Abstract
In teleosts, proper balance and hearing depend on mechanical sensors in the inner ear. These sensors include actin-based microvilli and microtubule-based cilia that extend from the surface of sensory hair cells and attach to biomineralized ‘ear stones’ (or otoliths)1. Otolith number, size and placement are under strict developmental control, but the mechanisms that ensure otolith assembly atop specific cells of the sensory epithelium are unclear. Here we demonstrate that cilia motility is required for normal otolith assembly and localization. Using in vivo video microscopy, we show that motile tether cilia at opposite poles of the otic vesicle create fluid vortices that attract otolith precursor particles, thereby biasing an otherwise random distribution to direct localized otolith seeding on tether cilia. Independent knockdown of subunits for the dynein regulatory complex and outer-arm dynein disrupt cilia motility, leading to defective otolith biogenesis. These results demonstrate a requirement for the dynein regulatory complex in vertebrates and show that cilia-driven flow is a key epigenetic factor in controlling otolith biomineralization.
Cilia are evolutionarily conserved organelles that perform motility, sensory and transport functions and are required for normal vertebrate development and physiology2–5. As such, cilium defects underlie a broad spectrum of human diseases4,5. Among the roles of ciliated organs in vertebrate embryogenesis, the contribution of cilia to inner-ear development remains poorly understood. In the zebrafish, Danio rerio, it has been proposed that beating cilia participate in the biogenesis of otoliths6, which are analogous to otoconia in the otolithic membrane of human ears. These biomineralized particles provide an inertial mass that facilitates deflection of underlying microvilli and cilia, thereby initiating signalling events that allow the brain to detect sound, gravity and linear acceleration1,7–9. During early development, nascent otoliths are formed from a pool of precursor particles and tethered to cilia in the otic vesicle6. So far, direct evidence for the necessity of ciliary motility in this process is lacking.
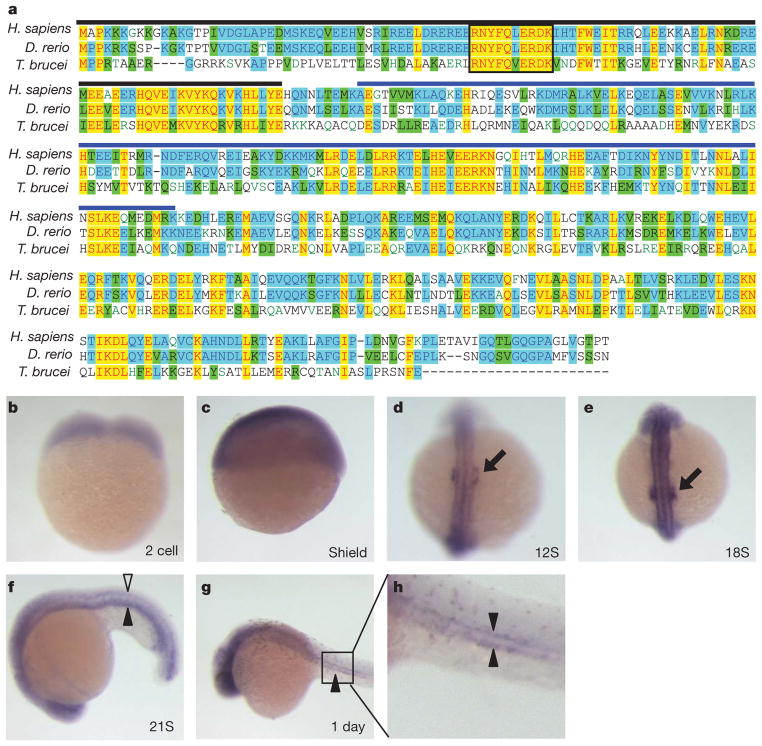
In protists, ciliary motility is controlled by the Dynein Regulatory Complex (DRC), which regulates axonemal dynein activity in response to signals from the radial spokes and central pair apparatus10–15. The DRC subunit trypanin is conserved across diverse phyla15–18 and the vertebrate (human) trypanin homologue, growth arrest-specific 8 (here called GAS8, in line with the HUGO database, but also, and originally, designated GAS11) is a microtubule-binding protein localized to regions of dynein regulation in mammalian cells19–21. So far, however, a requirement for GAS8 and the DRC in vertebrates has not been established. We identified a single trypanin homologue in zebrafish encoding a protein that is 63.8% identical to the human GAS8 protein and 32.0% identical to trypanin from Trypanosoma brucei (Fig. 1). The sequence identity and conserved genomic structure (Fig. 2a)15,22 indicate that this zebrafish protein, designated Gas8, is indeed a member of this conserved family of dynein regulatory proteins13,15. Maternal gas8 transcripts are ubiquitous throughout the embryo during early development (Fig. 1b, c). By the 12-somite stage, however, expression becomes concentrated in the developing ears (Fig. 1d, arrow) and this persists through the 18-to 20-somite stage (Fig. 1e, Supplementary Fig. 1). Transcripts are also clearly present in the brain, neural tube and pronephric ducts (Fig. 1f–h). Therefore, gas8 is expressed in ciliated tissues during zebrafish organogenesis.
Figure 1. gas8 is expressed in ciliated tissues.
a, Protein sequence alignment of trypanin/GAS8 from Homo sapiens, Danio rerio and Trypanosoma brucei. Yellow highlighting indicates absolutely conserved residues, blue indicates residues conserved in two homologues and green indicates residues that are conservative substitutions. The boxed region indicates a conserved RNYFQERDK stretch that is found in every known trypanin/GAS8 homologue17. The conserved microtubule binding domain ‘GMAD’ and the regulatory domain ‘IMAD’19 are indicated with blue and black overlines, respectively. b–h, In situ hybridizations show the gas8 expression pattern during the first 24 h of embryonic development. Developing ears (black arrows), neural tube (open arrowhead) and pronephric ducts (filled arrowheads) are shown. Developmental stages are indicated in each panel; S, somite. h is an enlargement of the boxed region in g.
