Abstract
Social environment and parental state affect stress responses in mammals, but their impact may depend on the social and reproductive strategy of the species. The influences of cohabitation with a male or female conspecific, and the birth of offspring, on the physiological and endocrine responses to chronic variable stress were studied in the monogamous and biparental California mouse (Peromyscus californicus). Adult male California mice were housed either with a male cage mate (virgin males, VM), a female cage mate (pair-bonded males, PBM), or a female cage mate and their first newborn litter (new fathers, NF). VM, PBM and NF underwent a 7-day chronic variable stress paradigm (CVS, three stressors per day at semi-random times, n=7-8 per housing condition). Compared to control males (CON, n=6-7 per housing condition), CVS caused loss of body mass, increased basal plasma corticosterone concentrations, and increased basal expression of arginine vasopressin (AVP) mRNA in the paraventricular nucleus of the hypothalamus (PVN). These effects were independent of housing condition. Neither CVS nor housing condition altered novel-stressor-induced corticosterone release, spleen or testis mass, or basal expression of corticotropin-releasing hormone (CRH) mRNA in the PVN. Although CVS appeared to increase adrenal mass and reduce thymus mass specifically in NF, these effects were explained by the lower adrenal mass and higher thymus mass of NF compared to PBM and VM under control conditions. These results suggest that neither engaging in a pair bond nor becoming a father attenuates typical responses to CVS, but that fatherhood may provide a buffer against transient mild stressors (i.e., weighing and blood sampling in the control groups) in this monogamous and biparental rodent.
Keywords: chronic variable stress, biparental, monogamous, corticosterone, Peromyscus californicus, CRH, AVP
Introduction
Chronic exposure to stress, eliciting repeated or continuous activation of the hypothalamic-pituitary adrenal (HPA) axis, is well known to cause deleterious effects on psychological, physiological and immunological parameters in mammals, including humans (de Kloet et al., 2005; Willner, 2005). In humans and other social species, the immediate social environment can be particularly important both in causing stress and in modulating its effects. For example, social conflict or social loss can cause or exacerbate the effects of chronic stress (Kiecolt-Glaser et al. 1998; Buckley et al., 2012), whereas a stable and affiliative social environment can provide a buffer against the effects of stress (Cohen and Wills, 1985; Uchino et al., 1996). In addition, chronically stressed individuals may disrupt their immediate social environment by damaging family bonds and neglecting or abusing their developing offspring (Wadsworth et al., 2005; Hackman et al., 2010).
Thorough elucidation of the complex interactions between chronic stress and the immediate social environment requires the use of effective animal models. The vast majority of stress research in non-human animals has thus far been performed in laboratory rats (Rattus norvegicus) and house mice (Mus musculus). The results of these studies have unequivocally demonstrated the important effects of social environment on HPA-axis (re)activity; for example, both long-term isolation and cohabitation with a dominant, same-sex conspecific cause symptoms of chronic stress (Blanchard et al., 1995; Fone and Porkess, 2008). In addition, parental state influences HPA-axis responses to stress, as demonstrated by the marked stress hyporesponsivity in lactating females (Slattery and Neumann, 2008). However, rats and house mice are predominantly polygynous and uniparental (i.e., pups are raised exclusively by mothers), and males form strong hierarchies when group-housed (Berdoy and Drickamer, 2007). In species with different social and reproductive strategies, for example species that form monogamous pair bonds and live in small, biparental family units, the males (and females) are likely to show altered stress responses under various social or reproductive conditions (DeVries et al., 2007; Bosch et al., 2008; Hennessy et al., 2009). Investigating the effects of chronic stress in such species may yield results that are potentially better translatable to many humans.
California mice (Peromyscus californicus) are a particularly suitable model for studying the interactions among social environment, parental state and stress. California mice are one of the relatively few (6-10%; Kleiman, 1977) monogamous and biparental mammalian species: under both natural and laboratory conditions, adult males and females form cohesive pair bonds (Ribble, 1991), and although lactating females are the only source of food for newborn pups, sires and dams spend equal amounts of time sheltering, warming, and retrieving the pups (Gubernick and Alberts, 1987a). Previous experiments have investigated whether housing condition (isolated, with a male cage mate or with a female pair mate) and parental state influence the stress-response system of male California mice. Social isolation was found to delay wound healing and to cause symptoms of chronic stress in male California mice (Glasper and De Vries, 2005; Chauke et al., 2012), indicating a role of social support comparable to other social rodent species (Hawkley et al., 2012). However, female pair mates did not provide more or less social support than male cage mates, since wound-healing processes or HPA reactivity in response to an acute challenge (predator-urine exposure or corticotropin-releasing hormone (CRH) injection) in adult male California mice did not differ depending on the sex of their cage mate (Martin et al., 2006; Chauke et al., 2011; Harris, 2012). In addition, it has been suggested that peripartum HPA hyporesponsiveness, which is thought to have evolved in female mammals to avoid the potentially disruptive effects of high levels of circulating glucocorticoids on parental care (Ivy et al., 2008; Slattery and Neumann, 2008; Saltzman and Abbott, 2009; Harris et al., 2011), evolved in the obligate paternal male California mouse for the same reason (Chauke et al., 2011). However, male California mice that had recently become fathers did not show altered corticosterone release in response to predator-urine exposure or acute CRH injection compared to non-fathers (Chauke et al., 2011; Harris, 2012).
