 (C1) and calsyntenin-2 (C2) represent different but similar amino acid sequence as compared with calsyntenin-3 (C3). (B) Quantification of the percentage of COS cells expressing the indicated construct that induced clusters of synapsin lacking MAP2 in the co-culture assay (left) and binding of neurexin1α-AP to the indicated constructs expressed in COS cells (right). ANOVA, p<0.0001, n= >30 cells each. *p<0.01 compared to negative control N-cadherin-CFP by post-hoc Bonferroni multiple comparison test. Data are presented as mean ± SEM. See also Figure S3.
We next tested tagged calsyntenin-1 and calsyntenin-2 for α-neurexin binding and, consistent with the lack of co-culture activity, found that these calsyntenin family members could not mediate binding of neurexin1α-AP (Figure 4). However, these constructs Myc-calsyntenin-1-CFP and Myc-calsyntenin-2-CFP showed poor surface trafficking as compared with Myc-calsyntenin-3-CFP (Figure S3). Thus, we generated variants with better surface expression. Myc-C1EX-CD8-CFP and Myc-C2EX-CD8-CFP containing the extracellular domains up to the primary cleavage sites trafficked well to the COS cell surface yet could not mediate binding of neurexin1α-AP nor presynaptic induction in co-culture, unlike the homologous Myc-C3EX-CD8-CFP (Figures 4 and S3). Thus, among the calsyntenins, calsyntenin-3 may be unique in its ability to bind α-neurexin and induce presynapse differentiation. Altogether, the correlation for multiple constructs between α-neurexin binding and co-culture activity (Figure 4) supports the idea that α-neurexin mediates the presynaptic inducing activity of calsyntenin-3.
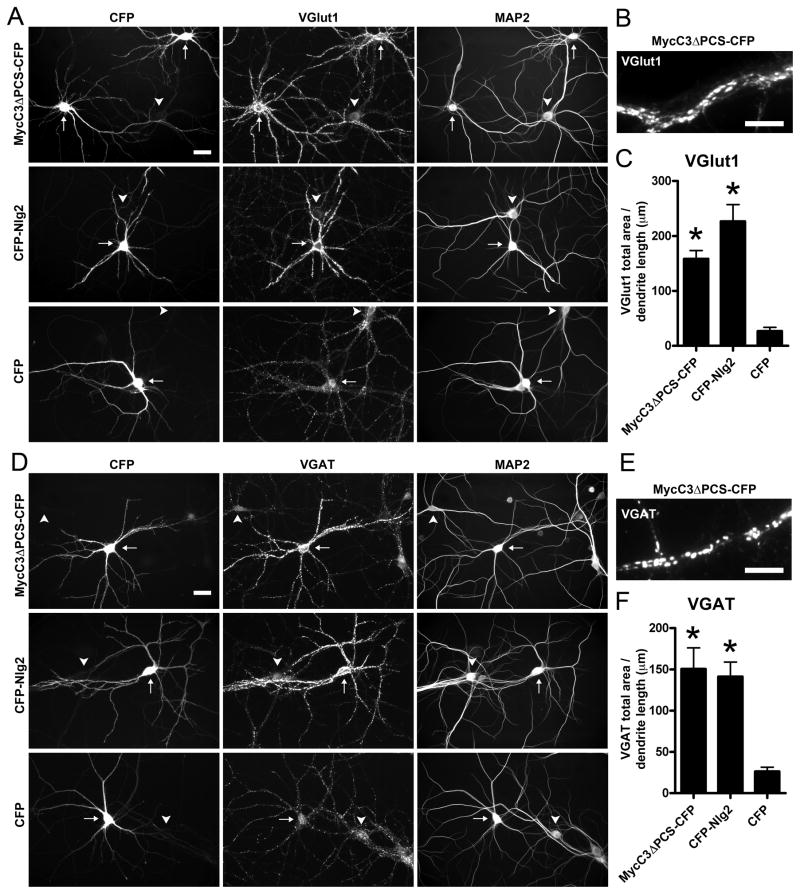
Overexpression of Calsyntenin-3ΔPCS in Neurons Promotes Glutamatergic and GABAergic Presynapse Differentiation
Next, we assessed the effect of elevating calsyntenin-3 levels on synaptic differentiation in cultured neurons. However, unlike in brain where the ratio of full-length to cleaved calsyntenin-3 is about equal (Figure 3A), endogenous calsyntenin-3 was primarily detected in cleaved form in cultured neurons (>90% cleaved; Figure S4C). Furthermore, recombinant Myc-calsyntenin-3 did not accumulate on the surface of cultured hippocampal neurons although it accumulated on the surface of COS cells. Myc-calsyntenin-3ΔPCS with a small deletion at the primary cleavage site showed significantly reduced cleavage allowing accumulation on the neuron surface (Figure S4A and S4B), and was therefore used to assess effects of overexpression. Similar to CFP-neuroligin-2, overexpression of Myc-calsyntenin-3ΔPCS significantly enhanced clustering of VGlut1, VGAT, and synapsin at inputs onto transfected dendrites as compared to neurons expressing only CFP or neighboring nontransfected neurons (Figures 5 and S4). Thus, dendritic surface expression of calsyntenin-3ΔPCS promotes excitatory and inhibitory presynapse differentiation in contacting axons. We then attempted to determine whether the non-cleavable Myc-C3EX-CD8-CFP also enhanced presynapse differentiation in cultured neurons but, unlike the partially cleavable Myc-C3EX-CD8-C3IN, this non-cleavable construct did not accumulate on the dendrite surface (Figure S4D–F).
Figure 5. Calsyntenin-3ΔPCS Overexpression Enhances Excitatory and Inhibitory Presynaptic Inputs in Cultured Neurons.
