Abstract
Background
Epoxyeicosatrienoic acids (EETs) are natural angiogenic mediators regulated by soluble epoxide hydrolase (sEH). Inhibitors of sEH can stabilize EETs levels and were reported to reduce atherosclerosis and inhibit myocardial infarction in animal models. In this work, we investigated wether increasing EETs with the sEH inhibitor t-AUCB would increase angiogenesis related function in endothelial progenitor cells (EPCs) from patients with acute myocardial infarction (AMI).
Methods and Results
EPCs were isolated from 50 AMI patients and 50 healthy subjects (control). EPCs were treated with different concentrations of t-AUCB for 24 hours with or without peroxisome proliferator activated receptor γ (PPARγ) inhibitor GW9662. Migration of EPCs was assayed in trans-well chambers. Angiogenesis assays were performed using a Matrigel-Matrix in vitro model. The expression of vascular endothelial growth factor (VEGF), hypoxia-inducible factor 1α (HIF-1α) mRNA and protein in EPCs were measured by real-time PCR or Western blot, respectively. Also, the concentration of EETs in the culture supernatant was detected by ELISA.
The activity of EPCs in the AMI patient group was reduced compared to healthy controls. Whereas increasing EET levels with t-AUCB promoted a dose dependent angiogenesis and migration in EPCs from AMI patients. Additionally, the t-AUCB dose dependently increased the expression of the angiogenic factors VEGF and HIF-α. Lastly, we showed that these effects were PPARγ dependent.
Conclusion
The results demonstrate that the sEH inhibitor positively modulated the functions of EPCs in patients with AMI through the EETs-PPARγ pathway. The present study suggests the potential utility of sEHi in the therapy of ischemic heart disease.
Keywords: soluble epoxide hydrolase inhibitor, acute myocardial infarction, endothelial progenitor cells, vascular endothelial growth factor
Introduction
Acute myocardial infarction (AMI) and subsequent ischemic heart failure are well known worldwide health problems and leading causes of morbidity and mortality. AMI is an ischemic disease; thus, therapy that promotes revascularization is beneficial. Endothelial progenitor cells (EPCs) are hematopoietic, bone-marrow-derived cells that express CD34 and vascular endothelial growth factor receptor 2 and differentiate into endothelial cells [1]. Evidence suggests that EPCs play important roles in vasculogenesis and re-endothelialization after ischemic injury by migrating and adhering to the site of injury and differentiating into vascular endothelial cells (ECs) [2–3].
Soluble epoxide hydrolase inhibitors (sEHi) have multiple protective effects on the cardiovascular system [4–6] and are thought to act by blocking the conversion of epoxyeicosatrienoic acids (EETs) to their less active and sometimes inflammatory corresponding diols by soluble epoxide hydrolase (sEH) [7–8].0 EETs have multiple biological functions, such as causing marked vasodilation, inhibiting migration of vascular smooth muscle cells, decreasing inflammatory reactions, inhibiting platelet aggregation, promoting fibrolysis, and decreasing the expression of adherence factor [9–11]. In addition, EETs can modulate lipid metabolism and insulin resistance [12]. Most importantly, EETs have been suggested to contribute to neovascularization by promoting angiogenesis [13]. Inhibiting sEH can increase the beneficial effects of EETs in animal models of hypertension, atherosclerosis, ischemic heart disease, myocardial hypertrophy, heart failure, diabetes and metabolic syndrome [14–19]. Therefore, we tested the hypothesis that the sEHi, t-AUCB (trans-4-[4-(3-Adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid]), will improve the angiogenic function of EPCs from AMI patients in order to explore the potential effect of sEHi on treating acute myocardial infarction. We further hypothesized that the EETs–PPARγ pathway is responsible for the mechanism underlying the effect of t-AUCB on EPCs.
1. Materials and methods
1.1 Reagents
t-AUCB was synthesized as described [21]. EBM-2 (Lonza); Ficoll-1077, human fibronectin, and FITC-UEA-I (Sigma); DiI-acLDL (Molecular Probes); Matrigel-Matrix (BD Biosciences); polyclonal rabbit anti-VEGF, HIF-α (Abcam) were used without further purification.
1.2 Study subjects
Fifty hospitalized primary ST segment-elevated AMI patients (29 males and 21 females) ranging in age from 55 to 75 years old (average age 58.42 ± 5.37 years) and having a Killip grade 1 heart function were enrolled in the study, which occurred May 2009 to December 2011. Fifty age and sex matched healthy subjects were recruited as controls.
The diagnosis of AMI was defined as the presence of severe chest pain for at least 20 minutes and electrocardiographic signs for AMI, which included ST-segment elevation of at least 0.1 mV in two or more limb leads or at least 2 mV in two or more contiguous precordial leads on the surface electrocardiogram. AMI diagnosis also included markers of myocardial necrosis, such as Troponin T, being elevated and creatine kinase-MB isoenzyme levels >2 times the upper limit of normal. Characteristics of the study subjects are shown in Table S1 in the Supplemental Material.
Patients were excluded from the study if they were >75 years old and had a severe disease(s), including heart failure, acute liver disease or hepatic dysfunction, primary hypothyroidism, and nephrotic syndrome. Patients with a history of malignancy, cerebrovascular diseases or using prohibited medication or immunosuppressive drugs were also excluded.
The study conformed to the Declaration of Helsinki, and all the study protocols were approved by the Ethical Board of Central South University. All enrolled subjects were given fully informed consent prior to entry in the study.
After enrollment, patients underwent a routine physical examination and lipoproteins, fasting blood glucose (FBS), and liver and kidney functions were measured.
1.3 EPCs isolation culture and identification
EPCs were obtained as previously described [22]. Briefly, human blood was collected from AMI patients immediately after admission. All enrolled patients were hospitalized within 24 hours after a heart attack. Blood from healthy subjects was taken as the control. Human peripheral mononuclear cells (PBMC) were isolated by subsequent purification over Ficoll gradients and cultured at a density of 5×105/cm in endothelial cell basal medium-2( EBM-2)medium with 10% FBS supplemented with vascular endothelial growth factor (VEGF), epidermal growth factor, fibroblast growth factor, insulin-like growth factor, ascorbic acid, hydrocortisone, heparin and antibiotics, according to the manufacturer’s instructions (Singlequots, Lonza). Cells were plated on human fibronectin coated culture dishes. After 4 days of culture, non-adherent cells were removed. Adherent cells were cultured for another 3 days.