These findings suggest that engagement in a pair bond with a female (compared to cohabitation with a male) and becoming a parent do not appreciably influence acute stress responses in male California mice. However, acute stress responses are considered beneficial for the survival of the individual, whereas persistent stress-induced glucocorticoid release is associated with dysregulation of the HPA-axis and can ultimately lead to diseases (McEwen, 2008). Thus, the social/parental buffering of stress responses may be more advantageous, and therefore more pronounced, in the context of chronic variable stress. This hypothesis has, to the best of our knowledge, not been tested thus far in biparental male mammals.
Chronic variable stress (CVS) is a widely implemented and highly effective paradigm in which individuals undergo a variety of stressors at unpredictable intervals over (typically) two to five weeks (Hill et al., 2012). CVS causes a number of well-characterized effects in male rats and house mice, including reduced body mass, dysregulation of the HPA axis (i.e., increased basal corticosterone levels, increased novel-stress-induced corticosterone release, increased adrenal mass, increased expression of CRH and arginine-vasopressin (AVP) mRNA in the paraventricular nucleus of the hypothalamus (PVN)) and dysregulation of the immune system (i.e., decreased thymus mass, altered spleen physiology) (Herman et al., 1995; Marin et al., 2007; You et al., 2011; Hill et al., 2012). In addition, chronic stress suppresses the hypothalamic-pituitary-gonadal-axis (Blanchard et al., 1995; Retana-Márquez at al., 2003).
We hypothesized that CVS would cause similar effects in adult virgin male California mice, and that at least some of these effects would be attenuated in males cohabiting with a female pair mate, compared to a male cage mate, and would be further blunted in males housed with both a female pair mate and their pups.
Materials and Methods
Animals
Male (n=42 experimental males and n=13 male cage mates) and female (n=29) California mice, descendants of mice purchased from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC, USA), were born in our laboratory colony, weaned at 27-33 days of age (prior to the birth of the next litter) and housed in same-sex groups of three or four animals (littermates and/or unrelated, age-matched mice) until they were paired for the experiment.
Mice were housed in transparent polycarbonate cages (LxWxH: 44x24x20 cm) containing aspen shavings as bedding and cotton for nest building (the same cages were used for the double-cage setup described below). The colony was maintained on a 14:10h day/night cycle (lights on at 0500h), with ambient temperature maintained at approximately 23°C and humidity maintained at approximately 65%, and had ad libitum access to chow (Purina 5001 Rodent Diet, PMI Nutrition International, St. Louis, MO) and water. All mice were weighed twice weekly, at 3- to 4-day intervals, to monitor health and pregnancies.
All procedures were approved by the University of California, Riverside Institutional Animal Care and Use Committee, and were conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals.
Experimental Design
Between 99 and 141 days of age (an age range that falls well within the prime fertile period in this species, Gubernick and Alberts, 1987a; unpublished observations), animals were randomly assigned to one of three housing conditions: males housed with another male (virgin males, VM, n=13), males housed with an unrelated, virgin, tubally ligated female (pair-bonded males, PBM, n=15) or males housed with an unrelated, intact virgin female and, ultimately, their first litter of pups (new fathers, NF, n=14). At least 31 days after pairing, intact females showing signs of imminent parturition (consistent weight gain over three consecutive weighings) and their male mates were transferred to double cages to facilitate a behavioral study running in parallel (manuscript in preparation). Double cages consisted of two regular, polycarbonate housing cages connected by a clear plastic tube approximately 10 cm in length with openings of approximately 5 cm in diameter, with bedding, food, and water available in both cages. An equal number of non-breeding pairs (tubally ligated females and their male mate) and virgin male dyads were transferred to double cages at the same time as the breeding pairs.
Each male either underwent a chronic variable stress (CVS) paradigm (n=7-8 per housing condition) or served as a control (CON, n=6-7 per housing condition). Due to the relatively unpredictable timing of fertilization and gestation length in California mice, combined with the complex and labor-intensive CVS paradigm, the experiment was repeated several times within six months, each time with a cohort of 1-3 animals from each of the six experimental groups (two stress conditions × three housing conditions). For each cohort, the CVS paradigm was started when the pups of the new fathers (both CVS and CON) were 1-3 days of age.
Tubal-ligation surgery
Female mice were tubally ligated using antiseptic techniques and standard surgical procedure. Briefly, mice were anesthetized with isoflurane gas, a ventral midline incision (approximately 1⁄2 cm) was made, the uterus was located and the ends of the right and left uterine horns were tied off using absorbable sutures (Monomend MT, Veterinary Products Laboratories, Phoenix, AZ). The oviducts were then located and severed using microscissors. All reproductive structures were repositioned back in the abdominal cavity, the abdominal incision was closed with absorbable sutures and the skin was sealed using tissue glue. Mice were given an injection of Ketoprofen (5 mg/kg, s.c.) to provide analgesia and allowed to recover in isolation for 7 days, after which time they were paired with a male for formation of non-breeding pairs.
