Abstract
Background
The hemostatic property of “fresh” whole blood (WB) has been observed in military application and cardiac surgery and is associated with reduced blood loss, transfusion requirements, and donor exposures. The time from donation to transfusion defining “fresh” has not been systematically studied. We undertook an in vitro study of coagulation properties of refrigerated WB stored for 31 days.
Study design and methods
Twenty-one WB units were obtained from healthy volunteer donors and stored under standard AABB refrigerated conditions. Samples were obtained on the day after donation and again on Days 2, 4, 7, 11, 14, 17, 21, 24, and 31. Tests included complete blood count, pH, pO2, pCO2, glucose, lactate, thromboelastography (TEG), and platelet function by light transmission aggregometry (LTA).
Results
There was progressive decline in pH, pO2, glucose, and sodium, but progressive increase in potassium, pCO2, and lactate. TEG variables in all units were normal through Day 11; abnormal values in some variables in some units began on Day 14. Final aggregation levels exhibited no change from Day 1 to Day 21 with adenosine diphosphate and epinephrine, but a decline with collagen (Day 7) and ristocetin (Day 17).
Conclusion
This in vitro study of coagulation properties demonstrates preservation of normal integrated coagulation function to a minimum of 11 days under standard conditions of refrigerated storage of WB for transfusion. These observations strongly suggest that the hemostatic quality of WB may extend beyond current transfusion practices. If confirmed clinically, this would increase availability and extend benefits of reduced donor exposure and transfusion requirements.
The replacement of large volumes of lost blood (massive transfusion) requires administration of red blood cells (RBCs), plasma, and platelets (PLTs) or, alternatively, whole blood (WB). There is a similar requirement with unique dilutional conditions, as in the use of extracorporeal circuits for pediatric cardiac surgery. The hemostatic properties and reduced transfusion requirements associated with “fresh” WB has been observed in military application (Vietnam, Iraq, and Afghanistan)1-5 and in pediatric cardiac surgery.6,7 WB use in military settings has been considered fresh up to 24 hours when stored at room temperature, after which it is considered neither fresh nor safe and is discarded.8 WB currently used in pediatric cardiac surgery is considered fresh for up to 48 hours of refrigerated storage. This limit reflects the minimum time from donation to administration required to complete routine processing, testing, and delivery from donor site to hospital for scheduled surgery.6,7,9 In hospitals that obtain fresh WB for pediatric cardiac surgery, it is often processed after 48 hours into RBCs, while plasma and PLTs are discarded; thus postponed surgeries currently result in waste. The limits of “freshness” in both military and civilian practices do not appear to be based on specific assessments of the duration of functional coagulation proteins and PLTs in stored WB. A literature search suggests the only serial assessment of storage longer than 24 hours on multiple coagulation properties of intact WB was in 2007 by Hughes and colleagues.10 They determined that a major degree of functionality was retained at least up to 72 hours of warm storage and urged further study. Reports of refrigerated storage on coagulation properties of WB are virtually limited to coagulation proteins but not PLTs,11 and there are no evaluations of integrated coagulation function. Storage effects have been studied extensively on refrigerated separated blood components of plasma and PLTs, suggesting that the integrity of plasma protein factors and PLT function may not fall below clinically useful levels during refrigerated storage until well beyond our current 48-hour limit.11-14 Stimulated by the reports from the military and our observations in pediatric cardiac surgery, we wanted to learn the limits of storage of WB with regard to preservation of its beneficial hemostatic properties. Therefore, as a first step we undertook an in vitro study of the coagulation and PLT function properties of refrigerated WB.
Materials and Methods
WB units
After approval by the institutional review board for the protection of human subjects, 21 blood units were obtained from healthy donors following standard clinical procedures, predonation screening, and written informed consent at the American Red Cross' Penn-Jersey Regional donor center. These donors were also screened for aspirin, nonsteroidal anti-inflammatory drugs, and herbal medications. Each unit contained 450 ± 50 mL of WB and 63 mL of citrate-phosphate-dextrose-adenine (CPDA-1) anticoagulant solution. All units were stored in the blood bank of the Children's Hospital of Philadelphia at 1 to 6°C in accordance with standard AABB storage procedure.
Sampling technique
Each unit was mixed by gently tilting the bag horizontally (2 min) and vertically (2 min) before sampling. Aliquots of 35 to 40 mL were placed into 50-mL plastic syringes via a sterile connection to maintain a closed system. Each aliquot was rationed into 7 mL red-top glass tubes, 2 × 2-mL samples for thromboelastography (TEG) and complete blood count (CBC), and 5 × 5-mL samples for light transmission aggregometry (LTA).