We were limited to 20 ml of blood per blood draw. For this reason it was necessary to pool PBMC from 4–5 patients’ blood to culture. We isolated EPCs from the pooled sample ten different times from the 50 AMI patients and repeated the same process for the controls to get ten independent cultures of EPCs per group.
To test whether or not these adherent cells were EPCs, positive double staining for both Dil-labeled acetylated low density lipoprotein (Dil-acLDL) (10µg/ml, Sigma) and FITC-labeled Ulex europaeus agglutinin I (FITC-UEA-I) (10µg/ml , Sigma) was visualized by fluorescence microscopy [23]. EPC expression of antigen CD34, CD133, CD31, and VEGFR2 were detected by flow cytometry [24–25]. Isotype controls were used as negative controls.
1.4 Method of challenging the EPCs
After starving EPCs from AMI patients for 24 hours, 0, 10−6,10−5,10−4 mol/L of t-AUCB were added to the cells for 24 hours. EPCs from healthy subjects were cultured in the same manner.
To further evaluate the functional role of the PPARγ pathway on the effects of t-AUCB on EPCs, we used the antagonist of PPARγ, GW9662. EPCs were preincubated with GW9662 (5 µmol/L) half an hour before 10−4 mol/L t-AUCB was added [26].
1.5 Migration assay
EPCs (5 × 104) were transferred into the upper chambers of a modified Boyden’s chamber (transwell, Millipore) filled with EBM-2 media, as described previously [27]. The lower chambers were loaded with phenol red free EBM alone or a different concentration of t-AUCB with or without 5 µmol/L of GW9662. EPCs were allowed to migrate for 10 hours, after which non-migrated EPCs were removed. Cells were fixed by paraformaldehyde and then stained with hematoxylin and eosin (H&E). The number of cells on the lower side of the membrane was counted at magnification ×200, and the mean value of ten different areas was determined for each sample.
1.6 In Vitro Angiogenesis assay
Briefly, EPCs were trypsinized and resuspended in medium to inactivate trypsin. After centrifugation, the medium was removed and the cells were resuspended in plain EBM-2. Reconstituted Matrigel-Matrix (BD Biosciences) was placed in a 48-well cell culture plate, and 5×104 EPCs were plated in each well with phenol red-free EBM-2 and various stimuli. After 48 hours of incubation, images of tube morphology were taken with an inverted microscope (Nikon). Capillary tube lengths were measured in 10 random low power fields ( ×100 ) per sample by investigators who were unaware of the study’s purpose. A capillary tube was defined as a tubular structure whose length was four times its width [28].
1.7 Real-Time PCR Analysis
Total RNA from 1×106 cells was harvested by TRIZOL (Invitrogen) according to the manufacturer’s instructions. The extracted RNA was dissolved in a final volume of 25 µL RNase free water, and concentrations of the total RNA were tested using a spectrophotometer. First-stranded cDNA was synthesized from 1µg total RNA (Fermentas Life Science). Real-time PCR was performed with the SYBR Premix Ex TaqII Kit (TaKaRa). The primers were: VEGF F: 5′-GCTACTGCCGTCCGATTGA-3’, R:5’-TGCTGGCTTTGGTGAGGTT-3’; HIF-α:F:5′-CTCGGCGAAGCAAAGAG-3’, R:5’-GCCATCTAGGGC TTTCAG-3’; GAPDH: F:5′-TGACCGGGTCACCCACACTGTGCCCATCTA-3′, R:5′-CTAGAAGCATTTGCG GTGGACGATGGAGGG-3′. PCR was performed in triplicate using a real time PCR machine (Applied Bioscience). The mRNA levels were estimated from the value of the threshold cycle (Ct) of the real-time PCR adjusted by that of GAPDH through the formula 2ΔCt (ΔCt=GAPDH Ct gene of interest Ct).
1.8 Western Blot
Protein samples were separated from the total cell lyates by SDS-PAGE and transferred to PVDF membranes (Amersham Biosciences, NJ). After incubating with primary and secondary antibodies, the immunoblots were exposed to Hyperfilm-ECL (Amersham-Pharmacia-Biotech, NJ) films and analyzed using an imaging system (Alpha Innotech, CA) to obtain densitometric values. β-actin was utilized as the internal control.
1.9 EETs measurement
Culture supernatants were collected, and the anti oxidant reagent triphenylphosphine (TPP) was added to the supernatant. Aliquots were kept at −80°C until analysis. The concentration of EETs was measured by the 14, 15-EETs/DHET Elisa kit (Detroit R&D) according to the protocol provided in the kit.
1.10 Statistical Analysis
All experiments were performed at least ten times in duplicate. Each experiment was considered to be independent. Results are expressed in mean ± SEMs. Comparison of continuous variables was performed by paired student’s t test. Differences in selected categorical variables between the respective comparison groups were analyzed with the χ2test of statistical significance. Comparison between the in vitro experimental groups was performed by using one way ANOVA. LSD and S-N-K post-hoc tests were used for comparison. A value of P<0.05 (2-sided) was considered statistically significant.
2 Results
2.1 Baseline Clinical Characteristics of the Patients
The baseline characteristics of the study population are summarized in Table S1 in supplemental material. Both AMI and healthy control groups were similar with regard to age, gender and body mass index. Furthermore, there were no significant differences in liver and kidney functions at baseline between the two groups (all p>0.05). The total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) in AMI patients was slightly higher than those in the control group, however there was no statistically difference between those two groups (all p>0.05). However, the FBS and white blood cell (WBC) count were substantially higher in the AMI than in control (p<0.05). The percentage of the smoker, diabetes and family history of coronary heart disease in AMI patients were high.
2.2 Culture results of EPCs from peripheral blood
Outgrowth colonies of adherent cells were found 2 to 3 days after plating (Figure S1a in supplement). After 5–6 days of culture, EPCs were differentiated into an adherent population characterized by a central cluster of round cells with many sprouts of elongated cells at the periphery defined as a colony-forming unit–EPC (Figure S1b–c in supplement). These colonies proliferated rapidly and exhibited spindle-shaped or cobblestone morphology. After 10–14 days, the cells formed a monolayer of spindle-shaped flat cells (Figure S1d in supplement).