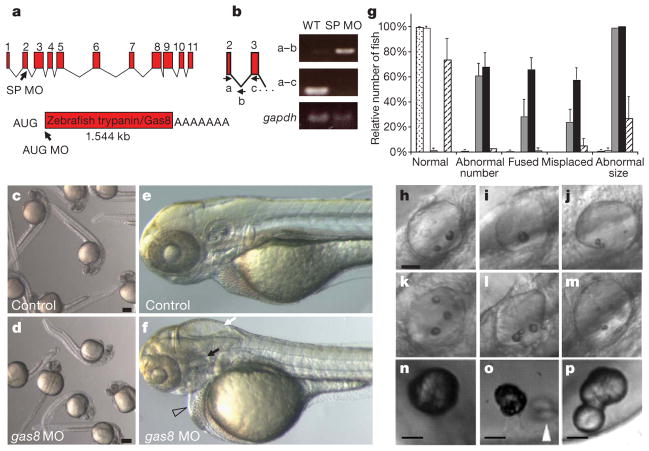
Figure 2. gas8 morphants exhibit developmental defects.
a, Intron/exon structure of the gas8 locus, which encodes a predicted 1.54-kb transcript. The positions of splice blocking (SP MO) and translation blocking (AUG MO) morpholino oligonucleotides are shown. b, RNA from wild-type (WT) and gas8 splice morphant embryos was analysed by PCR with reverse transcription (RT–PCR) using a forward primer (a) in the second exon and a reverse primer in either the second intron (b) or the third exon (c). In the morphant, blocking of the exon-2 splice donor site leads to a 315-bp RT–PCR product with the first primer set and no product with the second primer set. Controls for RT–PCR were provided by amplification of a 95-bp fragment of gapdh. c–f, gas8 morphants have a variety of defects: overall morphology of controls (c) and gas8 morphants (d) at 24 h.p.f.; detail of control (e) and morphant (f) embryos at 3 days post-fertilization (d.p.f.) showing hydrocephaly (white arrow), pericardial oedema (open arrowhead), disorganized somites and otolith abnormalities (black arrow). g, Quantitative analysis of otolith defects at 3 d.p.f. The relative number of fish having the indicated defect is shown for uninjected embryos (stippled bars; n = 324, five experiments), embryos injected with control MO (white bars; n = 167, two experiments), SP MO (grey bars; n = 89, two experiments), AUG MO (black bars; n = 96, two experiments) or co-injected with AUG MO and 250 pg in-vitro-transcribed gas8 mRNA (hatched bars; n = 225, two experiments). Error bars, s.d. h–p, Panels show the spectrum of otolith defects observed in gas8 morphants at 3 d.p.f. (h–m) and earlier times (n–p): normal otoliths (h); a single otolith (i); ectopic, fused and small otoliths (j–m); and nascent otoliths in control (n, 27 h.p.f.) and gas8 morphant (o, 24 h.p.f.; p, 27 h.p.f.) embryos. Scale bars, 30 μm (h–m); 20 μm (n–p). White arrowhead indicates ectopic and fused otoliths in the gas8 morphant. Embryos were injected with 6 ng (AUG MO), 4 or 5 ng (SP MO), 6 ng (standard control MO) or 6 ng (mismatch AUG MO) morpholinos.
To determine how loss of gas8 expression affects zebrafish development we employed antisense morpholino oligonucleotides (Fig. 2). Hydrocephaly, neural tube cell death and left–right axis defects are common in ciliary morphants and mutants3 and were evident in gas8 morphants (Fig. 2, Supplementary Fig. 2). Given the high expression of gas8 in the otic vesicle, we examined ear development more closely. By two days post-fertilization, inner-ear atrophy was evident gas8 morphants. The length along the antero-posterior axis was 30% less than in control embryos (morphants, 52 ± 6 μm, n = 15; control, 73 ± 7 μm, n = 8; 24–27 hours post-fertilization (h.p.f.); see, for example, Figs 2e, f, 3a, b). By three days post-fertilization, exactly two otoliths were present in control zebrafish, one at the anterior end and one at the posterior end of the otic vesicle, positioned ventrally to a semicircular canal6. By contrast, gas8 morphants had abnormal numbers of otoliths, fused otoliths, abnormally positioned otoliths and small otoliths (Fig. 2g–m). Examination of 24-h.p.f. and 27-h.p.f. embryos showed that the same spectrum of defects is evident during nascent otolith formation (Fig. 2n–p). The otolith phenotype is 95–100% penetrant and co-injection of in-vitro-transcribed gas8 messenger RNA carrying five base-pair mismatch mutations to prevent morpholino hybridization rescued the defect in a majority of embryos (Fig. 2g). Because inner ear patterning is tightly linked with neuraxis patterning23, we analysed markers for the hindbrain (egr2b), midbrain (eng2a), forebrain (otx2), inner-ear anterio-ventral area (fgf8a)24 and inner-ear anterior/posterior extremity (bmp4)25. We did not detect differences between controls and gas8 morphants (data not shown). Therefore, otolith defects are not due to abnormal neuraxis or inner-ear patterning.
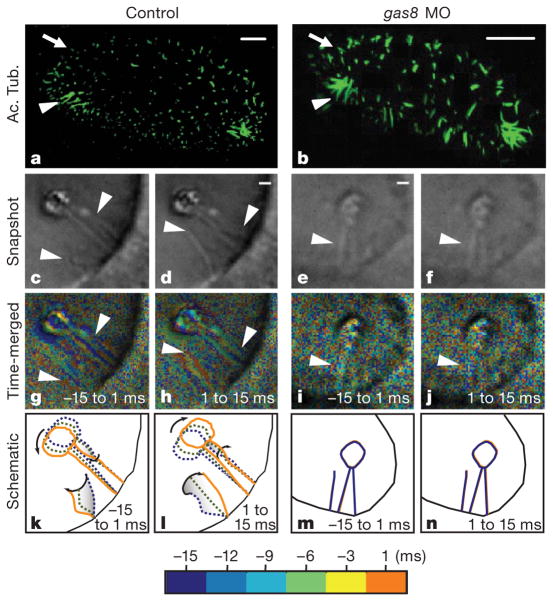
Figure 3. Gas8 is required for tether cilia motility.
