Abstract
African trypanosomes are devastating human and animal pathogens. Trypanosoma brucei rhodesiense and T. b. gambiense subspecies cause the fatal human disease known as African sleeping sickness. It is estimated that several hundred thousand new infections occur annually and the disease is fatal if untreated. T. brucei is transmitted by the tsetse fly and alternates between bloodstream-form and insect-form life cycle stages that are adapted to survive in the mammalian host and the insect vector, respectively. The importance of the flagellum for parasite motility and attachment to the tsetse fly salivary gland epithelium has been appreciated for many years. Recent studies have revealed both conserved and novel features of T. brucei flagellum structure and composition, as well as surprising new functions that are outlined here. These discoveries are important from the standpoint of understanding trypanosome biology and identifying novel drug targets, as well as for advancing our understanding of fundamental aspects of eukaryotic flagellum structure and function.
Keywords: flagellum, cilium, motility, axoneme, trypanosome, dynein, cytokinesis
INTRODUCTION
Trypanosoma brucei and related subspecies are protozoan parasites that cause African trypanosomiasis in humans and animals, a deadly disease with devastating health and economic consequences (72). The T. brucei flagellum and flagellar motility are central to disease pathogenesis in the mammalian host and parasite development in the tsetse fly vector. Recent functional studies have revealed that the T. brucei flagellum is an essential and multifunctional organelle with critical roles in motility, cellular morphogenesis, cell division, immune evasion, and potentially, sensory perception. Concurrently, genomic and proteomic studies have significantly expanded the inventory of known trypanosome flagellar proteins. Because the flagellum is an essential organelle, understanding unique aspects of the T. brucei flagellum may uncover novel drug targets. In addition, T. brucei is emerging as a powerful experimental system for studies of conserved aspects of flagellum/cilium biology (flagellum and cilium are interchangeable terms for the same organelle), with direct relevance to other eukaryotes, including humans, in which flagellum/cilium defects underlie many heritable, fatal, and debilitating diseases.
FLAGELLUM STRUCTURE AND COMPOSITION
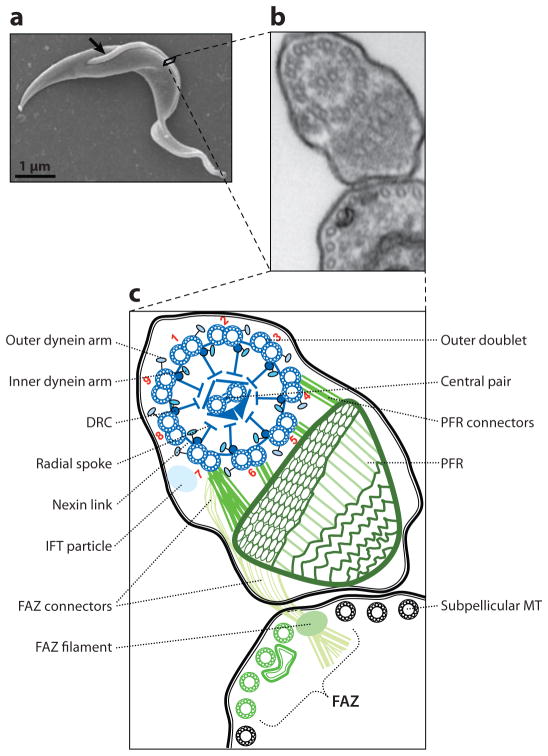
The T. brucei cell body has a vermiform shape with tapered ends. A single flagellum emerges from the basal body near the posterior end of the cell (Figure 1). The flagellum is surrounded by its own membrane and is attached along its length to the cell body, tracing a left-handed helical path from posterior to anterior, with the distal end extending a short distance beyond the cell body. A specialized membrane domain termed the flagellar pocket forms from an invagination of the cell membrane where the proximal end of the flagellum emerges from the basal body in the cytoplasm (Figure 2). Along the length of the cell, the flagellum and cell body are held in close apposition by a network of cytoskeletal and membranous connections that collectively make up the flagellum attachment zone (FAZ) (65, 145, 146). Within the flagellum are a canonical 9 + 2 microtubule axoneme and a lattice-like paraflagellar rod (PFR).
Figure 1.
The trypanosome flagellum. (a) Scanning electron micrograph (EM) of a procyclic trypanosome. The arrow indicates the single flagellum. (b) Transmission EM showing a cross-section of the flagellum and its attachment to the cell body as viewed looking from posterior toward anterior. (c) Schematic representation of the micrograph in panel b. Major flagellar substructures are indicated and outer doublet microtubules are numbered according to convention. Flagellar substructures that are broadly conserved among eukaryotes are in blue, and structures that are unique to trypanosomes and closely related organisms are in green. Abbreviations: DRC, dynein regulatory complex; FAZ, flagellum attachment zone; IFT, intraflagellar transport; MT, microtubule; PFR, paraflagellar rod. Adapted from Reference 113, with permission.
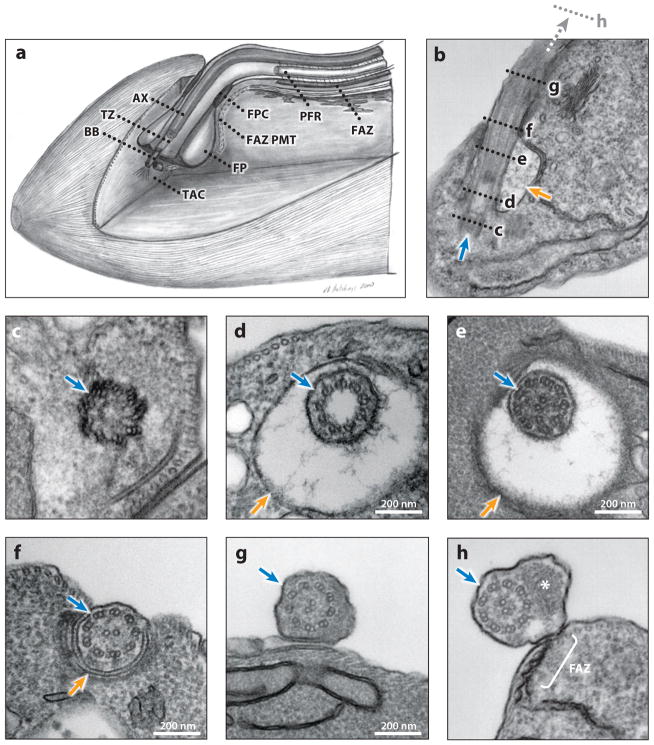
Figure 2.
Ultrastructure of the trypanosome flagellum. (a) Schematic cut-out view of the flagellar pocket area. Note that because the mitochondrion is not shown, only a partial TAC, consisting of the exclusion zone filaments extending from the basal body toward the mitochondrion, is represented in this schematic. (b–h) Electron micrographs of longitudinal (b) and transverse (c–h) sections of procyclic cells, from posterior (c) to anterior (h). (b) Longitudinal section showing the flagellar apparatus (blue arrow) and FP (orange arrow). The positions of the transverse sections in panels c–h are indicated with dashed lines, with panel h farther anterior than panel g. (c) The basal body apparatus. (d) The 9 + 0 transition zone within the FP; note the filaments of the ciliary necklace radiating from the doublet microtubules. (e) The 9 + 2 flagellar axoneme within the FP. (f) The flagellum exiting the FP through the FPC. (g) The flagellum outside of the FP but not yet having a PFR. (h) The flagellum outside of the FP, containing both an axoneme and a PFR (asterisk). Note that FAZ connections to the axoneme initiate proximal to the initiation of the PFR (not shown). Panel a is adapted from Reference 55, with permission. Abbreviations: AX, 9 + 2 axoneme; BB, basal body; FAZ, flagellum attachment zone; FAZ PMT, the four specialized subpellicular microtubules of the flagellum attachment zone; FP, flagellar pocket; FPC, flagellar pocket collar; PFR, paraflagellar rod; TAC, tripartite attachment complex; TZ, transition zone.
Basal Body
The basal body (Figure 2c) is a barrel-shaped structure comprising nine triplet microtubules analogous to the mammalian centriole. T. brucei cells in G1 have one mature basal body that nucleates the axoneme and one adjacent probasal body, positioned orthogonally to the mature basal body. At the G1/S boundary the probasal body matures, repositions to dock with the cell membrane, and extends to initiate assembly of a new flagellum while a new probasal body forms next to each mature basal body (129, 141). Basal body duplication is one of the earliest events in the cell cycle (129) and may function as a cell cycle checkpoint. Triplet microtubules of the mature basal body extend to become doublets, forming a 9 + 0 transition zone between the basal body and the basal plate (Figure 2d) that demarcates the beginning of the 9 + 2 axoneme (Figure 2e). In the transition zone, filaments connect microtubule doublets to the flagellar membrane, forming a ciliary necklace, first described by Gilula & Satir (45) in lamellibranch gill cilia and postulated to function as a filter to selectively control protein entry into flagellum (28, 124, 132).
The molecular composition of the T. brucei basal body has not been defined, but proteins identified in proteomic analyses of basal bodies from other organisms (62, 63) are encoded in the T. brucei genome and are present in the T. brucei extracted flagellar proteome, which includes the basal bodies (17). Several proteins have been experimentally localized to the basal body, including γ-tubulin and centrin (29, 79, 126, 154), the polycystic kidney disease protein seahorse/TbLRTP (84), and the NIMA-related kinase TbNRKC (109). The last two proteins function in basal body duplication, as RNA interference (RNAi) knockdown results in supranumerary basal bodies.
The tubulin-cofactor C (TBCC) domain-containing protein TbRP2 colocalizes with tyrosinated α-tubulin specifically at the mature basal body (132). Tyrosinated α-tubulin is associated with transitional fibers that radiate outward from the distal end of the mature basal body, delineating the boundary between the flagellum and cytoplasm and providing a docking site for proteins entering the flagellum (28, 132). TBCC proteins function in tubulin folding and assembly, leading to the idea that the basal bodies provide a quality-control checkpoint for tubulin and other proteins entering the flagellum. Supporting this hypothesis, knockdown of TbRP2 leads to shortened flagella and axonemal microtubule defects (132).
In mammalian cells the basal bodies/centrioles serve dual roles: They nucleate axonemal microtubules and the mitotic spindle. T. brucei undergoes a closed mitosis and basal bodies are not utilized for spindle formation (95). The basal bodies do, however, provide the unusual function of segregating the single-copy mitochondrial genome, the kinetoplast (119). The kinetoplast comprises a massive network of catenated DNA circles, kDNA, including protein coding genes whose mRNAs are posttranscriptionally edited, and guide RNA genes that guide the editing process (76). Hence, the kinetoplast is a complex genome that must be faithfully segregated during each round of cell division. A role for basal body migration in kDNA segregation was first revealed in pharmacological studies, in which inhibitors of basal body migration also blocked DNA segregation (119). Later work demonstrated that this is due to a network of filaments that extend from the proximal side of the basal body, through the mitochondrial inner and outer membranes, and connect to the kDNA (96). This physical connection, termed the tripartite attachment complex (TAC), is maintained through the cell division cycle, such that basal body segregation is directly coupled to partitioning of the replicated kDNA network into each daughter cell (96). Three proteins have been localized to the TAC, p166, alternatively edited protein 1 (AEP-1), and an unidentified antigen recognized by mAb22 (13, 94, 159). p166 and AEP-1 function in segregation and/or maintenance of the kinetoplast (94, 159). While p166 is nuclear encoded, AEP-1 is encoded by the mitochondrion and is produced through alternative editing of the COXIII mRNA (94). Hence, the TAC is a composite structure, containing proteins from both the nuclear and mitochondrial genomes. The basal bodies are also the point of origin for the four specialized subpellicular microtubules of the FAZ (Figure 2a) (50). Therefore, the basal bodies provide microtubule organizing centers for axonemal and subpellicular microtubules while controlling the position and segregation of the mitochondrial genome.
Axoneme
As in most motile eukaryotic flagella, the trypanosome axoneme consists of nine outer doublet microtubules (ODs) surrounding a central pair apparatus (CP) of singlet microtubules. Radial spokes extend inward from each outer doublet toward the CP. ATP-dependent dynein motor proteins attached to outer doublets generate the sliding forces that underlie flagellar movement. Nexin links connect outer doublets to one another (22, 75, 150) and may contribute to dynein regulation (74).
Outer doublets
Each outer doublet (Supplemental Figure 1b,c; follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org) consists of a complete A-tubule with 13 α/β-tubulin protofilaments and an incomplete B-tubule with 10 protofilaments plus one additional filament that may or may not be a bona fide protofilament (Supplemental Figure 1c) (133). The B-tubule is transected by an intratubule structure, first described in 1969 (145) and now called the ponticulus, which is assembled only in the mature flagellum (Supplemental Figure 1b) (143). Protofilament ribbons are an integral component of outer doublets, and the protofilament ribbon proteins Rib72 and Rib43 are present in the T. brucei axoneme (10, 17). Mutations in the human Rib72 homologue EFHC1 are linked to juvenile myoclonic epilepsy (59). The precise function of protofilament ribbons is unclear, but their requirement for motility was first demonstrated in T. brucei, through RNAi knockdown of Rib72, which impaired motility (10). Another protein important for outer doublet assembly is the parkin coregulated gene product PACRG. T. brucei contains two PACRG homologues, PACRG-A and PACRG-B (26). Knockdown of either PACRG gene individually produced no observable effect, but simultaneous knockdown led to defective motility and destabilization of outer doublet microtubules (26).