2.3 Characterization of human EPCs
EPCs incorporated acLDL and stained positive for Dil-acLDL (red color, Figure S2a in supplement). EPCs also were stained positive for FITC-labeled lectin (green color)(Figure S2b in supplement.) Most of the adherent cells (94% of all cells) were double stained positive (merged in yellow, Figure S2c in supplement). Flow cytometry quantified the expression CD34, CD133, CD31 and VEGFR2 on the surface of cells during their differentiation. As shown in Figure. S3a–b in supplement, after 7–8 days of culture, the EPCs cell-specific antigens CD34, CD133 were highly expressed (73.61 ±4.17)%, (70.53±5.29) respectively, antigens CD31, VEGFR2 were also detected as (65.31 ±2.74)% and (81.15±5.24)% respectively (Figure S3 c-d in supplement), which confirmed that those cells had an endothelial lineage. Based on those basic characterizations, we used EPCs after 7–8 days of culture.
2.4 Effects of t-AUCB on EPCs migration from AMI patients
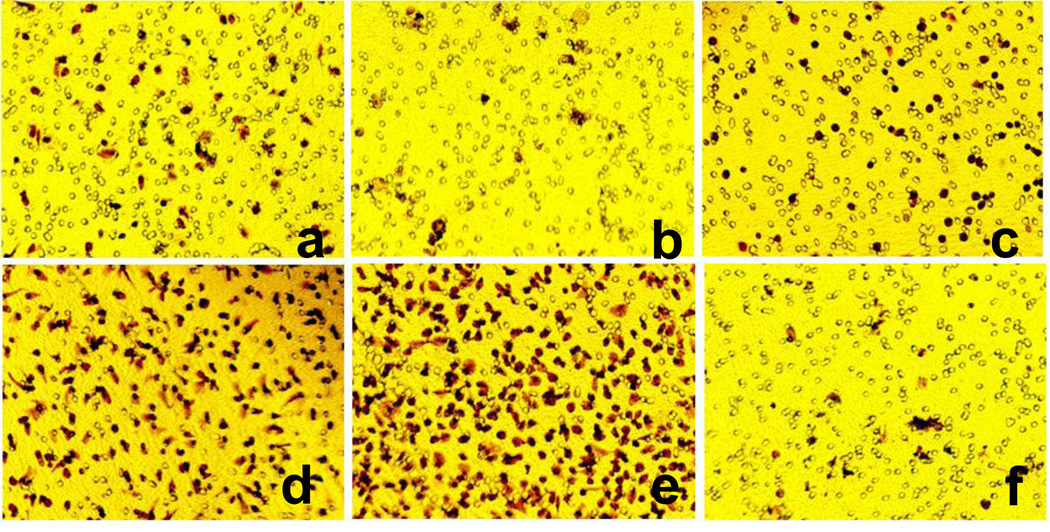
The first, critical step of the revascularization process is migration of EPCs to the site of injury. Therefore, the effect of t-AUCB on the migration activity of EPCs was assessed using a modified Boyden chamber apparatus in the presence or absence of t-AUCB. As shown in Figure 1b, the migration ability of EPCs from AMI patients without t-AUCB treatment (0 mol/L t-AUCB group) was markedly decreased as compared to those from healthy control (p<0.05) (Figure 1a). t-AUCB augmented the migratory activity of EPCs in a dose dependant manner , 10−6, 10−5, 10−4 mol/L of t-AUCB increased the migration activity of EPCs with statistical significance compared to the 0 mol/L t-AUCB group (p<0.05) (Figure 1b–e) (table 1). To further investigate the involvement of PPARγ pathway in the effects of t-AUCB on EPCs, we pretreated cells with PPARγ antagonist GW9662 (5µmol/L) before stimulation with 10−4 mol/L t-AUCB. As shown in Figure 1f, pretreatment with GW9662 markedly attenuated the migratory ability of EPCs from AMI patients responding to t-AUCB, (p<0.05) (Figure 1f) (table 1).
Figure 1. Effects of t-AUCB on the migratory activity of EPCs from AMI patients.
5 × 104 cells were kept into upper chambers of modified Boyden’s chamber with EBM-2 media. The lower chambers were loaded with phenol red free EBM alone or different concentration of t-AUCB with or without 5µmol/L of GW9662. EPCs were allowed to migrate for 10 h. a: migaration of EPCs from healthy control, b-e: migaration of EPCs from AMI patients by adding 0, 10−6, 10−5, 10−4 mol/L of t-AUCB to the lower chambers of transwell; f: migaration of EPCs from AMI patients by adding 10−4 mol/L t-AUCB+ 5µmol/L GW9662 to the lower chambers of transwell. Images are shown at ×300 magnification. The number of migrated EPCs per 10 microscopic fields (×200 magnification) was counted, and results are expressed as the mean ± SEM (n=10).
Table 1.