a, b, Cilia in control (a) and gas8 morphant (b) embryos at 24 h.p.f., visualized by immunofluorescence labelling with anti-acetylated tubulin antibodies (Ac. Tub.). Arrowheads indicate the location of the tether cilia and arrows indicate short cilia. Scale bars, 10 μm. c–n, Tether cilia are motile in control embryos, but not in gas8 morphants. Bright-field snapshot images from high-speed videos of cilia in controls (c, d) and gas8 morphants (e, f), showing two steps of the cilia beat cycle with a 15-ms interval (half the period of a beat). g–j, Time-to-colour merge of six frames encompassing 15 ms of the cilia motion immediately preceding the still images shown in c–f, respectively. Cilia position in time is marked by different colours following the colour bar. When merged, moving objects are visible in the corresponding colours, whereas immotile objects only show background and noise. k–n, Diagrams showing cilia and otolith motion in control (k, l) and immotility in gas8 morphant (m, n) embryos with three time points along half the period (15 ms) of the cilia beat cycle (see colour bar). Still images from control (c, d) and gas8 morphant (e, f) embryos are taken from Supplementary Movies 3 and 5, respectively. Scale bars, 1 μm (c–f). Arrowheads point to tether cilia.
We next asked whether otolith defects were due to improper formation or placement of cilia in the otic vesicle. By 24 h.p.f., two classes of cilia were visible in the otic vesicle6 (Fig. 3a, Supplementary Movie 1). Numerous short cilia were dispersed throughout the otic vesicle, and small patches of longer, ‘tether’ cilia were found exclusively at the anterior and posterior poles. Between 19 and 27 h.p.f., small precursor particles coalesced on tether cilia to form anterior and posterior otoliths6. We examined cilia distribution and size in morphant embryos during the critical developmental window of 19–27 h.p.f., when cilia are postulated to function in otolith assembly. At the 20-somite stage (corresponding to ~19 h.p.f. in wild type), control and morphant embryos had cilia in the developing otic vesicle (data not shown). gas8 morphants were slightly developmentally delayed, a common feature of morphant fish. Hence, we staged embryos based on developmental progression defined in ref. 26. By 24 h.p.f., both short cilia and tether cilia were distinguishable at the correct locations in control and gas8 morphant embryos (Fig. 3b, Supplementary Movie 2). Otic vesicle size was reduced as noted above, but we did not detect major length differences of cilia between control (tether, 5.9 ± 0.2 μm; short, 1.4 ± 0.1 μm; n = 5 embryos) and morphant (tether, 5.9 ± 0.4 μm; short, 1.2 ± 0.2 μm; n = 7 embryos) embryos. At later stages, tether cilia persisted whereas short cilia began to disappear as expected6 in both control and morphant embryos (data not shown). Cilia were also observed in the pronephric ducts and neural tube in gas8 morphants (data not shown). Therefore, loss of gas8 expression did not prevent formation, maintenance or correct positioning of cilia.
Because protist GAS8 homologues, trypanin in T. brucei and paralyzed flagella 2 in Chlamydomonas reinhardtii, are specifically involved in controlling ciliary beat13–15, we examined cilia motility directly using in vivo, high-speed video microscopy. In all control embryos, one to three beating tether cilia were detected near each nascent otolith (Fig. 3, Supplementary Fig. 3, Supplementary Movies 3–5). Beating cilia directly bore the forming otolith or were located nearby (5–10 μm distant), often causing the otolith itself to oscillate (Supplementary Movies 3, 5). These cilia beat with a frequency of 34 ± 6 Hz (n = 20) at 24 h.p.f. Short cilia were not motile (Supplementary Movie 6). This contrasts with a previous report suggesting that tether cilia are immotile whereas short cilia distributed throughout the ear are motile6. The reason for this discrepancy is not clear, but it is probably due to technical challenges associated with imaging cilia motility, which was inferred indirectly in the earlier work and imaged directly here. In gas8 morphants at every stage examined (19–27 h.p.f.), a majority of embryos displayed immotile tether cilia (60%, n = 30; Fig. 3, Supplementary Fig. 3, Supplementary Movies 7–8). Commonly, gas8 morphants displayed ectopic otoliths located at the base of non-motile tether cilia (Fig. 2o). In some cases, morphants harboured ectopic beating cilia (23%, n = 30). To confirm that the ear phenotype was due to cilia immotility and not loss of another, unknown gas8 function, we knocked down two genes directly involved in cilia motility: the gene for leucine-rich repeat-containing 50 protein, lrrc50, an outer-arm dynein subunit shown previously to be required for cilia motility27,28; and the left–right dynein gene dnah9, a well-characterized motor protein involved in cilia motility29. The same results were obtained upon knockdown of lrrc50 (Supplementary Figs 4, 5). Furthermore, simultaneous knockdown of lrrc50 and dnah9 had a synergistic effect, causing more significant motility and otolith defects than either single knockdown alone. These treatments neither affected brain development nor triggered neural tube cell death and pericardial oedema. In all cases, abnormal otolith size, number and positioning were directly correlated with defective ciliary motility in morphants (Supplementary Fig. 4).
Otoliths are composite crystals assembled from a common pool of small, precursor particles. We hypothesized that otolith defects in cilia morphants arise as a consequence of abnormal fluid flow and the concomitant failure to properly direct precursor particle movements. To test this hypothesis, we examined fluid flow patterns in the otic vesicle by tracking otolith precursors at high temporal resolution. A direct correlation between cilia beat and fluid flow was observed (Fig. 4, Supplementary Movies 9–11). In control embryos, the motion of precursor particles near the otolith was non-random, whereas those further away from the otolith exhibited Brownian motion (Fig. 4g, h, Supplementary Movies 9–10), consistent with a previous report6. Cilia beating triggers a local flow in the vicinity of the otolith, attracting precursors at the base of tether cilia and propelling them towards the otolith (Supplementary Fig. 6, Supplementary Movies 9–10). By contrast, in gas8 morphants, particles near tether cilia exhibited purely diffusive behaviour (Fig. 4, Supplementary Movie 11). Therefore, the absence of ciliary beating limits particles to random motion, leading to formation of ectopic aggregates.