(A, D) Hippocampal neurons transfected at 8–9 DIV with Myc-calsyntenin-3 with a small deletion at the primary cleavage site (Myc-C3ΔPCS-CFP) or CFP-neuroligin2 (CFP-Nlg2) but not CFP showed enhanced immunofluorescence for the excitatory input marker VGlut1 (A) and inhibitory input marker VGAT (D) as compared to non-transfected neighbor neurons at 14 DIV. Arrows indicate transfected neurons; arrowheads indicate non-transfected neurons.
(B, E) At high magnification, the strong VGlut1 and VGAT signals onto dendrites expressing MycC3ΔPCS resolve into individual puncta.
(C, F) Quantitation of VGlut1 (C) and VGAT (F) immunopositive area per transfected dendrite length. ANOVA, p < 0.0001, n = 20 cells each; *p < 0.001 compared to CFP by post-hoc Bonferroni multiple comparison test.
Scale bars: 30 μm in A and D, 10 μm in B and E. Data are presented as mean ± SEM. See also Figure S4.
The markedly different ratios of full-length to cleaved calsyntenin-3 in neuron culture as compared with in vivo (Figure S4C) and the opposing biological effects of these forms of the protein (Figure 3) prompted us to focus our attention next on assessing calsyntenin-3 distribution and function in vivo.
Calsyntenin-3 Shows Differential Expression Patterns In Vivo
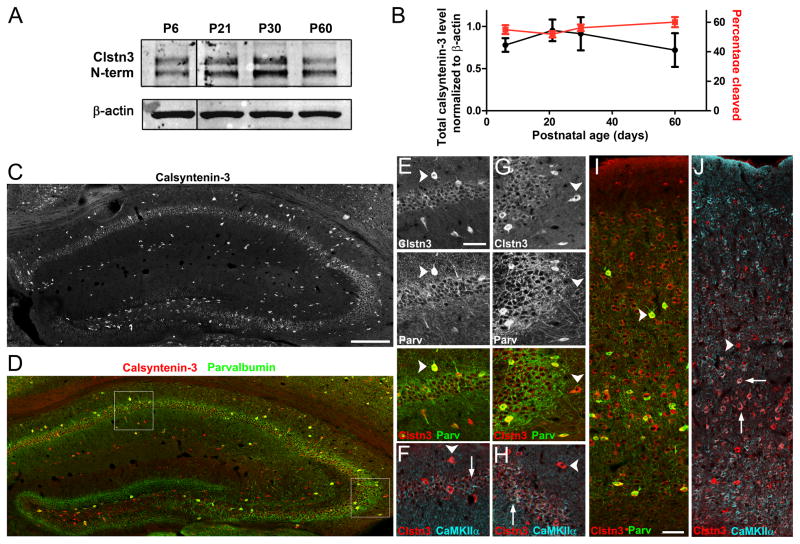
The developmental expression and cleavage profiles of calsyntenin-3 were determined between postnatal days P6 to P60 by Western blot analysis with the N-terminal antibody. Only modest non-significant changes in levels of calsyntenin-3 or in the ratio of cleaved to full-length forms were observed over this time frame (Figure 6A and 6B). The percentage of calsyntenin-3 in cleaved form was always in the range of 52–60%.
Figure 6. Calsyntenin-3 is Expressed by Neuron Subsets in Hippocampus and Cortex.
(A, B) Western blot and quantification of the expression level and fraction of total calsyntenin-3 present as the cleaved form during development (P, postnatal day).
(C–J) Distribution of calsyntenin-3 (Clstn3, with C-terminal antibody) in adult mouse hippocampus (C-H) and cortex (I,J). Co-labeling was performed for parvalbumin (D, E, G, I) and CaMKIIα (F, H, J).
(C–H) In hippocampus, calsyntenin-3 immunoreactivity was detected most strongly in interneurons, both parvalbumin-positive and parvalbumin-negative (arrowheads in E and G, respectively). Parvalbumin-negative interneurons were identified by their position outside the pyramidal cell layer and by the absence of immunoreactivity for CaMKIIα (arrowheads in F and H). By comparison with Clstn3 −/− tissue (see Figure 7), wild-type pyramidal neurons (arrows) in CA1 (E, F) and in CA3 (G, H) were also immunopositive for calsyntenin-3 whereas no immunoreactivity was detectable in dentate granule cells. (I, J) In neocortex, calsyntenin-3 was widely expressed, at highest levels by interneurons (arrowheads) including parvalbumin-positive interneurons (I) but also by some pyramidal neurons particularly in layer V (arrows in J).
Scale bars: 200 μm in C and D, 50 μm in E–J. Data are presented as mean ± SEM. See also Figure S5.
By immunofluorescence, calsyntenin-3 was detected widely in brain including hippocampus and cortex (Figure 6C–J) using an antibody raised against a C-terminal peptide that was validated using tissue lacking calsyntenin-3 (see Figures 7A and 7B). The N-terminal antibody gave similar results (Figure S5). Consistent with a previous report (Hintsch et al., 2002), calsyntenin-3 was detected most strongly in interneurons identified here by their location outside the main cell body layers of the hippocampus, by the absence of calcium-calmodulin activated protein kinase IIα (CaMKIIα), and by the presence of parvalbumin in a subset of interneurons. Calsyntenin-3 was also detected at lower levels in CA1 and CA3 pyramidal neurons in a pattern that was not detected in Clstn3 −/− tissue (Figures 6C–J and 7A). In the dentate gyrus, scattered hilar neurons and interneurons exhibited high levels of calsyntenin-3, while granule cells showed little or no calsysntenin-3. In neocortex, calsyntenin-3 was again most strongly expressed by interneurons including parvalbumin-positive neurons but also expressed by many pyramidal cells, particularly in layer V (Figures 6I, 6J and S6C). Calsyntenin-3 was detected in soma, proximal dendrites and additional processes. Although we did not detect clear synaptic puncta of calsyntenin-3, this may be due to limitations of antigen accessibility or antibody sensitivity as electron microscopic analyses detected calsyntenin-3 most prominently in postsynaptic specializations of asymmetric synapses in hippocampus, cortex, and cerebellum (Hintsch et al., 2002).