Laboratory measurements
All measurements were conducted in the clinical laboratory of the Children's Hospital of Philadelphia following standard clinical practice. Blood pH, pO2, pCO2, sodium, and potassium were determined using a blood gas and/or electrolytes analyzer (Rapidlab 1265, Siemens, Deerfield, IL). A standard cell counter (Advia 2120, Siemens) determined CBC. PLT function was determined by PFA-100 (Siemens) using collagen/adenosine diphosphate (ADP) and collagen/epinephrine agonist mixtures (Siemens) and standard LTA (PLT aggregometry profiler-4, Bio Data Corp., Horsham, PA) using ADP, collagen (Bio Data Corp.), epinephrine (CURA Pharmaceutical, Eatontown, NJ), and ristocetin (Helena Hemostasis, Beaumont, TX) agonists. TEG was conducted using a thromboelastograph (TEG 5000, Haemoscope Corp., Niles, IL) with kaolin activation.
Testing schedule
A pilot study assessing only TEG measurements from a single unit of WB daily for 31 days showed no change from Day 1 until Day 14 and the variables remained in the range of normal until Day 21. To minimize the potential effect of sampling frequency and to retain maximum volume of blood within each unit, a staggered testing schedule was created based on these observed changes. The day of the initial collection was designated as Day 0. Samples from all units were drawn on Days 1 (baseline), 7, 14, 21, and 31 and were subjected to TEG, CBC, blood gas, and PFA-100. Additional TEG and CBC tests were performed on samples drawn on Days 10, 17, and 24. PFA-100 measurements performed on samples from units older than 1 day yielded no values due to “flow obstruction” error. Therefore, an additional 11 units of blood were obtained to assess the effect of storage on PLT function using standard LTA and to evaluate correlation with TEG measurements. To minimize sample volume only TEG and LTA were performed in these units. Samples from these 11 units were drawn on Days 1, 2, 4, 7, 14, 17, and 21 for TEG and LTA. In addition only the TEG assay was performed on samples that were drawn on Days 10, 24, and 31 to parallel TEG data from the first phase of the study and minimize sample volume. Hemolysis was detected in one of the WB units starting on Day 1; thus this particular unit was not subjected to LTA assessment.
Teg
WB samples (340 μL) were treated with kaolin before testing. Calcium chloride (0.2 mol/L, 20 μL) was pipetted into the TEG sample cup before the sample was added. All tests were performed at 37°C. TEG was run in duplicate and results are reported as means of duplicate readings. The adult normal reference ranges were used as the threshold for change from normal to abnormal.
Light transmission PLT aggregometry
Approximately a total of 35 mL of blood (5 mL per tube) was drawn from each control donor by direct venipuncture into six to eight tubes (Vacutainer, Becton Dickenson, Cedex, France) containing 0.129 mol/L sodium citrate. PLT-rich plasma was obtained from each control and study unit sample by centrifugation at 800 rpm for 10 minutes. The remaining blood sample was further centrifuged at 3500 rpm for 10 minutes to attain PLT-poor plasma. Every PLT-rich plasma sample with a PLT count of more than 300 × 109/L was diluted using its autologous PLT-poor plasma to reach a count of 100 × 109 to 300 × 109/L. ADP (10 μmol/L), collagen (0.19 mg/mL), epinephrine (10 μmol/L), and ristocetin (1.5 mg/mL) were employed as agonists. All tests were performed at 37°C.
Statistical analysis
The data were analyzed using parametric methods for normally distributed data (analysis of variance for repeated measures) and nonparametric methods for non-normally distributed data (Kruskal-Wallis). Comparisons with p values of less than 0.05 were considered significant. Data management was accomplished using a computer spreadsheet (Microsoft Excel, Microsoft Corp., Redmond, WA) and statistical software (Stata SE 10.1, StataCorp, College Station, TX).