Figure 4. Tether cilia motility drives otolith biogenesis.
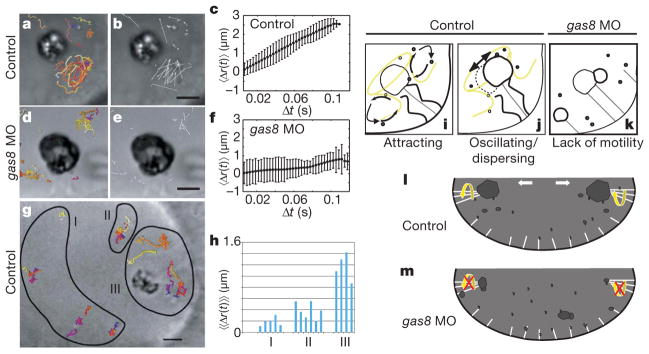
a–h, Particle tracking analysis demonstrates that cilium-dependent fluid dynamics drive precursor particle movement near the otolith. In control embryos, particle tracks (a) and displacements (b) show that particles near the otolith move by non-Brownian motion. Each track has a different colour. d, e, In gas8 morphants, particle tracks (d) and displacements (e) show decreased particle displacements in comparison with control. c, f, Mean particle displacement, 〈Δr(t)〉, plotted as a function of time. In control embryos, mean particle displacement is large and non-random, indicating diffusive transport. In gas8 morphants, mean particle displacement is small and random, indicating Brownian motion only. Error bars indicate the variance in the calculation of 〈Δr(t)〉. g, h, Particle tracking in control embryos shows that particle displacement is directly correlated with position relative to the otolith. g, particle tracks. h, Displacements of particles in regions I, II and III of g were calculated as a function of time and the average of the mean displacement, 〈〈Δr(t)〉〉, for each particle is shown. Net particle displacement decreases with increasing distance from tether cilia, indicating the reduction of the influence of ciliary beating. i–k, Diagrams depicting cilium-dependent otolith biogenesis. i, Tether cilia motility creates vortices that attract precursor particles. j, Cilia motility further serves to disperse particles locally and causes oscillation of the otolith, together facilitating uniform otolith growth. k, In gas8 morphants, absence of ciliary motility limits particles to Brownian motion. l, In wild-type embryos, the net consequence of tether cilia motility is that precursor particles are concentrated near the tethers, preferentially seeding otoliths at two poles of the otic vesicle. m, In gas8 morphants, loss of normal ciliary motility leads to formation of ectopic aggregates, non-uniform otolith growth and small particles that fail to coalesce into full-sized otoliths. Scale bars, 5 μm.
Our results demonstrate that Gas8 is required for normal motility of cilia in the otic vesicle and that ciliary motility is essential for normal ear development. The otic vesicle is a closed epithelial organ and fluid flow within this vesicle has been suggested to contribute to otolith formation6. Our study provides direct experimental evidence in support of this hypothesis. On the basis of high-speed video microscopy of cilia motility and quantitative analysis of precursor particle movements in wild-type and gas8 morphant embryos, we propose a new, cilium-dependent hydrodynamic mechanism for otolith biogenesis (Fig. 4). In this model, motility of tether cilia at the poles of the otic vesicle establishes a vortex that attracts otolith precursors (Fig. 4i, l), thereby biasing an otherwise random distribution of precursor particles and concentrating them near the two patches of tether cilia. This ensures preferential otolith seeding at the poles of the otic vesicle. At the otic vesicle poles, tether cilia motility further serves to disperse precursor particles locally and oscillation of the otolith increases effective contact area with precursors (Fig. 4j). Together, this prevents particles from sedimenting to form ectopic aggregates and promotes efficient uniform otolith growth. This model explains the different features of the otolith phenotype observed in gas8 morphants (Fig. 4k, m).
Our findings add to a growing list of developmental processes requiring fluid dynamic inputs for proper growth and patterning, further showing that epigenetic cues are part of the embryonic developmental program. In humans, hearing and balance defects are common among the elderly and are the most frequent sensory hereditary defects in newborns30. Although human patients with ciliopathies have not generally been observed to have obvious hearing loss4, our results should stimulate investigations to look for more subtle inner-ear changes. To conclude, our studies demonstrate a requirement for motile cilia in vertebrate ear development and suggest that DRC subunits should be considered as candidates for disease genes contributing to ciliopathies in humans.
METHODS
Zebrafish lines
Wild-type AB zebrafish were maintained and raised as described previously31. Dechorionated embryos were kept at 28.5 °C in E3 solution with or without 0.003% 1-phenyl-2-thiourea (PTU, Sigma) to suppress pigmentation. Embryos were staged according to somite number (S) or hours post-fertilization31.
gas8 riboprobe generation and in situ hybridization
The predicted gas8 gene transcript of zebrafish, Danio rerio (Ensembl Gene ID #ENSDARG0000004 0871), was obtained from the annotated Ensembl automatic analysis pipeline and predicted exon/intron boundaries, transcript size and predicted protein size were obtained as described previously32. D. rerio gas8 complementary DNA in pME18S-FL3 (clone IMAGE:5410961) was subcloned into the expression vector pcGlobin2 by PCR amplification of gas8 from pME18S-FL3 using SacII and XhoI flanked primers (5′ primer, CCCCGCGGGTGGAACAATTCAATGCATT; 3′ primer, CCGCTCGAGATTTGAAGAAGAAACAAACA). GAS8–pcGlobin2 was linearized using BamHI and the antisense digoxigenin-labelled full length riboprobe was transcribed using SP6 RNA polymerase (Promega). Whole-mount in situ hybridization was carried out essentially as described in ref. 33 using either the gas8 probe or one of the following probes: spaw, eng2a, otx2, egr2b, bmp4, fgf8a (provided by L. Trinh). Embryos were fixed with 4% para-formaldehyde, digested with proteinase K, and hybridized with the probe at 67 °C. Alkaline-phosphatase-conjugated anti-digoxigenin antibody (Roche) was used to detect the gas8 signals. After staining with NBT/X-phosphate (Roche), embryos were refixed with 4% paraformaldehyde and stored in PBS buffer. For imaging, embryos were mounted in 3.5% methyl cellulose and photographed on a Zeiss Axioplan microscope equipped with a Zeiss AxioCam digital camera.