Dyneins and dynein regulation
Outer arm and inner arm dyneins extend from each outer doublet A-tubule (Figure 1) and provide the driving force for motility. Dynein motors are composed of one to three catalytic heavy chains (HCs) and several associated light chains (LCs), intermediate chains (ICs), and light-intermediate chains (LICs) (64). Outer arm dyneins are generally uniform in composition and may contain either two or three distinct HCs, depending on the organism (64). The subunit composition of T. brucei axonemal dyneins has not been determined, but outer dyneins are predicted to be two-headed because the genome encodes only two predicted outer dynein HCs (Supplemental Table 1) (152). One of these is homologous to the Chlamydomonas reinhardtii gamma HC and the other groups are homologous to the C. reinhardtii alpha and beta HCs (Supplemental Table 1) (152). Inner arm dyneins are more diverse and may have one or two HCs. Recent phylogenetic analyses place inner arm dynein HCs into five groups and T. brucei encodes members of each group, indicating that the T. brucei axoneme contains the two-headed I1 dynein, as well as six presumably single-headed dyneins (Supplemental Table 1) (87, 152). Dynein-associated ICs, LCs, and LICs that were identified biochemically in C. reinhardtii (64) are well represented in the T. brucei genome (152), indicating a high degree of dynein subunit complexity.
Despite the central importance of flagellar motility to T. brucei biology and pathogenesis, little is known about individual dyneins and their contributions to axonemal motility. To date, no HCs, and only four noncatalytic subunits have been functionally characterized in T. brucei. RNAi knockdown of the outer arm dynein subunits IC78 (15) and LC1 (9) demonstrated a requirement for outer arm dyneins in controlling beat direction (9, 15). In both cases, outer arm dyneins were lost from the axoneme, and the tip-to-base beat that is a hallmark of trypanosome flagella was reversed to base-to-tip beating, causing cells to translocate backward. Notably, this illustrates a difference relative to C. reinhardtii, in which outer arm dyneins affect beat frequency and amplitude but not beat form. Depletion of a T. brucei TcTex2 homologue (15), or the p38/TAX1 inner arm dynein LIC (17), results in defective forward motility in procyclic cells.
Propagation of the flagellar waveforms along the axoneme requires coordinate spatial and temporal regulation of several thousand dynein motors. Regulation is partly mediated by the dynein regulatory complex (DRC), which was identified genetically in C. reinhardtii through isolation of extragenic suppressors of flagellar paralysis in CP and radial spoke mutants (57). The DRC functions in a pathway that transmits mechanochemical signals from the CP and radial spokes to axonemal dyneins and is conserved in T. brucei (57, 105, 106, 114, 122). The DRC subunit trypanin/PF2 is required for normal flagellar motility in T. brucei and C. reinhardtii (58, 114, 122) and is conserved among organisms with motile axonemes, but is absent in organisms that lack motile axonemes or that only assemble nonmotile cilia (10, 112). The DRC also functions in vertebrates, in which it is required for ciliary motility and inner ear development (23). The central importance of the DRC is evident from the observation that trypanin appears to be conserved in all extant organisms with motile axonemes, including those that lack outer arm dyneins and those that lack the CP, radial spokes, and inner arm dyneins (80, 112). Thus, the DRC appears to be a universal component of motile axonemes and was likely present in the last common ancestor of eukaryotes. Interestingly, T. brucei and other kinetoplastids possess two trypanin paralogues, whereas all other examined organisms have only one (112, 114), suggesting specialized functions for the DRC and dynein regulation in T. brucei.
Central pair apparatus
The CP micro-tubules originate at the basal plate and extend to the distal end of the flagellum (Figure 2e–h). Projections from each CP microtubule connect them to one another and to the radial spoke heads (Supplemental Figure 1d,e), imparting asymmetry to the CP apparatus, although unambiguous definition of a C1 or C2 micro-tubule as described for C. reinhardtii (56, 82, 97) has not been done for T. brucei. In some organisms, including C. reinhardtii, the CP rotates within the axoneme (81). In T. brucei, however, CP orientation is fixed relative to the outer doublet microtubules and this orientation is actively maintained because it is disrupted upon RNAi knockdown of CP proteins PF16, PF20, or hydin, but it is not disrupted in all motility mutants (10, 15, 27, 42, 112). The CP apparatus is required for motility in T. brucei, as ablation of the entire CP by RNAi knockdown of γ-tubulin causes severe motility defects, and knockdown of PF16, PF20, or hydin also inhibits flagellar motility (15, 27, 114).
Radial spokes
Extending inward from outer doublet microtubules are radial spokes (Supplemental Figure 1d,e), which are essential for normal motility of 9 + 2 axonemes (15, 114, 131). Radial spokes provide a platform for assembly of signaling proteins and are part of a mechanochemical signal transduction system that regulates dyneins (131, 156). Radial spoke proteins identified in other organisms are conserved in T. brucei. To date, however, only RSP3 has been investigated in T. brucei and it is required for radial spoke assembly and flagellar motility (15, 114). Flagellar beating in T. brucei exhibits many unique and striking features. Given the important role for the radial spokes as modifiers of dynein activity and flagellar beating, more direct and extensive studies of these structures in T. brucei are warranted.
Paraflagellar Rod
One of the most distinctive features of the trypanosome flagellum is the PFR, a large paracrystalline filament extending alongside the axoneme from the flagellar pocket to the flagellum tip (Supplemental Figure 1f). The PFR was first described 45 years ago (144) and is unique to kinetoplastids, euglenoids, and dinoflagellates (21). In cross-section the PFR has a lattice-like appearance with three distinct regions, proximal, intermediate, and distal, defined by their structural morphologies and positions relative to the axoneme (21). The proximal domain is connected to axonemal doublets 4–7 by electron-dense filaments of unknown composition.
The PFR consists of two major structural proteins, PFR1 and PFR2, along with several minor components (46, 77, 92, 108, 110), and is required for normal motility (11), although its precise function is unknown. Cells depleted of PFR2 retain only remnants of the proximal PFR and PFR-axoneme connectors (11, 78, 123). The ultrastructure of PFR1 knockdown mutants in T. brucei (31) has not been described, but a PFR1 knockout in Leishmania mexicana retains only the PFR-axoneme connectors, phenocopying a PFR1/2 double mutant (78). PFR2 knockdown mutants are immotile with reduced flagellar beat that is not sufficient to drive cellular rotation or translocation (11, 15, 48, 114).
PFR1 and PFR2 have been studied for many years, but additional PFR subunits have only recently been identified. Pullen and colleagues (110) identified two PFR-associated adenylate kinases, ADK-A and ADK-B. A third ADK (ADK-E) localizes to the T. brucei flagellum, but not to the PFR (46). There is precedence for flagellar ADKs associated with CP (157) and outer doublet (153) structures in C. reinhardtii. ADKs interconvert ADP, AMP, and ATP and might therefore function in energy homeostasis in the flagellum. Additionally, two cAMP phos-phodiesterases, PDEB1 and PDEB2, are PFR associated (92). PDEB1 is specific to the PFR, while PDEB2 is mostly cytoplasmic (92). cAMP is a well-known regulator of flagellar motility in many organisms, and flagellar cAMP phos-phodiesterases might therefore be part of a regulatory system acting in concert with flagellar adenylate cyclase to modulate flagellar motility. For a review of metabolic activities associated with the trypanosome flagellum see Reference 91. At present, the specific contribution of these proteins to flagellum function is unknown. Knockdown of flagellar ADKs or PDEBs did not impair growth of procyclic cells, but simultaneous ablation of PDEB1 and PDEB2 is lethal in bloodstream-form parasites (46, 92, 160). Simultaneous knockdown of ADK-A and ADK-B did not visibly affect motility in procyclic cells (46). The motility phenotypes of PDEB knockdown mutants were not described.
Portman et al. (108) recently provided a major advance in defining PFR subunit composition by employing quantitative mass spectrometry and two-dimensional differential in-gel electrophoresis (2D-DIGE) to systematically compare flagellar protein profiles of wild-type and PFR2 knockdown mutants. This analysis identified 20 novel proteins, denoted PFC1–PFC20. Epitope tagging of seven PFC proteins showed PFR localization for all seven (108). Domains associated with calcium signaling were prevalent in the PFC dataset, suggesting a role for calcium-mediated regulation as part of PFR function, consistent with the previous identification of calmodulin in the PFR (117). RNAi against PFC1 (containing an EF-hand) and PFC15 (containing an IQ motif) suggested potential interaction with PFR-associated ADKs but did not produce a detectable motility defect in procyclic trypanosomes (108).
Overall, these recent studies on PFR-associated proteins indicate that the PFR is more than a passive structural support to the axoneme and serves as a scaffold for assembly of regulatory and metabolic proteins that contribute to flagellum function. The importance of the flagellum and flagellar motility to T. brucei biology and pathogenesis, combined with the fact that the PFR is not found in the human host, makes the PFR an attractive target for therapeutic intervention.
Flagellar Pocket
A specialized membrane domain called the flagellar pocket forms from an invagination of the cell membrane where the proximal end of the flagellum emerges from the cytoplasm (Figure 2). The plasma membrane, flagellar pocket membrane, and flagellar membrane comprise three contiguous, but biochemically and functionally distinct, membrane domains (8, 39). Although the flagellar pocket is not completely membrane enclosed, electron-dense adhesion zones hold the flagellum in tight apposition to the cell membrane and demarcate the boundary of the flagellar pocket membrane (Figure 2f), causing a constriction termed the flagellar pocket neck or collar (14, 51). Hence, the lumen of the flagellar pocket is effectively an extracellular compartment that is secluded from hostile host environments.
The flagellar pocket makes up just 5% of the total cell surface, yet it is the sole site of endocytosis and exocytosis (98). As such, the sorting demands on this organelle are intense. The flagellar pocket must accommodate export of abundant variant surface glycoprotein (VSG) or procyclin surface proteins and simultaneously mediate uptake of extracellular macromolecules. By restricting receptors for host macromolecules, such as transferrin (85), to the flagellar pocket, these relatively invariant surface proteins are protected from exposure to the host immune system. Rates of endocytosis are developmentally regulated, such that endocytosis in the slender bloodstream form is approximately 10 times higher than in the procyclic form (85). This might reflect the need for removal of surface-bound antibodies, because flagellar beating influences trafficking through the pocket and contributes to the removal of VSG-Ig protein complexes from the cell surface (36) (see below). As the sole site of surface protein turnover and nutrient uptake, the flagellar pocket represents a key portal for host-parasite interaction, yet little is known about its protein and lipid composition. For a more complete review of vesicular trafficking in T. brucei see Reference 85.
Flagellar pocket duplication and segregation is coordinated with the cell cycle and is obligately linked with duplication and segregation of the flagellum. Until recently, the molecular machinery that mediates flagellar pocket biogenesis was completely unknown. The flagellar pocket forms independently of intraflagellar transport (IFT) (1, 25), demonstrating that axoneme extension is not required. Bonhivers et al. (14) identified a novel cytoskeletal flagellar pocket protein, Bilbo1. Bilbo1 is part of the ring-like flagellar pocket collar and is essential for flagellar pocket biogenesis and viability in both procyclic- and bloodstream-form cells (14). In the procyclic form, Bilbo1 mutants create new flagella that are mispositioned and lack a flagellar pocket or a detectable FAZ. A different phenotype is observed in bloodstream-form cells, where Bilbo1 mutants have spherical morphology and defects in flagellum attachment are not observed (14). Flagellar pocket biogenesis therefore affects the cytoskeleton and is required for positioning of the flagellum and formation of the FAZ in the procyclic form, whereas it plays a different and less clear role in bloodstream-form cells.
Flagellar Membrane and Matrix
Ciliary and flagellar membranes constitute specialized domains of the plasma membrane with distinct composition and biophysical properties (39, 40, 102, 124, 134, 137). In addition to their role in motility, eukaryotic flagella are sensory organelles that receive and transduce signals from the external environment (32, 158). As such, the flagellar membrane provides a scaffold for assembly of sensory proteins that transmit signals important for regulating motility and other cellular functions (102, 124). Although a sensory function for the T. brucei flagellum has not yet been directly demonstrated, the flagellar membrane represents a critical interface between the parasite and its vertebrate and invertebrate hosts (39, 151).