Effects of t-AUCB on the activity of migration and in vitro tube formation of EPCs from AMI patients (mean ± SEM)(n=10)
| t-AUCB mol/L | EPCs migration (cells/ χ200 fields) |
Length of tube formation ( mm/high power field) |
|
|---|---|---|---|
| Control | 90.36±1.95 | 21.8±0.85 | |
| AMI | 0 | 50.29 ±3.24* | 8.63±0.19* |
| 10−6 | 81.41±5.37Δ | 15.39±1.86Δ | |
| 10−5 | 138.5±79.14ΔΔ | 46.54±4.12ΔΔ | |
| 10−4 | 281.16±8.36 ΔΔ | 53.42±4.81ΔΔ | |
| 10−4+GW 9662 | 55.36±7.84 | 11.39±6.23 |
Compared with healthy control , p<0.05 ;
compared with AMI treated by 0 mol/L t-AUCB
p<0.05,
p<0.01
2.5 Effects of t-AUCB on In Vitro angiogenesis of EPCs from AMI patients
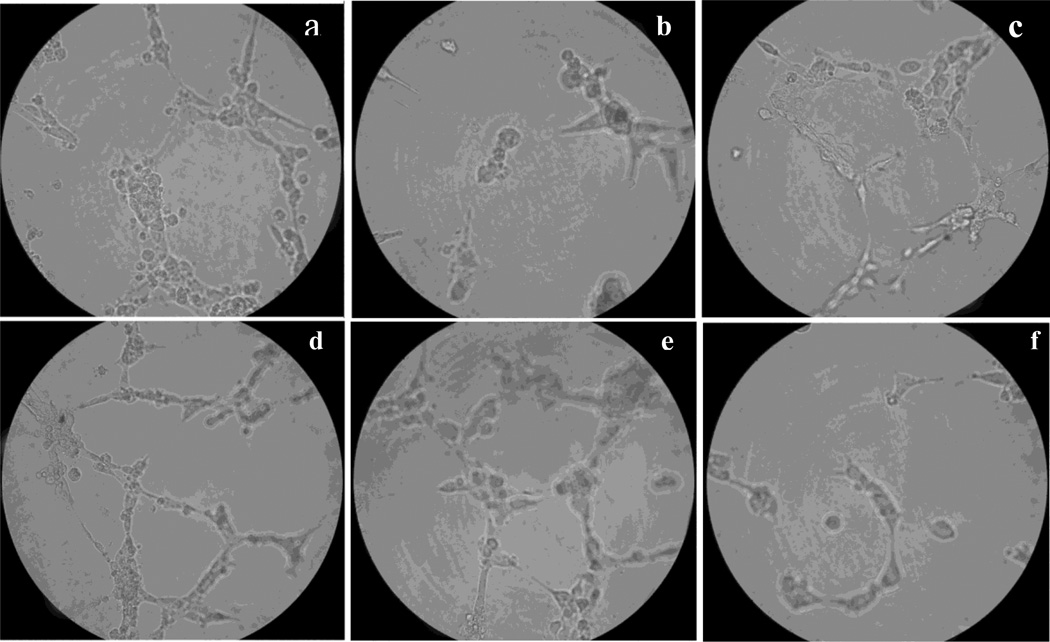
EPCs can differentiate into mature endothelial cells in situ and form capillary-like tubes. This process requires several biological activities, such as endothelial cell proliferation, cell migration, protease secretion and cell-to-cell interaction. The effect of t-AUCB on the in vitro angiogenesis activity of EPCs, namely in vitro capillary tube formation, was evaluated. Compared with those from healthy control (Figure 2a), the EPCs capillary tube formation of from AMI patients (0mol/L t-AUCB group) were markedly reduced compared to control patients (p<0.05, Figure 2b), whereas t-AUCB increased the activity of in vitro capillary tube formation in a dose dependent manner. 10−6, 10−5, 10−4 mol/L of t-AUCB significantly increased the activity of in vitro capillary tube formation of EPCs (Figure 2, b-e, p<0.05) (table 1), but tube formation was severely impaired by adding GW9662 with t-AUCB (p<0.05) (Figure 2f) (table 1).
Figure 2. Effects of t-AUCB on the activity of in vitro capillary tube formation of EPCs from AMI patients.
EPCs were trypsinized and resuspended in plain EBM-2. Reconstituted Matrigel-Matrix (BD Biosciences) was placed in a 48-well cell culture plate and 5×104 EPCs were plated in each well with phenol red-free EBM-2 and various stimuli. a: in vitro tube formation of EPCs from healthy control, b-e: in vitro tube formation of EPCs from AMI patients by adding 0,10−6,10−5,10−4 mol/L of t-AUCB to the cells. f: in vitro tube formation of EPCs from AMI patients by adding 10−4 mol/L t-AUCB+ 5µmol/L GW9662. Images are shown at ×300 magnification. Tube lengths were measured at random 10 high power fields per sample and results are expressed as the mean ± SEM. (n=10).
2.6 Effects of t-AUCB on the mRNA Expression of VEGF, HIF-1α
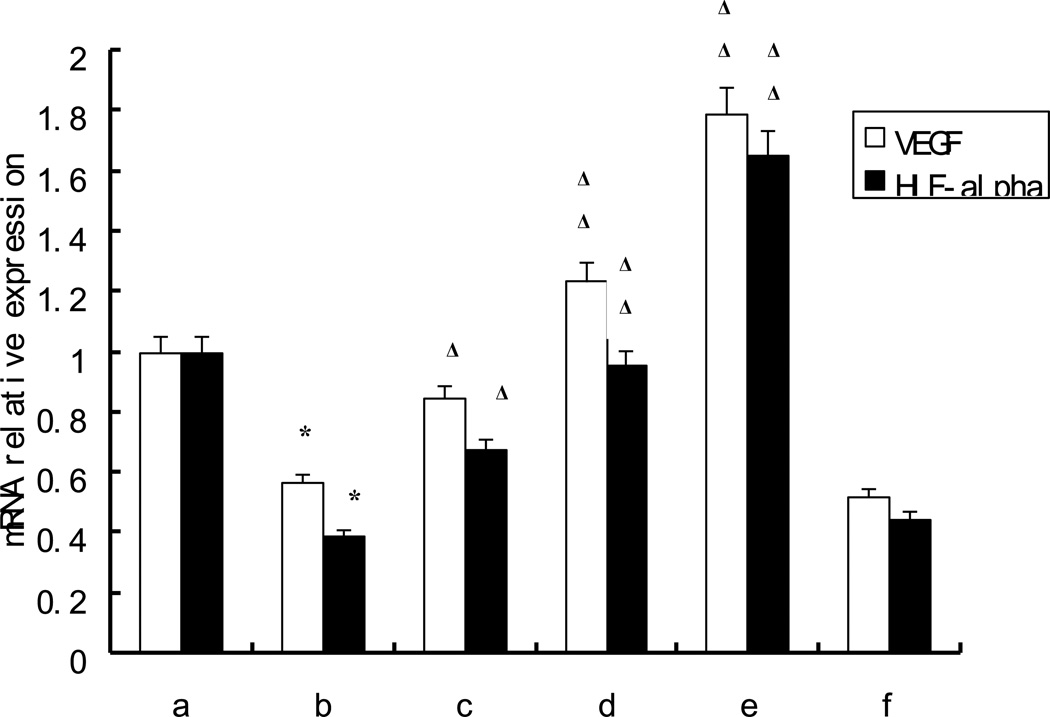
As shown in Figure 3, compared with those from healthy control, the mRNA expression of vascular endothelial growth factor (VEGF), hypoxia-inducible factor 1α (HIF- 1α) were decreased (p<0.05) in ischemic patients, whereas they were increased by t-AUCB in a dose dependent manner, 10−6, 10−5, 10−4 mol/L of t-AUCB increased the mRNA of VEGF, HIF-1α of EPCs from AMI patients with statistical significance (p<0.05). However, the effects of t-AUCB on the mRNA expression of VEGF and HIF-1α were significantly attenuated by adding GW9662 (p<0.05, Figure 3).