Results
Blood composition
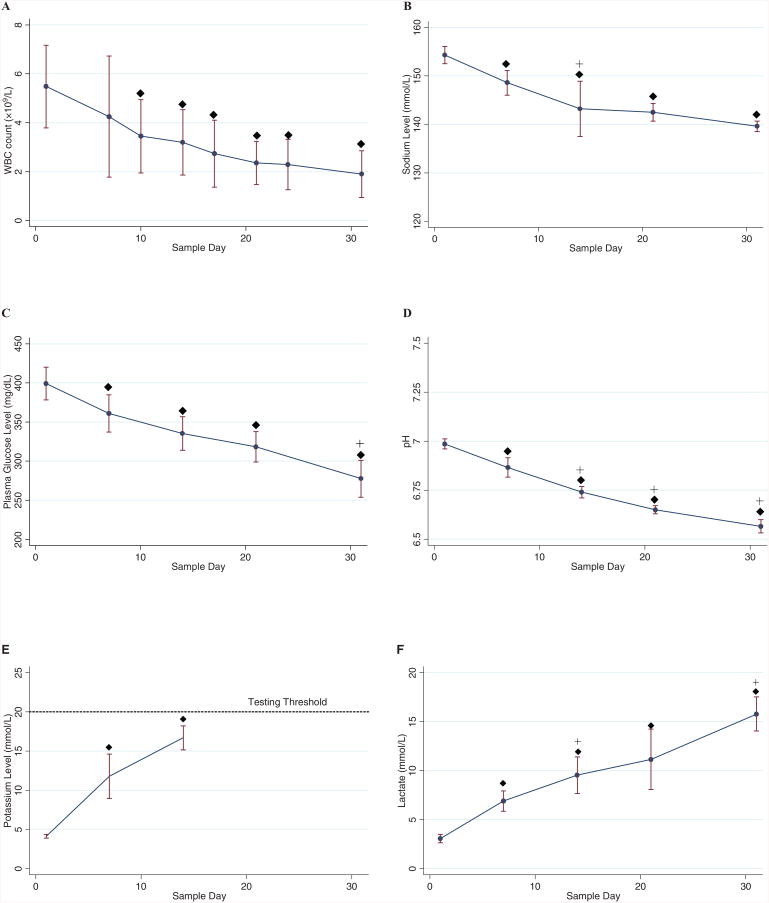
Blood gas assays were performed during the first phase of the study. Hematocrit via CBC was used as an indicator of consistency in sampling and showed minimal fluctuations over the sampling intervals and no significant differences were found between samples. Lower white blood cell (WBC) counts from baseline measurements were observed starting on Day 10 (Fig. 1A). As expected due to metabolic activity,15 sodium and plasma glucose levels decreased significantly from baseline (Fig. 1B and 1C). A progressive reduction in pH through Day 31 was observed (Fig. 1D). On the other hand, potassium and lactate concentrations increased over time (Fig. 1E and 1F). After Day 14, potassium readings exceeded the testing threshold (>20 mmol/L).
Fig. 1.
Blood composition by sample day shown as mean ± SD: (A) WBCs, (B) sodium, (C) glucose, (D) pH, (E) potassium, and (F) lactate. Values of potassium level after Day 14 exceeded the testing threshold. (◆) Values differ from baseline (Day 1), p < 0.05; (+) values differ from previous measurement day, p < 0.05. (●) Mean; (bars) ±1 SD. (E, —) Mean potassium.
WB coagulation function
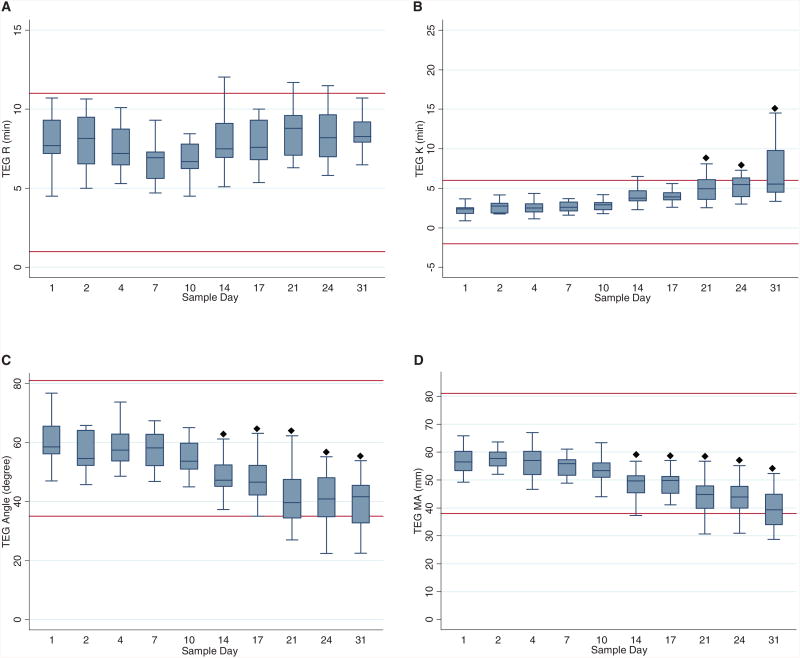
All TEG variables were within normal range for adult values at baseline for all units. Mean R showed no significant change from the baseline over time (Fig. 2A). No difference from baseline was observed for K up to Day 21 (Fig. 2B). The alpha values did not change until on or after Day 14 (Fig. 2C). Similarly, the maximum amplitude (MA) decreased on Day 14 and remained lower than baseline throughout the remainder of the study (Fig. 2D). The only variables that became consistently abnormal for any unit of blood were alpha, K, and MA, and abnormal results occurred only on Day 14 or later.