The T. brucei flagellar membrane is enriched in sterols, saturated fatty acids, and lipid rafts (34, 39, 40, 137). VSG and procyclin surface proteins of bloodstream-form and procyclic trypanosomes are present on the flagellar membrane, but our understanding of proteins specifically targeted to the flagellar membrane in T. brucei is limited. Paindavoine et al. (99) demonstrated that the adenylate cyclase encoded by the expression site associated gene 4 (ESAG4) is localized to the flagellar membrane. We speculate that ESAG4 may function in environmental sensing, since adenylate cylases are critical in cAMP-based signal transduction and additional flagellar cAMP signaling pathway components were recently discovered (91, 92). Currently, the function of ESAG4 and the mechanism of targeting to the flagellar membrane are unknown.
Inside of the flagellar membrane, the flagellar calcium binding protein FCaBP of T. cruzi is a dually acylated EF-hand calcium sensor that is localized to the flagellar membrane in a calcium-dependent and palmitoylation-dependent manner (20, 35). T. brucei possesses three FCaBP homologues, called calflagins Tb17, Tb24, and Tb44, that are also dually acylated (34, 137, 155). Recently, Emmer et al. (34) demonstrated that calflagins Tb24 and Tb44 are specifically localized to the flagellar membrane in T. brucei and that this localization requires palmitoylation by a single palmitoyl transferase. Myristoylation is required for palmitoylation and is sufficient to direct membrane association but not flagellar membrane localization, since singly myristoylated calflagin Tb44 is mislocalized to plasma membrane (34). Targeting of dually acylated calflagin to the flagellar membrane may be mediated by sequestration in lipid rafts that are enriched on the flagellar membrane, since depletion of lipid rafts results in redistribution of the calflagins to the plasma membrane (137). These important findings provide the first molecular analysis of flagellar membrane composition and protein targeting in T. brucei.
Like the flagellar membrane, the soluble flagellar matrix in T. brucei represents largely uncharted territory. This compartment is expected to contain the IFT machinery (101), and three IFT proteins examined biochemically, IFT20, IFT52, IFT172, exhibit a detergent-soluble pool and a detergent-insoluble, basal body associated pool (2). To date, however, no other soluble flagellar proteins have been identified. By comparison, proteomic analysis of the C. reinhardtii flagellum identified 146 proteins in the membrane + matrix fraction (101). Thus, many proteins in the T. brucei flagellar membrane and matrix remain to be identified, and this untapped area of research is rich with opportunity for important discoveries.
Flagellum Attachment Zone
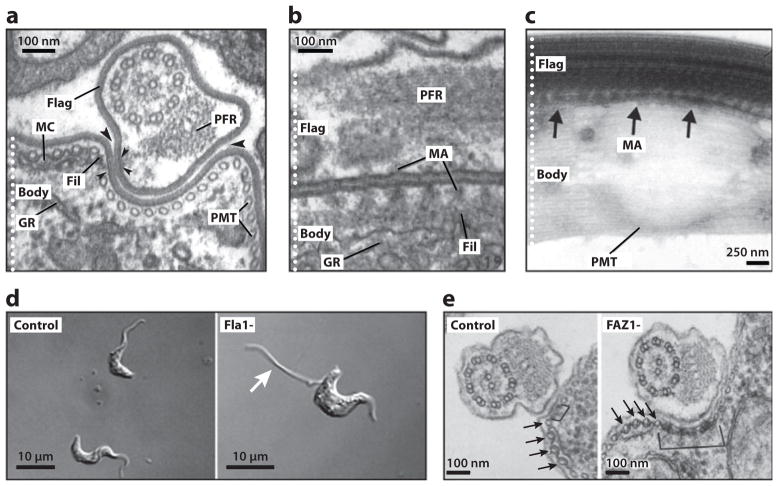
A unique feature of the T. brucei flagellum is lateral attachment to the cell body along the FAZ. Components of the T. brucei FAZ are mostly unknown, although the ultrastructure of the FAZ has been extensively characterized (Figure 3a–c) (50, 65, 129, 144, 145). On the cytoplasmic side, the FAZ is defined by an electron-dense filament of mostly unknown composition and by four microtubules of the subpellicular corset that are associated with a membranous compartment (Figure 3a). A network of regularly spaced filaments links the FAZ filament in the cell body to both the axoneme and the PFR in the flagellum, abutting the flagellar membrane and plasma membrane into macula adherens (Figure 3a–c) (145). The region of contact between the two membrane systems extends beyond the filamentous cross-links, indicating an additional membrane component to the attachment.
Figure 3.
The flagellum attachment zone (FAZ). (a) Transverse electron micrograph (EM) section of the Trypanosoma brucei rhodesiense bloodstream form, showing the flagellum (Flag) and cell body (Body). The electron-dense filament (Fil), macula adherens (small arrowheads) and membranous compartment (MC) of the FAZ are indicated. Large arrowheads point out the gap between the flagellar and cell body membranes. Also indicated are the subpellicular microtubules (PMT), the paraflagellar rod (PFR), and the granular endoplasmic reticulum (GR). (b) Longitudinal section of T. vivax, showing a series of macula adherens (MA) with their anchoring strands passing to longitudinally oriented filaments. (c) Whole mount of an intact, detergent-extracted T. brucei cytoskeleton, with the MA indicated with arrows. (d) RNAi knockdown of Fla1 in bloodstream-form T. brucei cells (Fla1-) leads to flagellar detachment (arrow). (e) RNAi knockdown of FAZ1 in procyclic T. brucei cells (FAZ1-) leads to abnormalities in FAZ ultrastructure, including unusually wide FAZ filaments (bracket). Arrows point to the four specialized PMTs of the FAZ. Panels a and b are adapted from Reference 145, panel c from Reference 58, panel d from Reference 69, and panel e from Reference 142, with permission.
Only a handful of FAZ proteins in T. brucei have been identified. Flagellum adhesion glycoprotein 1 (Fla1), the T. brucei homolog of T. cruzi GP72, localizes to the FAZ and is required for flagellum attachment (Figure 3d) (33, 69, 70, 90). Another FAZ component, FAZ1, was recently identified by screening an expression library with the monoclonal antibody L3B2 that recognizes the FAZ filament (142). FAZ1 is required for normal FAZ assembly (Figure 3e) and flagellum attachment and is conserved among kinetoplastids (142). Notably, flagellum assembly continues in the absence of attachment via the FAZ to the cell body (142). Given the role of the FAZ in cytokinesis (66), it is interesting that a T. brucei Polo-like kinase homologue, TbPLK, is localized to the FAZ (68), although a separate study reported that TbPLK localizes to the cytoplasm (53).
Flagellum Biogenesis
Flagellum assembly is one of the earliest stages of the T. brucei cell cycle (129) and is critical for cell division (66). Cells in early G1 have a single flagellum with a mature basal body and probasal body. At the G1/S boundary, the probasal body has docked to the flagellar pocket membrane and extended to form a transition zone, which then elongates to form the new axoneme (129). The new flagellum parallels the old flagellum as it grows, until it reaches a stop-point approximately halfway along old flagellum, at which point cytokinesis ensues, dividing the two cell bodies along a line between the new and old flagella (25, 83, 129).
In most eukaryotes flagellum assembly occurs extracytoplasmically, by addition of new subunits to the distal tip of the growing axoneme (121). This assembly is dependent upon IFT, a bidirectional transport system that delivers cytoplasmically synthesized flagellum subunits to the flagellum tip and returns the IFT machinery to the basal body (121). IFT particles, powered by heterotrimeric kinesin, transport flagellum proteins along the outside of ODs to the tip of the flagellum (67) and are then returned to the flagellum base by DHC1b dynein (103, 107). IFT has been studied most extensively in C. reinhardtii, where IFT particles can be separated biochemically into two sub-complexes, A and B, containing an estimated total of 17 proteins (24). Complex B and A proteins have been linked to anterograde and retrograde transport, respectively, although both complexes assemble together to form IFT particles in the flagellum (24). Sequences for 15 of 17 biochemically defined IFT proteins are published and four additional IFT genes have been identified through genetic studies in Caenorhabditis elegans (12).
The IFT pathway is conserved in organisms that build a flagellum extracytoplasmically and all 19 IFT genes are conserved in T. brucei (Supplemental Tables 2 and 3) (2, 25, 66). Functional analyses demonstrated that flagellar biogenesis in T. brucei requires IFT (2, 25, 66). This was first demonstrated by RNAi knockdown of the retrograde motor HC and IFT88 (66). In each case, knockdown led to the formation of cells with progressively shorter flagella, and ultimately nonflagellated cells (Supplemental Figure 2a,b) (66). Recently, Absalon et al. (2) used RNAi to ablate expression of 13 IFT genes and in all cases observed defects in flagellum biogenesis. Knockdown of complex A or B proteins supports the idea that these complexes have separable functions in T. brucei (2). T. brucei and other kinetoplastids uniquely possess two distinct homologues of the retrograde IFT motor heavy chain, DHC1b (4). Knockout studies in L. mexicana indicate different requirements for these two dynein HCs (4), although their distinct contributions to flagellum biogenesis in T. brucei have not been reported.
During the T. brucei cell cycle, cells have one mature flagellum and one nascent flagellum that grows alongside the old one (Figure 4). IFT proteins are localized to the detergent-soluble flagellum matrices and basal bodies of both the new and old flagellum (2), suggesting that IFT operates in both flagella. Consistent with this, IFT-like particles are visible by TEM in both old and new flagella (2). These particles are found almost exclusively adjacent to axonemal doublets 3/4 and 7/8 (Supplemental Figure 2c) (2), a preference that has not been reported in other organisms. By using an IFT52:GFP fusion protein, anterograde and retrograde intraflagellar movement were visualized in the old flagellum and new flagellum of live T. brucei cells (2). Knockdown of IFT genes blocked new flagellum growth, but the old flagellum was not visibly affected and retained IFT proteins (2, 25, 66). Therefore, IFT operates in the growing daughter flagellum and in the mature flagellum, but IFT proteins in the mature flagellum are not readily exchangeable with newly synthesized IFT proteins. IFT is required for growth of the new flagellum and presumably for maintenance of the mature flagellum, although the latter role has yet to be demonstrated experimentally. In T. brucei, compartmentalization of IFT proteins into distinct pools that are accessible only to the new or old flagellum may provide a means for independently controlling the length of the old flagellum while assembling the new flagellum.
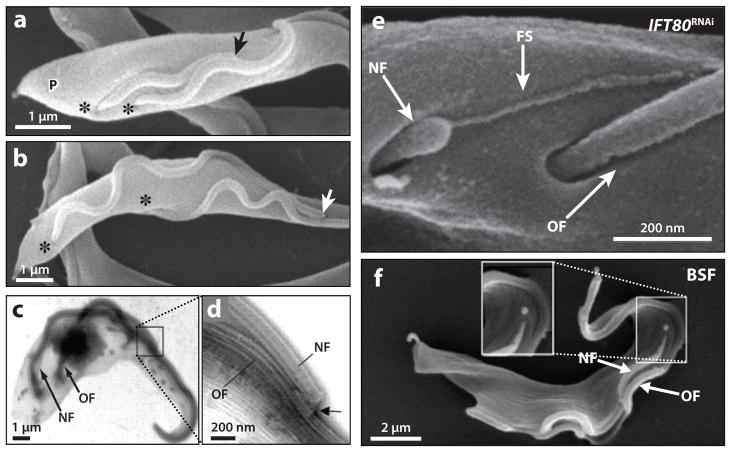
Figure 4.
Trypanosome flagellum biogenesis. (a,b) Scanning electron micrographs of procyclic-form cells show that the distal tip of the new flagellum (arrow) remains associated with the old flagellum during cell division. The distal tip of the new flagellum is indicated (arrow), the flagellar pockets are marked (asterisks), and the cell posterior is indicated (P). (c,d) Low (c) and high (d) magnification transmission electron micrographs of a detergent-extracted procyclic cytoskeleton showing the flagellar connector (arrow) that is present at the tip of the new flagellum (NF) and contacts the old flagellum (OF). (e) Scanning electron micrograph of a procyclic-form IFT80 RNAi knockdown mutant. A flagellar membrane sleeve (FS) of the new flagellum (NF) extends toward the lateral aspect of the old flagellum (OF). (f) Scanning electron micrograph of a bloodstream-form (BSF) cell during cell division, demonstrating that the tip of the new flagellum (NF) does not appear to connect to the old flagellum (OF) during elongation. Panels a–d are adapted from Reference 83, panel e from Reference 25, and panel f from Reference 16, with permission.
As the new flagellum extends, it traces a helical path alongside the existing flagellum. In procyclic cells this path of flagellum growth is directed by the flagellar connector (FC), a mobile, cytoskeletal structure that connects the tip of the new flagellum to outer doublets 7–9 of the old flagellum (Figure 4a–d) (16, 83). The FC forms within the flagellar pocket and moves with the tip of the new flagellum as it extends distally, maintaining attachment to the old flagellum until it reaches a point approximately halfway along the old flagellum (16, 83). Although the FC has been visualized by electron microscopy (Figure 4c,d) (2, 25, 66) and immunogold labeling (16, 25), the protein components are unknown. The mechanism for FC movement is also unclear but appears to involve a motor activity outside the new flagellum (25). The FC still forms in IFT mutants, connects an axoneme-less sleeve of flagellar membrane to the old flagellum, and moves distally along the old flagellum, moving the flagellar sleeve with it (Figure 4e) (2, 25). Therefore, the FC is apparently mobile in the absence of IFT in the new flagellum. The possibility that IFT in the old flagellum contributes to FC movement warrants further attention. The FC appears to be specific to the insect life cycle stage of T. brucei, since it is not detected in the bloodstream form or in other trypanosomatids (Figure 4f) (16).