Figure 3. Effects of t-AUCB on the mRNA expression of VEGF, HIF-1α.
EPCs were plated on fibronectin-coated 6-well plates, cultured until 90% confluent and then were pretreated with or without GW9662 (5 µmol/L) for 0.5h followed by the addition of 0,10−6,10−5,10−4 mol/L t-AUCB for 24 h. Results are shown as mean ± SEM. (n=10). a: healthy control, b-e: EPCs from AMI patients by adding 0,10−6,10−5,10−4 mol/L of t-AUCB to the cells. f: EPCs from AMI patients by adding 10−4 mol/L t-AUCB+ 5µmol/L GW9662. Compared with healthy control:* p<0.05 , compared with AMI (b) Δ p<0.05, ΔΔ p< 0.01.
2.7 Effects of t-AUCB on the protein expression of VEGF, HIF-1α
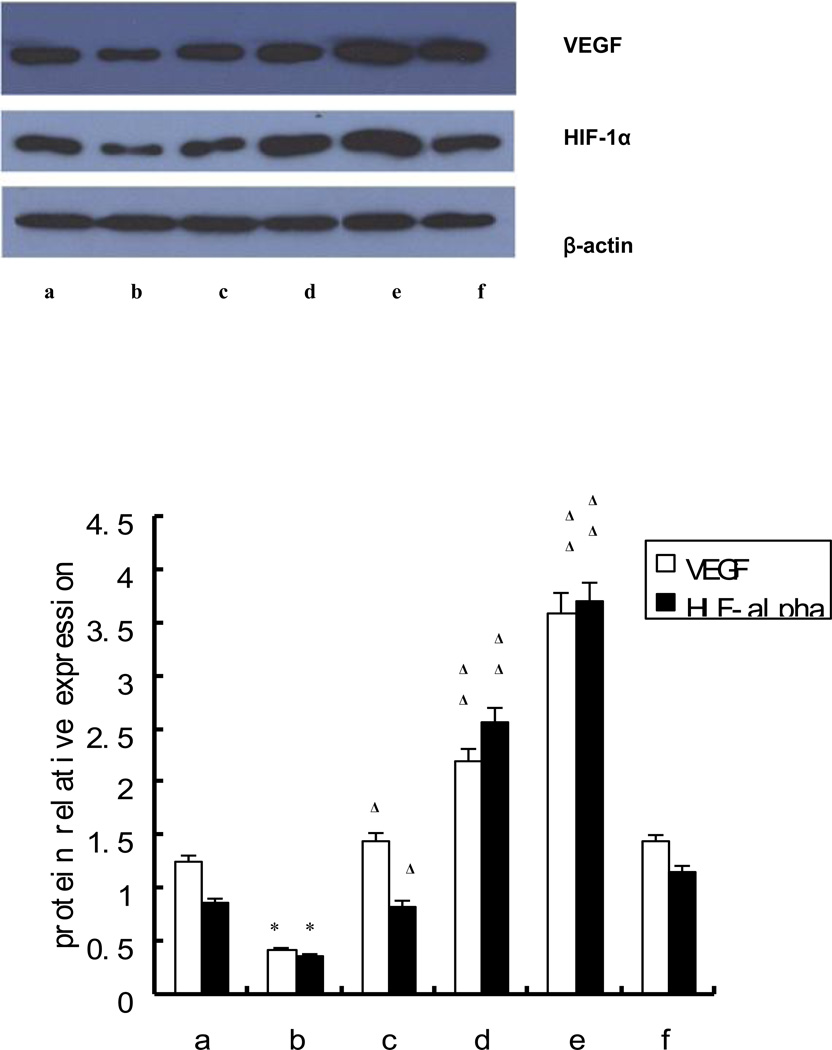
Consistent with the levels of mRNA expression, compared with those from healthy controls (Figure 4 column a), the protein expression of VEGF, and HIF-1α were attenuated in EPCs from AMI patients without t-AUCB treatment (p<0.05) (Figure 4 column b), whereas increased with t-AUCB treatment in a dose dependent manner (Figure 4 column b-e). 10−6, 10−5, 10−4 mol/L of t-AUCB increased the protein expression of VEGF and HIF-1α of EPCs with statistical difference (p<0.05) (Figure 4, Lower figure). However, as shown in Figure 4 column f, pre-treating EPCs with GW 9662 reduced the VEGF, HIF-1α protein expression.
Figure 4. Effects of t-AUCB on the protein expression of VEGF, HIF-1α.
EPCs were plated on fibronectin-coated 6-well plates, cultured until 90% confluent and then were pretreated with or without GW9662 (5 µmol/L) for 0.5 hr followed by the addition of 0,10−6,10−5,10−4 mol/L t-AUCB for 24 hr. Whole cell lysate were isolated for detecting VEGF, HIF-1α protein expression, whereas nuclear lysate were used to detect PPARγ protein expression. Results are shown as mean ± SEM. (n=10). Upper figure: image of western blot; a: control, b-e: 0, 10−6, 10−5, 10−4 mol/L t-AUCB, f: 10−4 mol/L t-AUCB+ 5µmol/L GW9662. Lower figure: relative expression level of each protein after normalized to β-actin. Compared with healthy control:* p<0.05; compared with AMI(b)Δ p<0.05, ΔΔ p<0.01.
2.8 Measurement of EETs concentration
The EETs concentration in the cell culture supernatant of EPCs from AMI patients without t-AUCB treated (0 mol/L t-AUCB group) were statistically lower than that of healthy control (p<0.05) (table 2), but the concentration of EETs were statistically increased in a dose dependent manner after treated by 10−6, 10−5, 10−4 mol/L of t-AUCB (p<0.05), whereas pretreating EPCs with 10−4 mol/L of t-AUCB by adding GW9662 reduced the EETs concentration (table 2).
Table 2.
