Abstract
Rationale
Pulmonary hypertension (PH) is characterized by progressive elevation in pulmonary pressure and loss of small pulmonary arteries. As bone morphogenetic proteins (BMPs) promote pulmonary angiogenesis by recruiting the Wnt/βcatenin pathway, we proposed that βcatenin activation could reduce loss and/or induce regeneration of small PAs and attenuate PH.
Objective
This study aims to establish the role of β–catenin in protecting the pulmonary endothelium and stimulating compensatory angiogenesis following injury.
Methods and Results
To assess the impact of β-catenin activation on chronic hypoxia-induced PH, we used the adenomatous polyposis coli (ApcMin/+) mouse, where reduced APC causes constitutive β–catenin elevation. Surprisingly, hypoxic ApcMin/+ mice displayed greater PH and small PA loss compared to control C57Bl6J (C57) littermates. Pulmonary artery endothelial cells (PAECs) isolated from ApcMin/+ demonstrated reduced survival and angiogenic responses along with a profound reduction in adhesion to laminin. The mechanism involved failure of APC to interact with the cytoplasmic domain of the α3 integrin, to stabilize focal adhesions and activate integrin-linked kinase (ILK-1) and pAkt. We found that PAECs from lungs of patients with idiopathic PH have reduced APC expression, decreased adhesion to laminin and impaired vascular tube formation. These defects were corrected in the cultured cells by transfection of APC.
Conclusions
We show that APC is integral to PAEC adhesion and survival and is reduced in PAECs from PH patient lungs. The data suggest that decreased APC may be a cause of increased risk or severity of PH in genetically susceptible individuals.
Keywords: Adenomatous poliposis coli, Wnt signaling, integrin signaling, angiogenesis, pulmonary hypertension
INTRODUCTION
Idiopathic pulmonary arterial hypertension (IPAH) is a rare but devastating condition in which progressive elevation in pulmonary arterial pressure and resistance to flow are associated with the loss and obliterative narrowing of small distal pulmonary arteries (PAs)1. The disease affects mostly women of reproductive age and, in the absence of treatment, results in worsening chronic right heart failure and death2. Since current PAH therapies are largely vasodilators, many patients ultimately need to be considered for lung transplantation1–3.
The discovery of the link between mutations in the bone morphogenetic protein (BMP) receptor 2 (BMPR2)3, 4 or reduced expression of this receptor5 and the development of IPAH led to efforts directed at understanding the role of BMP signaling in pulmonary blood vessel biology. Our group recently reported6 that activation of BMP signaling promotes pulmonary angiogenesis by simultaneously recruiting the Wnt/β-catenin and the Wnt/planar cell polarity signaling pathways, to induce pulmonary arterial endothelial cell (PAEC) proliferation and motility, respectively. Cellular levels of β-catenin (βC) are regulated by a cytoplasmic protein complex composed of Axin, adenomatous poliposis coli (APC) and glycogen synthase kinase (GSK) 3β̃ In human PAECs, we found that BMP-mediated phosphorylation of ERK inactivates GSK3β, and disassembles the Axin/APC/GSK3β complex resulting in βC accumulation and translocation to the nucleus, to regulate genes important in endothelial survival and growth6, 7. Human PAECs deficient in βC failed to form functional vessels in a murine model of angiogenesis6. Based upon our findings, we reasoned that constitutive activation of βC in a patient with dysfunctional BMPR2 signaling could protect the pulmonary endothelium against injury by promoting PAEC survival, and might induce regeneration of lost vessels by stimulating PAEC growth.
To test this hypothesis, we used the ApcMin/+ mouse in which constitutive elevation of βC in all tissues results from truncation and functional loss of an APC allele. Mice develop colonic polyps in a manner similar to that seen in patients that suffer from familial adenomatous poliposis (FAP), an autosomal dominant disease also associated with loss of function mutations in APC8. We exposed ApcMin/+ mice to chronic hypoxia, a stimulus that is known to produce pulmonary hypertension (PH) and loss of small distal PAs9. In this study we show that, contrary to our expectations, ApcMin/+ mice developed worse PH and demonstrated greater distal PA loss compared to control C57 littermates. In cell culture studies, we show that microvascular (mv) PAECs from ApcMin/+ compared to control C57Bl6J (C57) littermate mice, demonstrate reduced survival and tube formation associated with decreased adhesion to extracellular matrix proteins, in particular to laminin. We identify a novel interaction between APC and a cytoplasmic domain in the α3 integrin, and show that this interaction is required for activation of integrin linked kinase (ILK)-1, formation of focal adhesions and induction of pAkt. Furthermore, mvPAECs isolated from IPAH patients express less APC, and transfection of APC reverses impaired tube formation of these cells. Taken together, our findings reveal a novel role for APC in mediating PAEC adhesion and survival, suggesting that reduced expression or activity of APC could increase the risk of developing PH in individuals that may be susceptible due to specific environmental exposures and/or abnormalities in other genes, such as BMPR2.
METHODS
An expanded Methods section is available in the Online Data Supplement.
Hemodynamic and morphometric studies in mice
The Animal Care Committee at Stanford University approved all the experimental protocols used in this study. Animals used in the experiments were obtained by crossing a male C57 ApcMin/+ with a female C57 mouse. For hypoxia studies, mice were placed in a hypoxia chamber where they were exposed to 10% inspired O2 with access to food and water ad libitum for 3 weeks. Echocardiographic measurements of cardiac function and RVSP, LVEDP, RV and heart rate were measured under isoflurane anesthesia (1.5–2.5% in 2 L O2/min) in unventilated mice using a closed chest technique as previously described10.
Cell culture
Primary human mvPAECs and EC growth medium were obtained from Sciencell (Sciencell, Carlsbad, CA). Cells were grown in EC growth medium and used between passages 4–8. Cells were starved in EC starvation medium (0.2% FBS and gentamycin/amphotericin) for 24 hours prior to the experiment. For hypoxia studies, cells were placed in a hypoxia chamber (Biospherix, New York, NY) that provided 1% O2 concentration for 24 hours.
Mouse mvPAEC were isolated from lung tissue as previously described7. To ensure the purity of the culture, we re-purified these cultures with CD31 antibody coated beads after the first passage.
Adhesion assay
A 96-well plate coated with either fibronectin (FN), collagen IV (CIV) or laminin (LN) was seeded with human mvPAECs (20,000 cells/well) and incubated at 37°C for 30 minutes. The average number of adherent cells was calculated by counting the total number of cells in six random fields per well (200x magnification).
Integrin blockade assay
Cells were incubated with integrin blocking antibodies (α1, α2, α3, α4, α5, αV, α6, β1 and β4, Millipore, Billerica, MA) for one hour at 4°C and then seeded in FN, CIV or LN coated 96 well plates. The average number of adherent cells was calculated as described above.
Plasmids and transfection methods
A pCMV-Neo-Bam plasmid containing the H. sapiens WT APC sequence was a kind gift from Dr. Bert Vogelstein (Johns Hopkins University). Plasmids containing inserts with the integrin α3 and α4 sequences in a pBluescript II KS phagemid were obtained from ATCC. The siRNA duplexes (Dharmacon, Lafayette, CO) specific for β̃catenin (Dharmacon on-target plus; accession number NM_001012329, NM_020248), and APC (Ambion Validated siRNA, Ambion, Grand Island, NY), were transfected into human mvPAECs using a Nucleofector II (Program T-032) using the basic endothelial Nucleofection Kit (Lonza, Basel, Switzerland).
Generation of the ΔITGA3 and integrin chimeric mutants
For generation of the integrin chimeras, the cytoplasmic tail of α3 (3039G-3136A) or α4 (3129C-3225C) were excised from their native sequence and swapped followed by subcloning into a pcDNA 3.1 vector. For the generation of the ITGA3 mutant (Δα3), we substituted the serines found in the QPSXXE motif with alanines as illustrated below:
AGCCCAGCCGTCAGAGACAGA (Native Sequence)
AGaCCAGCCGgCAGAGACcGA (Mutant Sequence)
Site-directed mutagenesis and Chimera generation were performed at Mutagenex Labs (Mutagenex, Somerset, NJ).
Statistical analysis
The number of samples or animals studied per experiment is indicated in the Figure Legends. Values from multiple experiments are expressed as mean±SEM. Statistical significance was determined using unpaired t-test or one-way ANOVA followed by Dunnett’s or Bonferroni’s multiple comparison tests unless stated otherwise. A value of P<0.05 was considered significant.