Fig. 2.
Box plots of TEG variables by sample day shown as median, 25th, and 75th percentiles. Normal thresholds are represented by horizontal solid lines across each graph: (A) R, (B) K, (C) angle (α), and (D) MA. (◆) Values differ from baseline (Day 1), p < 0.05; (+) values differ from previous measurement day, p < 0.05.
PLT function
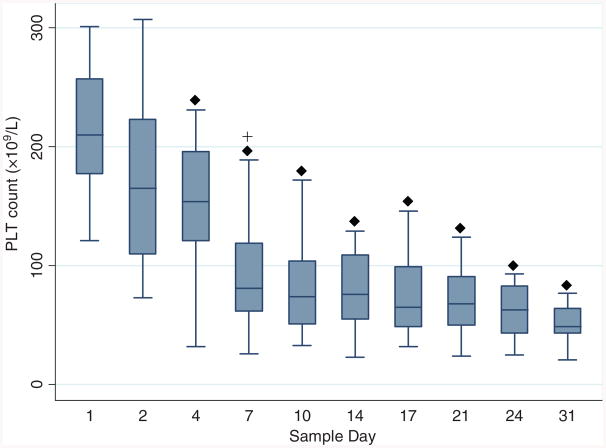
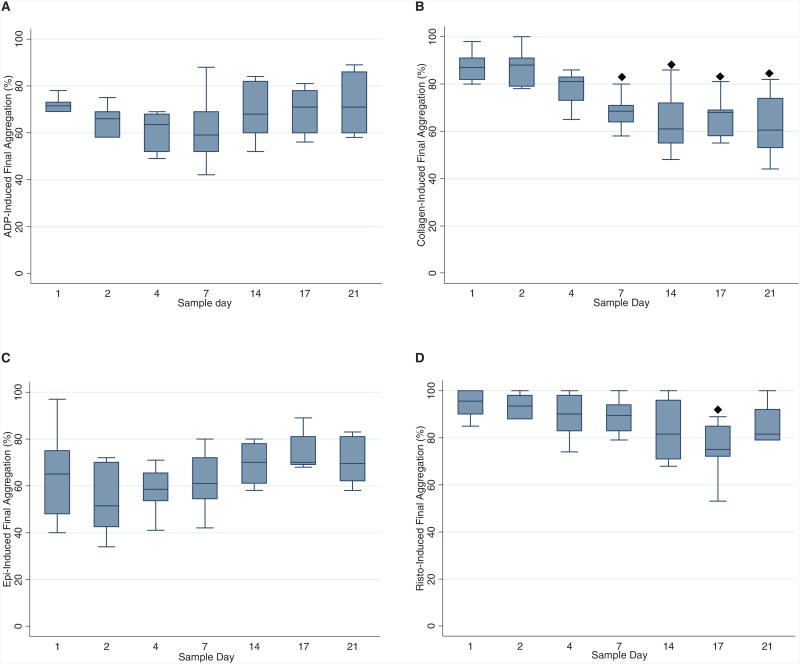
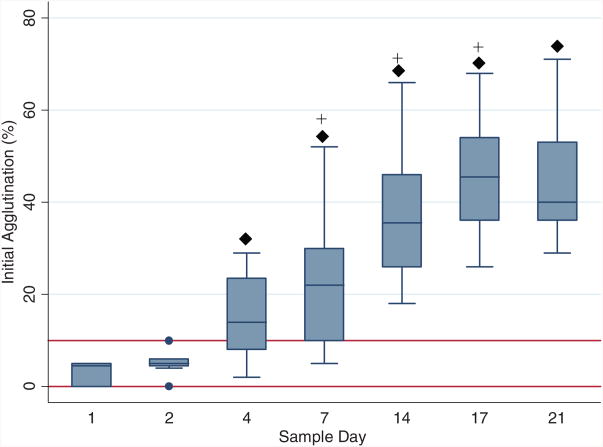
PLT count dropped significantly from baseline on Day 4, followed by further decline on Day 7 and remained unchanged for the remainder of the study period (Fig. 3). PLT function assays were performed on 10 WB units. No difference from baseline was observed for final aggregation level through Day 21 for ADP and epinephrine; ristocetin decreased on Day 17 (Fig. 4). Collagen-induced aggregation decreased over time, with change from baseline observed for Day 7 through Day 21. Spontaneous agglutination (clumping activity of PLTs without the addition of agonists) became more prominent as storage period increased. The level of spontaneous agglutination progressively increased from baseline after Day 4 (Fig. 5).