Upon termination of the experiment, tubally ligated females were sacrificed by CO2 and dissected to check for pregnancy. None of these females had visible embryos or fetuses at the time of sacrifice.
Chronic Variable Stress Paradigm
The CVS paradigm used in the present experiment (Table 1) was modified from commonly reported CVS paradigms as follows: 1) it was relatively short (7 days), in order to confine the paradigm to the most intensive phase of pup-rearing (Gubernick and Alberts, 1987b); 2) the number of stressors per day was three instead of two, because pilot tests suggested that two stressors per day did not consistently lead to the expected reduction in body mass or elevation of basal plasma corticosterone levels (unpublished observations); and 3) no stressors were used that required removal of animals from the home cage for more than 1h, in order to minimize separation from the cage mate and pups during the paradigm, which would interfere with our research question.
Table 1.
Experimental design including chronic variable stress (CVS) paradigm. Animals in the CVS condition underwent 21 stressor exposures over 7 days at unpredictable intervals of 6-10h. All CVS and control (CON) animals underwent blood sampling and weighing.
| Day | Data-collection procedures (CVS + CON) |
Stressors (CVS only) |
|---|---|---|
| 1 | 0900h: basal blood sample | |
| ∼0930h: body mass | ||
| 1000h: wet bedding | ||
| 1600h: forced swim | ||
|
| ||
| 2 | 0100h: hypertonic saline | |
| 1100h: cold exposure | ||
| 2000h: predator urine | ||
|
| ||
| 3 | ∼0930h: body mass | 0300h: shaker |
| 1300h: forced swim | ||
| 1900h: wet bedding | ||
|
| ||
| 4 | 0900h: basal blood sample | 0200h: shaker |
| 0900h: hypertonic saline (immed. after blood sample) | ||
| 1500h: cold exposure | ||
| 2200h: predator urine | ||
|
| ||
| 5 | ∼0930h: body mass | 0700h: restraint |
| 1700h: cold exposure | ||
| 2300h: shaker | ||
|
| ||
| 6 | 0800h: restraint | |
| 1800h: hypertonic saline | ||
|
| ||
| 7 | ∼0930h: body mass | 0000h: restraint |
| 0600h: predator urine | ||
| 1400h: restraint | ||
| 2000h: wet bedding | ||
|
| ||
| 8 | 0900h: basal blood sample | |
| ∼0900h: oil injection | ||
| ∼0910h: blood sample | ||
| ∼0940h: decapitation, trunk blood | ||
| collection, dissection of brains/organs | ||
Each CVS male underwent a total of 21 stressor exposures (involving 7 different stressors) over a 7-day period, following the schedule in Table 1. The stressors were as follows: 1) wet bedding: a mixture of wood shavings and tap water (24-26°C) was spread on the bottom of a clean cage, and a mouse was placed in the cage for 1 h; 2) forced swim: each male was placed in a 1L cylindrical plastic Baxter container filled with 850mL tap water (24-26°C) and was forced to swim for 5 min; 3) hypertonic saline: each male was injected IP with 1.5M NaCl (15 ml/kg body mass); 4) cold exposure: each male was placed in a closed, ventilated plastic container (volume: 700 ml) in a refrigerator (inside temperature: 4°C) for 15 min; 5) predator urine: each male was placed in a clean cage with wood shavings on the bottom, and exposed for 8 min to a small (1 cubic inch) stainless steel tea ball containing a cotton ball saturated with 1mL of predator urine (bobcat, coyote, wolf, or mountain lion; Maine Outdoor Solutions, Herman, MA); 6) shaker: each male was placed in a closed, ventilated plastic container (volume: 700 ml) on a lab shaker rotating at 200 rpm for 15 min; and 7) restraint: each male was placed in a DC M200 decapicone (Braintree Scientific, Braintree, MA, USA), which was folded tightly, shut with a clip and hung on a horizontal wire so that the male was facing downward for 15 min. All of the stressors, except hypertonic saline, were performed in a separate room from the one in which the experimental animals were housed. All stressors were verified to elicit pronounced, acute corticosterone release in California mice (forced swim, predator urine, shaker, restraint: Harris et al., 2012; wet bedding, hypertonic saline, cold exposure: unpublished observations).
Both CVS and CON males were weighed and their blood was sampled at regular intervals (see Table 1). Body mass was measured between 0930h and 1000h on day 1 (immediately prior to the start of the CVS paradigm) and between 0930h and 1100h on day 3, 5 and 7 of the paradigm. Blood samples (ca. 75 μl per sample) were taken on day 1 (between 0841h and 0940h, prior to the start of the CVS paradigm), on day 4 (between 0854h and 0924h, approximately 7h after exposure to a stressor for CVS animals), and on day 8 (between 0854h and 0924h, approximately 12h after exposure to a stressor for CVS animals). Mice were anesthetized briefly with isoflurane, and blood was collected from the orbital sinus within 3 min of cage disturbance to avoid stress-induced elevations of corticosterone.