RESULTS
ApcMin/+ mice demonstrate greater right ventricular systolic pressure (RVSP), right ventricular hypertrophy and reduction in small distal PAs after chronic hypoxia and recovery in room air
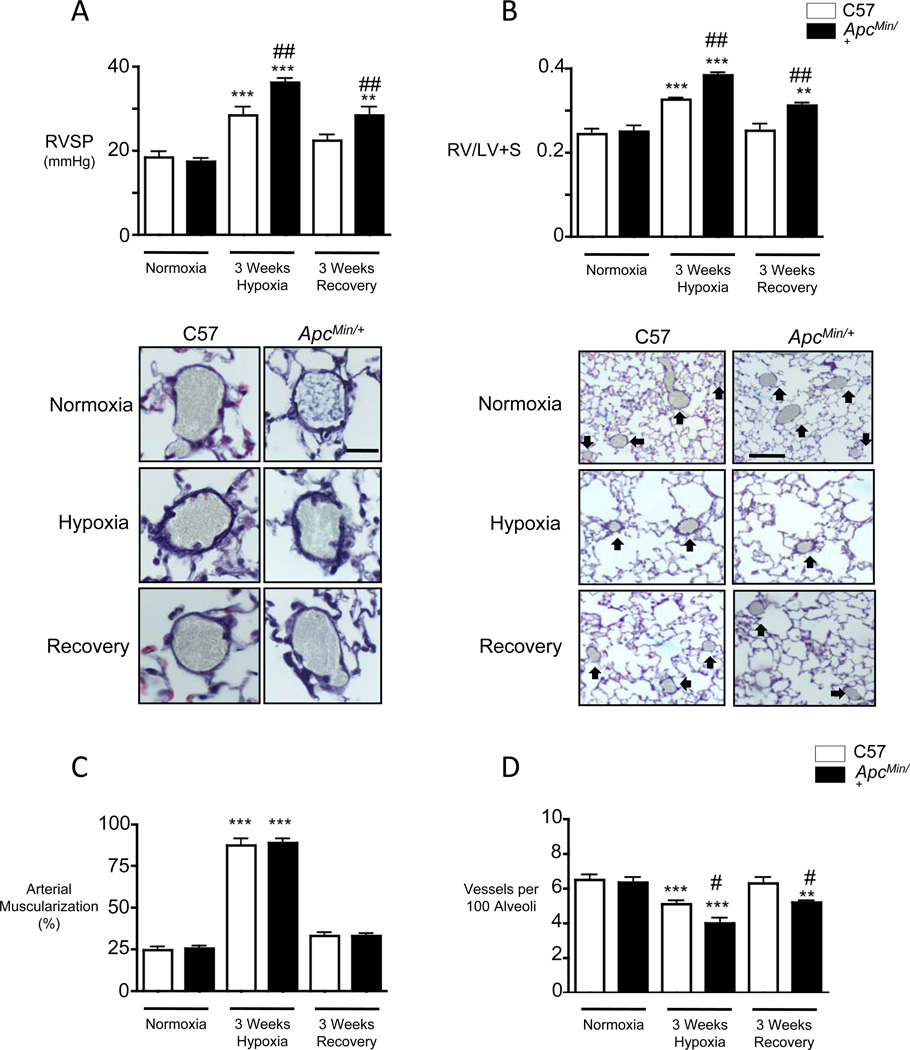
To determine whether increased levels of total βC in the pulmonary circulation protects against small distal PA loss and development of PH, we placed 15 week old male and female ApcMin/+ and C57Bl6J wild type littermate control mice (C57) in a hypoxia chamber (FiO2: 10%) for three weeks, and compared their structural and functional response immediately following exposure, and after three weeks of recovery in room air, as in our previous studies11. Compared to C57, the ApcMin/+ mice of both genders developed more severe PH as judged by significantly higher right ventricular systolic pressure (RVSP) after three weeks of hypoxia not attributable to a change in left ventricular end diastolic pressure (LVEDP). Moreover, the elevation in RVSP persisted after three weeks of recovery in room air (Figure 1A). Consistent with these findings, ApcMin/+ mice, when compared to C57, had greater right ventricular hypertrophy (RVH) as judged by Fulton index (the ratio of the right ventricle to left ventricle and septum), both after chronic hypoxia and following the room air recovery period (Figure 1B). Of note, differences in Fulton index were due solely to increase in RV mass as no significant differences in LV+S mass was seen between C57 and ApcMin/+ mice in any of the experimental conditions. Similar values for RVSP and RVH were observed in C57 and ApcMin/+ mice maintained over the same period of time exclusively in room air (Figure 1A and B).
Figure 1. ApcMin/+ mice in chronic hypoxia demonstrate increased right ventricular systolic pressure (RVSP) and RV hypertrophy and small vessel loss compared to wild type C57 littermate controls.
Measurements of (A) RVSP, (B) right ventricular weight relative to that of left ventricle and septum (RV/LV+S), (C) muscularization of peripheral arteries at alveolar wall and duct level and (D) number of peripheral alveolar duct and wall arteries per 100 alveoli in mice exposed to room air (Normoxia), three weeks of 10% O2 (Hypoxia) and three weeks of recovery in room air (Recovery) as described in the Methods. Representative images of muscularized pulmonary arteries (C) and vessel number (D) are shown above the corresponding measurements. Bars represent mean ±SEM from experiments involving 10 animals per group. **P<0.001, ***P<0.0001 vs. normoxia, #P<0.01, ##P<0.001 vs. C57, one way ANOVA with Bonferroni’s post-test. Scale bar=25µm (C) and 100µm (D).
Chronic hypoxia-induced PH is associated with muscularization of normally non-muscular small distal alveolar duct and wall arteries, and ApcMin/+ and C57 mice demonstrated a similar increase in the muscularization of these vessels following chronic hypoxia and recovery (Figure 1C). However, we observed a significant reduction in the number of these distal vessels in the ApcMin/+ versus C57 mice in chronic hypoxia that, in contrast to the control group, failed to normalize at the end of the recovery period (Figure 1D).
Echocardiographic analyses in the C57 and ApcMin/+ mice after chronic hypoxia and recovery in room air revealed similar values for left ventricular function as judged by fractional shortening, cardiac output, ejection fraction and heart rate (Supplement Figure IA-D). Systemic blood pressure (Supplement Figure IE), LVEDP (Supplement Figure IF) and pulmonary artery acceleration times (PAAT, Supplement Figure IG) were also similar in both genotypes under all conditions of study. Similar values for LVEDP and cardiac output, suggested that the elevated RVSP could represent an increase in pulmonary vascular resistance. However, the hematocrit levels were lower in the ApcMin/+ vs. C57 mice in room air (30±5 vs. 40±10%) and hypoxia (54±10 vs. 65±8%). We then set out to investigate how, despite the heightened βC expression in ApcMin/+ mice, there could be increased hypoxia-mediated loss of small distal PAs and impaired recovery in room air.
ApcMin/+ mvPAECs demonstrate reduced survival following serum withdrawal but a preserved proliferative response under normoxia and hypoxia
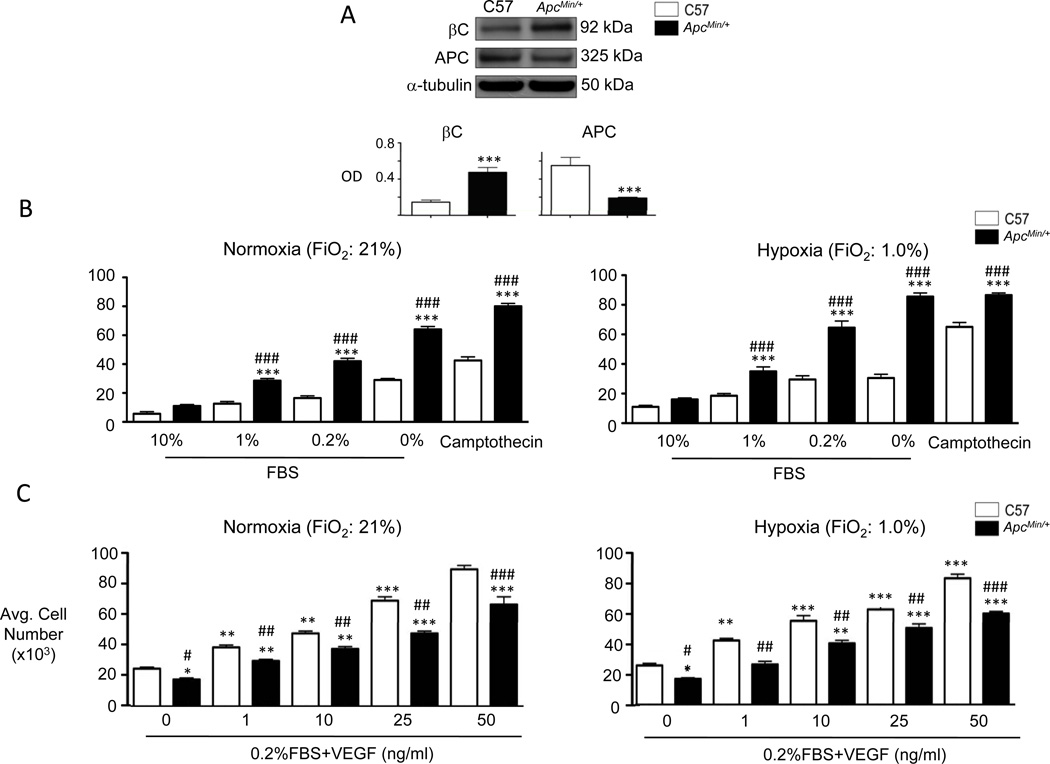
We harvested mvPAECs using CD31 antibody-coated beads (see Methods) to determine if the impaired survival of these cells when exposed to hypoxia could be a cause of loss of vessels in the ApcMin/+ mice. Compared to C57, ApcMin/+ mvPAECs demonstrated a >50% reduction in APC protein, accompanied by an increase in total βC (Figure 2A). ApcMin/+ and C57 cells were then either incubated in room air (FiO2: 21%) or in a hypoxia chamber (FiO2: 1%) for 24 hours, as described in Methods, and both genotypes were exposed to decreasing serum concentrations ranging from 10% (full growth medium) to 0% (serum free). Impaired cell survival or apoptosis, judged by active caspase 3/7, was significantly greater in ApcMin/+ vs. C57 mvPAECs, and correlated with decreasing serum supplementation in normoxia (Figure 2B, left panel) that was further aggravated by hypoxia (Figure 2B, right panel).