Clstn3 −/− Mice Are Deficient in Inhibitory and Excitatory Synapse Development
To determine the function of calsyntenin-3 in vivo, we generated Clstn3 −/− mice (Figures S6A and S6B). Loss of calsyntenin-3 protein was confirmed by loss of calsyntenin-3 immunofluorescence in brain sections (Figure 7A) and by Western blotting of brain homogenates (Figure 7B). Levels of α-neurexin were normal in Clstn3 −/− mouse brain crude synaptosome fractions (Figure 7C). Clstn3 −/− mice showed no obvious deficits in survival or fertility. The overall brain morphology of Clstn3 −/− mice was normal, as was the overall organization of the excitatory synapse marker VGlut1 and the inhibitory synapse marker glutamic acid decarboxylase GAD65 (Figures 7A, 7E and S6). However, quantitative high-resolution confocal analysis of hippocampal CA1 and dentate gyrus regions revealed significant regional reductions in VGlut1 and global reductions in GAD65 punctate immunofluorescence in mice lacking calsyntenin-3 as compared to wild-type littermate controls (Figure 7D).
To determine whether the reductions in punctate VGlut1 and GAD65 immunofluorescence in CA1 of Clstn3 −/− mice represent reductions in excitatory and inhibitory synapse numbers, we assessed asymmetric and symmetric synapse density by transmission electron microscopy (Figure 7F). Indeed, asymmetric synapse density was significantly reduced in CA1 stratum radiatum and symmetric synapse density was significantly reduced in CA1 stratum pyramidale in mice lacking calsyntenin-3 as compared to wild-type littermate controls (Figure 7G). Non-significant reductions of >30% were also seen for asymmetric synapses in stratum pyramidale and symmetric synapses in stratum radiatum, regions of low synapse density. Thus, loss of calsyntenin-3 results in reductions in both excitatory and inhibitory synapse density.
Clstn3 −/− Mice Are Deficient in Inhibitory and Excitatory Transmission
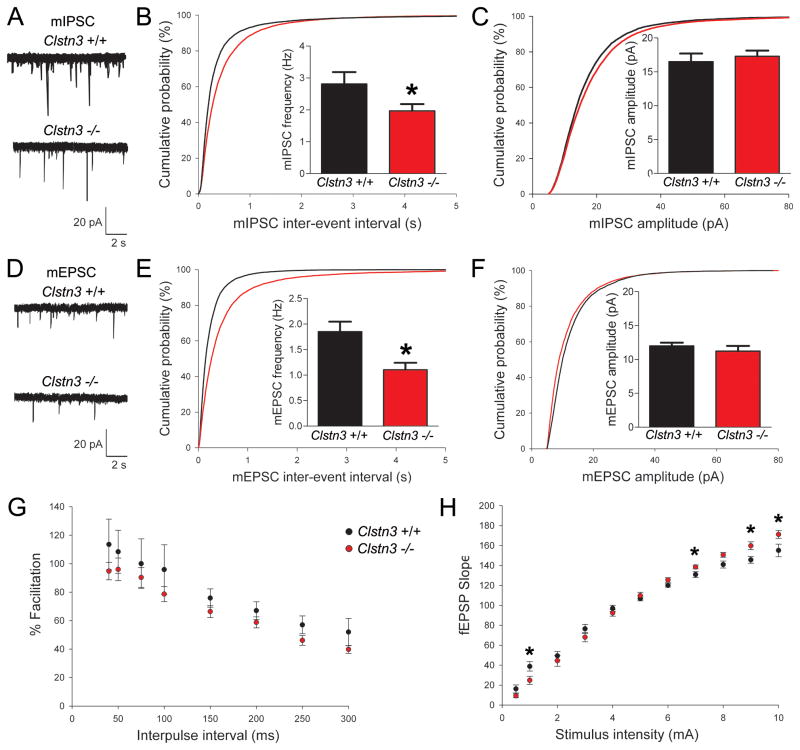
We next sought to determine if synaptic transmission is altered in the absence of calsyntenin-3. We performed whole-cell recordings from CA1 pyramidal cells in Clstn3 −/− and wild-type littermate hippocampal slices to quantify spontaneous miniature inhibitory postsynaptic currents (mIPSCs) and miniature excitatory postsynaptic currents (mEPSCs). Relative to wild-type littermates, mIPSC recordings from Clstn3 −/− slices revealed a reduction in mIPSC frequency but not amplitude (corresponding to an increased inter-event interval, Figure 8A–C). Similarly, comparisons of mEPSCs from Clstn3 −/− and wild-type mice revealed reductions in frequency but not amplitude (Figure 8D–F). Taken together, these data indicate that calsyntenin-3 is required for normal development of functional inhibitory and excitatory synapses.
Figure 8. Inhibitory and Excitatory Neurotransmission is Compromised inClstn3 −/− Mice.
(A) Representative mIPSC recordings from hippocampal CA1 pyramidal neurons in acute slices from Clstn3 +/+ and Clstn3 −/− adult mice.