On the first morning after the end of the CVS paradigms (day 8), immediately after collection of basal blood samples, all males (both CVS and CON) were exposed to a novel stressor: subcutaneous injection of 0.2 ml sesame oil, which had been verified to cause a marked release of corticosterone in adult male California mice (unpublished observations). Blood samples were collected from the orbital sinus, 10 min (mean ± s.e.m.: 612.40 ± 4.19s; range: 557 – 681s) after oil injection, within 3 min of cage disturbance. Males were decapitated 40 min (mean ± s.e.m.: 2423.44 ± 5.63s; range: 2381 – 2567s) after oil injection, and trunk blood was collected in plastic weigh boats containing 0.1ml heparin and processed as described below. Brains were dissected and flash-frozen in dry ice within 90 s after decapitation in order to characterize the expression of CRH and AVP mRNA in the paraventricular nucleus of the hypothalamus (PVN). Since acute stressors do not affect mRNA levels of these neuropeptides for at least 60 min (Ma et al., 1999), the results putatively reflect baseline expression. Thymus, adrenals, spleen, and testes were carefully dissected, cleaned of fat and collected in cold 0.9% saline, then blotted three times on a paper towel and weighed to the nearest 0.0001 g.
Corticosterone Assays
Immediately after collection, blood samples were centrifuged for 12 min (13,300 rpm, 4°C), and plasma was removed and stored at -80°C until assay. Plasma was assayed in duplicate for corticosterone using an 125I double-antibody radioimmunoassay (RIA) kit (#07-120102, MP Biomedicals, Costa Mesa, CA) previously validated for this species (Chauke et al., 2011). Samples from each experimental condition were balanced evenly across three assays; however, all samples from an individual mouse were always analyzed in a single assay run. The standard curve ranged from 12.5 ng/ml (91% bound) to 1000 ng/ml (20% bound), and plasma samples were assayed using dilutions ranging from 1:100 to 1:800 depending on anticipated corticosterone concentrations. Inter- and intra-assay coefficients of variation (CVs) were 11.2% and 4.7%, respectively (N = 45 assays).
In Situ Hybridization
A total of 30 brains (N=5 from each of the six experimental groups) were randomly selected for in situ hybridization procedures. Each frozen brain was sliced on a cryostat into five series of 20 μm thick coronal sections containing the PVN, and thaw-mounted on gelatin/chrome-alum coated glass slides. The first series of each brain was thawed, air-dried and stained with Quick Stain (American MasterTech, Lodi, CA, USA) to determine the exact location of the PVN; the remaining series were kept frozen until in situ hybridization procedures were started.
Expression levels of AVP and CRH mRNA were quantified as described previously (de Jong et al., 2012), using 35S-labeled deoxyoligonucleotide probes synthesized by Sigma Genosys (The Woodlands, TX, USA). In situ hybridizations for both CRH and AVP probes were performed in three consecutive rounds, each round containing four selected brain sections from 10 animals (N=1-2 from each of the six experimental groups). Brain sections were fixed in freshly made 4% buffered paraformaldehyde for 20 min, followed by dehydration and rehydration through graded ethanols. Sections were exposed to 0.25% acetic anhydride and 0.1 M triethanolamine (pH=8) for 8 min and were dehydrated through graded ethanols. Sections were hybridized overnight (20h) in a humidified chamber at 42°C, with 0.20 × 106 CPM of labeled probe dissolved in a buffer solution (50% formamide, 5X SET, 0.2% SDS, 5X Denhart's, 0.5 mg/ml salmon sperm DNA, 0.25 mg/ml yeast tRNA, 100 mM dithiothreitol and 10% dextran sulfate; 30 μl per section). After hybridization, sections underwent serial washes of saline sodium citrate (SSC): 4X SSC for 5 min at RT, 2X SSC for two times 30 min at 55°C, and 1X SSC and 0.3X SSC for 30 min each at RT. Sections were then dehydrated through graded ethanols containing 0.3 m ammonium acetate, followed by 95% and 100% ethanol, and air-dried.
All sections were placed in autoradiography cassettes (two per round per probe) and apposed to film (Kodak BioMax MR Film, Eastman Kodak Co., NY, USA) for 1 day (AVP) or 10 days (CRH). Subsequently, the two sections displaying the strongest signal were selected for each animal and each probe, placed in autoradiography cassettes (one per round per probe), and re-apposed to film together with a 14C-standard (American Radiolabeled Chemicals, St. Louis, MO, USA) for 1 day (AVP) or 10 days (CRH). Developed films were digitized and analyzed using ImageJ software from the National Institutes of Health. Gray levels of the 14C-standard on each film were measured and fitted to a 4th degree polynomial curve expressed in nCi/g, and hybridization and background signals on the same film were quantified using that curve. Each positive signal in the PVN (average of five unilateral outlines) minus background signal (average of three random outlines immediately adjacent to the PVN) yielded values representing the amount of CRH or AVP in the PVN in nCi/g. The high inter-film and intra-individual variability in signal strengths required normalization and selection, which we did as follows: control pair-bonded males (CON-PBM) were chosen as an artificial control group based on their low inter- and intra-individual variability in signal strength. For each film, all unilateral values of all individuals within this control group were averaged, and all unilateral values of all individuals on that film were then expressed as a percentage of this average. The highest number (percentage) was selected for each individual, and these percentages were used for analysis of interaction and group effects.