Figure 2. ApcMin/+ mvPAECs demonstrate reduced survival and growth.
(A) Representative western immunoblots for βC and APC in lysates from C57 littermate and ApcMin/+ mvPAECs. Densitometric values are shown relative to α-tubulin. ***P<0.0001, unpaired t-test. (B) Apoptosis was measured by the Caspase 3/7 assay in C57 and ApcMin/+ mvPAECs exposed to a range of serum concentrations (0–10%) under either normoxia (left panel) or hypoxia (right panel). After 24 hours, lysates were analyzed for luciferase activity (LU) as described in the Methods. Camptothecin was used as a positive control. (C) Proliferation was assesed by cell count assays in C57 and ApcMin/+ mvPAECs exposed to a range of VEGF concentrations (0–50ng/ml) under either normoxia (left panel) or hypoxia (right panel). Cell numbers were measured 72 hours after the addition of VEGF as described in the Methods. Bars represent mean ±SEM from N=3 experiments. *P<0.01, **P<0.001, ***P<0.0001 vs. C57 at 10% FBS (apoptosis assay) or baseline (VEGF proliferation assay); #P<0.01, ##P<0.001, ###P<0.0001 vs. C57, one way ANOVA with Bonferroni’s post-test.
Next, we compared the proliferative response of the ApcMin/+ vs. C57 mvPAEC in response to an angiogenic stimulus. We counted the cells 24h following stimulation with vascular endothelial growth factor (VEGF) at concentrations ranging from 0 to 50ng/ml12. We observed a lower initial number of adherent ApcMin/+ vs. control mvPAECs cells, but the rate of proliferation of the ApcMin/+ cells that did adhere was comparable to that of C57 mvPAECs in both normoxia (Figure 2C, left panel) and hypoxia (Figure 2C, right panel).
While most studies show that elevated βC is a survival factor in mammalian cells, there are reports that excessive βC can also be linked to apoptosis13. We therefore investigated whether the impaired survival of the ApcMin/+ mvPAEC could be linked to elevated βC levels. ApcMin/+ cells were transfected with either scrambled or βC-specific siRNA, with the goal of reducing endogenous βC levels to those seen in C57 cells (See Figure 2A). After confirming that βC levels were reduced to the target range (Supplement Figure IIA), we starved the cells and measured active caspase 3/7 as an indication of apoptosis (see Methods). We found no difference in apoptosis in ApcMin/+ mvPAEC treated with βC siRNA vs. scrambled siRNA after 24 hours (Supplement Figure IIB), suggesting that elevated βC levels are not responsible for the reduced survival of ApcMin/+ mvPAEC.
APC deficiency is associated with reduced tube formation in matrigel
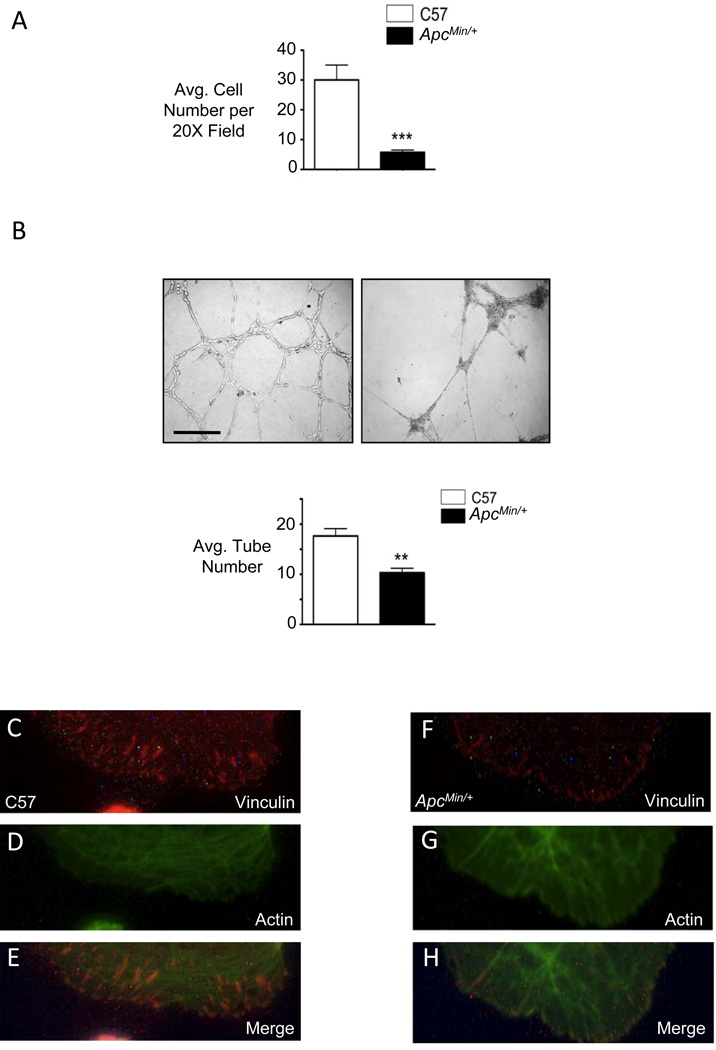
To further understand the consequences of reduced APC on angiogenesis, we seeded ApcMin/+ and C57 mvPAECs in wells coated with matrigel, a biological matrix similar in composition to the endothelial basement membrane14. We found that, after 30 minutes, significantly fewer ApcMin/+ vs. C57 cells attached to matrigel (Figure 3A), and ApcMin/+ mvPAECs formed less complex tube networks when assessed 6 hours after seeding (Figure 3B). In association with reduced adhesion of ApcMin/+ mvPAECs to matrigel, we observed impaired formation of focal adhesions in these cells, as assessed by vinculin and actin staining (Figure 3, F-H vs. C-E) as well as reduced clustering of microtubules at the cell periphery (Supplement Figure III).
Figure 3. ApcMin/+ mvPAECs demonstrate reduced adhesion to matrigel resulting in decreased tube formation.
(A) Adhesion assay was performed by counting the number of C57 littermate and or ApcMin/+ mvPAECs attached to a matrigel coated surface 30 minutes after seeding. Bars represent mean ±SEM from N=3 experiments. ***P<0.0001, unpaired t-test. (B) Representative photomicrographs of tube formation assays in matrigel at six hours. Scale bar=150µm. Tube number was analyzed as described in the Methods. Bars represent mean ±SEM from N=3 experiments. **P<0.001, unpaired t-test. (C) Representative immunofluorescence microscopy photomicrographs demonstrate abundant focal adhesions in controls (C-E) but not in ApcMin/+ mvPAECs (F-H) as judged by vinculin (red) at the tips of actin (green) fibers at the cell periphery. Scale bar=30µm.