(B,C) Cumulative distributions of mIPSC inter-event intervals (B) and amplitudes (C) in Clstn3 +/+ and Clstn3 −/− neurons (Kolmogorov-Smirnov test, p < 0.001 for inter-event intervals). Insets display mean ± SEM for mIPSC frequency (B) and amplitude (C). mIPSC frequency but not amplitude was significantly reduced in Clstn3 −/− neurons (Student’s t-test, *P < 0.05; n = 11 cells, Clstn3 +/+; n = 21 cells, Clstn3 −/−).
(D) Representative mEPSC recordings from Clstn3 +/+ and Clstn3 −/− hippocampal CA1 pyramidal neurons.
(E,F) Cumulative distributions of mEPSC inter-event intervals (E) and amplitudes (F) in Clstn3 +/+ and Clstn3 −/− neurons (Kolmogorov-Smirnov test, p < 0.001 for inter-event intervals). Insets display mean ± SEM for mEPSC frequency (E) and amplitude (F). mEPSC frequency but not amplitude was significantly reduced in Clstn3 −/− neurons (Student’s t-test, *P < 0.01; n = 20 cells, Clstn3 +/+; n = 22 cells, Clstn3 −/−).
(G) Paired-pulse facilitation was assessed as the ratio of fEPSP slopes elicited by paired pulses in hippocampal CA1 stratum radiatum in response to stimulation of Schaffer collateral fibers. No significant difference was observed between Clstn3 +/+ (n = 12) and Clstn3 −/− (n = 8) mouse hippocampal slices. (H) Basal evoked synaptic responses were measured through comparison of input/output responses. At higher stimulation intensities, Clstn3 −/− fEPSP responses were enhanced relative to Clstn3 +/+ littermate controls (Student’s t-test, *p < 0.05, n = 8 slices each).
To determine if the loss of calsyntenin-3 affects neurotransmitter release probability, we measured paired-pulse facilitation with field EPSP (fEPSP) recordings in CA1 stratum radiatum in response to stimulation of Schaffer collateral fibers. No significant differences between mutant and control slices were detected (Figure 8G). These data suggest that calsyntenin-3 does not alter transmitter release probability and, along with the mPSC recordings (Figure 8A–F) and confocal and ultrastructural imaging data (Figure 7D–F), are consistent with the idea that loss of calsyntenin-3 reduces excitatory and inhibitory synapse density. We next monitored input/output curves in response to step-wise increases in stimulation intensity to determine if basal evoked synaptic responses are altered in Clstn3 −/− mice. At the higher end of the stimulation range, fEPSP responses from Clstn3 −/− hippocampal slices were significantly enhanced relative to wild-type littermate controls (Figure 8H). These data suggest that the net deficit in inhibitory transmission in Clstn3 −/− hippocampus may slightly outweigh the deficit in excitatory transmission to alter overall circuit excitability.
DISCUSSION
From an unbiased expression screen, we identified calsyntenin-3 as a novel synaptogenic protein. Calsyntenin-3, but not the related calsyntenin-1 or calsyntenin-2, can trigger excitatory and inhibitory presynapse differentiation in contacting axons. We show with cell-based binding and recruitment assays and pull-down from brain that calsyntenin-3 specifically binds α-neurexins and not β-neurexins. Calsyntenin-3 undergoes prominent ectodomain shedding with at least half of calsyntenin-3 in mouse brain present in the cleaved form. Full-length calsytenin-3 is synaptogenic in co-culture and a small deletion at the primary cleavage site allows calsyntenin-3 to accumulate on the dendritic surface of cultured neurons and to enhance presynapse differentiation. However, the released ectodomain of calsyntenin-3 not only lacks synaptogenic activity but inhibits the presynaptic inducing activity of multiple α-neurexin partners. Calsyntenin-3 is highly expressed by interneurons throughout hippocampus and cortex and is expressed at lower levels by many pyramidal neurons. Clstn3 −/− mice show reduced punctate immunofluorescence for GAD65 and VGlut1, reduced density of symmetric and asymmetric synapses, and reduced mIPSC and mEPSC frequency in hippocampal CA1 neurons. Together, these data suggest that an α-neurexin-calsyntenin-3 complex is essential for normal inhibitory and excitatory synapse development and function.
Unlike the binding specificity of calsyntenin-3 for α-neurexins, other neurexin partners neuroligins, LRRTMs, cerebellin-GluRδ, dystroglycan, and latrophilin bind α- and β-neurexins or just β-neurexins (Boucard et al., 2005; Boucard et al., 2012; Matsuda and Yuzaki, 2011; Siddiqui et al., 2010; Sugita et al., 2001; Uemura et al., 2010). Neurexin splice site 4, a key molecular switch which affects interactions with many of these partners, did not appear to affect the interaction with calsyntenin-3 (Figures 2 and S2). Mice lacking all three α-neurexins but with normal expression of β-neurexins die at birth due to impaired synaptic transmission linked to deficits in presynaptic calcium channel currents (Missler et al., 2003). Clstn3 −/− mice exhibit relatively normal survival and lack the profound reductions in evoked synaptic transmission that were detected in mice lacking α-neurexins (Figures 7 and 8). Thus, it seems likely that neurexin partners other than calsyntenin-3 mediate its presynaptic calcium channel recruitment function. However, we found a similar reduction in density of inhibitory synapses in mice lacking calsyntenin-3 as found in mice lacking α-neurexins. The small but significant increase in input-output curve in Clstn3 −/− CA1 region compared with wild-type controls supports the idea that the contribution of calsyntenin-3 to inhibitory synapse development is important for circuit function. However, the near normality of the input-output curve may be misleading given the parallel reductions in mEPSC and mIPSC frequency which indicate significant deficits in excitatory and inhibitory transmission in the absence of calsyntenin-3.