Statistics
All data were analyzed by SPSS 19.0 software, and statistical significance was set at P ≤ 0.05 (two-tailed). Apart from a few specified cases, non-significant findings are not described in the results.
Baseline values measured prior to the start of the CVS paradigm (number of pups, age, body mass and plasma corticosterone levels on the morning of day 1 of the paradigm) were compared across groups using one-way ANOVAs.
Changes over time in body mass as well as in basal and stress-induced plasma corticosterone levels were evaluated using a general linear model for repeated measures, including time (day of the paradigm or time after oil injection) as a within-subjects factor, and stress condition (CVS or CON) and housing condition (VM, PBM, or NF) as between-subjects factors. All plasma corticosterone values were log10-transformed prior to analysis to meet normality requirements. The assumption of sphericity (as determined by Mauchly's W) was not met in the analysis of body mass and basal corticosterone levels; therefore the degrees of freedom were adjusted by the Huynh-Feldt approximation of ε. In case of significant main or interaction effects, post-hoc pairwise comparisons were made using Fisher's LSD (comparison of stress conditions) or Bonferroni tests (comparison of housing conditions).
Area under the curve was calculated in two ways (Pruessner et al., 2003) to quantify total corticosterone release over time following subcutaneous oil injection. AUCg represents the total amount of hormone produced over time with respect to a starting value of zero, thus not accounting for variability in baseline levels of circulating hormone, whereas AUCi characterizes the responsiveness of the HPA axis to oil injection by evaluating the amount of hormone produced above the starting baseline level. Since AUCg and AUCi levels correlated highly in the present experiment (r=0.978, n=41, P<0.001), we discuss only the analysis of AUCg. AUCg as well as the relative expression of CRH and AVP mRNA in the PVN were analyzed using two-way ANOVAs, with stress condition and housing condition as independent factors; all data were log10-transformed to meet normality assumptions. In case of significant main or interaction effects, post-hoc pairwise comparisons were made using Fisher's LSD (comparison of stress conditions) or Bonferroni tests (comparison of housing conditions).
Wet organ masses (adrenals, thymus, spleen and testes) were analyzed by ANCOVAs, using body mass on day 1 (just prior to the start of the CVS paradigm) as the covariate and both stress condition and housing condition as fixed factors. Since right and left testis and adrenal masses were highly and significantly correlated (adrenals: r=0.876, n=41, P<0.001; testes: r=0.894, n=41, P<0.001), only total masses (right + left adrenal; left + right testis) were used for analysis. Total adrenal masses, thymus masses, and spleen masses were log10-transformed to meet normality assumptions. A separate ANCOVA was run for each organ of interest. Interaction terms with the covariate were excluded if P-values for homogeneity of slopes were greater than 0.15, Levene's test was used to evaluate homogeneity of variance. Significant main or interaction effects were followed up by post-hoc pairwise comparisons, using Fisher's LSD (comparison of stress conditions) or Bonferroni tests (comparison of housing conditions).
Results
Starting Values
All starting values are expressed as expressed as mean ± s.e.m. Breeding pairs (n=7 per stress condition) gave birth to 1.9 ± 0.1 pups (CVS: 1.9 ± 0.1; CON: 2.0 ± 0.2; P=0.594), as is typical for this species (Gubernick and Alberts, 1987a). One-way ANOVA showed that on the morning of day 1, just prior to the start of the CVS paradigm, males from the six experimental groups did not significantly differ in age (156.17 ± 1.87 days; range: 137-200 days, P=0.762), body mass (40.84 ± 0.93g; range: 31.95 – 55.12g; P=0.764) or basal plasma corticosterone levels (48.57 ± 7.41 ng/ml; range: 10.24 - 239.85 ng/ml; P=0.361).