Fig. 3.
Box plots of PLT counts by sample day shown as median, 25th, and 75th percentiles. (◆) Values differ from baseline (Day 1), p < 0.05; (+) values differ from previous measurement day, p < 0.05.
Fig. 4.
Box plots of final aggregation percentages by sample day shown as median, 25th, and 75th percentiles. Agonists: (A) ADP, (B) collagen, (C) epinephrine, and (D) ristocetin. (◆) Values differ from baseline (Day 1), p < 0.05; (+) values differ from previous measurement day, p < 0.05.
Fig. 5.
Box plots of initial agglutination percentages by sample day shown as median, 25th, and 75th percentiles. Normal initial agglutination percentages are indicated by solid horizontal lines. (◆) Values differ from baseline (Day 1), p < 0.05; (+) values differ from previous measurement day, p < 0.05.
Discussion
The efficacy of WB to treat or prevent coagulopathic bleeding has traditionally been based on minimal time from donation to administration, hence the term “fresh” WB. The question of how long WB can be stored and retain normal coagulation and PLT function properties from an integrated functional perspective has received little attention. The primary objective of our study was to investigate the functional coagulation and PLT function properties of WB units stored in CPDA-1 anticoagulant at 4°C over a period of 31 days, which closely approximates the industry standard shelf life of 35 days. As an individual component of coagulation, PLTs retain normal responsiveness to agonists for 7 to 21 days. However, integrated coagulation function as determined by TEG remains normal up to 14 days of refrigerated storage.
The integrated function of coagulation proteins and PLTs was measured by TEG. TEG has been used to diagnose abnormal coagulation states in surgical and other settings of blood loss and replacement.16-18 A normal TEG has been shown to have a high negative predictive value for inadequate hemostasis due to coagulopathy.16,19-22 We postulated that TEG measurements of stored blood would be analogous to clinical use in detecting abnormalities or, if within the range of normal, would suggest an intact coagulation state. The R value in TEG is the time required for initial fibrin formation; R values in TEG remained unchanged throughout the duration of our study.
Significant departure of other TEG variables from adult normal values did not occur until Day 14. Both K and alpha variables quantify the rate of clotting; K is mostly influenced by the fibrinogen level, whereas alpha reflects PLT activity in stimulating fibrin polymerization to a greater extent. MA measures the maximum strength (amplitude) of the blood clot and correlates with the aggregation function of PLT contribution to clot formation. The significant departure of TEG alpha and MA values from baseline indicates that PLT contribution to clot development begins to decline at 14 days.
The LTA results imply that normal agonist-induced PLT function (reaching >60% light transmittance)23 is preserved for at least 17 days, with the exception of collagen-induced aggregation, which was reduced beginning on Day 7. However, there was no concomitant effect on alpha or MA on Day 7; both remained unchanged until Day 14. Also notable is spontaneous agglutination before the addition of agonists, which progressively increases. While agglutination increases from Day 4, no TEG change is observed until Day 14, suggesting no significant impairment of PLT contribution to clotting. Refrigeration of PLT concentrates at 4°C has been known to cause changes in PLT structure and function. Cold-induced morphologic change on the PLT membrane leads to clearance of PLTs from the circulation as early as 1 to 2 days after transfusion.24,25 However, it is important to note that the reduced duration of transfused PLTs in the circulation is entirely separable from the ability to contribute to the blood clotting process.24,26 While it is true that in vivo survivability of refrigerated PLTs is compromised when compared to room temperature–stored PLTs, their aggregation functionality is, in fact, arguably better. Several studies have shown that refrigerated PLTs perform better than room temperature–stored PLTs in aggregation assays.27-29 A clinical trial in pediatric cardiac surgical patients comparing PLT aggregation test results up to 3 hours after transfusion concluded that PLTs of refrigerated WB demonstrated better functionality compared to standard PLT concentrates and was associated with reduced bleeding and transfusion requirements.7
A review of the literature reveals that most coagulation proteins are not significantly depleted during 4°C storage. Factor (F)II, FVII, FIX, FX, FXII, and FXIII were found to remain above the lower limit of normal when stored in WB at 4°C through 35 days.11 Furthermore, fibrinogen and plasminogen activity did not change. FV and FVIII are known to be the most labile coagulation factors exhibiting greater reduced concentration during storage. Even so, results from previous studies suggest that FVIII activity remains above bleeding threshold (30%) for 14 to 42 days and FV (10%) for up to 28 days.11,30-33 In our study TEG R value remained normal up to Day 31, suggesting that although likely reduced, the protein concentrations are functionally adequate.