FLAGELLUM FUNCTION
The contribution of the flagellum to parasite motility and host cell attachment has been appreciated for many years. Functional analyses of flagellar proteins have now revealed surprising new roles for the flagellum in cell morphogenesis, cell division, and immune evasion. We will outline the importance for motility and host cell attachment, and then review new studies leading to an appreciation of the multifunctional nature of the flagellum. Details of these new studies have been reviewed recently (47, 113), so the main findings are summarized and the reader is referred to earlier reviews for details.
Motility
The prominent role of motility in trypanosome biology is evidenced by the genus name Trypanosoma, derived from the Greek trypanon “auger” and soma “body,” which describes the auger-like movement of trypanosomes first noted by Gruby over 160 years ago (49). This auger-like motion is due to the tip-to-base spiral waveform of the flagellum, which wraps around the cell body (Figure 1). Until recently, we knew little about the molecular mechanisms of flagellar beat in T. brucei and this knowledge was based almost exclusively on analogy to other organisms. While such analogies are helpful, unique features of T. brucei motility require direct investigation and this is an area needing further effort. In addition to the structural peculiarities discussed above, the T. brucei flagellum moves rapidly in three dimensions with a spiral waveform and highly variable beat parameters. The dynamic, complex, and rapid movement of these parasites has defied traditional efforts at quantitative analysis of flagellar beating, and our current understanding is therefore mostly qualitative. By contrast, detailed quantitative descriptions of flagellar beating in related kinetoplastids have been completed (18, 41).
African trypanosomes are highly motile, moving at speeds of up to 20 um s−1 (58). Wild-type cells exhibit alternating periods of translational cell movement and tumbling, which causes reorientation (Figure 5) (58), reminiscent of the run-and-tumble behavior of bacteria. The dominant waveform in T. brucei and other trypanosomatids is a tractile beat that initiates at the tip of the flagellum and propagates toward the base (148, 149), which is opposite of that observed in most organisms. This tractile beat is interrupted intermittently with a base-to-tip beat that appears to be associated with parasite reorientation (9, 15). As discussed above, the loss of outer arm dyneins results in the loss of the dominant tractile beat and the appearance of a steady base-to-tip beat that drives cell movement in reverse. These findings illustrate an interesting contrast with C. reinhardtii, in which outer arm dyneins modify beat parameters, but not beat form (60). RNAi knockdown of several other axonemal proteins, including components of the CP, radial spokes, DRC, outer doublets, dynein motors, and PFR, demonstrated a requirement for each of these structures in normal motility (Figure 5b). However, the lack of a quantitative description of flagellar beating has limited our ability to define proteins that are responsible for more T. brucei–specific features. We also have not yet taken full advantage of facile systems for inducible ectopic gene expression to dissect the structure and function of specific flagellar proteins.
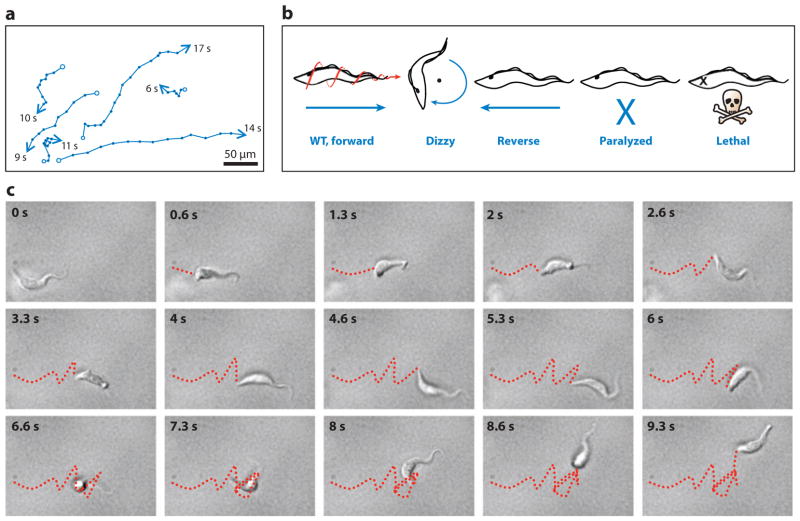
Figure 5.
Trypanosoma brucei cell motility. (a) Motility traces of wild-type procyclic cells. The positions of individual cells are plotted at 1-s intervals (indicated by dots). Starting positions are marked with open circles; ending positions are marked with arrowheads, and the time in seconds that each cell was within the field of view is indicated. (b) Schematic representations of motility phenotypes in procyclic motility mutants. Wild-type (WT) cell movement is helical with the flagellum tip leading. Dizzy mutants, such as trypanin knockdown mutants (58), retain a vigorously beating flagellum but are unable to move directionally. Reverse mutants, such as DNAI1 (15) or LC1 (9) knockdown mutants, exhibit a reverse, base-to-tip beat, and move backward, with the flagellum tip trailing. Paralyzed mutants, such as central pair and radial spoke mutants (15, 114), are incapable of movement at the cellular level. Lethal defects arise in mutants with severe flagellar paralysis (10). (c) Time series (0.66-s intervals) of a WT procyclic cell moving in liquid medium. The movements of the posterior tip of the cell body are traced with a red dashed line. Note that periods of running (0–4.66 and 8–9.33 s) alternate with periods of tumbling (5.33–7.33 s). Panel a is adapted from Reference 58, and panel b from Reference 113, with permission.
Motility in Disease Pathogenesis and Parasite Transmission
African trypanosomes are transmitted to the bloodstream of a mammalian host through the bite of an infected tsetse fly. In both hosts, parasites are extracellular and depend upon their own motility for migration through tissues. In the mammalian host, T. brucei initially circulates in the bloodstream but ultimately penetrates the blood vessel endothelium, spreading into connective tissues and the central nervous system (CNS). Clinical manifestations of sleeping sickness are divided into an early stage, with parasites circulating in the blood and lymph, and a late stage, when parasites have invaded the CNS (104). These stages are characterized by distinct clinical manifestations and respond differently to antiparasitic drugs. If untreated, sleeping sickness is always fatal and this fatal outcome is directly linked to parasite migration into the CNS. The CNS is a privileged environment and migration of parasites into this compartment may additionally provide a population that is resistant to chemotherapy and a source of relapse infection. Thus, pathogenic features of sleeping sickness are directly linked to migration of the parasite to specific host tissues and parasite traversal of the blood brain barrier is a critical and defining step of disease progression.
In the insect vector, trypanosomes taken up during a blood meal initially reside in the midgut, where they develop into procyclic forms adapted for survival within the fly. Pro-cyclic trypanosomes are not infectious to mammals and must undergo a complex series of developmental transformations and directional migrations to complete their development into mammalian infectious forms. Procyclic parasites migrate through the peritrophic membrane into the ectoperitropic space and differentiate into mesocyclic trypomastigotes, which migrate through the proventriculus into the foregut and differentiate into postmesocyclic epimastigotes (138). These epimastigotes complete the journey through the proboscis and hypopharynx to reach the lumen of the salivary glands, where the final stage of development occurs and where the parasites are in the opportune location for transmission to a new host. Attachment to the salivary gland epithelium via the flagellum stimulates the final stage of development into mammalian infectious forms (135, 145). Specific fly tissues are heavily infected, whereas adjacent tissues are devoid of parasites, indicating that parasite migration is not random (43, 44). Hence, in both tsetse and mammalian hosts trypanosome migration to specific host tissues is central to parasite development and disease pathogenesis.
Host Cell Attachment
The final step in maturation to infectious bloodstream forms, including acquisition of the VSG coat, occurs following parasite attachment to the tsetse fly salivary gland. This attachment is mediated by the parasite flagellum, which forms elaborate outgrowths of membrane and cytoskeletal material that penetrate into the spaces between microvilli of the salivary gland epithelium (Figure 6) (135, 145). Flagellar and host cell membranes are held in close apposition by hemidesmosome-like attachment plaques (Figure 6a–c) (135, 145). Attached epimastigotes then cease division and lose the extensive flagellar outgrowths but retain the attachment plaques. It is at this point that the parasites acquire the VSG coat and undergo additional morphogenetic changes (Figure 6d). Ultimately, VSG-coated meta-cyclic parasites are released into the lumen of the salivary gland for transmission to a new mammalian host. The molecular machinery that underlies modification of the flagellum into an attachment organelle and the signaling pathways that drive these critical developmental changes are unknown. Of interest is a recent report that trypanosomes completely lacking pro-cyclin exhibit dramatically reduced interaction with the salivary gland epithelium (139).
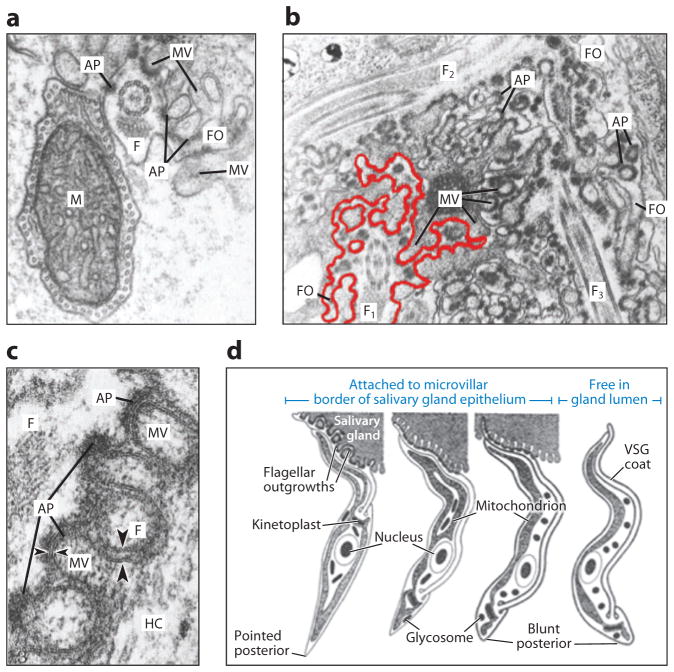
Figure 6.
The flagellum mediates attachment to the tsetse fly salivary gland. (a) Transverse electron micrograph section of a Trypanosoma brucei epimastigote showing the flagellum (F) with flagellar outgrowths (FO) intercalating with the microvilli (MV) of the tsetse fly salivary gland epithelium. Note the attachment plaques (APs) that form junctions with MV. The mitochondrion (M) is indicated. (b) Electron micrograph section showing three T. brucei flagella (F1, F2, and F3), each with extensive flagellar outgrowths. For emphasis, the outgrowths of F1 are outlined in red. (c) Detail of the attachment between the flagellum and the host cell (HC). Note that APs are found on the flagellar membrane only where the membrane is indented by the MV, leading to a much smaller gap between host and parasite membranes in this region (small arrowheads) than in the spaces that lack APs (large arrowheads). (d) Diagram of the developmental changes that occur while the parasite is attached to the salivary gland epithelium. Cells from left to right are epimastigote, premetacyclic, nascent metacyclic, and metacyclic forms. VSG, variant surface glycoprotein. Adapted from Reference 135, with permission.
Endocytosis and Immune Evasion
The flagellar pocket is the sole site of endocytosis and secretion (151), and access to the pocket lumen is restricted by close contacts between the flagellar and flagellar pocket membranes. As such, it has long been speculated that flagellum beating influences entry into the flagellar pocket, although experimental evidence was lacking. In an elegant set of experiments designed to track fluorescent protein trafficking on the surface of live cells, Engstler et al. (36) have now directly demonstrated a requirement for normal flagellar beating in endocytosis of VSG complexed with immunoglobulin (Ig) in bloodstream-form T. brucei (Supplemental Figure 3). Clearance of VSG-Ig protein complexes from the parasite surface is part of an integrated strategy for avoiding destruction by the host immune system and is disrupted in flagellar motility mutants. Therefore, flagellar motility is expected to contribute to immune evasion and persistent infection.
Sensory Perception
To be successful, trypanosomes must integrate host-derived and parasite-derived signals to direct parasite movements and developmental transformations within specific host compartments. While most evident in the tsetse (138), signaling also operates in the mammalian host, where autocrine signaling stimulates transformation of proliferating long-slender to nonproliferating short-stumpy forms (30, 116, 140). Penetration of the microvascular endothelium of the blood brain barrier involves paracrine signaling between parasite and host (88, 89). Recent studies have localized cyclic nucleotide and calcium signaling pathways to the flagellum (91, 92, 99, 108), suggesting that the flagellum provides a signaling platform for environmental sensing. Flagella are well known for their sensory roles in other organisms. In mammalian sperm, flagellar cyclic nucleotide and calcium signaling are crucial for chemotaxis and maturation (32, 38, 61). During C. reinhardtii mating, interaction of flagellar surface molecules stimulates flagellar cAMP signaling, leading to fusion and zygote formation (158). Analysis of T. brucei flagellar signaling pathways, including ligands and downstream effectors, is an important area of research that demands further attention and will surely yield important insights into parasite biology and pathogenesis.