In the simplest model, full-length calsyntenin-3 may act as a postsynaptic cell adhesion and synapse organizing protein, similar to neuroligins and LRRTMs and other presynaptic inducing proteins identified from this screen. This model is consistent with the ability of calsyntenin-3 to induce presynaptic specializations when presented to axons in trans on COS cells or upon dendritic overexpression of calsyntenin-3ΔPCS (Figures 1 and 5). Although we were unable to detect specific synaptic localization, electron microscopy showed prominent enrichment of calsyntenin-3 in the postsynaptic specializations of asymmetric synapses in hippocampus, cortex and cerebellum (Hintsch et al., 2002). The deficits observed in excitatory synapses could be explained by this simple model: VGlut1 puncta, asymmetric synapse density and mEPSC frequency were reduced in Clstn3 −/− CA1 neurons, which normally express calsyntenin-3, while VGlut1 immunofluorescence was unchanged at inputs onto dentate granule cells, which express little or no calsyntenin-3. Even in this simplest model, extensive calsyntenin-3 cleavage differentiates it from other synaptogenic proteins, and our data suggest the cleaved form may be important in buffering the synaptogenic activity of α-neurexin partners, perhaps balancing overall synapse development.
However, considering the multiple unique characteristics of calsyntenin-3, its prominent intracellular pools detected with our N- and C-terminal antibodies (Figures 6 and S5) and its functional importance for both inhibitory and excitatory synapse development (Figures 7 and 8) as well as its high degree of cleavage (Figure 6), calsyntenin-3 may act instead or in addition by a distinct mechanism from other known neurexin ligands. We detected Myc-calsyntenin-3ΔPCS on the surface of transfected axons as well as dendrites. Although the level of α-neurexin in crude synaptosomes was not affected by loss of calsyntenin-3 (Figure 7C), the possibility remains that calsyntenin-3 might interact in cis with α-neurexin to regulate neurexin trafficking or function. Indeed, loss of calsyntenin-3 resulted in widespread deficits in inhibitory synapses, evidenced by loss of GAD65 even onto dentate granule cells that normally express little or no calsyntenin-3, possibly resulting from loss of calsyntenin-3 in interneurons. Further work is needed to assess subcellular distribution and circuit contributions of full-length and cleaved calsyntenin-3 in interneurons as compared with pyramidal cells and to determine the mechanisms by which calsyntenin-3 regulates synapse development, whether as inducer or modulator.
Calsyntenin-3 undergoes proteolytic processing in a manner similar to amyloid precursor protein, involving primary cleavage that releases a large extracellular fragment, followed by secondary intramembranous cleavage by γ-secretase (Hata et al., 2009). Neuroligin-1 was also recently found to undergo regulated ectodomain cleavage (Peixoto et al., 2012; Suzuki et al., 2012). However, while the vast majority of neuroligin-1 in brain is present as the full-length form (Peixoto et al., 2012), less than half of the calsyntenin-3 in brain is present as full-length form and over half as the shed ectodomain (Figure 6). Recombinant calsyntenin-3 can be partially cleaved by ADAM-10 and ADAM-17 (Hata et al., 2009), although the identity of the primary proteases in brain has not been determined. In cultured neurons, nearly all calsyntenin-3 was cleaved and we were unable to inhibit cleavage despite assaying a large range of protease inhibitors (data not shown). Perhaps like the related calsyntenin-1 (Maruta et al., 2012), calsyntenin-3 may be largely cleaved en route to the cell surface. We suspect that calsyntenin-3 cleavage and trafficking in neurons may be linked as the partially cleavable Myc-C3ΔPCS-CFP and Myc-C3EX-CD8-C3IN accumulated on the dendrite surface but the non-cleaved Myc-C3EX-CD8-CFP did not (Figures 3 and S4). A better understanding of the regulation of calsyntenin-3 proteolytic processing will be important considering our finding that full-length calsyntenin-3 and the released ectodomain can have opposing functions (Figure 3).
Calsyntenin-1 and -2 have a similar domain structure as calsyntenin-3, undergo similar cleavage (Araki et al., 2004; Hata et al., 2009), and were also found to be enriched at postsynaptic sites (Hintsch et al., 2002; Vogt et al., 2001). However, calsyntenin-1 and -2 lack synaptogenic activity and we could detect no interaction with neurexin (Figures 4 and S1). Calsyntenin-1 was found to act as an organelle adaptor that links kinesin-1 light chain to transport vesicles (Araki et al., 2007; Konecna et al., 2006; Vagnoni et al., 2011). No role for calsyntenin-3 has been demonstrated in organelle transport. Indeed, such a function may be unlikely as calsyntenin-3 lacks one of the kinesin light chain binding sites and has a variant residue in the other which reduces the affinity of kinesin interaction (Konecna et al., 2006). These differences in their proposed functions, calsyntenin-3 in synapse development and calsyntenin-1 in vesicle transport, are also consistent with the higher COS7 cell surface expression we observed for tagged calsyntenin-3 as compared with calsyntenin-1 (Figure S3). Synapse organizing function is typically but not always shared among family members; for example, TrkC but not TrkA or TrkB was found to interact with PTPσ to organize excitatory synapses (Takahashi et al., 2011).