Biochemical changes in WB units during storage are collectively termed as the storage lesion. Progressive reductions in pH, ATP, and 2,3-diphosphoglycerate acid and an increase in plasma hemoglobin and potassium have been documented.15 Cell count and metabolic assay data of our study demonstrated signs of storage lesions as early as Day 4. Levels of potassium and lactate were elevated, whereas blood pH, WBC, and PLT counts gradually declined. Interestingly, the onset of these metabolic alterations did not result in changes in TEG variables or PLT aggregation. CPDA-1 solution and the storage time of samples of WB units may have affected results of some laboratory tests. Blood samples obtained by venipuncture from patients for CBC (PLT count) are collected in ethylenediaminetetraacetate anticoagulant because PLTs tend to progressively clump in citrate, resulting in lower than true counts. Thus, our observations of declining PLT count may be related to clumping in citrate anticoagulant. Similarly, we believe that PLT clumping was responsible for the failure (“flow obstruction error”) of the PFA-100 assay. Standard laboratory practice for PFA-100 dictates that the assay must be performed within 4 hours after the blood sample is collected from a patient in acitrated tube. We documented the presence of clumped PLTs using standard light microscopy. The results of TEG and LTA on stored blood are not yet known to predict clinical efficacy. However, a comparison of in vitro PLT function with an in vivo animal model of hemostatic efficacy suggests that in vivo function may be satisfactory despite in vitro test changes.16
While the fractionation of WB is clearly efficient and advantageous for the majority who require only a component(s), there are specific applications where patients may be better served by using WB. The use of nonrefrigerated WB versus components in cardiac surgery demonstrated qualitative and quantitative laboratory evidence of improved coagulation profile in infants34 and increased PLT count and function in adults.35 Transfusion of freshly drawn WB in combat casualties compared to components was associated with improved survival.2 An observational study of refrigerated WB (stored 8-28 days; mean, 14 days) demonstrated no evidence of coagulopathic bleeding until 20+ units were transfused.5 Prospective randomized trials demonstrated decreased blood loss after pediatric cardiac surgery when refrigerated WB (48 hr) compared to components is used during and immediately after surgery.6,7 Secondary outcomes in those studies include improved PLT function,7 lower inotropic score, fewer donor exposures, less time on ventilator support, and shorter hospital length of stay.6 Similarly, stored WB versus components during liver transplantation resulted in reduced donor exposures.36 Unfortunately, there are no clinical studies addressing the effect of refrigerated storage between 48 hours6,7 and 8 to 20 days.5
A significant limitation in improving the utilization of WB is its availability, which is directly related to current restrictive guidelines for use, shelf life, and difficulty in obtaining WB from blood centers. Establishing a longer duration of hemostatic quality (shelf life) would increase availability and utility. This would be particularly beneficial in locations where donation and refrigeration are available but component separation is not, such as in military or disaster relief scenarios and remote locations.37 Other certain elective surgical patients who require large-volume blood replacement such as pediatric craniofacial reconstruction, liver transplant, or emergency major vascular surgery and trauma patients would also benefit.37-39 Attempts to determine optimum ratios of components for clinical efficacy in large-volume transfusion have not surprisingly concluded that WB should be replicated, that is, should be administered in a 1:1:1 ratio of RBCs:fresh-frozen plasma (FFP):PLTs.38,40 The continuous immediate availability of a “transfusion package” (5 units of RBCs, 5 units of thawed FFP, 2 units of pooled PLTs) for unexpected massively bleeding patients reduced transfusion requirements and increased survival significantly.38 Substituting WB in such a program would be more efficient resulting in fewer donor exposures and less wastage of outdated plasma and PLTs. We believe that there is sufficient direct and indirect evidence from this and other studies to warrant clinical trials of refrigerated WB stored longer than 48 hours for selected patient populations.
Acknowledgments
The authors thank Mindy Einarson and Dr Ralph Vassalo of the American Red Cross' Penn-Jersey Region donor center for procuring the donors. The Cardiac Center provided partial funding and the Department of Pathology and Laboratory Medicine contributed laboratory resources.We also recognize the assistance of the clinical laboratory staff of The Children's Hospital, especially Maria Tanzer, Linda Cimorelli, and Susan Shibutani of the coagulation section.