Adhesion defect of APC deficient mvPAECs is prominent on laminin
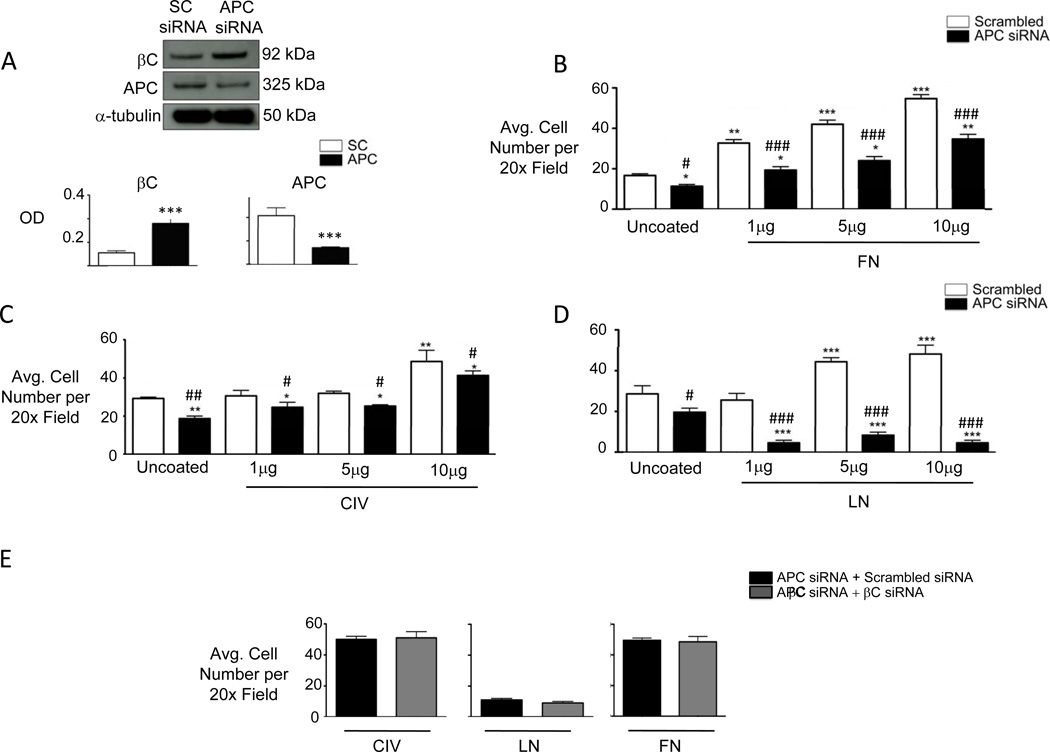
It has been proposed that APC is required for cell adhesion to the extracellular matrix by promoting the activation of integrin complexes and their association with the underlying actin cytoskeleton15, 16. To determine whether the adhesion defect of ApcMin/+ mvPAECs is selective to a specific component of the extracellular matrix, we used human mvPAECs transfected with either scrambled or APC-targeting siRNA and measured APC expression using an antibody in which the specificity for APC was validated using mass spectrometry (see Methods). A greater than 50% reduction in APC protein was documented 48 hours after transfection, associated with a greater than two-fold increase in βC (Figure 4A). Similar to ApcMin/+ mvPAECs, APC siRNA treated mvPAECs demonstrated a preserved growth response to VEGF in reduced serum conditions (Supplement Figure IVA) but reduced survival when incubated in hypoxia (Supplement Figure IVB). Reduced expression of APC by siRNA resulted in a mild (10–20%) decrease in adhesion to fibronectin (FN; Figure 4B) and a somewhat more impaired adhesion (30–40%) to type IV collagen (CIV; Figure 4C) at all tested concentrations of substrate. Adhesion of human APC siRNA-transfected mvPAECs to laminin (LN) was, however, severely reduced (>80%) relative to adhesion to an uncoated substrate, and failed to improve despite an increase in the amount of laminin coating (Figure 4D). The reduced adhesion to all three substrates was unrelated to elevated levels of βC, as shown in experiments using βC-targeting siRNA to reduce βC levels in ApcMin/+ mvPAECs (Figure 4E).
Figure 4. APC deficiency in mvPAECs reduces adhesion to laminin in a βC independent manner.
(A) Representative western immunoblots for APC and βC in human mvPAECs lysates treated with scrambled (SC) or APC siRNA. Densitometric values are shown relative to α-tubulin. Bars represent mean ±SEM of n=3. ***P<0.0001, unpaired t-test. (B, C, D) Adhesion of APC and SC siRNA treated human mvPAEC on increasing amounts of fibronectin (FN, panel B), collagen IV (CIV, panel C), and laminin (LN, panel D). (E) Adhesion to all three substrates (10µg per well) was measured in cells co-transfected with APC siRNA and scrambled or βC siRNA. The average number of cells was calculated by counting the total number of cells in six random fields per well (200x magnification). Bars represent mean ±SEM from N=3 experiments. *P<0.01, **P<0.001, ***P<0.0001 vs. uncoated, #P<0.01,##P<0.001, ###P<0.0001 vs. SC, one way ANOVA with Bonferroni’s post-test.
Laminin interaction with α3β1 integrin triggers an interaction with cytoplasmic APC in mvPAECs
To determine which integrin receptor complex17 regulates adhesion of human mvPAECs to LN, we incubated cells with blocking antibodies against specific α and β integrins prior to seeding (see Methods). While adhesion to LN was reduced with high concentrations of α1, α4, α5 and αV blocking antibodies, and with lower concentrations of α6 and β4 blocking antibodies, the most profound effect was noted with blockade of α3 and β1 integrins (Supplement Figure VA).
Binding to extracellular matrix proteins can result in conformational changes that facilitate the formation of active signaling complexes on α and β integrin cytoplasmic tails18 that can impact cell adhesion and survival. To establish whether LN promotes recruitment of APC to the cytoplasmic tail of the β3 integrin to enable signaling, we first immunoprecipitated human mvPAEC lysates after one hour of exposure to LN using antibodies specific to this integrin, followed by western immunoblot for APC. We also assessed APC interaction with the α1 ιντεγριν in cells adherent to CIV, or with τηε α5 ιντεγριν in cells plated on FN. We found that, while APC can associate with all of these α integrins, there was a striking increase in the formation of a α3-APC complex following seeding of the mvPAECs on LN, likely due to clustering of α3 integrins on laminin (Supplement Figure VB).
Taken together, our studies suggest that binding to LN induces recruitment of APC to the α3 (likely β1) integrin complex. We next sought to examine the nature of this interaction and why recruitment of APC to the α3 integrin is required for adhesion to LN.
Laminin-dependent APC binding to α3 integrin facilitates focal adhesion complex formation by selectively activating integrin-linked kinase-1
Binding of the integrin receptor complex to the extracellular matrix triggers a sequence of intracellular events that not only stabilizes cell-matrix interactions but also controls cell survival. Central to these biological events is the recruitment of integrin-linked kinase (ILK)-1, a 52 kDa protein composed of three structurally distinct domains: three ankyrin repeats near the N terminus, a short linker sequence, and a kinase domain at the C terminus19, 20. Activation of cytoplasmic adaptor proteins, such as paxillin and vinculin, via direct or indirect binding to ILK-1 is necessary for the formation of mature integrin-actin focal adhesion complexes. Thus, a reduction in ILK-1 expression or activity can change cell shape and decrease the strength and number of focal adhesions20. Indeed both proteins colocalize at the cell periphery at the site of focal adhesions19, 20 (Supplement Figure VI), suggesting that they may cooperatively interact in the mechanism responsible for cell adhesion to the extracellular environment.
To determine whether APC is required for the recruitment of ILK-1 to integrin receptors upon binding to extracellular substrates, we performed co-immunoprecipitation (IP) studies. Using α integrin antibodies, we precipitated protein complexes in cell lysates from human mvPAECs treated with either scrambled or APC siRNA following binding to CIV, FN and LN, and then carried out western immunoblotting for ILK-1. We found that the amount of ILK-1 precipitated using the α3 integrin antibody was independent of the extracellular matrix substrate (LN, CIV or FN) to which the cells adhered (Figure 5A) and was preserved despite reducing levels of APC (Figure 5A). Thus, recruitment of ILK-1 by the α3 integrin is independent of its interaction with APC, even on laminin.
Figure 5. Laminin induces APC dependent ILK-1 and Akt activation.
(A) Immunoprecipitation with α3 integrin antibody and immunoblotting with APC antibody in whole cell lysates of human mvPAECs following adhesion to CIV, LN and FN. (B) ILK-1 kinase assay performed in scrambled and APC siRNA treated human mvPAECs seeded on CIV, LN and FN coated surfaces. Phosphorylation of the GSK3β substrate was used as a measure of ILK kinase activity as decribed in the Methods. ILK-1 activity was measured by densitometry against total ILK-1 levels. Total GSK3β levels in whole lysates are also shown. **P<0.001, one way ANOVA with Bonferroni’s post-test Bars represent mean ±SEM from N=3 experiments. (C) Akt phosphorylation in whole cell lysates recovered from scrambled or APC siRNA treated human mvPAEC seeded on CIV, LN and FN coated surfaces. Densitometric values are shown relative to total Akt. Bars represent mean ±SEM from N=3 experiments. ***P<0.0001, scrambled vs. corresponding APC siRNA, one way ANOVA with Bonferroni’s post-test.
We next determined whether APC deficiency could influence ILK-1 activation. We incubated ILK-1 precipitated from lysates of human mvPAEC seeded on FN, CIV and LN with GSK3β, a known substrate of ILK-1 (see Methods). We found that GSK3β phosphorylation by ILK-1 was higher in cells cultured on LN compared to CIV and FN. Moreover, ILK-1 activity was reduced in cells treated with APC-targeting siRNA when cultured on LN, but not in cells cultured on FN or CIV (Figure 5B). Thus, the increase in APC associated with the α3 integrin in mvPAECs seeded on laminin is a requirement for the activation of ILK-1.
ILK-1 activation also increases cell survival via the PKB/Akt signaling pathway21. Given the poor survival response seen in APC deficient mvPAECs (see Figure 2), we investigated whether loss of APC by APC-targeting siRNA reduced activation of PKB/Akt in mvPAECs cultured on LN, versus cells cultured on CIV or FN. While there was considerable variability in levels of phospho (i.e. active) Akt (pAkt) in mvPAECs on the different substrates, reducing APC decreased pAkt only in LN-bound cells (Figure 5C).