Body Mass
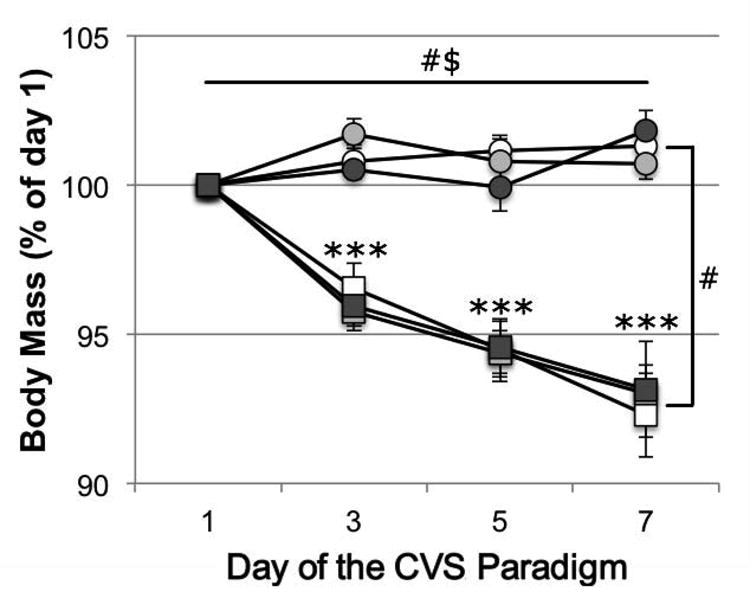
For relative body mass during the CVS/CON paradigm (calculated as the percentage of body mass on the morning of day 1 and expressed as mean ± s.e.m; Fig. 1), a general linear model for repeated measures revealed significant main effects of time (day of the stress paradigm; F=6.948, df=1.983[72.86], P=0.002) and an interaction effect of time × stress condition (F=11.722, df=1.983[72.86], P<0.001). Between-subjects tests showed a main effect of stress condition (F=67.931, df=1[36], P<0.001), and post-hoc pairwise comparisons showed that relative body mass was significantly lower in CVS animals compared to CON animals (both pooled over all housing conditions) on day 3 (t=5.050, P<0.001), day 5 (t=6.086, P<0.001) and day 7 (t=8.359, P<0.001).
Fig. 1.
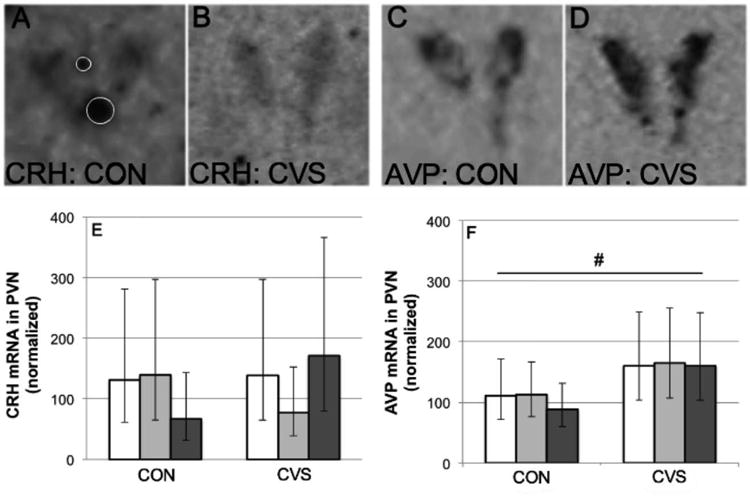
Effects of chronic variable stress (CVS) compared to control conditions (CON) on the relative expression (see text for explanation) of CRH mRNA (A, B, E) and AVP mRNA (C, D, F) in the PVN of adult male California mice living as virgin males (white bars), pair-bonded males (grey bars) or new fathers (black bars). Photographs depict CRH and AVP mRNA signals from one pair-bonded male under control conditions (A, C) and one pair-bonded male under CVS conditions (B, D); white circles in A signify artifacts that were excluded from the analyzed area of the PVN. Data are geometric means and 95% CI. #Main effect of stress condition (P=0.013).
Plasma Corticosterone Concentrations
For basal plasma corticosterone levels during the CVS/CON paradigm (presented as geometric means and 95% CI, Fig. 2A), a general linear model for repeated measures revealed a significant main effect of time (day of the stress paradigm, F=3.938, df=1.940 [65.96], P=0.025) and an interaction effect of time × stress condition (F=4.823, df=1.940[65.96], P=0.012). Between-subjects tests revealed a main effect of stress condition (F=13.384, df=1[34], P=0.001), and post-hoc pairwise comparisons revealed that basal plasma corticosterone levels were significantly increased in CVS males compared to CON males on day 4 (t=-0.511, P<0.001), but not on day 1 (t=-0.102, P=0.287) or on day 8 (t=-0.222, P=0.106).
Fig. 2.
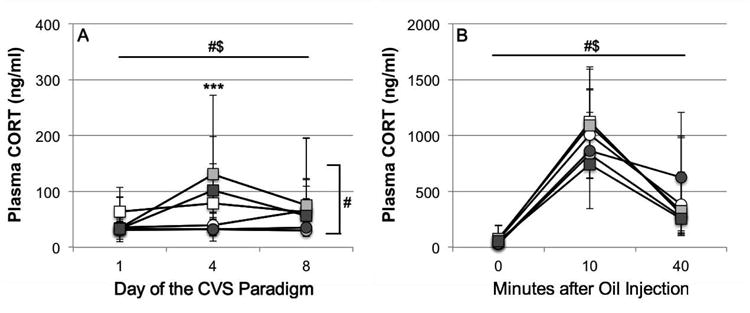
Effects of chronic variable stress (CVS, squares) compared to control conditions (CON, circles) on (A) basal plasma corticosterone concentrations and (B) plasma corticosterone concentrations in response to a subcutaneous oil injection on day 8 in adult male California mice living as virgin males (white markers), pair-bonded males (light grey markers) or new fathers (dark grey markers). Data are geometric means and 95% CI. Note that the y-axis scale differs between the two graphs. #Significant main effects: time (above graph in A/B, P<0.05) and stress condition (right side of graph in A, P=0.001). $Significant interaction effect: time × stress condition (P<0.05) ***Significant difference between CVS and CON (pooled over housing conditions, P<0.001).