Morpholino antisense oligonucleotides
Morpholino antisense oligonucleotides were designed to either target the translation start site of the gas8 mRNA (AUG MO) or to target the splice donor site of the second coding exon (SP MO) and were obtained from Gene Tools. Gas8 AUG MO: GCGACGATTTTCTTTTTGGTGGCAT; gas8 SP MO: CGTTACCGACAAAATACCTGCTCTT; gas8 5-bp mismatch AUG MO, used for rescue experiments: GCCACCATTTTGTTTTTGCTGGGAT; standard control MO: CCTCTTACCTCAGTTACAATTTATA. lrrc50 SP MO: AATGTAGACACTAAAGTTACCTGCT; lrrc50 5-bp mismatch MO: AATcTAGACAgTAAAcTTAgCTcCT; dnah9 AUG MO: CGGTTCCTGCTCCTCCATCGCGCCG. The morpholinos were diluted in 5 mM HEPES buffer, pH 7.6. Embryos were injected at the one- to four-cell stage with either 6 ng AUG MO, 4 or 5 ng SP MO, or 6 ng standard control MO or mismatch AUG MO. For morpholino amounts used in lrrc50 and dnah9 injections, see Supplementary Fig. 5. The first MO (SP MO) targets the splice junction between exons 2 and 3, causing abnormal RNA processing and yielding a predicted transcript with an early stop codon (Fig. 2a). The second MO (AUG MO) targets the translational start site (Fig. 2a). Splice interference by the SP MO was demonstrated by RT–PCR (Fig. 2b).
Microinjection of either MO consistently produced hydrocephaly, brain and neural tube cell death, pericardial oedema, curved body axis and otolith defects (Fig. 2 and data not shown). Left–right axis determination was also affected, as demonstrated by in situ hybridization with the southpaw protein, a nodal-related protein that is normally expressed in the left lateral plate mesoderm34 (Supplementary Fig. 1).
RT–PCR
Primer pairs used in RT–PCR to investigate the knockdown efficiency of gas8 SP MO are 5′-AATCGTCGCCGAAGGGCAAAAC-3′(forward in exon 2) and 5′-CTGCATTGTTGTGGCTGCAG-3′ (reverse in intron 2–3) or 5′-CTTGGTGGCGTTCCTCTACTTC-3′ (reverse in exon 3); these pairs yield no product (normal splicing) or 315 bp (abnormal splicing with intron 1 retained), and 258 bp (normal splicing) or 1,957 bp (abnormal splicing with intron 1 retained; too large to be amplified under conditions used), respectively. Primers used for lrrc50 were (1) 5′-GATCGTATCCACATCGATGAACT-3′ (‘lrrc50 – Forward’), (2) 5′-CTCCATTAGGCATTTTACATG-3′ (‘lrrc50 – Intron 1’), and (3) 5′-CTGGGGCCTGAAGGCTTTATG-3′ (‘lrrc50 – Exon 2’). Control primer pairs for amplification of gapdh transcripts were also used to amplify a 95-bp region: 5′-TGTGATGGGAGTCAACCAGGACAA-3′ (forward); 5′-TTAGCCAGAGGAGCCAAGCAGTTA-3′ (reverse).
Immunofluorescence
Embryos were fixed in 4% formaldehyde in PBS buffer for a minimum of 2 h, rinsed in methanol, permeabilized by proteinase K treatment, blocked in 10% goat serum in PBS buffer containing 1% DMSO and 0.1% Tween 20 for at least 1 h, and incubated with a 1:500 dilution of anti-acetylated alpha tubulin antibody (Sigma) overnight. Secondary antibody, anti-mouse IgG Alexa-Fluor 488 (Molecular Probes), was used at 1:200 dilution. Embryos were then embedded in 1% low melting agarose and imaged on a Zeiss LSM 510 confocal microscope using a water immersion objective with numerical aperture 1.1 and magnification either ×40 or ×63. Confocal images were collected using Zeiss LSM 510 software. Images were processed using Imaris (Bitplane AG).
High-speed video microscopy
For video microscopy, fish were anaesthetized with 0.0175% tricaine and embedded in agarose wells using 0.7% low-melt agarose. As a test, imaging of embryos without anaesthetic did not affect cilia beating relative to anaesthetized animals. Particle tracking was performed using Imaris (Bitplane AG). Data were further analysed using Matlab scripts. One hallmark of purely diffusive systems is a non-zero mean displacement 〈Δr(t)〉 over extended periods of time. Thus, a marker for non-diffusive transport is a changing mean displacement over time. We measured 〈Δr(t)〉 over a range of elapsed times t. To determine with a single parameter whether the mean displacement changes over time, we calculated the average of this quantity, 〈〈Δr(t)〉〉. If the particle is freely diffusing then 〈〈Δr(t)〉〉 ≈ 0; if it is driven then 〈〈Δr(t)〉〉 > 0. Cilia motion displayed in Fig. 3g–j was obtained using the time-to-colour Matlab script (provided by M. Liebling). Time-to-colour visualizes moving objects by assigning a colour according to the point in the cycle from which the image was taken. The resulting coloured images are obtained by superimposing six frames of the image sequence plus a greyscale frame. The first frame is assigned blue and the last frame, which corresponds to the still frame, is assigned orange. Non-moving objects, therefore, look like noise whereas moving objects display coherent patterns.
Supplementary Material
Supplemental Figure S1. High magnification image of gas11 expression in the otic vesicle. In situ hybridization shows gas11 is expressed throughout the otic vesicle at 8 to 13-somite stages, then becomes concentrated at the poles of the otic vesicle (white arrows) at 15 to 18-somite stages. Beyond the 18-somite stage, the signal in the otic vesicle is not detectable above that in surrounding tissues (not shown). The dorsal/ventral and anterior/posterior axis are shown.