Cell Morphogenesis
The T. brucei cell is highly polarized with single-copy organelles whose positions must be maintained, and then duplicated and segregated during cell division. The flagellum is connected to several subcellular organelles and structures, e.g., FAZ, flagellar pocket, kinetoplast, and mitochondrion, and dictates their arrangement. Through the flagellar connector, the old flagellum directs the path of new flagellum growth (Figure 4a,b), translating positional information to flagellum-associated organelles and thereby organizing them for segregation during cytokinesis (83). This arrangement provides a dramatic example of cytotaxis, whereby positional information is inherited from existing cellular structures. Recent RNAi studies of individual flagellum proteins have illustrated the importance of this concept and the flagellum in T. brucei morphogenesis and division.
Flagellum mutants display defects in cell morphology and polarity (2, 14, 17, 112). Commonly, the new kinetoplast fails to migrate posteriorly prior to cell division (3, 15, 26, 114). This is due to the connection between the kinetoplast and the basal body (119), though the mechanism by which the flagellum drives posterior basal body/kinetoplast movement is unclear. The defect is not strictly correlated with motility, since PFR mutants segregate the kinetoplast (3). It has been proposed that the FC reaches a stop point on the old flagellum, causing a reaction force to be transmitted posteriorly, moving the basal body (3, 25). Currently, it remains unknown what transmits the reaction force or how it overcomes resistance by FAZ connections along the cell body. Alternatively, the connector stop point might signal initiation of basal body migration independent of a reaction force.
Cell Division
The T. brucei cleavage furrow initiates between the tips of the new and old flagella/FAZ and progresses posteriorly (Figure 7c), suggesting an important role for the flagellum/FAZ in directing cleavage furrow formation (120). Supporting this hypothesis, knockdown of IFT88 or the retrograde IFT motor halts new flagellum/FAZ growth, and cytokinesis initiates at a point that correlates with the position of the truncated FAZ (Supplemental Figure 4a–c) (66). This yields short and ultimately nonviable aflagellate cells with severely abnormal morphology and polarity. The potential to control cell size based on flagellum/FAZ positioning is directly relevant in vivo, where transformation from trypomastigotes to epimastigotes is associated with an asymmetric cell division that yields one long cell and one short cell (Supplemental Figure 4d) (138). Reduced cell size accompanying this differentiation is correlated with the position of a short new flagellum (127). Thus, the flagellum/FAZ are critical determinants of cleavage furrow formation, but many interesting questions remain, particularly regarding the signals that control flagellum/FAZ length and the cues for the initiation of cytokinesis and asymmetric division in vivo.
Figure 7.
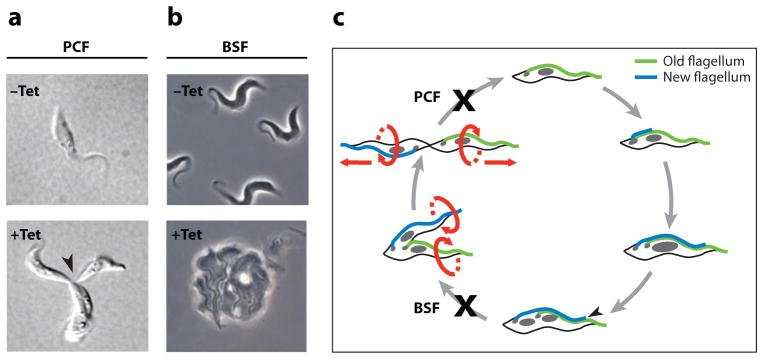
There is a dichotomy in the requirement of the flagellum in procyclic versus bloodstream-form cells. Panels a and b show phenotypes commonly observed in procyclic (PCF) and bloodstream-form (BSF) cells when flagellum proteins are knocked down. (a) Differential interference contrast (DIC) images of uninduced (−Tet) and tetracycline-induced (+Tet) procyclic pfr2 knockdown mutants (PCF Mot −). Induced cells fail in the completion of cytokinesis and accumulate as clusters of distinct cell bodies that remain physically attached at their posterior ends (arrowhead). (b) Phase-contrast images of −Tet and +Tet bloodstream-form trypanin knockdown mutants (BSF Mot −). Induced cells fail in the initiation of cytokinesis and accumulate as amorphous masses with multiple flagella. (c) Schematic representation of the cell cycle and the differing cell cycle blocks in procyclic versus bloodstream flagellum mutants. The cell cycle begins with replication of the basal body and initiation of new flagellum biogenesis. The new flagellum extends along a path defined by the old flagellum, while the basal bodies and associated kinetoplasts (small gray circles) migrate away from each other. In a closed mitotic cycle, the nucleus (large gray circle) is replicated and the new nucleus assumes a position between the new and old kinetoplast/basal body apparatus. Cytokinesis initiates at the tip of the new flagellum (black arrowhead), and cleavage furrow ingression proceeds to the cell posterior, separating the two daughter cells between the new and old flagella. Ultimately, daughter cells are oriented in opposite directions, with their flagella exerting rotational and pulling forces that facilitate final cell separation. Panel a is adapted from Reference 114, panel b from Reference 112, and panel c from Reference 113, with permission.
Motility and Cytokinesis
Perhaps more surprising than a structural requirement for the flagellum in cell division was the discovery that flagellar motility mutants with full-length flagella have cytokinesis defects (9, 10, 15, 17, 112, 114, 115). The effect is observed in procyclic and bloodstream forms, though the terminal phenotype differs and is more pronounced in bloodstream forms (Figure 7) (17, 112). In procyclic cells, motility mutants fail in the final stage of cell separation, yielding clusters of cells connected at their posterior (Figure 7a) (15, 114, 115). This phenotype has been observed in 33 independent mutants and the defect is rescued by mechanical agitation (9, 10, 15, 114, 115). Some mutants are reported not to exhibit the defect (17) and the reason for this discrepancy is not yet known. Nonetheless, the combined data indicate that pulling and rotational forces supplied by flagellar beating contribute to normal cytokinesis in procyclic cells. A similar phenomenon, termed rotokinesis, operates in Tetrahymena (19).
In bloodstream-form trypanosomes, all motility mutants so far examined are nonviable, indicating an exquisite sensitivity to perturbing the flagellum (15, 17, 112). There are distinct phenotypic differences between procyclic and bloodstream flagellum mutants (Figure 7) (112, 113). While procyclic mutants fail during progression and/or completion of cell cleavage (Figure 7a), bloodstream mutants ultimately fail to initiate cytokinesis (Figure 7b) (112). Furthermore, many knockdowns that are lethal bloodstream cells are viable in the procyclic form (26, 58). These results demonstrate life cycle stage-specific flagellum functions and emphasize the emerging concept (52, 136) of a dichotomy in the control of cell division in bloodstream and procyclic forms.
While the above studies show that flagellar proteins are essential in the bloodstream life cycle stage, they do not demonstrate that motility itself is essential. For example, lethality might result from structural defects rather than motility defects. Notably, all mutants examined thus far have known or suspected structural defects in the flagellum, as directly shown in T. brucei or in analogous C. reinhardtii mutants (113). Because the flagellum is critically linked to cell division, alterations to the flagellum or associated structures might preclude cell division. Consistent with this idea, mechanical agitation in vitro does not rescue the cell division defect of bloodstream motility mutants (K. Hill, unpublished results), nor do physical forces associated with bloodstream circulation (48). The situation is therefore different than that for pro-cyclic motility mutants. Furthermore, lethality in bloodstream-form mutants does not strictly correlate with the severity of motility defects in the corresponding procyclic mutants (113).
Flagellum assembly/disassembly is linked to cell cycle control in other organisms (100, 111, 118), and it is therefore possible that perturbing flagellum fidelity triggers a bona fide cytokinesis checkpoint in bloodstream-form trypanosomes. All mutants generated so far are RNAi knockdowns, and it will be informative to employ site-directed mutants that disrupt motility without disrupting flagellum assembly/structure to further address this issue. Regardless of the precise reason for lethality in bloodstream-form flagellum mutants, these studies raise the exciting possibility that the flagellum, and its estimated 700+ protein components, might be exploited as a novel target for therapeutic intervention in African sleeping sickness.
FLAGELLUM COMPOSITION REVEALED THROUGH GENOMIC AND PROTEOMIC STUDIES
Recent genomic and proteomic approaches have identified novel flagellar proteins in several organisms (6, 10, 17, 54, 73, 80, 101). In T. brucei Baron et al. (10) identified 50 components of motile flagella, or CMF genes, that are conserved in eukaryotes with motile flagella but are absent in organisms that contain only non-motile flagella or lack flagella entirely. RNAi knockdown of 41 CMF genes, 30 of which were novel, demonstrated a motility function for the majority (10). Biochemical studies on select CMF proteins demonstrated specific and stable association with the flagellum (10). Broadhead et al. (17) and Hart et al. (54) used proteomic analysis of salt-extracted flagella to identify 499 proteins that constitute a T. brucei extracted flagellum proteome, here referred to as TbEFP, including the axoneme, basal body, and FAZ. RNAi knockdown demonstrated flagellum function for eight novel TbEFP proteins (17). Two hundred eight TbEFP proteins are kinetoplastid specific and 238 were annotated as hypothetical, emphasizing the unexplored nature of the trypanosome flagellum.
In summary, these studies identified novel flagellum- and kinetoplastid-specific proteins (6, 10, 17, 54, 73, 80, 101), identified candidate subunits of the elusive nexin links (10), added to the inventory of human ciliary disease genes (6, 10, 17, 73, 101), and provided some of the first demonstrations that these genes have bona fide flagellum functions. These studies further revealed flagellar proteins that are encoded by expanded gene families in T. brucei, including components of the DRC, protofilament ribbons, dynein LCs, the retrograde IFT motor (4), and three novel protein families (10). Trypanosome-specific expansion of flagellar genes might reflect unique aspects of flagellar beat or constraints imposed by the unusual architecture of the trypanosome flagellum.
T. BRUCEI AS A MODEL SYSTEM FOR FLAGELLUM BIOLOGY.
Cilia and flagella drive movement of human pathogens such as T. brucei, Trichomonas vaginalis, Giardia lamblia and microgametes of Plasmodium spp. (37, 47, 130, 147). Cilia and flagella are also essential for normal human development and physiology, and ciliary defects cause a variety of heritable diseases (7). The flagellar axoneme is conserved among flagellated eukaryotes, and tools available in T. brucei make it an excellent system for dissecting flagellum biology. T. brucei offers facile systems for reverse genetics, including inducible RNAi, targeted gene knockouts, and inducible expression of recombinant proteins, as well as systems for forward genetics (71, 86). Epitope and tandem affinity purification tags are readily integrated into any endogenous locus using single-step PCR (93, 125, 128). Recent studies in T. brucei identified novel flagellar genes and demonstrated function of putative flagellar components. For some human ciliary disease genes, studies in T. brucei provided the first connection to human disease and, in some cases, the first demonstration that these genes have bona fide flagellum functions (10, 17). T. brucei is therefore emerging as a powerful experimental system for investigating eukaryotic flagellum structure and function, and it will become even more valuable as the era of gene discovery comes to a close and the focus turns to functional analysis.
We compiled an extensive inventory of flagellar and putative flagellar proteins in T. brucei (Supplemental Table 4; Supplemental Figure 5) using the CMF and TbEFP datasets (10, 17, 54), and the T. brucei homologues of C. reinhardtii flagellar proteome and motility cut proteins (80, 101). Details of these datasets are provided in the corresponding references. Our flagellar protein inventory includes 749 putative flagellar proteins. Interestingly, 43% of these proteins are specific to kinetoplastids (Supplemental Figure 5) and the vast majority (75%) have not been investigated individually, underscoring the need for additional functional studies.
SUMMARY AND FUTURE CONSIDERATIONS
The flagellum of African trypanosomes is now established as an essential and multifunctional organelle, moving well beyond its canonical role in parasite motility. Continued investigation is needed to determine the role of flagellar motility in vivo and to examine the exciting possibility that the flagellum may function in sensory perception and chemotaxis. Analysis of the membrane compartment is likely to be rewarding in this regard. There is also a need for more detailed examination of the flagellum in the bloodstream life cycle stage and more detailed ultrastructural analysis of the flagellum in both procyclic and bloodstream stages. Additional surprises surely await continued investigation of flagellar pocket, flagellar membrane and matrix composition, as well as flagellar biogenesis. In a broader context, expanded utilization of T. brucei as an experimental system to investigate flagellum biology will provide further insights into flagellum structure and function in humans and other organisms (Figure 8) (see sidebar, T. brucei as a Model System for Flagellum Biology). Although numerous flagellar proteins have been identified in genomic and proteomic studies, functional analysis is needed to determine how these proteins contribute individually and collectively to flagellum function. The hundreds of recently identified flagellar proteins represent novel targets for therapeutic intervention in African sleeping sickness, as well as other infectious and heritable diseases. Much work is needed to capitalize on this possibility and continued studies of the trypanosome flagellum provide an opportunity to move in this direction.