EETs concentration in the EPCs from AMI patients (mean ± SEM) (n=10)
| t-AUCB mol/L | EETs concentration (ng/ml) | |
|---|---|---|
| Control | 9.01 ± 3.06 | |
| AMI | 0 | 8.07 ± 2.60* |
| 10−6 | 9.14± 1.07Δ | |
| 10−5 | 11.37±2.44ΔΔ | |
| 10−4 | 15.49± 4.19ΔΔ | |
| 10−4+GW 9662 | 8.34±0.53 |
Compared with healthy control ,p<0.05, :
compared with AMI treated by 0 mol/L t-AUCB
p<0.05,
p<0.01
3 Discussion
These results demonstrate a potential role of the sEH inhibitor t-AUCB in promoting angiogenesis and migration of EPCs. The present findings also show that t-AUCB can increase the expression of angiogenic factors VEGF and HIF-1α and that these effects appear to be PPARγ dependent.
Acute myocardial infarction (AMI) is an acute ischemic event; therefore, restoring coronary blood circulation by stimulating new blood vessel formation is of primary importance [29]. Circulating bone marrow derived cells, defined as early endothelial progenitor cells (EPCs), can proliferate multiple times and differentiate into endothelial cells [30–31]. Recent studies have shown that mobilization and differentiation of EPCs play an important role in augmenting neovascularization and endothelial replacement in the ischemic and injured heart [32–34]. Increased numbers and mobility of circulating EPCs have been reported after ischemia, vascular injury, and in the early phase after AMI [35–39]. In contrast, other studies have demonstrated that the mean circulating level and migratory activity of EPCs is notably lower in AMI patients than in normal control subjects [40–43].
In the present study, we found that the migratory and angiogenic properties of EPCs from AMI patients were markedly attenuated as compared with healthy controls. A reasonable explanation for this result is that the patients in the present study had more risk factors compared to the controls, such as smoking and hypertension (Table S1 in Supplement Material), the number and migratory activity of circulating EPCs were inversely correlated with the number of risk factors for coronary artery disease [42]. Another reason could be that the blood samples were drawn before early revascularization caused by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) were used, which have positive effects on EPCs [44–45]. Although those AMI patients who had high risk factors in the present study routinely used anti-hypertension drugs such as angiotensin converting enzyme inhibitors (ACEI)/ angiotensin receptor inhibitors(ARB), calcium channel blockers (CCB) and hypoglycemic agents or insulin were, which was reported had positive effects on the function of EPCs [46–48], the results of the present study still showed ability of EPCs in AMI patients were markedly attenuated which suggested the ability of EPCs in AMI patients was ineffective and insufficient to match the demanding of the neovascularization of the ischemic issues after injury.
EPCs augment angiogenesis not only by providing a source of progenitor cells that can differentiate into mature vascular endothelial cells, but also by stimulating the secretion of angiogenic growth factors at the site of incorporation, which enhance growth and differentiation of EPCs in an autocrine manner [49–51]. VEGF and HIF-1α are two of the most powerful angiogenic factors for blood vessels [52]. VEGF is crucial for preservation of the microvasculature [53], whereas HIF-1α is the most important transcription factor driving VEGF mRNA expression and production [54–55]. In this regard, increasing VEGF and HIF-1α in EPCs has the potential to be beneficial to AMI patients. The present study showed the VEGF and HIF-1α mRNA and protein expressions in AMI patients EPCs were much lower than in the healthy controls, which might partly explain the inadequate angiogenic ability of EPCs in the AMI patients. Most importantly, our study showed that the mRNA and protein expression of VEGF and HIF-1α were dose dependently increased by t-AUCB and the migration and angiogenesis ability of EPCs were markedly increased at the same time. These results suggest that t-AUCB positively modulates the functions of EPCs in AMI patients, showing its possible pivotal role in therapeutic vasculogenesis.
EETs are endogenous lipid epoxides and act as lipid mediators of many important biological functions in the cardiovascular system [56–57]. They are catalytically produced from arachidonic acid by epoxygenase or CYP enzymes [58]. Conversion of EETs to their corresponding diols (dihydroxyeicosatrienoic acids, DHETs) by sEH is responsible for decreasing EETs levels and diminishing EETs beneficial cardiovascular properties. Accordingly, we evaluated the effects of sEH inhibitor t-AUCB on EETs concentrations. EETs were found to be dose dependently increased by t-AUCB, which indicated that the effect of t-AUCB on EPCs is through the effective inhibition of sEH.
EETs have been shown to increase VEGF and have angiogenic properties [59], and they are endogenous activators and ligands of PPARγ [26]. Therefore, we hypothesized that the EETs – PPARγ pathway is responsible for the mechanism underlying the effect of t- AUCB on EPCs. First, we found that VEGF and HIF-1α expression were dose dependently increased by t-AUCB. However, GW9662, an antagonist of PPARγ ligands, abolished t-AUCB induced expression of VEGF and HIF-1α. Moreover, GW9662 markedly attenuated the concentration of EETs induced by t-AUCB. Thus, these results indicate that the angiogenic effects of the sEH inhibitor are facilitated by activating the EETs- PPARγ pathway, which in turn increases VEGF and HIF-1α and triggers EPC migration and proliferation, eventually leading to angiogenesis. Therefore, the study not only shows the mechanism of sEHi angiogenesis, but also suggests that endogenous angiogenic EETs act in a PPARγ dependent fashion.
This study test the effects of sEH inhibition in EPCs isolated from AMI patients. The in vitro nature of the study presents several limitations. Whether the sEH inhibitor will be able to increase EETs to an effective dose to increase EPC angiogenic properties and whether that will translate in improved outcomes in AMI patients remains unknown. Additionally, it must be noted that the enrolled number of patients in the study was relatively small and the patients had high cardiovascular risk factors, which might cause bias.
Future work is needed to address these limitations and translate sEH inhibitor treatment to AMI patients. First, it must be demonstrated that sEHi has positive effects on EPCs in vivo. Currently, we are investigating this in a mouse model of AMI. Second, the effect of sEH inhibitor treated EPCs on the revascularization of coronary vessel in AMI patients needs to be observed. Third, the effect of sEH inhibitor on the numbers and activities of EPCs should be confirmed following sEH inhibitor treatment by AMI patients. Lastly, the usage of sEH inhibitor on preventing AMI occurrence or reoccurrence should be explored.