Supplemental Figure S2. gas11 knockdown results in left-right axis defects. In situ hybridizations using southpaw riboprobe demonstrate laterality defects in gas11 morphants at the 16–18 somite stage. The relative numbers of uninjected (n = 119) and gas11 morphant (n = 138) embryos having each staining pattern are shown.
Supplemental Figure S3. gas11 is required for tether cilia motility. Brightfield images taken from high-speed videos of tether cilia in control (top) and gas11 morphant (bottom) embryos. Panels are consecutive frames extracted at 16 ms intervals from supplemental movies 5 (control), and 8 (gas11 morphant). Lines trace the cilium and are colorized (violet to red) in time sequence. These are then aligned at the base of the cilium and overlayed in the right-most panel to generate the “combined” image.
Supplemental Figure S4. Morpholino knockdown of lrrc50 and lrdr1 disrupts tether cilia motility and causes otolith defects. (a) Table summarizing the effect of lrrc50 and lrdr1 knock down. To confirm the specificity of the lrrc50 morpholino, a control morpholino (5 base pairs mismatch) was injected. Error bars show standard deviation. (b–f) phenotypic comparison between control (b) and morpholino injected embryos (c–f) at 27 hpf. Among multiple otolith defects the most common are: (c) fused otolith, (d) smaller otoliths, (e) mis-positioned otolith, (f) single otolith. Left otic vesicle, anterior to the right. Scale bar: 20 microns.
Supplemental Figure S5. The lrrc50 splice morpholino interferes with lrrc50 splicing. (A) RT-PCR with two different primer sets confirms that the lrrc50 splicing morpholino interferes with lrrc50 splicing. mRNA from control embryos (Ctrl2) or embryhos injected with 10 ng of splicing MO (Mo-2) were subjected to RT-PCR with the indicated primers for lrrc50, or GAPDH as a control. The positions of lrrc50 primers are shown relative to the intron/exon positions of the lrrc50 mRNA (not to scale). (B) RT-PCR with lrrc50 primers (1) x (3) showing a decrease in the abundance of the product from the spliced transcript (147 bp, open arrow) and the appearance of a band corresponding to the size expected for the product from the unspliced transcript in the injected sample (1241 bp, black arrow). GAPDH mRNA levels were unaffected. (C) RT-PCR with lrrc50 primers (1) x (2) shows the appearance of a band corresponding to the size predicted from the unspliced transcript in the injected sample (1147 bp, red arrow). The same results were obtained with an independent set of mRNA samples (not shown).
Supplemental Figure S6. Particle displacement in control embryo. White arrows indicate particle direction, illustrating the attractive flow at the base of the cilium. These particle displacements correspond to the particle traces shown in figure 4G.
Supplemental movie 1. Three dimensional display of a control inner ear at stage 27 hpf after immunofluorescence labeling of cilia with acetylated tubulin antibody. Notice the two clusters of tether cilia on the lateral side of the inner ear. Side view, anterior on the right. (QuickTime: 1.27 MB)
Three dimensional display of a gas11 morphant inner ear at stage 27 hpf after acetylated tubulin immunofluorescence labeling. The two clusters of tether cilia are properly located. Side view, anterior on the right. (QuickTime: 2.30 MB)
Video shows tether cilia motility in control embryo. This video corresponds to snapshots shown in figure 3A, B. One cilium is attached to the otolith and beating of this cilium causes the otolith to move. A second motile cilium is also evident. Embryo is 20 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 1.30 MB)
Video shows tether cilia motility in control embryo. Embryo is 24 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 1.50 MB)
Video shows tether cilia motility in control embryo. Cilium beating causes otolith to move. This video corresponds to snapshots shown in supplemental figure 2 (control). Embryo is 20–22 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 2.95 MB)
Video shows short cilia in control embryo are not motile. Embryo is 20–22 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 5.9 MB)
Video shows tether cilia in a gas11 morphant embryo are not motile. Embryo is 20 hpf. This video corresponds to snapshots shown in figure 3C,D. (QuickTime: 0.83 MB)
Video shows tether cilia in a gas11 morphant embryo are not motile. Embryo movement during the video is due to normal embryonic activity. This video corresponds to snapshots shown in supplemental figure 2 (gas11 MO). Embryo is 20–22 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 8.05 MB)
High speed video microscopy of tether cilia in a control embryo at 24 hpf showing vortices in the vicinity of the tether cilia and particle propelling along the growing otolith. Acquisition rate: 322 frames/sec played at 50 frames/sec. (QuickTime: 2.94 MB)
Same as 9a with particle tracking. This video corresponds to figure 4A, B. Acquisition rate: 64 frames/sec played at 20 frames/sec. (QuickTime: 10.69 MB)
High speed video microscopy of the inner ear in a control embryo at 23 hpf showing high displacement of otolith precursors in the vicinity of tether cilia and low displacement away from tether cilia. Acquisition rate: 322 frames/sec played at 20 frames/sec. (QuickTime: 3.11 MB)
Same as supplemental movie 10a with particle tracking. This video corresponds to figure 4G and supplemental Figure S3. Acquisition rate: 64 frames/sec played at 50 frames/sec. (Quicktime: 1.59 MB)
High speed video microscopy of tether cilia in a gas11 morphant at 25 hpf showing very low particle displacement in the vicinity of tether cilia. Acquisition rate: 322 frames/sec played at 20 frames/sec. (QuickTime: 1.36 MB)
Same as 11a with particle tracking. This video corresponds to figure 4D, E. Acquisition rate 64 frames/sec played at 50 frames/sec. (QuickTime: 14.79 MB)
Acknowledgments
We are grateful to R. Crosbie for discussions and encouragement throughout the course of the project. We thank I. Drummond and C. Nguyen for discussions and comments on the work. We are grateful to L. Trinh, O. Bricaud and A. Collazo for sharing reagents and for providing probes, as well as to all the members of the Fraser laboratory for discussions, in particular M. Liebling and W. Supatto for sharing Matlab scripts and comments. We are grateful to Z. P. Kabututu for performing the lrrc50 reverse transcriptase PCR experiments. J.V. was supported by a fellowship from the Human Frontier Science Program, D.W. was supported by the NIH Medical Scientist Training Program at UCLA/Caltech. J.R.C. was supported by NIH RSDA training grant no. M07185 and a Warsaw Fellowship from the MIMG Department at UCLA. A.D.L is supported by an NSF fellowship. This work was supported by NIH grant R01 HL081799 (J.C.), NIH grant R01AI52348 and Beckman Young Investigator Award (K.L.H.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions J.R.C., J.V., D.W. and K.L.H. designed the experiments and interpreted the results. J.R.C, J.V. and D.W. conducted the experiments. J.V. and D.W. developed the equipment and systems for and performed in vivo video imaging for the quantitative flow study and analyzed the data with J.R.C., S.F. and K.L.H. A.D.L. assisted with in situ hybridization. A.D.L. and J.C. provided guidance on gas8 morpholino injections. The manuscript was written by J.R.C., J.V., D.W. and K.L.H. All authors discussed the results and commented on the manuscript.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Sollner C, Nicolson T. In: Biomineralization: From Biology to Biotechnology and Medical Application. 2. Bauerlein E, editor. Wiley-VCH; 2004. pp. 229–242. [Google Scholar]