Figure 8.
Trypanosoma brucei as a model system for flagellum structure and function studies. Artistic representation of the trypanosome, emphasizing the evolutionarily conserved character of the 9 + 2 flagellar axoneme, with various ciliated organisms representing the radial spokes. (Clockwise from top: Ciona intestinalis, Danio rerio, Drosophila melanogaster, Gallus gallus, Chlamydomonas reinhardtii, Homo sapiens, Caenorhabditis elegans, Mus musculus, and Asterias forbesi.) The paraflagellar rod, unique to trypanosomes, is not shown.
Supplementary Material
SUMMARY POINTS.
Flagellar motility plays a central role in parasite development and disease pathogenesis.
The T. brucei flagellum is an essential and multifunctional organelle with critical roles beyond motility, including host-parasite interaction, cell morphogenesis, cell division and immune evasion.
Flagellar mutants have defective cell division in both the bloodstream form and procyclic form, but there are fundamental differences in the phenotypes observed, indicating life cycle stage-specific flagellum functions. This point speaks to the broader concept of stage-specific control of the cell division cycle and understanding the underlying mechanisms represents a major unresolved question.
All motility mutants so far examined in the bloodstream life cycle stage are inviable. However, it remains to be determined whether this lethal phenotype is due to loss of motility per se, or to another aspect of flagellum function.
Structure of the T. brucei flagellum exhibits conserved and unique features compared to other eukaryotes and further ultrastructural work is needed to precisely define these. Unique features may be exploited as drug targets, while conserved features are broadly relevant to understanding flagellum/cilium structure and function.
The motility behavior of T. brucei and the presence of sensory/signaling pathways in the flagellum indicate that the flagellum provides a platform for the assembly of environmental signaling systems.
Trypanosomes provide an excellent model system for investigating fundamental aspects of eukaryotic flagellar biology. Already this has led to novel insights into flagellum function, as well as discovery and functional analysis of several human disease genes.
Acknowledgments
Our apologies to those whose work was not covered in this review owing to space limitations. We thank Dr. Paul Webster (House Ear Institute, Los Angeles, CA) for the basal body electron micrograph and Randy Nessler (U. Iowa, Iowa City, IA) for assistance with electron microscopy. We thank Desiree Baron and Zachary Matheny for the drawing in Figure 8. We thank all members of our laboratory for thoughtful comments on the work and colleagues for providing information prior to publication. Katherine Ralston is the recipient of a USPHS National Research Service Award (GM07104) and a Dissertation Year Fellowship from the UCLA graduate division. Jason Melehani is a MARC Scholar (T34GM-08563–14). Work in the authors’ laboratory is supported by grants from the National Institutes of Health (R01AI52348), the Arnold and Mabel Beckman Foundation, and the Burroughs Wellcome Fund.
Glossary
- Flagellum/cilium
a hair-like projection extending from the cell surface that provides motility, sensory and transport functions
- Basal body
a microtubule organizing center that nucleates the microtubules of the flagellar axoneme
- Flagellar pocket
a specialized invagination of the trypanosome plasma membrane formed at the site where the flagellum emerges from the cytoplasm
- Axoneme
an evolutionarily-conserved microtubule-based structure that provides the scaffold for all eukaryotic flagella
- FAZ
flagellum attachment zone
- Paraflagellar rod (PFR)
a large paracrystaline filament that is attached to the axoneme of kinetoplastids and provides a scaffold for assembly of regulators of axonemal motility
- RNAi
RNA interference
- TAC
tripartite attachment complex
- OD
outer doublet microtubule
- CP
central pair apparatus
- Dynein
a AAA-type, microtubule-based molecular motor composed of one or more catalytic heavy chains and several associated noncatalytic subunits, that power flagellar motility and function as the motor for retrograde IFT
- Dynein regulatory complex (DRC)
a central regulator of dynein activity likely found in all extant organisms with motile axonemes
- Intraflagellar transport (IFT)
a bidirectional, microtubule-dependent transport system that transports flagellum components into the flagellum and returns transport machinery to the flagellum base, powered by kinesin and dynein
- Flagellar connector (FC)
a T. brucei–specific mobile cytoskeletal structure that connects the tip of the new flagellum to the side of the old flagellum
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Absalon S, Blisnick T, Bonhivers M, Kohl L, Cayet N, et al. Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. J Cell Sci. 2008;121:3704–16. doi: 10.1242/jcs.035626. [DOI] [PubMed] [Google Scholar]
- 2.Absalon S, Blisnick T, Kohl L, Toutirais G, Doré G, et al. Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol Biol Cell. 2007;19:929–44. doi: 10.1091/mbc.E07-08-0749. Completed functional analysis of 13 IFT genes and provided the first direct visualization of IFT transport in the flagellum of live trypanosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Absalon S, Kohl L, Branche C, Blisnick T, Toutirais G, et al. Basal body positioning is controlled by flagellum formation in Trypanosoma brucei. PLoS ONE. 2007;2:e437. doi: 10.1371/journal.pone.0000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhiambo C, Forney JD, Asai DJ, LeBowitz JH. The two cytoplasmic dynein-2 isoforms in Leishmania mexicana perform separate functions. Mol Biochem Parasitol. 2005;143:216–25. doi: 10.1016/j.molbiopara.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Deleted in proof
- 6.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, et al. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–39. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 7.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 8.Balber AE. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit Rev Immunol. 1990;10:177–201. [PubMed] [Google Scholar]
- 9.Baron DM, Kabututu ZP, Hill KL. Stuck in reverse: Loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J Cell Sci. 2007;120:1513–20. doi: 10.1242/jcs.004846. [DOI] [PubMed] [Google Scholar]
- 10.Baron DM, Ralston KS, Kabututu ZP, Hill KL. Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J Cell Sci. 2007;120:478–91. doi: 10.1242/jcs.03352. Provided a comprehensive genomic and functional analysis of flagellar proteins to identify 50 genes that are uniquely conserved in organisms with motile flagella. [DOI] [PubMed] [Google Scholar]
- 11.Bastin P, Sherwin T, Gull K. Paraflagellar rod is vital for trypanosome motility. Nature. 1998;391:548. doi: 10.1038/35300. [DOI] [PubMed] [Google Scholar]
- 12.Blacque OE, Li C, Inglis PN, Esmail MA, Ou G, et al. The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol Biol Cell. 2006;17:5053–62. doi: 10.1091/mbc.E06-06-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonhivers M, Landrein N, Decossas M, Robinson DR. A monoclonal antibody marker for the exclusion-zone filaments of Trypanosoma brucei. Parasit Vectors. 2008;1:21. doi: 10.1186/1756-3305-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonhivers M, Nowacki S, Landrein N, Robinson DR. Biogenesis of the trypanosome endoexocytotic organelle is cytoskeleton mediated. PLoS Biol. 2008;6:e105. doi: 10.1371/journal.pbio.0060105. Identified Bilbo1 as the first component of the ring-shaped flagellar pocket collar and demonstrated its necessity for flagellar pocket biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branche C, Kohl L, Toutirais G, Buisson J, Cosson J, Bastin P. Conserved and specific functions of axoneme components in trypanosome motility. J Cell Sci. 2006;119:3443–55. doi: 10.1242/jcs.03078. [DOI] [PubMed] [Google Scholar]
- 16.Briggs LJ, McKean PG, Baines A, Moreira-Leite F, Davidge J, et al. The flagella connector of Trypanosoma brucei: an unusual mobile transmembrane junction. J Cell Sci. 2004;117:1641–51. doi: 10.1242/jcs.00995. [DOI] [PubMed] [Google Scholar]
- 17.Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–27. doi: 10.1038/nature04541. Provided a comprehensive proteomic and functional analysis of extracted flagellar skeletons that identified numerous flagellar proteins and showed many are essential in bloodstream trypanosomes. [DOI] [PubMed] [Google Scholar]
- 18.Brokaw CJ. Thinking about flagellar oscillation. Cell Motil Cytoskelet. 2008;66:426–36. doi: 10.1002/cm.20313. [DOI] [PubMed] [Google Scholar]
- 19.Brown JM, Hardin C, Gaertig J. Rotokinesis, a novel phenomenon of cell locomotion-assisted cytokinesis in the ciliate Tetrahymena thermophila. Cell Biol Int. 1999;23:841–48. doi: 10.1006/cbir.1999.0480. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, et al. A flagellum-specific calcium sensor. J Biol Chem. 2005;280:40104–11. doi: 10.1074/jbc.M505777200. [DOI] [PubMed] [Google Scholar]
- 21.Cachon J, Cachon M, Cosson M-P, Cosson JC. The paraflagellar rod: a structure in search of a function. Biol Cell. 1988;63:169–81. [Google Scholar]
- 22.Cibert C. Elastic extension and jump of the flagellar nexin links: a theoretical mechanical cycle. Cell Motil Cytoskelet. 2001;49:161–75. doi: 10.1002/cm.1030. [DOI] [PubMed] [Google Scholar]
- 23.Colantonio JR, Vermot J, Wu D, Langenbacher AD, Fraser S, et al. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature. 2009;457:205–9. doi: 10.1038/nature07520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–42. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 25.Davidge JA, Chambers E, Dickinson HA, Towers K, Ginger ML, et al. Trypanosome IFT mutants provide insight into the motor location for mobility of the flagella connector and flagellar membrane formation. J Cell Sci. 2006;119:3935–43. doi: 10.1242/jcs.03203. [DOI] [PubMed] [Google Scholar]
- 26.Dawe HR, Farr H, Portman N, Shaw MK, Gull K. The Parkin coregulated gene product, PACRG, is an evolutionarily conserved axonemal protein that functions in outer-doublet microtubule morphogenesis. J Cell Sci. 2005;118:5421–30. doi: 10.1242/jcs.02659. [DOI] [PubMed] [Google Scholar]
- 27.Dawe HR, Shaw MK, Farr H, Gull K. The hydrocephalus inducing gene product, hydin, positions axonemal central pair microtubules. BMC Biol. 2007;5:33. doi: 10.1186/1741-7007-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–90. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 29.Dilbeck V, Berberof M, Van Cauwenberge A, Alexandre H, Pays E. Characterization of a coiled coil protein present in the basal body of Trypanosoma brucei. J Cell Sci. 1999;112:4687–94. doi: 10.1242/jcs.112.24.4687. [DOI] [PubMed] [Google Scholar]
- 30.Domenicali Pfister D, Burkard G, Morand S, Renggli CK, Roditi I, Vassella E. A mitogen-activated protein kinase controls differentiation of bloodstream forms of Trypanosoma brucei. Eukaryot Cell. 2006;5:1126–35. doi: 10.1128/EC.00094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durand-Dubief M, Kohl L, Bastin P. Efficiency and specificity of RNA interference generated by intra- and intermolecular double stranded RNA in Trypanosoma brucei. Mol Biochem Parasitol. 2003;129:11–21. doi: 10.1016/s0166-6851(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 32.Eisenbach M, Giojalas LC. Sperm guidance in mammals—an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–85. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 33.El-Sayed NM, Alarcon CM, Beck JC, Sheffield VC, Donelson JE. cDNA expressed sequence tags of Trypanosoma brucei rhodesiense provide new insights into the biology of the parasite. Mol Biochem Parasitol. 1995;73:75–90. doi: 10.1016/0166-6851(95)00098-l. [DOI] [PubMed] [Google Scholar]
- 34.Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J Cell Sci. 2009;122:867–74. doi: 10.1242/jcs.041764. Provided the first functional analysis of flagellar membranes and membrane-targeted proteins in T. brucei. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engman DM, Krause KH, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. A novel flagellar Ca2+-binding protein in trypanosomes. J Biol Chem. 1989;264:18627–31. [PubMed] [Google Scholar]
- 36.Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, et al. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell. 2007;131:505–15. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson D. Toxoplasma gondii and sex: essential or optional extra? Trends Parasitol. 2002;18:351. [PubMed] [Google Scholar]
- 38.Fraser LR, Adeoya-Osiguwa S, Baxendale RW, Mededovic S, Osiguwa OO. First messenger regulation of mammalian sperm function via adenylyl cyclase/cAMP. J Reprod Dev. 2005;51:37–46. doi: 10.1262/jrd.51.37. [DOI] [PubMed] [Google Scholar]