Conclusions
In conclusion, sEH inhibitors have great potential to treat cardiovascular diseases, as advocated in Nature Reviews Drug Discovery by Dr. Imig [60] and the translation of sEH inhibitor treatment to the clinical setting has begun with phase II trials to target hypertension and type 2-diabetes [61]. The findings of this study open up new avenues of exploration for the use of sEH inhibitors as a novel therapy to stimulate EPC dependent revascularization in AMI patients. Lastly, we provide evidence of the underlying mechanism of these effects and insight into the mechanism of an endogenous angiogenesis pathway.
Supplementary Material
Acknowledgement
The authors of this manuscript certify that they have complied with the Principles of Ethical Publishing in the International Journal of Cardiology [62].
Funding Sources:
This work was supported by the following funding sources: National Nature Scientific Funding of China (No. 30770856, 81170190), Program for New Century Excellent Talents in University (NCET-08-0566), Natural Science Foundation of Hunan Province (10JJ3026), Institute of Metabolism and Endocrine of Central South University (DY-2008-02-04) to D.X, Tobacco-Related Disease Research Program (18KT-0037) to B. D, and NIH R01 ES002710 and NIH R01 ES013933 to B.D.H, and NIH R01 HL85727, HL85844, and VA Merit Review to N.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
BDH, NC, and CM are at the University of California, which holds composition of matter and use patents regarding sEH and EET.
Reference
- 1.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 2.Hung HS, Shyu WC, Tsai CH, Hsu SH, Lin SZ, Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc Res. 2010;79(3):200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung HS, Shyu WC, Tsai CH, Hsu SH, Lin SZ. Transplantation of endothelial progenitor cells as therapeutics for cardiovascular diseases. Cell Transplant. 2009;18(9):1003–1012. doi: 10.3727/096368909X12483162196683. [DOI] [PubMed] [Google Scholar]
- 4.Ingraham RH, Gless RD, Lo HY. Soluble epoxide hydrolase inhibitors and their potential for treatment of multiple pathologic conditions. Curr Med Chem. 2011;18(4):587–603. doi: 10.2174/092986711794480212. [DOI] [PubMed] [Google Scholar]
- 5.Simpkins AN, Rudic RD, Roy S, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide hydrolase inhibition modulates vascular remodeling. Am J Physiol Heart Circ Physiol. 2010;298(3):H795–H806. doi: 10.1152/ajpheart.00543.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, Gless R, Webb HK, Wang YX. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol. 2009;29(9):1265–1270. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- 7.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43(1):55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 8.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem. 1993 Mar 25;268(9):6402–6407. [PubMed] [Google Scholar]
- 9.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292(3):C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 10.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44(1):1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Miller AW, Dimitropoulou C, Han G, White RE, Busija DW, Carrier GO. Epoxyeicosatrienoic acid-induced relaxation is impaired in insulin resistance. Am J Physiol Heart Circ Physiol. 2001;281:H1524–H1531. doi: 10.1152/ajpheart.2001.281.4.H1524. [DOI] [PubMed] [Google Scholar]
- 12.Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, Morisseau C, Wang CY, Inscho EW, Hammock BD, Wang MH. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther. 2010;334(2):430–438. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellien J, Joannides R, Richard V, Thuillez C. Modulation of cytochrome-derived epoxyeicosatrienoic acids pathway: a promising pharmacological approach to prevent endothelial dysfunction in cardiovascular diseases? Pharmacol Ther. 2011 Jul;131(1):1–17. doi: 10.1016/j.pharmthera.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50(3):225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 15.Oguro A, Fujita N, Imaoka S. Regulation of soluble epoxide hydrolase (sEH) in mice with diabetes: high glucose suppresses sEH expression. Drug Metab Pharmacokinet. 2009;24(5):438–445. doi: 10.2133/dmpk.24.438. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary KR, Abukhashim M, Hwang SH, Hammock BD, Seubert JM. Inhibition of soluble epoxide hydrolase by trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is protective against ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2010;55(1):67–73. doi: 10.1097/FJC.0b013e3181c37d69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu H, Li N, Liu JY, Harris TR, Hammock BD, Chiamvimonvat N. Soluble Epoxide Hydrolase Inhibitors and Heart Failure. Cardiovasc Ther. 2010;23 doi: 10.1111/j.1755-5922.2010.00150.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, Chiamvimonvat N. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103(49):18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Liu JY, Timofeyev V, Qiu H, Hwang SH, Tuteja D, Lu L, Yang J, Mochida H, Low R, Hammock BD, Chiamvimonvat N. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: Insight gained using metabolomic approaches. J Mol Cell Cardiol. 2009;47(6):835–845. doi: 10.1016/j.yjmcc.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50(16):3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeter MR, Leifheit M, Sudholt P, Heida NM, Dellas C, Rohm I, Alves F, Zientkowska M, Rafail S, Puls M, Hasenfuss G, Konstantinides S, Schäfer K. Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ Res. 2008;103(5):536–344. doi: 10.1161/CIRCRESAHA.107.169375. [DOI] [PubMed] [Google Scholar]
- 23.Qian C, Tio RA, Roks AJ, Boddeus KM, Harmsen MC, van Gilst WH, Schoemaker RG. A promising technique for transplantation of bone marrow-derived endothelial progenitor cells into rat heart. Cardiovasc Pathol. 2007 May-Jun;16(3):127–135. doi: 10.1016/j.carpath.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003 Jun;9(6):702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 25.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001 Aug;108(3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2005;102(46):16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, Aoki M, Kondo T, Kobayashi K, Okumura K, Komori K, Murohara T. Therapeutic angiogenesis with intramuscular injection of low-dose recombinant granulocyte-colony stimulating factor. Arterioscler Thromb Vasc Biol. 2005 Dec;25(12):2535–2541. doi: 10.1161/01.ATV.0000190609.28293.17. [DOI] [PubMed] [Google Scholar]
- 28.Cid MC, Hernández-Rodríguez J, Esteban MJ, Cebrián M, Gho YS, Font C, Urbano-Márquez A, Grau JM, Kleinman HK. Tissue and serum angiogenic activity is associated with low prevalence of ischemic complications in patients with giant-cell arteritis. Circulation. 2002 Sep 24;106(13):1664–1671. doi: 10.1161/01.cir.0000030185.67510.c0. [DOI] [PubMed] [Google Scholar]