- 2.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 3.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 4.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 5.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nature Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 6.Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev Biol. 1997;191:191–201. doi: 10.1006/dbio.1997.8736. [DOI] [PubMed] [Google Scholar]
- 7.Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Hughes I, Thalmann I, Thalmann R, Ornitz DM. Mixing model systems: using zebrafish and mouse inner ear mutants and other organ systems to unravel the mystery of otoconial development. Brain Res. 2006;1091:58–74. doi: 10.1016/j.brainres.2006.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- 10.Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982;28:115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- 11.Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol. 1992;118:1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner LC, O’Toole E, Perrone CA, Giddings T, Porter ME. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J Cell Biol. 1994;127:1311–1325. doi: 10.1083/jcb.127.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralston KS, Lerner AG, Diener DR, Hill KL. Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot Cell. 2006;5:696–711. doi: 10.1128/EC.5.4.696-711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchings NR, Donelson JE, Hill KL. Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J Cell Biol. 2002;156:867–877. doi: 10.1083/jcb.200201036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupp G, Porter ME. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J Cell Biol. 2003;162:47–57. doi: 10.1083/jcb.200303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron DM, Ralston KS, Kabututu ZP, Hill KL. Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J Cell Sci. 2007;120:478–491. doi: 10.1242/jcs.03352. [DOI] [PubMed] [Google Scholar]
- 17.Hill KL, Hutchings NR, Grandgenett PM, Donelson JE. T lymphocyte triggering factor of African trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J Biol Chem. 2000;275:39369–39378. doi: 10.1074/jbc.M006907200. [DOI] [PubMed] [Google Scholar]
- 18.Ralston KS, Hill KL. Trypanin, a component of the flagellar dynein regulatory complex, is essential in bloodstream form African trypanosomes. PLoS Pathogens. 2006:2. doi: 10.1371/journal.ppat.0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekker JM, et al. Direct interaction of Gas11 with microtubules: Implications for the dynein regulatory complex. Cell Motil Cytoskeleton. 2007;64:461–473. doi: 10.1002/cm.20196. [DOI] [PubMed] [Google Scholar]
- 20.Colantonio JR, et al. Expanding the role of the dynein regulatory complex to non-axonemal functions: association of GAS11 with the golgi apparatus. Traffic. 2006;7:538–548. doi: 10.1111/j.1600-0854.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 21.Yeh SD, et al. Isolation and properties of Gas8, a growth arrest-specific gene regulated during male gametogenesis to produce a protein associated with the sperm motility apparatus. J Biol Chem. 2002;277:6311–6317. doi: 10.1074/jbc.M106941200. [DOI] [PubMed] [Google Scholar]
- 22.Whitmore SA, et al. Characterization and screening for mutations of the growth arrest-specific 11 (GAS11) and C16orf3 genes at 16q24.3 in breast cancer. Genomics. 1998;52:325–331. doi: 10.1006/geno.1998.5457. [DOI] [PubMed] [Google Scholar]
- 23.Bok J, Brunet LJ, Howard O, Burton Q, Wu DK. Role of hindbrain in inner ear morphogenesis: analysis of Noggin knockout mice. Dev Biol. 2007;311:69–78. doi: 10.1016/j.ydbio.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 25.Mowbray C, Hammerschmidt M, Whitfield TT. Expression of BMP signalling pathway members in the developing zebrafish inner ear and lateral line. Mech Dev. 2001;108:179–184. doi: 10.1016/s0925-4773(01)00479-8. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel CB, Ballard WW, Kimmel SR, Ullman B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan-Brown J, et al. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev Biol. 2008;314:261–275. doi: 10.1016/j.ydbio.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rooijen E, et al. LRRC50, a conserved ciliary protein implicated in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1128–1138. doi: 10.1681/ASN.2007080917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawakami Y, Raya A, Raya RM, Rodriguez-Esteban C, Belmonte JC. Retinoic acid signalling links left–right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature. 2005;435:165–171. doi: 10.1038/nature03512. [DOI] [PubMed] [Google Scholar]
- 30.Shastry BS. Mammalian cochlear genes and hereditary deafness. Microb Comp Genomics. 2000;5:61–69. doi: 10.1089/10906590050179747. [DOI] [PubMed] [Google Scholar]
- 31.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) Univ. Oregon Press; 1993. [Google Scholar]
- 32.Curwen V, et al. The Ensembl automatic gene annotation system. Genome Res. 2004;14:942–950. doi: 10.1101/gr.1858004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JN, Fishman MC. Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development. 1996;122:3809–3816. doi: 10.1242/dev.122.12.3809. [DOI] [PubMed] [Google Scholar]
- 34.Drees BL, et al. A protein interaction map for cell polarity development. J Cell Biol. 2001;154:549–576. doi: 10.1083/jcb.200104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. High magnification image of gas11 expression in the otic vesicle. In situ hybridization shows gas11 is expressed throughout the otic vesicle at 8 to 13-somite stages, then becomes concentrated at the poles of the otic vesicle (white arrows) at 15 to 18-somite stages. Beyond the 18-somite stage, the signal in the otic vesicle is not detectable above that in surrounding tissues (not shown). The dorsal/ventral and anterior/posterior axis are shown.