Based on their similar proteolytic processing as amyloid precursor protein (Araki et al., 2004), calsyntenins/alcadeins have been suggested as biomarkers for Alzheimer’s disease. Indeed, presenilin I mutations linked to familial Alzheimer’s disease alter processing of all calsyntenins (Hata et al., 2009), and altered levels of calsyntenin-1 and calsyntenin-3 products were found in Alzheimer’s disease patients (Hata et al., 2011; Vagnoni et al., 2012) and in a mouse model (Uchida et al., 2011), respectively. Multiple mechanisms have been suggested for a role of calsyntenins in Alzheimer’s disease including reduced transport mediated by a calsyntenin kinesin-1 light chain complex, and reduced stabilization of amyloid precursor protein by a triple complex with X11L/Mint2 and calsyntenins (Araki et al., 2003). The data presented here suggest an additional possibility: loss of the synaptic organizing function of calsyntenin-3 may contribute to Alzheimer’s disease, particularly to the associated cognitive decline.
The single calsyntenin ortholog in C. elegans, CASY-1, is essential for multiple forms of associative learning, through mechanisms that are not well understood but require the extracellular domain of CASY-1 (Hoerndli et al., 2009; Ikeda et al., 2008). Similar to our mapping showing the importance of calsyntenin-3 cadherin and LNS domains for neurexin binding and synaptogenic function (Figure 4), the cadherin and LNS domains of CASY-1 were required to rescue chemotaxis learning (Ikeda et al., 2008). Of the many variants in neurexins linked to cognitive and psychiatric disorders, the majority affect α-neurexins and not β-neurexins (Ching et al., 2010; Schaaf et al., 2012), and calsyntenin-3 is the first α-neurexin-specific extracellular partner of which we are aware other than the small neurexophilins. We propose that calsyntenin-3 may contribute to cognitive performance through its effects on synapse development and function and suggest that further studies may be warranted on the role of calsyntenin-3 in psychiatric disorders.
EXPERIMENTAL PROCEDURES
Additional details are provided in Supplemental Information
Cell Culture and Transfection
Dissociated primary hippocampal neuron cultures were prepared from embryonic day 18 rat embryos as described previously (Kaech and Banker, 2006). Neurons were plated at a density of 300,000 cells per 60 mm dish on poly-L-lysine coated coverslips and inverted over a feeder layer of glia. To prevent overgrowth of glia, cytosine arabinoside (5 μM) was added to neuron cultures at 2 d. Serum-free media was also supplemented with 100 μM APV (Research Biochemicals) to prevent excitotoxicity during transfection or co-culture. For overexpression studies, neurons were transfected with 5–8 μg of DNA per dish at day in vitro (DIV) 8–9 using the ProFection Mammalian Transfection System (Promega) and fixed at 14 DIV.
COS7 and HEK293T cells were cultured in DMEM-H media with 10% fetal bovine serum. Transfections of COS7 cells were performed using TransIT-LT1 Transfection Reagent (Mirus). Co-cultures of primary hippocampal neurons with COS7 cells were performed as described previously (Graf et al., 2004). Briefly, transfected COS7 cells were trypsinized on the day after transfection and resuspended in conditioned neuron culture media. Neurons grown for 9–10 DIV were inverted in their home dish and COS cells were added. After 2 h, the neuron coverslips were flipped back over so the neurons and COS cells were facing the glial feeder layer. After 20–24 h of co-culture, cells were fixed for analysis.
Expression Screen
An unamplified full-length size-selected cDNA expression library from rat brain postnatal day 11 was custom-generated and screening performed as described (Linhoff et al., 2009). Briefly, pools of plasmid DNA were generated from 200 colony forming units grown in ultralow gelling agarose to promote uniform amplification. Each pool of cDNA was transfected into COS cells which were harvested by trypsinization after 7 or 24 h and seeded onto neuron coverslips pre-grown for 8–9 DIV. Co-cultures were fixed 20 h later for screening. Pools that generated COS cells with overlying axon-associated clustering of synapsin lacking PSD-95 or gephyrin were tested by PCR for neuroligins, netrin-G ligands, and LRRTMs. Positive pool PC151 lacked these known synaptogenic factors. PC151 glycerol stock was used to prepare and screen sub-pools from 100 colony forming units each. The sub-pool with strongest activity was arrayed into 384-well plates of individual colonies and plasmid DNA prepared and screened in rows and then by individual clone.
Immunofluorescence
The C-terminal antibody against calsyntenin-3 was raised in rabbits against the peptide DSPSSDERRIIESPPHRY with an N-terminal cysteine for conjugation to the carrier protein keyhole limpet hemocyanin and affinity purified against the peptide (RAP Affinity Purification Kit; Zymed). The N-terminal antibody against calsyntenin-3 was raised in rabbits against a GST fusion with the cadherin repeat region (KANKHKPW…QVKPTCKP) expressed from the pGEX-4T-1 vector and purified from bacteria. All image analysis was performed blind to experimental condition.
Binding Assays and Pull-Down
For cell-based binding assays, COS7 cells were transfected with the indicated expression vectors and grown for 24 h. Live cells were incubated with fusion proteins (at 50 nM unless otherwise indicated) either for 1 hour at 20°C or for 45 min at 4°C. Fc fusions were visualized with FITC-conjugated anti-human IgG and AP fusions with anti-alkaline phosphatase antibody.
For the pull-down from brain, cow synaptosomes were prepared following a previously described method (Villasana et al., 2006). Pull-down was performed with hexahistidine-tagged neurexin1α ectodomain coupled to Talon beads or with neurexin1β ectodomain fused to GST or GST control coupled to glutathione beads. Elutions were performed with buffer containing 2% SDS. Samples were analyzed by Western blot with the C-terminal antibody against calsyntenin-3 or with anti-neuroligin antibody (Synaptic Systems #129011).