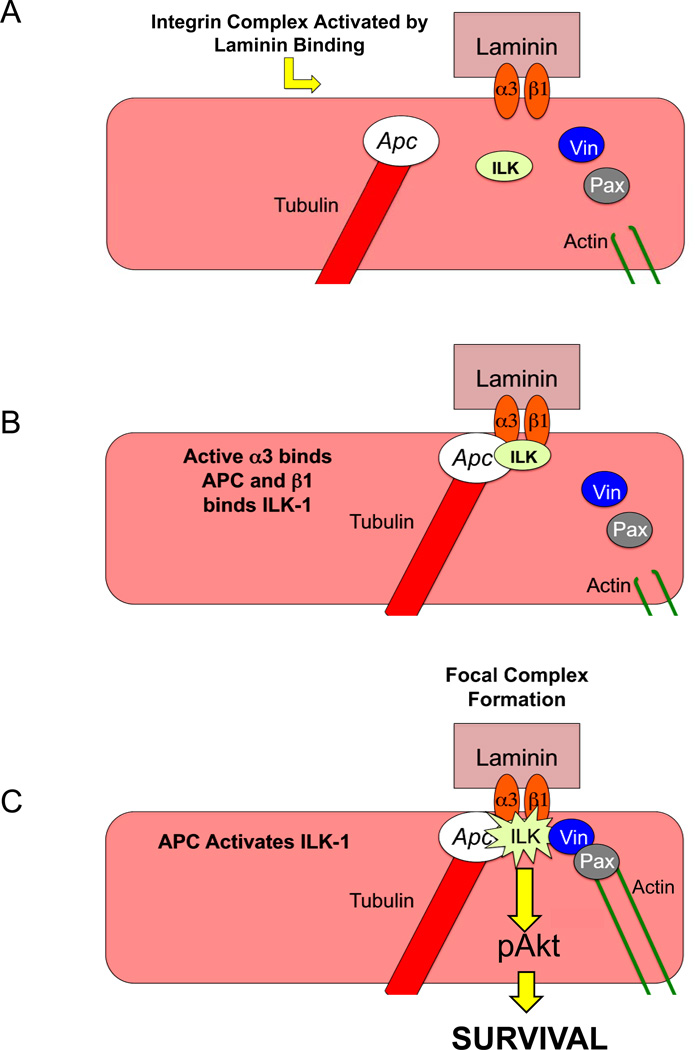
Taken together, these studies in human mvPAECs cultured on LN show that the interaction between APC and the α3 integrin is required for the activation of ILK-1, and that ILK-1 activity promotes both adhesion of mvPAEC on LN and survival via PKB/Akt (Figure 8).
Figure 8. Proposed model for involvement of APC in laminin adhesion and pAkt mediated mvPAEC survival.
(A) Upon binding laminin, α3β1 integrin complex recruits APC to the α3 cytoplasmic tail (B). Once there, APC facilitates ILK-1 activation, formation of focal adhesion complexes by recruitment of vinculin, paxillin and actin, followed by activation of pAkt leading to cell survival (C).
Adhesion to laminin is dependent on the QPSXXE motif of α3 integrin
The next series of experiments was carried out to determine whether the specific interaction of APC with the α3 integrin that is required for ILK-1 activity is also required for cell adhesion to LN. The cytoplasmic portion of the α3 integrin is composed of 52 amino acids and can undergo a series of post-translational modifications. One such modification is the phosphorylation of serine 1042 within the QPSXXE motif that strengthens adhesion to laminin via paxillin and focal adhesion kinase (FAK) activity22.
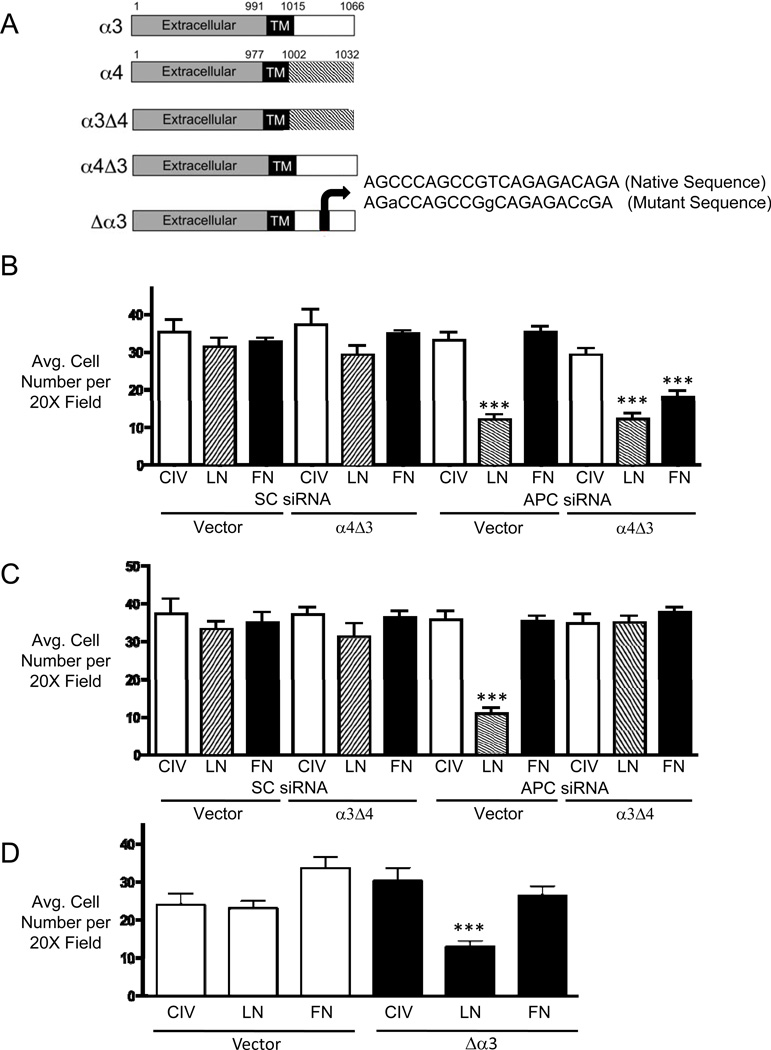
Given the specificity of our findings, we hypothesized that the association of APC with the α3 cytoplasmic tail would promote cell adhesion independent of the extracellular substrate. To this end, we engineered an integrin chimera in which the cytoplasmic tail of the α3 integrin was switched with that of the FN specific integrin, α4 (α3Δ4)23–25 and another in which the α4 cytoplasmic tail was switched with that of the α3 integrin (α4Δ3) (Schema in Figure 6A). We transfected these constructs individually into mvPAECs co-transfected with either scrambled or APC siRNA. We found that cells transfected with the α4 chimera (α4Δ3) demonstrated loss of adhesion to FN following treatment with APC siRNA (Figure 6B) while the α3 chimera (α3Δ4) demonstrated preserved laminin adhesion despite transfection with APC siRNA (Figure 6C). As expected, under conditions of reduced APC, we also observed reduced LN binding in both vector and α4Δ3 transfected human mvPAECs (Figure 6B).
Figure 6. The α3 cytoplasmic tail regulates matrix (ECM) binding specificity and requires preservation of the QPSXXE motif.
(A) Diagram illustrating the WT and mutant α3 and α4 integrin constructs. Details of the their construction can be found in the Methods. (B and C) Adhesion to CIV, LN and FN was measured in human mvPAECs transfected with the mutant α4 chimera containing the α3 cytoplasmic tail (α4Δ3) (B) or the corresponding α3 chimera containing the α4 cytoplasic tail (α3Δ4) (C) following SC or APC siRNA treatment. (D) Adhesion to CIV, LN and FN in mvPAECs transfected with either vector or mutant α3 integrin construct containing mutations in the amino acids within the QPSXXE motif (Δα3) as described in the Methods. Bars represent mean ±SEM from N=3 experiments. ***P<0.0001 versus CIV, one way ANOVA with Bonferroni’s post-test.
We also investigated whether disruption of the QPSXXE sequence in the α3 integrin cytoplasmic tail, which contains the serine residue critical to laminin binding (S1042), could reproduce the adhesion defect seen with APC deficiency. To this end, we mutated the QPSXXE motif in the α3 integrin cDNA sequence by substituting alanines for serines within the motif (Figure 6A). Consistent with our expectation, we found that cells transfected with the mutant α3 integrin construct (Δα3) demonstrated reduced adhesion to LN, compared to cells transfected with an empty vector (Figure 6D).
Taken together, our results demonstrate that when human mvPAECs bind LN, a functional QPSXXE motif in the cytoplasmic tail of the α3 integrin recruits APC to activate ILK-1, stabilize focal adhesions and activate pAkt (Figure 8). This property is not unique to mvPAECs, as we also documented reduced adhesion to LN compared to FN and CIV in the SW480 colon cancer cell line that expresses a truncated version of APC missing the cytoplasmic tail (Supplement Figure VII).