For stress-induced plasma corticosterone concentrations (presented as geometric means and 95% CI, Fig. 2B), a general linear model for repeated measures revealed a significant main effect of time (0 min (basal sample on day 8), 10 min and 40 min after s.c. oil injection; F=168.223, df=2[70], P<0.001) and a significant interaction effect of time × stress condition (F=3.615, df=2[70], P=0.032). Post-hoc pairwise comparisons (based on the significant interaction effect of time × stress condition) showed that plasma corticosterone levels did not differ significantly between CVS and CON animals (both pooled over all housing conditions) at any time point after oil injection.
For time-integrated plasma corticosterone release (area under the curve, AUCg) in response to subcutaneous oil injection, two-way ANOVA revealed no significant main or interaction effects.
Organ Masses
For total adrenal mass (presented as body-mass-corrected geometric means and 95% CI; Fig 3A), interaction terms of the covariate (body mass on day 1) with either stress condition or housing condition were not significant (P>0.511) and therefore were removed from the model. The subsequent model showed significant main effects of body mass on day 1 (F=9.467, df=1[34], P=0.004), stress condition (F=4.297, df=1[34], P=0.046), and housing condition (F=4.279, df=2[34], P=0.022) and a non-significant trend toward an interaction effect of stress condition × housing condition (F=2.738, df=2[34], P=0.079). Based on the combination of the two significant main effects and the trend toward an interaction effect, explorative Bonferroni post-hoc pairwise comparisons were performed which indicated that among CON males, total adrenal masses were significantly lower in NF compared to VM (t=0.173, P=0.006). Explorative Fisher's LSD post-hoc pairwise comparisons between CVS and CON males indicated that CVS caused a significant increase in total adrenal masses in NF (t=-0.150, P=0.003), but not in PBM or VM.
Fig. 3.
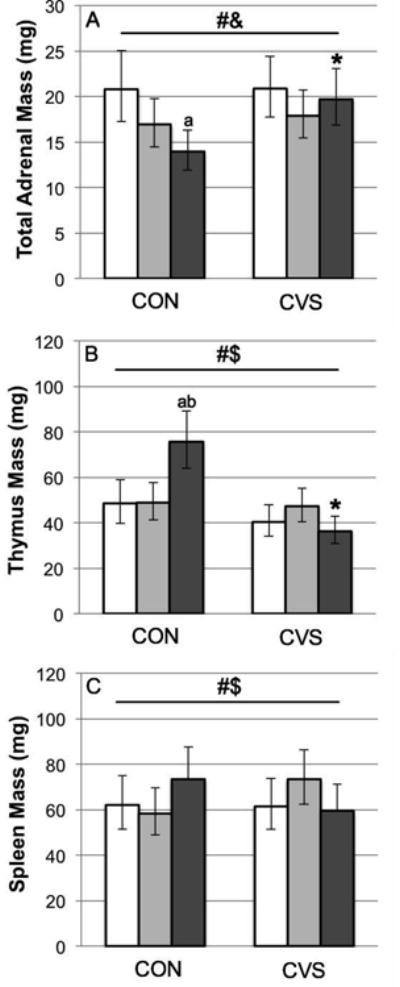
Effects of chronic variable stress (CVS) compared to control conditions (CON) on body-mass-corrected adrenal mass (A; sum of left and right adrenals), thymus mass (B), and spleen mass (C) of adult male California mice living as virgin males (white bars), pair-bonded males (light grey bars) or new fathers (dark grey bars). Data are geometric means and 95% CI. #Significant main effect of stress (adrenals: P=0.046; thymus: P<0.001; spleen: P=0.052) &Significant main effect of housing condition (P=0.022) $Significant interaction effect: stress × housing condition (thymus: P<0.001; spleen: P=0.050) a,bSignificant difference between new fathers and (a) virgin males (P<0.01) and (b) pair-bonded males (P=0.004) *Significant difference between CVS and CON (only in new fathers: P<0.01).
For thymus mass (presented as body-mass-corrected geometric means and 95% CI; Fig 3B), interaction terms of the covariate (body mass on day 1) with either stress condition or housing condition were not significant (P>0.393) and therefore were removed from the model. The subsequent model revealed significant main effects of body mass on day 1 (F=28.516, df=1[34], P<0.0001) and stress condition (F=20.864, df=1[34], P<0.001), and a significant interaction between stress condition and housing condition (F=10.228, df=1[34], P<0.001). Bonferroni post-hoc pairwise comparisons showed that among CON males, thymus mass was significantly increased in NF compared to PBM (t=-0.190, P=0.004) and VM (t=-0.193, P=0.002). Fisher's LSD post-hoc pairwise comparisons between CVS and CON males showed that CVS caused a significant decrease in thymus mass in NF (t=0.317, P<0.001), but not in PBM or VM.