This study was supported by grants from the Cardiac Center at The Children's Hospital of Philadelphia and the Departmental Fund of Pathology and Laboratory Medicine at The Children's Hospital of Philadelphia.
Abbreviations
- CBC
complete blood count
- LTA
light transmission aggregometry
- MA
maximum amplitude
- TEG
thromboelastography
- WB
whole blood
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.Spinella PC. Warm fresh whole blood transfusion for severe hemorrhage: U.S. military and potential civilian applications. Crit Care Med. 2008;36:S340–5. doi: 10.1097/CCM.0b013e31817e2ef9. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66:S69–76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Azarow K, Holcomb JB. Fresh whole blood transfusions in coalition military, foreign national, and enemy combatant patients during Operation Iraqi Freedom at a U.S. combat support hospital. World J Surg. 2008;32:2–6. doi: 10.1007/s00268-007-9201-5. [DOI] [PubMed] [Google Scholar]
- 4.Miller RD. Massive blood transfusions: the impact of Vietnam military data on modern civilian transfusion medicine. Anesthesiology. 2009;110:1412–6. doi: 10.1097/ALN.0b013e3181a1fd54. [DOI] [PubMed] [Google Scholar]
- 5.Miller RD, Robbins TO, Tong MJ, Barton SL. Coagulation defects associated with massive blood transfusions. Ann Surg. 1971;174:794–801. doi: 10.1097/00000658-197111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruenwald CE, McCrindle BW, Crawford-Lean L, Holtby H, Parshuram C, Massicotte P, Van Arsdell G. Reconstituted fresh whole blood improves clinical outcomes compared with stored component blood therapy for neonates undergoing cardiopulmonary bypass for cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2008;136:1442–9. doi: 10.1016/j.jtcvs.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manno CS, Hedberg KW, Kim HC, Bunin GR, Nicolson S, Jobes D, Schwartz E, Norwood WI. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991;77:930–6. [PubMed] [Google Scholar]
- 8.Lounsburry D, Bellammy RF. Emergency war surgery. 3rd. Washington, DC: Borden Institute; 2004. [Google Scholar]
- 9.Mou SS, Giroir BP, Molitor-Kirsch EA, Leonard SR, Nikaidoh H, Nizzi F, Town DA, Roy LC, Scott W, Stromberg D. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351:1635–44. doi: 10.1056/NEJMoa041065. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JD, Macdonald VW, Hess JR. Warm storage of whole blood for 72 hours. Transfusion. 2007;47:2050–6. doi: 10.1111/j.1537-2995.2007.01429.x. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson L, Hedner U, Nilsson IM, Robertson B. Shelf-life of bank blood and stored plasma with special reference to coagulation factors. Transfusion. 1983;23:377–81. doi: 10.1046/j.1537-2995.1983.23584018713.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman RM. Uncommon cold: could 4 degrees C storage improve platelet function? Transfusion. 2005;45:1407–12. doi: 10.1111/j.1537-2995.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman RM. Platelets: testing, dosing and the storage lesion—recent advances. Hematology. 2006;2006:492–6. doi: 10.1182/asheducation-2006.1.492. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell SW, Maglasang P, Reid TJ, Gorogias M, Krishnamurti C. Correlation of in vivo and in vitro functions of fresh and stored human platelets. Transfusion. 2000;40:988–93. doi: 10.1046/j.1537-2995.2000.40080988.x. [DOI] [PubMed] [Google Scholar]
- 15.Roback JD, Combs MR, Grossman BJ, Hillyer CD, editors. AABB technical manual. 16th. Bethesda (MD): AABB; 2008. [Google Scholar]
- 16.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–9. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez LJ, Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. Br J Anaesth. 2009;103:i14–22. doi: 10.1093/bja/aep318. [DOI] [PubMed] [Google Scholar]
- 18.Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. 2009;18:277–88. doi: 10.1016/j.hlc.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Cammerer U, Dietrich W, Rampf T, Braun SL, Richter JA. The predictive value of modified computerized thromboelastography and platelet function analysis for postoperative blood loss in routine cardiac surgery. Anesth Analg. 2003;96:51–7. doi: 10.1097/00000539-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Essell JH, Martin TJ, Salinas J, Thompson JM, Smith VC. Comparison of thromboelastography to bleeding time and standard coagulation tests in patients after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1993;7:410–5. doi: 10.1016/1053-0770(93)90161-d. [DOI] [PubMed] [Google Scholar]