Reduced APC expression in mvPAECs from IPAH patients is related to decreased adhesion and tube formation
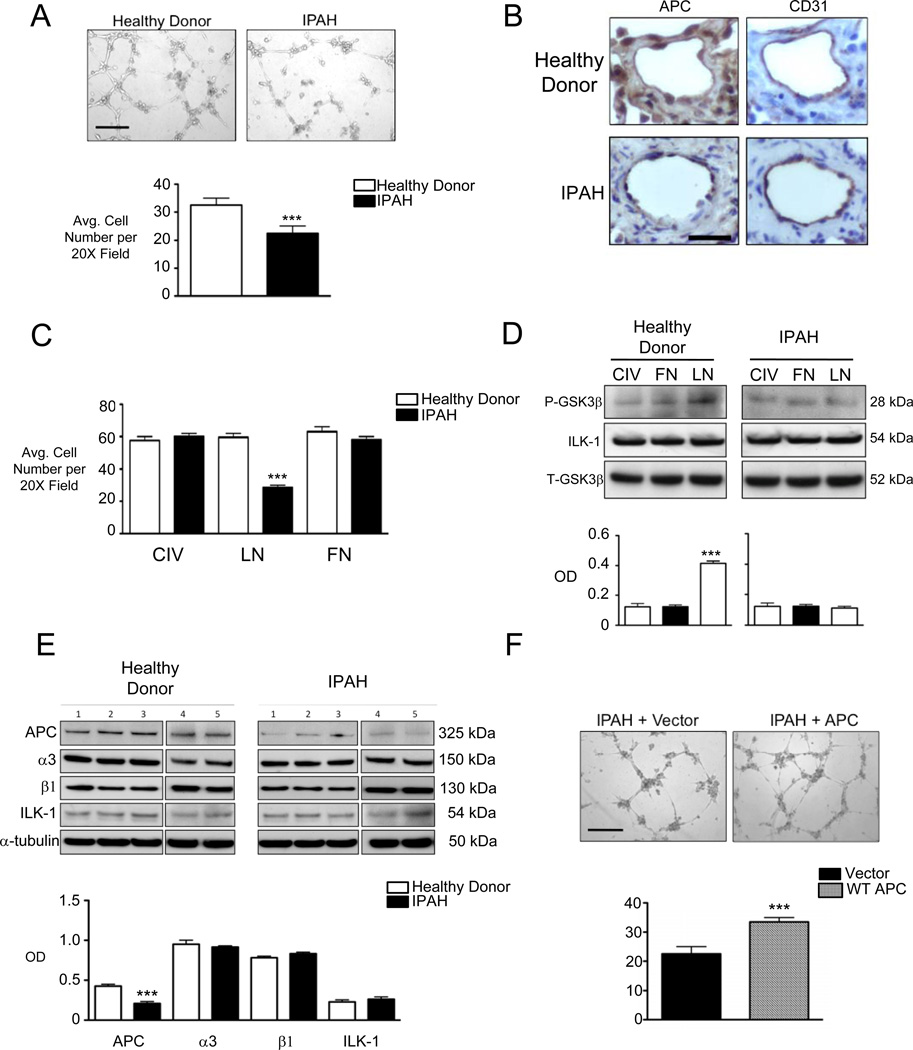
Previous studies showed that PAECs isolated from IPAH patients demonstrate reduced survival and form smaller vascular tube networks compared to those of healthy donors26, but the mechanism involved was poorly understood. To determine whether the reduced angiogenic potential of IPAH mvPAECs could be related to decreased expression of APC, we isolated cells from the lungs of 5 healthy subjects (unused donor lungs) and lungs explanted from 5 IPAH patients undergoing lung transplant (see Supplement Table I for patient characteristics). These cells were obtained from tissues procured through the Pulmonary Hypertension Breakthrough Initiative (PHBI) (see Methods). We confirmed reduced tube formation in matrigel in mvPAECs from IPAH patients compared to those from healthy donors (Figure 7A). IPAH mvPAECs exhibited decreased adhesion to LN-coated surfaces (Figure 7B) compared to healthy donor mvPAECs, associated with lower levels of APC as demonstrated by western immunoblot (Figure 7C) and immunohistochemistry (Figure 7B). As with APC siRNA treated mvPAECs (Figure 7B), we found evidence of reduced ILK-1 activation in IPAH mvPAECs incubated with laminin (Figure 7D). To determine if the APC deficiency is directly responsible for the adhesion defect seen in IPAH mvPAECs, we transfected these cells with an APC expression construct. When transfected in healthy donor cells, WT APC protects against apoptosis secondary to hypoxia supporting its role as a pro-survival factor (Supplement Figure VIII). APC transfected IPAH mvPAECs recovered the ability to adhere to LN (Supplement Figure IX) and, when seeded in matrigel, were able to form vascular networks of similar size and density as those produced by healthy donor mvPAECs (Figure 7D).
Figure 7. Microvascular PAECs from IPAH patients demonstrate adhesion defects and reduced APC protein expression.
(A) Tube formation in matrigel, (B) immunohistochemistry for APC and CD31 in patient microvessels, (C) adhesion to CIV, LN and FN, (D) ILK-1 activation assay and (E) WB for APC, ILK-1, α3 and β1 integrin in IPAH versus healthy donor mvPAECs. Lysates from mvPAECs were isolated from five healthy donors and five IPAH patients were used for western immunoblotting. Line separating first three samples from samples 4+5 in (E) was placed to indicate that samples were run in different gels. (D) Transfection of a WT APC expression vector versus empty plasmid and tube formation in IPAH mvPAECs. Bars represent mean ±SEM from N=5 experiments or patients. ***P<0.0001, unpaired t-test in A and F. ***P<0.0001 one way ANOVA with Bonferroni’s post-test versus all other groups in C and D. ***P<0.0001 versus corresponding control in E. Scale bar=150 µm in A and F. Scale bar=25µm in B.
DISCUSSION
Based on our findings, we propose that in mvPAECs, APC promotes cell survival in response to formation of α3 integrin-rich focal adhesions upon binding to LN (Figure 8). Interaction of QPSXXE motif of the α3 integrin cytoplasmic tail with APC, is a pre-requisite for the activation of ILK-1, formation of focal adhesions and phosphorylation of pAkt to mediate cell survival. To the best of our knowledge, this is the first demonstration that APC can promote mvPAEC survival independent of its role in regulating Wnt signaling. Moreover, a reduction in APC appears to contribute to the decreased angiogenic potential of mvPAECs in IPAH patients. While the current findings appear to contradict our previous work on the pro-angiogenic effects of βC in BMP stimulated PAECs6, in fact we have discovered a novel function of APC that also promotes cell survival.
Since its original characterization, studies of the ApcMin/+ mouse established a firm link between increased Wnt/βC activation and development of colonic polyps. However, few studies have investigated whether there is a vascular phenotype in these animals, or whether APC might play a role in tumor pathogenesis independent of Wnt signaling. A study by Bhandaru et al27 demonstrated that, compared to C57 controls, ApcMin/+ mice have higher than normal blood pressure and blood volume as a consequence of increased aldosterone levels and reduced glomerular filtration. In our study, we found only a trend towards higher systemic blood pressures in ApcMin/+ mice using tail cuff measurements. Our results may be related to lower salt content in the diet, the timing of the blood pressure measurements (early morning vs. afternoon), and a smaller sample size. Furthermore, hypoxia is known to trigger systemic vasodilatation and to inhibit the release of aldosterone from the adrenal glands, thus promoting a reduction in systemic pressure28.
We show that ApcMin/+ mice exposed to chronic hypoxia develop more severe PH in association with greater loss of peripheral PAs and impaired ability to regenerate these vessels during the recovery period. A recent study in mammalian cells revealed that APC mRNA and protein levels fall in response to hypoxia as a result of repression of the APC promoter by hypoxia inducible factor (HIF)-1α29, a phenomenon that we were able to reproduce in human mvPAECs incubated in hypoxia (FiO2: 1%) for 24 hours (Supplement Figure X). It is surprising that the hypoxia-mediated endothelial apoptosis and loss of vessels and the exaggerated PH in the ApcMin/+ vs. C57 mice were not accompanied by greater muscularization of peripheral arteries as is seen in other murine models of PH. This may, however be related to a confounding effect of loss of APC in PA smooth muscle cells. Future studies creating a transgenic mouse with EC- or SMC-specific deletion of APC would allow us to better understand the nature of the SMC response to APC deletion. Finally, it is also intriguing that mice develop PH only when exposed to hypoxia even though they can develop malignant tumors at baseline.
A prominent finding in our studies in APC deficient mvPAECs was the relative specificity of the adhesion defect to LN and LN-enriched substrates such as matrigel. Since the basement membrane of blood vessels is rich in LN, inability to properly attach to this substrate would substantially impair the physiological angiogenic response to a vascular injury30, 31. In HeLAS3 cells, microtubule-associated APC is recruited to focal adhesions by Disheveled (Dvl) following the binding of Wnt5a to the Wnt receptor Fzd216, 32. In this setting, it was thought that APC is brought in close proximity to the integrin receptors by its association with Dvl and Fzd2, and that this fosters cytoskeletal changes that influence cell adhesion, polarity and directed migration. Our studies demonstrate not only that there is a physical interaction between APC and the α3 integrin, but that this interaction is required for ILK-1 activity and stabilization of focal adhesions. Moreover, this interaction can only occur if the QPSXXE motif in the α3 cytoplasmic tail is preserved22.
At the center of the interaction between LN, α3 integrin and APC is the activation of ILK-1, a protein that facilitates integrin-actin interactions and activates numerous signaling pathways in response to integrin binding to the extracellular matrix33, 34. Our studies show that the α3 integrin can recruit ILK-1 independent of APC, but that the APC interaction with α3 integrins clustering on laminin is required to activate ILK-1. Other intermediary proteins like APC can promote a functional interaction between integrin receptors and ILK-1, and are likely associated with mvPAEC binding to other substrates. An example is the calponin homology ILK binding protein (CH-ILKBP or α-parvin/actopaxin), an integrin adaptor protein that binds β1 integrin and recruits ILK-1 by interacting with its C-terminal35, 36. This event also ensures the activation of ILK-1 and consequent or concomitant downstream signaling via PKB/Akt.