Electron Microscopy
Clstn3 −/− and wild-type littermate mice were perfused transcardially with 4% paraformaldehyde and then PBS. Brain tissue was collected and post-fixed with 4% paraformaldehyde for 24 h. Hippocampal CA1 region was blocked and fixed with osmium oxide, dehydrated, embedded in a JEMBED/Spurr’s mixture and 70 nm sections were generated and stained with 2% uranyl acetate and Reynold’s lead. Images of stratum pyramidale (>100) and stratum radiatum (>50) were obtained on a Hitachi H7600 transmission electron microscope. Symmetric and asymmetric synapses were visually identified and counted.
Electrophysiology
Hippocampal slices were prepared from 6- to 10-week-old Clstn3 −/− and wild-type littermates. Whole-cell recordings of CA1 neurons were performed with a MultiClamp 700B amplifier using WinLTP software. mEPSCs were recorded under voltage clamp at −60 mV with a Cs-methanesulfonate-based intracellular solution in the presence of tetrodotoxin (TTX) and bicuculline. mIPSCs were recorded at −70 mV with a CsCl-based intracellular solution in the presence of TTX, CNQX and DL-AP5. Analyses for frequency and amplitude were conducted using MiniAnalysis software. Statistical analyses were completed using GraphPad InStat and SigmaPlot. Paired-pulse and input-output data were obtained by extracellular recordings in CA1 stratum radiatum while stimulating Schaffer collateral fibers. The initial slope of the fEPSP was measured to quantify synaptic strength (Johnston and Wu, 1995).
Supplementary Material
Highlights.
calsyntenin-3 triggers excitatory and inhibitory presynapse differentiation
calsyntenin-3 binds α-neurexins but not β-neurexins
shed calsyntenin-3 ectodomain has broad anti-synaptogenic activity
Clstn3 −/− mice are deficient in excitatory and inhibitory synapse development
Acknowledgments
We thank Xiling Zhou and Nazarine Fernandes for excellent technical assistance, Vivian Lam and Sarah Au-Yeung for contributions for the Western blot analysis, Michiko Takeda for contributions to mouse colony management, Mika Kishimoto-Suga for experimental advice with antibody generation, and Dr. Robert Holt and team at the Michael Smith Genome Sciences Centre for arraying the cDNA sub-pool and preparing DNA in 384-well format. This work was supported by the National Institutes of Health (MH070860) and Canada Research Chair salary award to A.M.C., a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship to K.L.P., Michael Smith Foundation for Health Research Fellowships to K.L.P. and T.J.S., the German Research Foundation (SPP1365/KA3423/1-1) to H.K and N.B., the Fritz Thyssen Foundation to H.K., and the National Institutes of Health (MH077303) to G.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki Y, Kawano T, Taru H, Saito Y, Wada S, Miyamoto K, Kobayashi H, Ishikawa HO, Ohsugi Y, Yamamoto T, et al. The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport. Embo J. 2007;26:1475–1486. doi: 10.1038/sj.emboj.7601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Miyagi N, Kato N, Yoshida T, Wada S, Nishimura M, Komano H, Yamamoto T, De Strooper B, Yamamoto K, et al. Coordinated metabolism of Alcadein and amyloid beta-protein precursor regulates FE65-dependent gene transactivation. J Biol Chem. 2004;279:24343–24354. doi: 10.1074/jbc.M401925200. [DOI] [PubMed] [Google Scholar]
- Araki Y, Tomita S, Yamaguchi H, Miyagi N, Sumioka A, Kirino Y, Suzuki T. Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid beta-protein precursor metabolism. J Biol Chem. 2003;278:49448–49458. doi: 10.1074/jbc.M306024200. [DOI] [PubMed] [Google Scholar]
- Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha-and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Boucard AA, Ko J, Sudhof TC. High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J Biol Chem. 2012;287:9399–9413. doi: 10.1074/jbc.M111.318659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo SY, Hanson E, Hundley R, et al. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:937–947. doi: 10.1002/ajmg.b.31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafreniere RG, et al. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum Genet. 2011;130:563–573. doi: 10.1007/s00439-011-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Fujishige S, Araki Y, Kato N, Araseki M, Nishimura M, Hartmann D, Saftig P, Fahrenholz F, Taniguchi M, et al. Alcadein cleavages by amyloid beta-precursor protein (APP) alpha-and gamma-secretases generate small peptides, p3-Alcs, indicating Alzheimer disease-related gamma-secretase dysfunction. J Biol Chem. 2009;284:36024–36033. doi: 10.1074/jbc.M109.057497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Fujishige S, Araki Y, Taniguchi M, Urakami K, Peskind E, Akatsu H, Araseki M, Yamamoto K, Martins RN, et al. Alternative processing of gamma-secretase substrates in common forms of mild cognitive impairment and Alzheimer’s disease: evidence for gamma-secretase dysfunction. Ann Neurol. 2011;69:1026–1031. doi: 10.1002/ana.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintsch G, Zurlinden A, Meskenaite V, Steuble M, Fink-Widmer K, Kinter J, Sonderegger P. The calsyntenins--a family of postsynaptic membrane proteins with distinct neuronal expression patterns. Mol Cell Neurosci. 2002;21:393–409. doi: 10.1006/mcne.2002.1181. [DOI] [PubMed] [Google Scholar]