- 39.Fridberg A, Buchanan KT, Engman DM. Flagellar membrane trafficking in kinetoplastids. Parasitol Res. 2007;100:205–12. doi: 10.1007/s00436-006-0329-2. [DOI] [PubMed] [Google Scholar]
- 40.Fridberg A, Olson CL, Nakayasu ES, Tyler KM, Almeida IC, Engman DM. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J Cell Sci. 2008;121:522–35. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 41.Gadelha C, Wickstead B, Gull K. Flagellar and ciliary beating in trypanosome motility. Cell Motil Cytoskelet. 2007;64:629–43. doi: 10.1002/cm.20210. [DOI] [PubMed] [Google Scholar]
- 42.Gadelha C, Wickstead B, McKean PG, Gull K. Basal body and flagellum mutants reveal a rotational constraint of the central pair microtubules in the axonemes of trypanosomes. J Cell Sci. 2006;119:2405–13. doi: 10.1242/jcs.02969. [DOI] [PubMed] [Google Scholar]
- 43.Gibson W, Bailey M. The development of Trypanosoma brucei within the tsetse fly midgut observed using green fluorescent trypanosomes. Kinetoplastid Biol Dis. 2003;2:1. doi: 10.1186/1475-9292-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson W, Peacock L, Ferris V, Williams K, Bailey M. Analysis of a cross between green and red fluorescent trypanosomes. Biochem Soc Trans. 2006;34:557–59. doi: 10.1042/BST0340557. [DOI] [PubMed] [Google Scholar]
- 45.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginger ML, Ngazoa ES, Pereira CA, Pullen TJ, Kabiri M, et al. Intracellular positioning of isoforms explains an unusually large adenylate kinase gene family in the parasite Trypanosoma brucei. J Biol Chem. 2005;280:11781–89. doi: 10.1074/jbc.M413821200. [DOI] [PubMed] [Google Scholar]
- 47.Ginger ML, Portman N, McKean PG. Swimming with protists: perception, motility and flagellum assembly. Nat Rev Microbiol. 2008;6:838–50. doi: 10.1038/nrmicro2009. [DOI] [PubMed] [Google Scholar]
- 48.Griffiths S, Portman N, Taylor PR, Gordon S, Ginger ML, Gull K. RNA interference mutant induction in vivo demonstrates the essential nature of trypanosome flagellar function during mammalian infection. Eukaryot Cell. 2007;6:1248–50. doi: 10.1128/EC.00110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruby M. Recherches et observations sur une nouvelle espèce d’hèmatozoaire, Trypanosoma sanguinis. C R Acad Sci Paris. 1843;17:1134–36. [Google Scholar]
- 50.Gull K. The cytoskeleton of trypanosomatid parasites. Annu Rev Microbiol. 1999;53:629–55. doi: 10.1146/annurev.micro.53.1.629. [DOI] [PubMed] [Google Scholar]
- 51.Gull K. Host-parasite interactions and trypanosome morphogenesis: a flagellar pocketful of goodies. Curr Opin Microbiol. 2003;6:365–70. doi: 10.1016/s1369-5274(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 52.Hammarton TC, Clark J, Douglas F, Boshart M, Mottram JC. Stage-specific differences in cell cycle control in Trypanosoma brucei revealed by RNA interference of a mitotic cyclin. J Biol Chem. 2003;278:22877–86. doi: 10.1074/jbc.M300813200. [DOI] [PubMed] [Google Scholar]
- 53.Hammarton TC, Kramer S, Tetley L, Boshart M, Mottram JC. Trypanosoma brucei Polo-like kinase is essential for basal body duplication, kDNA segregation and cytokinesis. Mol Microbiol. 2007;65:1229–48. doi: 10.1111/j.1365-2958.2007.05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hart SR, Lau KW, Hao Z, Broadhead R, Portman N, et al. Analysis of the trypanosome flagellar proteome using a combined electron transfer/collisionally activated dissociation strategy. J Am Soc Mass Spectrom. 2009;20:167–75. doi: 10.1016/j.jasms.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Hill KL, Hutchings NR, Grandgenett PM, Donelson JE. T lymphocyte triggering factor of African trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J Biol Chem. 2000;275:39369–78. doi: 10.1074/jbc.M006907200. [DOI] [PubMed] [Google Scholar]
- 56.Hopkins JM. Subsidiary components of the flagella of Chlamydomonas reinhardii. J Cell Sci. 1970;7:823–39. doi: 10.1242/jcs.7.3.823. [DOI] [PubMed] [Google Scholar]
- 57.Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell. 1982;28:115–24. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- 58.Hutchings NR, Donelson JE, Hill KL. Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J Cell Biol. 2002;156:867–77. doi: 10.1083/jcb.200201036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikeda T, Ikeda K, Enomoto M, Park MK, Hirono M, Kamiya R. The mouse ortholog of EFHC1 implicated in juvenile myoclonic epilepsy is an axonemal protein widely conserved among organisms with motile cilia and flagella. FEBS Lett. 2005;579:819–22. doi: 10.1016/j.febslet.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 60.Kamiya R. Exploring the function of inner and outer dynein arms with Chlamydomonas mutants. Cell Motil Cytoskelet. 1995;32:98–102. doi: 10.1002/cm.970320205. [DOI] [PubMed] [Google Scholar]
- 61.Kaupp UB, Kashikar ND, Weyand I. Mechanisms of sperm chemotaxis. Annu Rev Physiol. 2008;70:93–117. doi: 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- 62.Keller LC, Romijn EP, Zamora I, Yates JR, 3rd, Marshall WF. Proteomic analysis of isolated Chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr Biol. 2005;15:1090–98. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 63.Kilburn CL, Pearson CG, Romijn EP, Meehl JB, Giddings TH, Jr, et al. New Tetrahymena basal body protein components identify basal body domain structure. J Cell Biol. 2007;178:905–12. doi: 10.1083/jcb.200703109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King SM. Organization and regulation of the dynein microtubule motor. Cell Biol Int. 2003;27:213–15. doi: 10.1016/s1065-6995(02)00337-2. [DOI] [PubMed] [Google Scholar]
- 65.Kohl L, Gull K. Molecular architecture of the trypanosome cytoskeleton. Mol Biochem Parasitol. 1998;93:1–9. doi: 10.1016/s0166-6851(98)00014-0. [DOI] [PubMed] [Google Scholar]
- 66.Kohl L, Robinson D, Bastin P. Novel roles for the flagellum in cell morphogenesis and cytokinesis of trypanosomes. EMBO J. 2003;22:5336–46. doi: 10.1093/emboj/cdg518. First demonstrated that the flagellum is an essential organelle in T. brucei and directs positioning of the cleavage furrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–27. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar P, Wang CC. Dissociation of cytokinesis initiation from mitotic control in a eukaryote. Eukaryot Cell. 2006;5:92–102. doi: 10.1128/EC.5.1.92-102.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LaCount DJ, Barrett B, Donelson JE. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J Biol Chem. 2002;277:17580–88. doi: 10.1074/jbc.M200873200. [DOI] [PubMed] [Google Scholar]
- 70.LaCount DJ, Bruse S, Hill KL, Donelson JE. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol Biochem Parasitol. 2000;111:67–76. doi: 10.1016/s0166-6851(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 71.Leal S, Acosta-Serrano A, Morris J, Cross GA. Transposon mutagenesis of Trypanosoma brucei identifies glycosylation mutants resistant to concanavalin A. J Biol Chem. 2004;279:28979–88. doi: 10.1074/jbc.M403479200. [DOI] [PubMed] [Google Scholar]
- 72.Legros D, Ollivier G, Gastellu-Etchegorry M, Paquet C, Burri C, et al. Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect Dis. 2002;2:437–40. doi: 10.1016/s1473-3099(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 73.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–52. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 74.Lindemann CB. Testing the geometric clutch hypothesis. Biol Cell. 2004;96:681–90. doi: 10.1016/j.biolcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Lindemann CB, Kanous KS. A model for flagellar motility. Int Rev Cytol. 1997;173:1–72. doi: 10.1016/s0074-7696(08)62475-4. [DOI] [PubMed] [Google Scholar]
- 76.Lukes J, Hashimi H, Zikova A. Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates. Curr Genet. 2005;48:277–99. doi: 10.1007/s00294-005-0027-0. [DOI] [PubMed] [Google Scholar]
- 77.Maga JA, LeBowitz JH. Unraveling the kinetoplastid paraflagellar rod. Trends Cell Biol. 1999;9:409–13. doi: 10.1016/s0962-8924(99)01635-9. [DOI] [PubMed] [Google Scholar]
- 78.Maga JA, Sherwin T, Francis S, Gull K, LeBowitz JH. Genetic dissection of the Leishmania paraflagellar rod, a unique flagellar cytoskeleton structure. J Cell Sci. 1999;112:2753–63. doi: 10.1242/jcs.112.16.2753. [DOI] [PubMed] [Google Scholar]
- 79.McKean PG, Baines A, Vaughan S, Gull K. Gamma-tubulin functions in the nucleation of a discrete subset of microtubules in the eukaryotic flagellum. Curr Biol. 2003;13:598–602. doi: 10.1016/s0960-9822(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 80.Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–50. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell DR, Nakatsugawa M. Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J Cell Biol. 2004;166:709–15. doi: 10.1083/jcb.200406148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitchell DR, Sale WS. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol. 1999;144:293–304. doi: 10.1083/jcb.144.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreira-Leite FF, Sherwin T, Kohl L, Gull K. A trypanosome structure involved in transmitting cytoplasmic information during cell division. Science. 2001;294:610–12. doi: 10.1126/science.1063775. Reported the discovery of the flagellar connector and articulated the concept of cytotaxis in relationship to organelle inheritance and cellular morphogenesis in T. brucei. [DOI] [PubMed] [Google Scholar]
- 84.Morgan GW, Denny PW, Vaughan S, Goulding D, Jeffries TR, et al. An evolutionarily conserved coiled-coil protein implicated in polycystic kidney disease is involved in basal body duplication and flagellar biogenesis in Trypanosoma brucei. Mol Cell Biol. 2005;25:3774–83. doi: 10.1128/MCB.25.9.3774-3783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morgan GW, Hall BS, Denny PW, Carrington M, Field MC. The kinetoplastida endocytic apparatus. Part I: a dynamic system for nutrition and evasion of host defences. Trends Parasitol. 2002;18:491–96. doi: 10.1016/s1471-4922(02)02391-7. [DOI] [PubMed] [Google Scholar]
- 86.Morris JC, Wang Z, Drew ME, Englund PT. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. EMBO J. 2002;21:4429–38. doi: 10.1093/emboj/cdf474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris RL, Hoffman MP, Obar RA, McCafferty SS, Gibbons IR, et al. Analysis of cytoskeletal and motility proteins in the sea urchin genome assembly. Dev Biol. 2006;300:219–37. doi: 10.1016/j.ydbio.2006.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nikolskaia OV, de ALAP, Kim YV, Lonsdale-Eccles JD, Fukuma T, et al. Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J Clin Investig. 2006;116:2739–47. doi: 10.1172/JCI27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nikolskaia OV, Kim YV, Kovbasnjuk O, Kim KJ, Grab DJ. Entry of Trypanosoma brucei gambiense into microvascular endothelial cells of the human blood-brain barrier. Int J Parasitol. 2006;36:513–19. doi: 10.1016/j.ijpara.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Nozaki T, Haynes PA, Cross GA. Characterization of the Trypanosoma brucei homologue of a Trypanosoma cruzi flagellum-adhesion glycoprotein. Mol Biochem Parasitol. 1996;82:245–55. doi: 10.1016/0166-6851(96)02741-7. [DOI] [PubMed] [Google Scholar]
- 91.Oberholzer M, Bregy P, Marti G, Minca M, Peier M, Seebeck T. Trypanosomes and mammalian sperm: one of a kind? Trends Parasitol. 2007;23:71–77. doi: 10.1016/j.pt.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Oberholzer M, Marti G, Baresic M, Kunz S, Hemphill A, Seebeck T. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. FASEB J. 2007;21:720–31. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- 93.Oberholzer M, Morand S, Kunz S, Seebeck T. A vector series for rapid PCR-mediated C-terminal in situ tagging of Trypanosoma brucei genes. Mol Biochem Parasitol. 2006;145:117–20. doi: 10.1016/j.molbiopara.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Ochsenreiter T, Anderson S, Wood ZA, Hajduk SL. Alternative RNA editing produces a novel protein involved in mitochondrial DNA maintenance in trypanosomes. Mol Cell Biol. 2008;28:5595–604. doi: 10.1128/MCB.00637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogbadoyi E, Ersfeld K, Robinson D, Sherwin T, Gull K. Architecture of the Trypanosoma brucei nucleus during interphase and mitosis. Chromosoma. 2000;108:501–13. doi: 10.1007/s004120050402. [DOI] [PubMed] [Google Scholar]
- 96.Ogbadoyi EO, Robinson DR, Gull K. A high-order trans-membrane structural linkage is responsible for mitochondrial genome positioning and segregation by flagellar basal bodies in trypanosomes. Mol Biol Cell. 2003;14:1769–79. doi: 10.1091/mbc.E02-08-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olson GE, Linck RW. Observations of the structural components of flagellar axonemes and central pair microtubules from rat sperm. J Ultrastruct Res. 1977;61:21–43. doi: 10.1016/s0022-5320(77)90004-1. [DOI] [PubMed] [Google Scholar]
- 98.Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol. 2004;53:735–44. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]