In contrast to the strong APC-dependent activation of ILK-1 seen in laminin-bound mvPAECs, the extent of ILK-1 activation seen in cells cultured on FN or CIV was minimal and did not correlate with pAkt activation. One possible explanation for this observation is that FN and CIV specific integrin receptors preferentially utilize focal adhesion kinase (FAK), to ensure maturation of focal adhesion complexes and to trigger activation of pAkt. Indeed, studies performed in mesenchymal stem cells, intestinal epithelial cells and human umbilical vein endothelial cells (HUVECs)37, 38 have demonstrated that both CIV and FN promote focal adhesion formation and pAkt through activation of FAK39, 40. It was interesting that transfection of a constitutively active Akt expression construct failed to reverse or improve survival of APC deficient cells (Supplement Figure XI). Based upon previous studies, it is probable that recruitment of APC to the laminin bound α3β1 integrin complex initiates a signaling cascade involving additional survival pathways responsible for protecting mvPAECs during stress and injury. Alternatively, recruitment of constitutively active Akt to the integrin cytoplasmic scaffold is necessary to properly target signaling to downstream effectors of survival41.
Reduced APC levels in SW480 colon cancer cells42 could contribute to heightened metastatic behavior by allowing the cells to detach from the laminin-based ECM and assume an invasive phenotype. In a study investigating 22 colorectal cancer cell lines with different levels of APC expression, a strong correlation was found between CpG methylation in two regions of the APC promoter and the degree of reduced APC expression43. Moreover, treatment of colon cancer cells with 5-aza-2’-deoxycytidine, a known DNA methyltransferase inhibitor, reduced CpG methylation and concomitantly increased levels of APC. Other agents with demethylating properties, such as selenite, also increase levels of APC in prostate cancer cells and reduce tumor growth and metastasis44. It is possible that these strategies to increase APC could be utilized to reverse the angiogenic defect in IPAH mvPAECs, as we showed, by transfecting the cells with an APC construct.
While the mechanism leading to a reduction in APC in IPAH mvPAECs is unknown, several possibilities could be explored. Using a microRNA microarray to analyze colorectal cancer cells exposed to the novel antineoplastic agent CM-1, Li et al. discovered that CM-1 dependent suppression of miR-135a/b was associated with an increase in APC expression and anticancer activity45. Also, Nagel et al found that untreated colorectal cancer cells exhibit higher level of miR-135a/b that was inversely related to APC expression46. Other microRNAs, such as miR-27, suppress APC expression in osteoblasts and this regulates their differentiation47. Thus, it is possible that in IPAH cells there is an increase in miR-135a/b or in miR27. Caruso et al described a different miRNA expression profile in IPAH compared to control lungs48, but miR-135a/b and miR27 were not mentioned in their study.
Given the potential clinical relevance of our findings, the fact that there is no reported link between familial adenomatous poliposis (FAP) and PAH is surprising. Recent reports describe increased vascularity in the oral mucosa of patients with FAP, which contrasts with our findings in the lungs of ApcMin/+ mouse49. A possible explanation for this discrepancy may be related to an inherent difference in the sub-endothelial matrix of systemic vs. pulmonary microvessels. Alternatively environmental hypoxia may also cause systemic microvessel dropout. That is, specific environmental and genetic modifiers may be necessary to unmask FAP or PAH. It is conceivable that patients who are carriers of the BMPR2 mutation may be at a higher risk of developing IPAH when they have loss of function of APC. It is also possible that some patients with FAP have mild or moderate PH, but their symptoms are masked by the underlying pathology of their colonic condition, or that some patients with BMPR2 mutations and PAH have mild colonic disease. Given the rarity of both conditions, i.e., the lack of penetrance of the APC mutation in causing colonic disease50, 51, and the BMPR2 mutation in causing PAH, it is likely that screening FAP patients with APC mutations for PAH may be necessary to further characterize the presence of PAH in FAP as well as the contribution of reduced APC to IPAH, particularly in patients with BMPR2 dysfunction.
Supplementary Material
Novelty and Significance.
What Is Known?
Idiopathic pulmonary arterial hypertension (IPAH) is associated with progressive loss of small vessels and impaired regeneration following genetic and/or environmental injury.
Activation of Wnt/β-catenin (βC) signaling in pulmonary artery endothelial cells (PAECs) promotes angiogenesis by targeting expression of βC specific genes involved in proliferation and survival.
Loss of adenomatous poliposis coli (APC) leads to increased proliferation and survival of cancer cells by increasing both the amount and transcriptional activity of βC.
What New Information Does This Article Contribute?
Despite increasing βC levels in PAECs, partial loss of APC leads to significant small vessel loss and more severe PAH in transgenic (ApcMin/+) mice exposed to chronic hypoxia when compared to controls.
Loss of APC results in reduced α3 integrin-dependent laminin adhesion and increased endothelial cell apoptosis.
APC deficiency is seen in PAECs isolated from IPAH patients and correlates with reduced adhesion and impaired angiogenesis. Restoring APC in IPAH results in normalization of PAEC phenotype.
Pulmonary arterial hypertension (IPAH) is characterized by progressive elevation in pulmonary pressures and loss of small pulmonary arteries. As BMPs promote pulmonary angiogenesis by recruiting the Wnt/βC pathway, we proposed that βC activation could reduce loss and/or induce regeneration of small PAs and attenuate PAH. To assess the impact of βC activation on chronic hypoxia-induced PAH, we used the adenomatous poliposis coli (ApcMin/+) mouse, where reduced APC causes constitutive βC elevation. Surprisingly, hypoxic ApcMin/+ mice displayed greater elevation in pulmonary pressures and small pulmonary artery loss compared to control littermates. PAECs isolated from ApcMin/+ demonstrated reduced survival and angiogenic responses along with profound decrease in adhesion to the laminin component of the basement membrane. We discovered that APC is required to interact with the laminin α3 integrin cytoplasmic domain to stabilize focal adhesions and trigger pAkt by activating integrin-linked kinase (ILK-1). This signaling mechanism is clinically relevant as PAECs from IPAH patients also have reduced APC expression, impaired laminin adhesion and vascular tube formation, that were corrected by restoration of APC levels. Thus, we propose that APC is integral to endothelial cell adhesion and survival and a reduction in APC could increase the risk of IPAH in genetically susceptible individuals.
ACKNOWLEDGEMENTS
Lung tissues from IPAH and control patients were provided by the Pulmonary Hypertension Breakthrough Initiative (PHBI), which is funded by the Cardiovascular Medical Research and Education Fund (CMREF). The tissues were procured at the Transplant Procurement Centers at Stanford University, Cleveland Clinic and Allegheny General Hospital and de-identified patient data were obtained via the Data Coordinating Center at the University of Michigan.
We would like to express our gratitude to Dr. Michal Bental Roof, for edits and helpful suggestions regarding the manuscript and for administrative assistance and Dr. Chris Adams for his help with the methods and performance of Mass Spectrometry to validate our APC antibody.
SOURCES OF FUNDING
This work was supported by a Robert Wood Johnson Foundation Harold Amos Career Development Award and by an NIH-NHLBI K12 RFA-HL-07-004:CDP to V.A. de JP, by an NIH-NHLBI grant R01 HL074186 and by an endowment of the Wall Center for Pulmonary Vascular Disease at Stanford University to MR. Mass Spectrometry was supported by award number S10RR027425 from the NIH National Center for Research Resources.