- 29.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45(4):530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albiero M, Menegazzo L, Avogaro A, Fadini GP. Pharmacologic targeting of endothelial progenitor cells [J] Cardiovasc Hematol Disord Drug Targets. 2010;10(1):16–32. doi: 10.2174/187152910790780087. [DOI] [PubMed] [Google Scholar]
- 31.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005 Sep 8;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 32.Li Calzi S, Neu MB, Shaw LC, Kielczewski JL, Moldovan NI, Grant MB. EPCs and pathological angiogenesis: when good cells go bad. Microvasc Res. 2010;79(3):207–216. doi: 10.1016/j.mvr.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58(2):148–151. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78(3):413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 35.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L. Vascular trauma induces rapid but transient mobilization of VEGFR2+ AC331+ endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 37.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 38.Wojakowiski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslsnkiewicz K. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 39.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R. Increased circulating hematopoietic and endothelial progenitorcells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 40.Liguori A, Fiorito C, Balestrieri ML, Crimi E, Bruzzese G, Williams-Ignarro S, D'Amora M, Sommese L, Grimaldi V, Minucci PB, Giovane A, Farzati B, Ignarro LJ, Napoli C. Functional impairment of hematopoietic progenitor cells in patients with coronary heart disease. Eur J Haematol. 2008;80(3):258–264. doi: 10.1111/j.1600-0609.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 41.Briguori C, Testa U, Riccioni R, Colombo A, Petrucci E, Condorelli G, Mariani G, D'Andrea D, De Micco F, Rivera NV, Puca AA, Peschle C, Condorelli G. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. FASEB J. 2010;24(6):1981–1988. doi: 10.1096/fj.09-138198. [DOI] [PubMed] [Google Scholar]
- 42.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001 Jul 6;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 43.G Chang LT, Yuen CM, Sun CK, Wu CJ, Sheu JJ, Chua S, Yeh KH, Yang CH, Youssef AA, Yip HK. Role of stromal cell-derived factor-1alpha, level and value of circulating interleukin-10 and endothelial progenitor cells in patients with acute myocardial infarction undergoing primary coronary angioplasty. Circ J. 2009 Jun;73(6):1097–1104. doi: 10.1253/circj.cj-08-0497. [DOI] [PubMed] [Google Scholar]
- 44.Baran C, Durdu S, Dalva K, Zaim C, Dogan A, Ocakoglu G, Gürman G, Arslan O, Akar AR. Effects of Preoperative Short Term Use of Atorvastatin on Endothelial Progenitor Cells after Coronary Surgery: A Randomized, Controlled Trial. Stem Cell Rev. 2011 Nov 11; doi: 10.1007/s12015-011-9321-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Chang HW, Leu S, Sun CK, Hang CL, Youssef AA, Hsieh YK, Yang CH, Cheng CI, Chen SM, Chen CJ, Chua S, Chang LT, Wu CJ, Yip HK. Level and value of circulating endothelial progenitor cells in patients with acute myocardial infarction undergoing primary coronary angioplasty: in vivo and in vitro studies. Transl Res. 2010 Oct;156(4):251–263. doi: 10.1016/j.trsl.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Min TQ, Zhu CJ, Xiang WX, Hui ZJ, Peng SY. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004 May;18(3):203–209. doi: 10.1023/B:CARD.0000033641.33503.bd. [DOI] [PubMed] [Google Scholar]
- 47.Kito T, Shibata R, Kondo M, Yamamoto T, Suzuki H, Ishii M, Murohara T. Nifedipine Ameliorates Ischemia-Induced Revascularization in Diet-Induced Obese Mice. Am J Hypertens. 2012 Jan 5; doi: 10.1038/ajh.2011.239. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Dong L, Kang L, Ding L, Chen Q, Bai J, Gu R, Li L, Xu B. Insulin modulates ischemia-induced endothelial progenitor cell mobilization and neovascularization in diabetic mice. Microvasc Res. 2011 Nov;82(3):227–236. doi: 10.1016/j.mvr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Smythe J, Fox A, Fisher N, Frith E, Harris AL, Watt SM. Measuring angiogenic cytokines, circulating endothelial cells, and endothelial progenitor cells in peripheral blood and cord blood: VEGF and CXCL12 correlate with the number of circulating endothelial progenitor cells in peripheral blood. Tissue Eng Part C Methods. 2008;14(1):59–67. doi: 10.1089/tec.2007.0251. [DOI] [PubMed] [Google Scholar]
- 50.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005 Jul;46(1):7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 51.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004 Jun 8;109(22):2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 52.Mac Gabhann F, Qutub AA, Annex BH, Popel AS. Systems biology of pro-angiogenic therapies targeting the VEGF system. Wiley Interdiscip Rev Syst Biol Med. 2010 Nov-Dec;2(6):694–707. doi: 10.1002/wsbm.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu JX, Huang XF, Lv WM, Ye CS, Peng XZ, Zhang H, Xiao LB, Wang SM. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg. 2009;50(3):608–616. doi: 10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 54.Jiang M, Wang B, Wang C, He B, Fan H, Guo TB, Shao Q, Gao L, Liu Y. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem. 2008;103(1):321–334. doi: 10.1002/jcb.21416. [DOI] [PubMed] [Google Scholar]
- 55.Hoenig MR, Bianchi C, Sellke FW. Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis. Curr Drug Targets. 2008;9(5):422–435. doi: 10.2174/138945008784221215. [DOI] [PubMed] [Google Scholar]
- 56.Nithipatikom K, Gross GJ. Review article: epoxyeicosatrienoic acids: novel mediators of cardioprotection. J Cardiovasc Pharmacol Ther. 2010;15(2):112–119. doi: 10.1177/1074248409358408. [DOI] [PubMed] [Google Scholar]
- 57.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292(3):C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 58.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webler AC, Michaelis UR, Popp R, Barbosa-Sicard E, Murugan A, Falck JR, Fisslthaler B, Fleming I. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol. 2008;295(5):C1292–C1301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen D, Whitcomb R, Macintyre E, Tran V, Do ZN, Sabry J, Patel DV, Anandan SK, Gless R, Webb HK. Pharmacokinetics and Pharmacodynamics of AR9281, an Inhibitor of Soluble Epoxide Hydrolase, in Single- and Multiple-Dose Studies in Healthy Human Subjects. J Clin Pharmacol. 2011 Mar 21; doi: 10.1177/0091270010397049. Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.