- 21.Spiess BD. Thromboelastography and cardiopulmonary bypass. Semin Thromb Hemost. 1995;21(4):27–33. [PubMed] [Google Scholar]
- 22.Johansson PI. The blood bank: from provider to partner in treatment of massively bleeding patients. Transfusion. 2007;47:176S–81S. doi: 10.1111/j.1537-2995.2007.01381.x. discussion 82S-83S. [DOI] [PubMed] [Google Scholar]
- 23.Yee DL, Sun CW, Bergeron AL, Dong JF, Bray PF. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood. 2005;106:2723–9. doi: 10.1182/blood-2005-03-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 25.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability—deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–8. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmeister KM, Josefsson EC, Isaac NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of chilled blood platelets. Science. 2003;301:1531–4. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 27.Babic AM, Josefsson EC, Bergmeier W, Wagner DD, Kaufman RM, Silberstein LE, Stossel TP, Hartwig JH, Hoffmeister KM. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion. 2007;47:442–51. doi: 10.1111/j.1537-2995.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi JW, Pai SH. Influence of storage temperature on the responsiveness of human platelets to agonists. Ann Clin Lab Sci. 2003;33:79–85. [PubMed] [Google Scholar]
- 29.Hornsey VS, Drummond O, McMillan L, Morrison A, Morrison L, MacGregor IR, Prowse CV. Cold storage of pooled, buffy-coat-derived, leucoreduced platelets in plasma. Vox Sang. 2008;95:26–32. doi: 10.1111/j.1423-0410.2008.01052.x. [DOI] [PubMed] [Google Scholar]
- 30.Laposata M, Connor AM, Hicks DG, Phillips D. The clinical hemostasis handbook. Chicago (IL): Year Book Medical Publishers Inc.; 1989. [Google Scholar]
- 31.Picker SM, Sturner SS, Oustianskaja L, Gathof BS. Leucodepletion leads to component-like storage stability of whole blood—suggesting its homologous use? Vox Sang. 2004;87:173–81. doi: 10.1111/j.1423-0410.2004.00570.x. [DOI] [PubMed] [Google Scholar]
- 32.Riggert J. Improvement of the coagulation potential in whole blood through leukocyte depletion. Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35:645–8. doi: 10.1055/s-2000-7368-2. [DOI] [PubMed] [Google Scholar]
- 33.von Bormann B, Wirtz S, Weiler J, von Bormann C, Tro-bisch H. Quality of whole blood as a result of storage and preparation (inline-leukocyte depletion) Evidence for autologous predeposit. Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35:326–32. doi: 10.1055/s-2000-323. [DOI] [PubMed] [Google Scholar]
- 34.Friesen RH, Perryman KM, Weigers KR, Mitchell MB, Friesen RM. A trial of fresh autologous whole blood to treat dilutional coagulopathy following cardiopulmonary bypass in infants. Paediatr Anaesth. 2006;16:429–35. doi: 10.1111/j.1460-9592.2005.01805.x. [DOI] [PubMed] [Google Scholar]
- 35.Lavee J, Martinowitz U, Mohr R, Goor DA, Golan M, Langsam J, Malik Z, Savion N. The effect of transfusion of fresh whole blood versus platelet concentrates after cardiac operations. A scanning electron microscope study of platelet aggregation on extracellular matrix. J Thorac Cardiovasc Surg. 1989;97:204–12. [PubMed] [Google Scholar]
- 36.Laine E, Steadman R, Calhoun L, Blackall D, Levin P, Braunfeld M, Nourmand H, Neelakanta G, Ting L, Gornbein J, Busuttil R, Petz L. Comparison of RBCs and FFP with whole blood during liver transplant surgery. Transfusion. 2003;43:322–7. doi: 10.1046/j.1537-2995.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 37.Repine TB, Perkins JG, Kauvar DS, Blackborne L. The use of fresh whole blood in massive transfusion. J Trauma. 2006;60:S59–69. doi: 10.1097/01.ta.0000219013.64168.b2. [DOI] [PubMed] [Google Scholar]
- 38.Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96:111–8. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stricker PA, Shaw TL, DeSouza DG, Hernandez SV, Bartlett SP, Friedman DF, Sesok-Pizzini DA, Jobes DR. Blood loss, replacement, and associated morbidity in infants and children undergoing cariofacial surgery. Paediatr Anaesth. 2010;20:150–9. doi: 10.1111/j.1460-9592.2009.03227.x. In press. [DOI] [PubMed] [Google Scholar]
- 40.Johansson PI, Hansen MB, Sorensen H. Transfusion practice in massively bleeding patients: time for a change? Vox Sang. 2005;89:92–6. doi: 10.1111/j.1423-0410.2005.00668.x. [DOI] [PubMed] [Google Scholar]