- 99.Paindavoine P, Rolin S, Van Assel S, Geuskens M, Jauniaux JC, et al. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol Cell Biol. 1992;12:1218–25. doi: 10.1128/mcb.12.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–57. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 101.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–13. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Mamm Dev. 2008;85:111–45. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 103.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–81. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pepin J, Donelson JE. African trypanosomiasis (sleeping sickness) In: Guerrant R, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens and Practice. Philadelphia, PA: Churchill Livingstone; 1999. pp. 774–84. [Google Scholar]
- 105.Piperno G, Mead K, LeDizet M, Moscatelli A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J Cell Biol. 1994;125:1109–17. doi: 10.1083/jcb.125.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol. 1992;118:1455–63. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Portman N, Lacomble S, Thomas B, McKean PG, Gull K. Combining RNA interference mutants and comparative proteomics to identify protein components and dependencies in a eukaryotic flagellum. J Biol Chem. 2008;284:5610–19. doi: 10.1074/jbc.M808859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pradel LC, Bonhivers M, Landrein N, Robinson DR. NIMA-related kinase TbNRKC is involved in basal body separation in Trypanosoma brucei. J Cell Sci. 2006;119:1852–63. doi: 10.1242/jcs.02900. [DOI] [PubMed] [Google Scholar]
- 110.Pullen TJ, Ginger ML, Gaskell SJ, Gull K. Protein targeting of an unusual, evolutionarily conserved adenylate kinase to a eukaryotic flagellum. Mol Biol Cell. 2004;15:3257–65. doi: 10.1091/mbc.E04-03-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ralston KS, Hill KL. Trypanin, a component of the flagellar dynein regulatory complex, is essential in bloodstream form African trypanosomes. PLoS Pathog. 2006;2:e101. doi: 10.1371/journal.ppat.0020101. Showed that the DRC is uniquely conserved eukaryotes with motile flagella and is essential in bloodstream trypanosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ralston KS, Hill KL. The flagellum of Trypanosoma brucei: new tricks from an old dog. Int J Parasitol. 2008;38:869–84. doi: 10.1016/j.ijpara.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ralston KS, Lerner AG, Diener DR, Hill KL. Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot Cell. 2006;5:696–711. doi: 10.1128/EC.5.4.696-711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ralston KS, Lerner AG, Hill KL. Flagellar motility is essential for cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system (Meet Abstr.) Am J Trop Med Hyg Suppl. 2005;73(90060):365. [Google Scholar]
- 116.Reuner B, Vassella E, Yutzy B, Boshart M. Cell density triggers slender to stumpy differentiation of Trypanosoma brucei bloodstream forms in culture. Mol Biochem Parasitol. 1997;90:269–80. doi: 10.1016/s0166-6851(97)00160-6. [DOI] [PubMed] [Google Scholar]
- 117.Ridgley E, Webster P, Patton C, Ruben L. Calmodulin-binding properties of the paraflagellar rod complex from Trypanosoma brucei. Mol Biochem Parasitol. 2000;109:195–201. doi: 10.1016/s0166-6851(00)00246-2. [DOI] [PubMed] [Google Scholar]
- 118.Robert A, Margall-Ducos G, Guidotti JE, Bregerie O, Celati C, et al. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in nonciliated cells. J Cell Sci. 2007;120:628–37. doi: 10.1242/jcs.03366. [DOI] [PubMed] [Google Scholar]
- 119.Robinson DR, Gull K. Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature. 1991;352:731–33. doi: 10.1038/352731a0. [DOI] [PubMed] [Google Scholar]
- 120.Robinson DR, Sherwin T, Ploubidou A, Byard EH, Gull K. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J Cell Biol. 1995;128:1163–72. doi: 10.1083/jcb.128.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 122.Rupp G, Porter ME. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J Cell Biol. 2003;162:47–57. doi: 10.1083/jcb.200303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Santrich C, Moore L, Sherwin T, Bastin P, Brokaw C, et al. A motility function for the paraflagellar rod of Leishmania parasites revealed by PFR-2 gene knockouts. Mol Biochem Parasitol. 1997;90:95–109. doi: 10.1016/s0166-6851(97)00149-7. [DOI] [PubMed] [Google Scholar]
- 124.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 125.Schimanski B, Nguyen TN, Gunzl A. Highly efficient tandem affinity purification of trypanosome protein complexes based on a novel epitope combination. Eukaryot Cell. 2005;4:1942–50. doi: 10.1128/EC.4.11.1942-1950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Selvapandiyan A, Kumar P, Morris JC, Salisbury JL, Wang CC, Nakhasi HL. Centrin1 is required for organelle segregation and cytokinesis in Trypanosoma brucei. Mol Biol Cell. 2007;18:3290–301. doi: 10.1091/mbc.E07-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharma R, Peacock L, Gluenz E, Gull K, Gibson W, Carrington M. Asymmetric cell division as a route to reduction in cell length and change in cell morphology in trypanosomes. Protist. 2008;159:137–51. doi: 10.1016/j.protis.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Shen S, Arhin GK, Ullu E, Tschudi C. In vivo epitope tagging of Trypanosoma brucei genes using a one step PCR-based strategy. Mol Biochem Parasitol. 2001;113:171–73. doi: 10.1016/s0166-6851(00)00383-2. [DOI] [PubMed] [Google Scholar]
- 129.Sherwin T, Gull K. The cell division cycle of Trypanosoma brucei brucei: timing of event markers and cytoskeletal modulations. Philos Trans R Soc London Ser B. 1989;323:573–88. doi: 10.1098/rstb.1989.0037. [DOI] [PubMed] [Google Scholar]
- 130.Sinden RE. Sexual development of malarial parasites. Adv Parasitol. 1983;22:153–216. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 131.Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskelet. 2004;57:8–17. doi: 10.1002/cm.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stephan A, Vaughan S, Shaw MK, Gull K, McKean PG. An essential quality control mechanism at the eukaryotic basal body prior to intraflagellar transport. Traffic. 2007;8:1323–30. doi: 10.1111/j.1600-0854.2007.00611.x. Identified a tubulin assembly cofactor, TBCC, localized to the basal body transitional fibers, leading to the idea that this site represents a quality control checkpoint for proteins entering the flagellum. [DOI] [PubMed] [Google Scholar]
- 133.Sui H, Downing KH. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature. 2006;442:475–78. doi: 10.1038/nature04816. [DOI] [PubMed] [Google Scholar]
- 134.Tetley L, Coombs GH, Vickerman K. The surface membrane of Leishmania mexicana mexicana: comparison of amastigote and promastigote using freeze-fracture cytochemistry. Z Parasitenkd. 1986;72:281–92. doi: 10.1007/BF00928737. [DOI] [PubMed] [Google Scholar]
- 135.Tetley L, Vickerman K. Differentiation in Trypanosoma brucei: host-parasite cell junctions and their persistence during acquisition of the variable antigen coat. J Cell Sci. 1985;74:1–19. doi: 10.1242/jcs.74.1.1. [DOI] [PubMed] [Google Scholar]
- 136.Tu X, Wang CC. The involvement of two cdc2-related kinases (CRKs) in Trypanosoma brucei cell cycle regulation and the distinctive stage-specific phenotypes caused by CRK3 depletion. J Biol Chem. 2004;279:20519–28. doi: 10.1074/jbc.M312862200. [DOI] [PubMed] [Google Scholar]
- 137.Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, et al. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2008;122:859–66. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Den Abbeele J, Claes Y, van Bockstaele D, Le Ray D, Coosemans M. Trypanosoma brucei spp. development in the tsetse fly: characterization of the postmesocyclic stages in the foregut and proboscis. Parasitology. 1999;118:469–78. doi: 10.1017/s0031182099004217. [DOI] [PubMed] [Google Scholar]
- 139.Vassella E, Oberle M, Urwyler S, Renggli CK, Studer E, et al. Major surface glycoproteins of insect forms of Trypanosoma brucei are not essential for cyclical transmission by tsetse. PLoS ONE. 2009;4:e4493. doi: 10.1371/journal.pone.0004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110(Pt. 21):2661–71. doi: 10.1242/jcs.110.21.2661. [DOI] [PubMed] [Google Scholar]
- 141.Vaughan S, Gull K. The structural mechanics of cell division in Trypanosoma brucei. Biochem Soc Trans. 2008;36:421–24. doi: 10.1042/BST0360421. [DOI] [PubMed] [Google Scholar]
- 142.Vaughan S, Kohl L, Ngai I, Wheeler RJ, Gull K. A repetitive protein essential for the flagellum attachment zone filament structure and function in Trypanosoma brucei. Protist. 2008;159:127–36. doi: 10.1016/j.protis.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 143.Vaughan S, Shaw M, Gull K. A postassembly structural modification to the lumen of flagellar microtubule doublets. Curr Biol. 2006;16:R449–50. doi: 10.1016/j.cub.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 144.Vickerman K. The mechanism of cyclical development in trypanosomes of the Trypanosoma brucei subgroup: an hypothesis based on ultrastructural observations. Trans R Soc Trop Med Hyg. 1962;56:487–95. doi: 10.1016/0035-9203(62)90072-x. [DOI] [PubMed] [Google Scholar]
- 145.Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci. 1969;5:163–93. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- 146.Vickerman K. The fine structure of Trypanosoma congolense in its bloodstream phase. J Protozool. 1969;16:54–69. doi: 10.1111/j.1550-7408.1969.tb02233.x. Provided some of the earliest descriptions of trypanosome flagellum ultrastructure. [DOI] [PubMed] [Google Scholar]
- 147.Vlachou D, Schlegelmilch T, Runn E, Mendes A, Kafatos FC. The developmental migration of Plasmodium in mosquitoes. Curr Opin Genet Dev. 2006;16:384–91. doi: 10.1016/j.gde.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 148.Walker PJ. Organization of function in trypanosome flagella. Nature. 1961;189:1017–18. doi: 10.1038/1891017a0. [DOI] [PubMed] [Google Scholar]
- 149.Walker PJ, Walker JC. Movement of trypanosome flagella. J Protozool. 1963;10(Suppl):32. [Google Scholar]
- 150.Warner FD. Ciliary intermicrotubule bridges. J Cell Sci. 1976;20:101–14. doi: 10.1242/jcs.20.1.101. [DOI] [PubMed] [Google Scholar]
- 151.Webster P, Russell DG. The flagellar pocket of trypanosomatids. Parasitol Today. 1993;9:201–6. doi: 10.1016/0169-4758(93)90008-4. [DOI] [PubMed] [Google Scholar]
- 152.Wickstead B, Gull K. Dyneins across eukaryotes: a comparative genomic analysis. Traffic. 2007;8:1708–21. doi: 10.1111/j.1600-0854.2007.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wirschell M, Pazour G, Yoda A, Hirono M, Kamiya R, Witman GB. Oda5p, a novel axonemal protein required for assembly of the outer dynein arm and an associated adenylate kinase. Mol Biol Cell. 2004;15:2729–41. doi: 10.1091/mbc.E03-11-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Woodward R, Carden MJ, Gull K. Immunological characterization of cytoskeletal proteins associated with the basal body, axoneme and flagellum attachment zone of Trypanosoma brucei. Parasitology. 1995;111:77–85. doi: 10.1017/s0031182000064623. [DOI] [PubMed] [Google Scholar]
- 155.Wu Y, Deford J, Benjamin R, Lee MG, Ruben L. The gene family of EF-hand calcium-binding proteins from the flagellum of Trypanosoma brucei. Biochem J. 1994;304:833–41. doi: 10.1042/bj3040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang P, Diener DR, Yang C, Kohno T, Pazour GJ, et al. Radial spoke proteins of Chlamydomonas flagella. J Cell Sci. 2006;119:1165–74. doi: 10.1242/jcs.02811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang H, Mitchell DR. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci. 2004;117:4179–88. doi: 10.1242/jcs.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Y, Snell WJ. Flagellar adhesion-dependent regulation of Chlamydomonas adenylyl cyclase in vitro: a possible role for protein kinases in sexual signaling. J Cell Biol. 1994;125:617–24. doi: 10.1083/jcb.125.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao Z, Lindsay ME, Roy Chowdhury A, Robinson DR, Englund PT. p 166, a link between the trypanosome mitochondrial DNA and flagellum, mediates genome segregation. EMBO J. 2008;27:143–54. doi: 10.1038/sj.emboj.7601956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zoraghi R, Seebeck T. The cAMP-specific phosphodiesterase TbPDE2C is an essential enzyme in bloodstream form Trypanosoma brucei. Proc Natl Acad Sci USA. 2002;99:4343–48. doi: 10.1073/pnas.062716599. [DOI] [PMC free article] [PubMed] [Google Scholar]
RELATED RESOURCES
- Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–49. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- Vickerman K, Preston TM. The diversity of the kinetoplastid flagellates. In: Lumsden WHR, Evans DA, editors. Biology of the Kinetoplastida. London: Academic; 1976. pp. 35–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.