Supplemental Figure S2. gas11 knockdown results in left-right axis defects. In situ hybridizations using southpaw riboprobe demonstrate laterality defects in gas11 morphants at the 16–18 somite stage. The relative numbers of uninjected (n = 119) and gas11 morphant (n = 138) embryos having each staining pattern are shown.
Supplemental Figure S3. gas11 is required for tether cilia motility. Brightfield images taken from high-speed videos of tether cilia in control (top) and gas11 morphant (bottom) embryos. Panels are consecutive frames extracted at 16 ms intervals from supplemental movies 5 (control), and 8 (gas11 morphant). Lines trace the cilium and are colorized (violet to red) in time sequence. These are then aligned at the base of the cilium and overlayed in the right-most panel to generate the “combined” image.
Supplemental Figure S4. Morpholino knockdown of lrrc50 and lrdr1 disrupts tether cilia motility and causes otolith defects. (a) Table summarizing the effect of lrrc50 and lrdr1 knock down. To confirm the specificity of the lrrc50 morpholino, a control morpholino (5 base pairs mismatch) was injected. Error bars show standard deviation. (b–f) phenotypic comparison between control (b) and morpholino injected embryos (c–f) at 27 hpf. Among multiple otolith defects the most common are: (c) fused otolith, (d) smaller otoliths, (e) mis-positioned otolith, (f) single otolith. Left otic vesicle, anterior to the right. Scale bar: 20 microns.
Supplemental Figure S5. The lrrc50 splice morpholino interferes with lrrc50 splicing. (A) RT-PCR with two different primer sets confirms that the lrrc50 splicing morpholino interferes with lrrc50 splicing. mRNA from control embryos (Ctrl2) or embryhos injected with 10 ng of splicing MO (Mo-2) were subjected to RT-PCR with the indicated primers for lrrc50, or GAPDH as a control. The positions of lrrc50 primers are shown relative to the intron/exon positions of the lrrc50 mRNA (not to scale). (B) RT-PCR with lrrc50 primers (1) x (3) showing a decrease in the abundance of the product from the spliced transcript (147 bp, open arrow) and the appearance of a band corresponding to the size expected for the product from the unspliced transcript in the injected sample (1241 bp, black arrow). GAPDH mRNA levels were unaffected. (C) RT-PCR with lrrc50 primers (1) x (2) shows the appearance of a band corresponding to the size predicted from the unspliced transcript in the injected sample (1147 bp, red arrow). The same results were obtained with an independent set of mRNA samples (not shown).
Supplemental Figure S6. Particle displacement in control embryo. White arrows indicate particle direction, illustrating the attractive flow at the base of the cilium. These particle displacements correspond to the particle traces shown in figure 4G.
Supplemental movie 1. Three dimensional display of a control inner ear at stage 27 hpf after immunofluorescence labeling of cilia with acetylated tubulin antibody. Notice the two clusters of tether cilia on the lateral side of the inner ear. Side view, anterior on the right. (QuickTime: 1.27 MB)
Three dimensional display of a gas11 morphant inner ear at stage 27 hpf after acetylated tubulin immunofluorescence labeling. The two clusters of tether cilia are properly located. Side view, anterior on the right. (QuickTime: 2.30 MB)
Video shows tether cilia motility in control embryo. This video corresponds to snapshots shown in figure 3A, B. One cilium is attached to the otolith and beating of this cilium causes the otolith to move. A second motile cilium is also evident. Embryo is 20 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 1.30 MB)
Video shows tether cilia motility in control embryo. Embryo is 24 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 1.50 MB)
Video shows tether cilia motility in control embryo. Cilium beating causes otolith to move. This video corresponds to snapshots shown in supplemental figure 2 (control). Embryo is 20–22 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 2.95 MB)
Video shows short cilia in control embryo are not motile. Embryo is 20–22 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 5.9 MB)
Video shows tether cilia in a gas11 morphant embryo are not motile. Embryo is 20 hpf. This video corresponds to snapshots shown in figure 3C,D. (QuickTime: 0.83 MB)
Video shows tether cilia in a gas11 morphant embryo are not motile. Embryo movement during the video is due to normal embryonic activity. This video corresponds to snapshots shown in supplemental figure 2 (gas11 MO). Embryo is 20–22 hpf. Acquisition rate 322 frames/sec played at 20 frame/sec. (QuickTime: 8.05 MB)
High speed video microscopy of tether cilia in a control embryo at 24 hpf showing vortices in the vicinity of the tether cilia and particle propelling along the growing otolith. Acquisition rate: 322 frames/sec played at 50 frames/sec. (QuickTime: 2.94 MB)
Same as 9a with particle tracking. This video corresponds to figure 4A, B. Acquisition rate: 64 frames/sec played at 20 frames/sec. (QuickTime: 10.69 MB)
High speed video microscopy of the inner ear in a control embryo at 23 hpf showing high displacement of otolith precursors in the vicinity of tether cilia and low displacement away from tether cilia. Acquisition rate: 322 frames/sec played at 20 frames/sec. (QuickTime: 3.11 MB)
Same as supplemental movie 10a with particle tracking. This video corresponds to figure 4G and supplemental Figure S3. Acquisition rate: 64 frames/sec played at 50 frames/sec. (Quicktime: 1.59 MB)
High speed video microscopy of tether cilia in a gas11 morphant at 25 hpf showing very low particle displacement in the vicinity of tether cilia. Acquisition rate: 322 frames/sec played at 20 frames/sec. (QuickTime: 1.36 MB)
Same as 11a with particle tracking. This video corresponds to figure 4D, E. Acquisition rate 64 frames/sec played at 50 frames/sec. (QuickTime: 14.79 MB)