Non-standard Abbreviations
- APC
Adenomatous poliposis coli
- BMP
Bone morphogenetic protein
- βC
β-catenin
- C57Bl6J
C57
- ILK-1
Integrin linked kinase 1
- PH
Pulmonary hypertension
- mvPAEC
microvascular pulmonary artery endothelial cell
- EC
endothelial cell
- pAkt
phospho Ak thymoma
- ERK
Extracellular signal regulated kinase
- GSK3β
Glycogen synthase kinase 3 β
- BMPR2
Bone morphogenetic protein receptor 2
- VEGF
Vascular endothelial growth factor
- PA
Pulmonary artery
- FAP
Familial adenomatous poliposis
- CIV
Collagen IV
- LN
Laminin
- FN
Fibronectin
- siRNA
Short interfering RNA
- Co-IP
Co-immunoprecipitation
- ANOVA
Analysis of variance
- RVSP
Right ventricular systolic pressure
- RVH
Right ventricular hypertrophy
- FAK
Focal adhesion kinase
- Dvl
Disheveled
- CH-ILKBP
calponin homology ILK binding protein
- Fzd
Frizzled
- HUVEC
Human umbilical vein endothelial cells
- HIF
Hypoxia inducible factor
- FiO2
Fractional inspired oxygen
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: Baseline characteristics from the reveal registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 3.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA, 3rd, Newman J, Williams D, Galie N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L, Loyd JE, Trembath RC, Nichols WC. Bmpr2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in bmpr2, encoding a tgf-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type ii bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 6.de Jesus Perez VA, Alastalo TP, Wu JC, Axelrod JD, Cooke JP, Amieva M, Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via wnt-beta-catenin and wnt-rhoa-rac1 pathways. J Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alastalo TP, Li M, Perez Vde J, Pham D, Sawada H, Wang JK, Koskenvuo M, Wang L, Freeman BA, Chang HY, Rabinovitch M. Disruption of ppargamma/beta-catenin-mediated regulation of apelin impairs bmp-induced mouse and human pulmonary arterial ec survival. J Clin Invest. 2011;121:3735–3746. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the apc gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 9.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 10.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes pdgf receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1082–L1090. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes pdgf receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1082–L1090. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Pang KM, Evans M, Hay ED. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with lef-1 or the involvement of major g1 cell cycle regulators. Mol Biol Cell. 2000;11:3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 15.Langford KJ, Askham JM, Lee T, Adams M, Morrison EE. Examination of actin and microtubule dependent apc localisations in living mammalian cells. BMC Cell Biol. 2006;7:3. doi: 10.1186/1471-2121-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto S, Fumoto K, Okamoto T, Kaibuchi K, Kikuchi A. Binding of apc and dishevelled mediates wnt5a-regulated focal adhesion dynamics in migrating cells. EMBO J. 2010;29:1192–1204. doi: 10.1038/emboj.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 18.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian Y, Zhong X, Flynn DC, Zheng JZ, Qiao M, Wu C, Dedhar S, Shi X, Jiang BH. Ilk mediates actin filament rearrangements and cell migration and invasion through pi3k/akt/rac1 signaling. Oncogene. 2005;24:3154–3165. doi: 10.1038/sj.onc.1208525. [DOI] [PubMed] [Google Scholar]
- 20.Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ilk), ch-ilkbp, and the tumor suppressor pten. Mol Biol Cell. 2003;14:4813–4825. doi: 10.1091/mbc.E03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: Implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XA, Bontrager AL, Stipp CS, Kraeft SK, Bazzoni G, Chen LB, Hemler ME. Phosphorylation of a conserved integrin alpha 3 qpsxxe motif regulates signaling, motility, and cytoskeletal engagement. Mol Biol Cell. 2001;12:351–365. doi: 10.1091/mbc.12.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez VA, Ali Z, Alastalo TP, Ikeno F, Sawada H, Lai YJ, Kleisli T, Spiekerkoetter E, Qu X, Rubinos LH, Ashley E, Amieva M, Dedhar S, Rabinovitch M. Bmp promotes motility and represses growth of smooth muscle cells by activation of tandem wnt pathways. J Cell Biol. 2011;192:171–188. doi: 10.1083/jcb.201008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornberger LK, Singhroy S, Cavalle-Garrido T, Tsang W, Keeley F, Rabinovitch M. Synthesis of extracellular matrix and adhesion through beta(1) integrins are critical for fetal ventricular myocyte proliferation. Circ Res. 2000;87:508–515. doi: 10.1161/01.res.87.6.508. [DOI] [PubMed] [Google Scholar]
- 25.Molossi S, Elices M, Arrhenius T, Rabinovitch M. Lymphocyte transendothelial migration toward smooth muscle cells in interleukin-1 beta-stimulated co-cultures is related to fibronectin interactions with alpha 4 beta 1 and alpha 5 beta 1 integrins. J Cell Physiol. 1995;164:620–633. doi: 10.1002/jcp.1041640321. [DOI] [PubMed] [Google Scholar]
- 26.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 27.Bhandaru M, Kempe DS, Rotte A, Rexhepaj R, Kuhl D, Lang F. Hyperaldosteronism, hypervolemia, and increased blood pressure in mice expressing defective apc. Am J Physiol Regul Integr Comp Physiol. 2009;297:R571–R575. doi: 10.1152/ajpregu.00070.2009. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence DL, Skatrud JB, Shenker Y. Effect of hypoxia on atrial natriuretic factor and aldosterone regulation in humans. Am J Physiol. 1990;258:E243–E248. doi: 10.1152/ajpendo.1990.258.2.E243. [DOI] [PubMed] [Google Scholar]
- 29.Newton IP, Kenneth NS, Appleton PL, Nathke I, Rocha S. Adenomatous polyposis coli and hypoxia-inducible factor-1{alpha} have an antagonistic connection. Mol Biol Cell. 2010;21:3630–3638. doi: 10.1091/mbc.E10-04-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer RH, Bensch KG, Davison PM, Karasek MA. Basal lamina formation by cultured microvascular endothelial cells. J Cell Biol. 1984;99:692–698. doi: 10.1083/jcb.99.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurie GW, Leblond CP, Martin GR. Localization of type iv collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982;95:340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iizuka-Kogo A, Shimomura A, Senda T. Colocalization of apc and dlg at the tips of cellular protrusions in cultured epithelial cells and its dependency on cytoskeletons. Histochem Cell Biol. 2005;123:67–73. doi: 10.1007/s00418-004-0729-2. [DOI] [PubMed] [Google Scholar]
- 33.Gary DS, Milhavet O, Camandola S, Mattson MP. Essential role for integrin linked kinase in akt-mediated integrin survival signaling in hippocampal neurons. J Neurochem. 2003;84:878–890. doi: 10.1046/j.1471-4159.2003.01579.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko Y, Kitazato K, Basaki Y. Integrin-linked kinase regulates vascular morphogenesis induced by vascular endothelial growth factor. J Cell Sci. 2004;117:407–415. doi: 10.1242/jcs.00871. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda T, Guo L, Shi X, Wu C. Ch-ilkbp regulates cell survival by facilitating the membrane translocation of protein kinase b/akt. J Cell Biol. 2003;160:1001–1008. doi: 10.1083/jcb.200212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C. The pinch-ilk-parvin complexes: Assembly, functions and regulation. Biochim Biophys Acta. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Sanders MA, Basson MD. Collagen iv-dependent erk activation in human caco-2 intestinal epithelial cells requires focal adhesion kinase. J Biol Chem. 2000;275:38040–38047. doi: 10.1074/jbc.M003871200. [DOI] [PubMed] [Google Scholar]
- 38.Paik JY, Ko BH, Jung KH, Lee KH. Fibronectin stimulates endothelial cell 18f-fdg uptake through focal adhesion kinase-mediated phosphatidylinositol 3-kinase/akt signaling. J Nucl Med. 2009;50:618–624. doi: 10.2967/jnumed.108.059386. [DOI] [PubMed] [Google Scholar]
- 39.Zebda N, Dubrovskyi O, Birukov KG. Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res. 2012;83:71–81. doi: 10.1016/j.mvr.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wary KK, Kohler EE, Chatterjee I. Focal adhesion kinase regulation of neovascularization. Microvasc Res. 2012;83:64–70. doi: 10.1016/j.mvr.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 42.Faux MC, Ross JL, Meeker C, Johns T, Ji H, Simpson RJ, Layton MJ, Burgess AW. Restoration of full-length adenomatous polyposis coli (apc) protein in a colon cancer cell line enhances cell adhesion. J Cell Sci. 2004;117:427–439. doi: 10.1242/jcs.00862. [DOI] [PubMed] [Google Scholar]
- 43.Deng G, Song GA, Pong E, Sleisenger M, Kim YS. Promoter methylation inhibits apc gene expression by causing changes in chromatin conformation and interfering with the binding of transcription factor ccaat-binding factor. Cancer Res. 2004;64:2692–2698. doi: 10.1158/0008-5472.can-03-3000. [DOI] [PubMed] [Google Scholar]
- 44.Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–2181. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birnbaum T, Hildebrandt J, Nuebling G, Sostak P, Straube A. Glioblastoma-dependent differentiation and angiogenic potential of human mesenchymal stem cells in vitro. J Neurooncol. 2011;105:57–65. doi: 10.1007/s11060-011-0561-1. [DOI] [PubMed] [Google Scholar]
- 46.Nagel R, le Sage C, Diosdado B, van der Waal M, Oude Vrielink JA, Bolijn A, Meijer GA, Agami R. Regulation of the adenomatous polyposis coli gene by the mir-135 family in colorectal cancer. Cancer Res. 2008;68:5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Q, Xue Y, Liu ZD, Li H, Wang JF, Li ZJ, Wang YM, Dong P, Xue CH. Differential effects of sulfated triterpene glycosides, holothurin a1, and 24-dehydroechinoside a, on antimetastasic activity via regulation of the mmp-9 signal pathway. J Food Sci. 2010;75:H280–H288. doi: 10.1111/j.1750-3841.2010.01837.x. [DOI] [PubMed] [Google Scholar]
- 48.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microrna profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 49.Edelstein DL, Giardiello FM, Basiri A, Hylind LM, Romans K, Axilbund JE, Cruz-Correa M, Ramella-Roman JC. A new phenotypic manifestation of familial adenomatous polyposis. Fam Cancer. 2011;10:309–313. doi: 10.1007/s10689-011-9432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, Bicknell D, Bodmer WF, Tomlinson IP. Apc mutations in sporadic colorectal tumors: A mutational "hotspot" and interdependence of the "two hits". Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J, Frayling I, Efstathiou J, Pack K, Payne S, Roylance R, Gorman P, Sheer D, Neale K, Phillips R, Talbot I, Bodmer W, Tomlinson I. The type of somatic mutation at apc in familial adenomatous polyposis is determined by the site of the germline mutation: A new facet to knudson's 'two-hit' hypothesis. Nat Med. 1999;5:1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.