- Hoerndli FJ, Walser M, Frohli Hoier E, de Quervain D, Papassotiropoulos A, Hajnal A. A conserved function of C. elegans CASY-1 calsyntenin in associative learning. PLoS ONE. 2009;4:e4880. doi: 10.1371/journal.pone.0004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda DD, Duan Y, Matsuki M, Kunitomo H, Hutter H, Hedgecock EM, Iino Y. CASY-1, an ortholog of calsyntenins/alcadeins, is essential for learning in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:5260–5265. doi: 10.1073/pnas.0711894105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito-Ishida A, Miyazaki T, Miura E, Matsuda K, Watanabe M, Yuzaki M, Okabe S. Presynaptically Released Cbln1 Induces Dynamic Axonal Structural Changes by Interacting with GluD2 during Cerebellar Synapse Formation. Neuron. 2012;76:549–564. doi: 10.1016/j.neuron.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu S-S. Foundations of cellular neurophysiology. Cambridge: MIT Press; 1995. [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64:791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecna A, Frischknecht R, Kinter J, Ludwig A, Steuble M, Meskenaite V, Indermuhle M, Engel M, Cen C, Mateos JM, et al. Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell. 2006;17:3651–3663. doi: 10.1091/mbc.E06-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Tuffy LP, Papadopoulos T, Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol. 2012;22:412–422. doi: 10.1016/j.conb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Kozorovitskiy Y, Oh WJ, Peixoto RT, Akhtar N, Saulnier JL, Gu C, Sabatini BL. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat Neurosci. 2012 doi: 10.1038/nn.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta C, Saito Y, Hata S, Gotoh N, Suzuki T, Yamamoto T. Constitutive cleavage of the single-pass transmembrane protein alcadeinalpha prevents aberrant peripheral retention of Kinesin-1. PLoS One. 2012;7:e43058. doi: 10.1371/journal.pone.0043058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Yuzaki M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur J Neurosci. 2011;33:1447–1461. doi: 10.1111/j.1460-9568.2011.07638.x. [DOI] [PubMed] [Google Scholar]
- Missler M, Sudhof TC. Neurexophilins form a conserved family of neuropeptide-like glycoproteins. J Neurosci. 1998;18:3630–3638. doi: 10.1523/JNEUROSCI.18-10-03630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Sudhof TC, Biederer T. Synaptic cell adhesion. Cold Spring Harb Perspect Biol. 2012;4:a005694. doi: 10.1101/cshperspect.a005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;424:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, Philpot BD, Ehlers MD. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron. 2012;76:396–409. doi: 10.1016/j.neuron.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Takahashi H, Ge Y, Craig AM. Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J Cell Biol. 2013;200:321–336. doi: 10.1083/jcb.201206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf CP, Boone PM, Sampath S, Williams C, Bader PI, Mueller JM, Shchelochkov OA, Brown CW, Crawford HP, Phalen JA, et al. Phenotypic spectrum and genotype-phenotype correlations of NRXN1 exon deletions. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shen K, Scheiffele P. Genetics and cell biology of building specific synaptic connectivity. Annu Rev Neurosci. 2010;33:473–507. doi: 10.1146/annurev.neuro.051508.135302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr Opin Neurobiol. 2011;21:132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30:7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Fuccillo MV, Ko J, Sudhof TC, Malenka RC. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc Natl Acad Sci U S A. 2011;108:16502–16509. doi: 10.1073/pnas.1114028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Hayashi Y, Nakahara S, Kumazaki H, Prox J, Horiuchi K, Zeng M, Tanimura S, Nishiyama Y, Osawa S, et al. Activity-dependent proteolytic cleavage of neuroligin-1. Neuron. 2012;76:410–422. doi: 10.1016/j.neuron.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Arstikaitis P, Prasad T, Bartlett TE, Wang YT, Murphy TH, Craig AM. Postsynaptic TrkC and presynaptic PTPsigma function as a bidirectional excitatory synaptic organizing complex. Neuron. 2011;69:287–303. doi: 10.1016/j.neuron.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Craig AM. Protein tyrosine phosphatases PTPdelta, PTPsigma, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 2013 doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Nakano S, Gomi F, Takahashi H. Up-regulation of calsyntenin-3 by beta-amyloid increases vulnerability of cortical neurons. FEBS Lett. 2011;585:651–656. doi: 10.1016/j.febslet.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Vaags AK, Lionel AC, Sato D, Goodenberger M, Stein QP, Curran S, Ogilvie C, Ahn JW, Drmic I, Senman L, et al. Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am J Hum Genet. 2012;90:133–141. doi: 10.1016/j.ajhg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnoni A, Perkinton MS, Gray EH, Francis PT, Noble W, Miller CC. Calsyntenin-1 mediates axonal transport of the amyloid precursor protein and regulates Abeta production. Hum Mol Genet. 2012;21:2845–2854. doi: 10.1093/hmg/dds109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnoni A, Rodriguez L, Manser C, De Vos KJ, Miller CC. Phosphorylation of kinesin light chain 1 at serine 460 modulates binding and trafficking of calsyntenin-1. J Cell Sci. 2011;124:1032–1042. doi: 10.1242/jcs.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Villasana LE, Klann E, Tejada-Simon MV. Rapid isolation of synaptoneurosomes and postsynaptic densities from adult mouse hippocampus. J Neurosci Methods. 2006;158:30–36. doi: 10.1016/j.jneumeth.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt L, Schrimpf SP, Meskenaite V, Frischknecht R, Kinter J, Leone DP, Ziegler U, Sonderegger P. Calsyntenin-1, a proteolytically processed postsynaptic membrane protein with a cytoplasmic calcium-binding domain. Mol Cell Neurosci. 2001;17:151–166. doi: 10.1006/mcne.2000.0937. [DOI] [PubMed] [Google Scholar]
- Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, Kim E, Kim CH, Ko J. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2013;110:4057–4062. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rohlmann A, Sargsyan V, Aramuni G, Hammer RE, Sudhof TC, Missler M. Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci. 2005;25:4330–4342. doi: 10.1523/JNEUROSCI.0497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.