For spleen mass (presented as body-mass-corrected geometric means and 95% CI; Fig 3C), a significant interaction between the covariate (body mass on day 1) and stress condition (P=0.068), but not between the covariate and housing condition (P=0.838), was detected; thus, the significant interaction term as well as the interaction between stress condition and housing condition (P=0.057) remained in the model. The subsequent analysis showed a significant main effect of body mass on day 1 (F=18.692, df=1[34], P<0.001), a marginally significant main effect of stress condition (F=4.038, df=1[34], P=0.052), and marginally significant interactions between stress condition and housing condition (F=3.276, df=2[34], P=0.050), and stress condition and body mass on day 1 (F=4.095, df=1[34], P=0.051). Subsequent post-hoc pairwise comparisons showed no significant differences between the three housing conditions under separated or pooled stress conditions, or between the two stress conditions under separated or pooled housing conditions.
For total testis mass (range: 0.154-0.523g, no further data shown), neither interaction term with the covariate (body mass on day 1) was significant (P>0.164); therefore, both terms were removed from the model. Subsequent analysis revealed that there were no significant main or interaction effects of body mass on day 1, housing condition, or stress condition.
Neuropeptide mRNA Expression in the PVN
The levels of CRH and AVP mRNA in the PVN (presented as geometric means and 95% CI; Fig. 4), calculated as the average over all highest values (one per animal), were 522.35 ± 72.35 nCi/g and 6527.11 ± 821.78 nCi/g, respectively. Since inter-film and intra-individual variability was high, data were normalized as described in the methods section and only the highest value (percentage) of each individual was selected for analysis. For the relative density of CRH mRNA expression in the PVN (Fig. 4A, 4B and 4E), two-way ANOVA log10-transformed revealed no significant main or iteraction effects of stress condition and housing condition. For the relative density of AVP mRNA expression in the PVN (Fig. 4C, 4D and 4F), two-way ANOVA on log10-transformed values revealed a significant main effect of stress condition (F=7.357, df=1[20], P=0.013). Fisher's LSD post-hoc pairwise comparisons between the CON and CVS conditions (pooled over housing conditions) showed that CVS caused a significant increase in AVP mRNA expression in the PVN (t=-0.195, P=0.013).
Fig. 4.
Effects of chronic variable stress (CVS, squares) compared to control conditions (CON, circles) on relative body mass (calculated as percentage of body mass on day 1, prior to the start of the CVS paradigm) of adult male California mice living as virgin males (white markers), pair-bonded males (light grey markers) or new fathers (dark grey markers). Data are means ± s.e.m. #Significant main effects: time (P=0.002) and stress condition (P<0.001), $Significant interaction effect: time × stress condition (P<0.001), ***Significant difference between CVS and CON (pooled over housing conditions, P<0.001)
Discussion
The present experiment aimed to validate a chronic variable stress (CVS) paradigm in adult male California mice, and to determine the effects of social environment and parental state on responses to this stress paradigm. The modified, relatively short (7 days) but intensive (3 unpredictable stressors per day) CVS paradigm caused a persistent loss of body mass and a transient increase in basal plasma corticosterone levels in adult male California mice, consistent with findings in adult male rats and house mice in response to various CVS paradigms (Willner, 2005; Cox et al., 2011). These effects of CVS occurred independently of housing condition.
In addition, CVS caused an increased expression of AVP mRNA in the PVN while not affecting the expression of CRH mRNA in the PVN, again independent of housing condition. Although these neuropeptide results are consistent with studies using chronic homotypic stressors in adult male rats, such as repeated restraint (Ma et al., 1999) or chronic inflammation (Chowdrey et al., 1995), most chronic variable stress studies performed in adult male rats find an increased CRH transcription and no changes in AVP transcription (Hill et al., 2012). It is possible that adult male California mice show a stronger habituation to CVS, and thus do not maintain the stress-induced CRH (and associated ACTH) release followed by CRH transcription, while AVP turnover and the associated proliferation of corticotrophs and remodeling of the pituitary tissue remains high (Aguilera et al., 2008). However, future studies with larger group sizes and multiple time points are needed to rule out any changes in CRH mRNA in response to CVS in male California mice, preferably with concomitant monitoring of stress-induced ACTH release.
Other common effects of chronic variable stress were not found in the present study: basal corticosterone levels were no longer elevated on the day after cessation of CVS (in contrast to Ostrander et al., 2006 and Solomon et al., 2010, but similar to Simpkiss and Devine, 2003; Marin et al., 2007; Jankord et al., 2010, all studies performed in adult male rats), and corticosterone release in response to a novel stressor was not facilitated at that time point (in contrast to Marin et al., 2007; Jankord et al., 2010, but similar to Simpkiss and Devine, 2003; Ostrander et al., 2006; Solomon et al., 2010, all studies performed in adult male rats).
Although adult male California mice did show increased total adrenal masses and reduced thymus masses in the CVS condition compared to the CON condition, consistent with results from CVS studies in adult male rats (Simpkiss and Devine, 2003; Ulrich-Lai et al, 2006; Ostrander et al., 2006; Jankord et al., 2010; Solomon et al. 2010), closer scrutiny of the data suggested that this effect was caused exclusively by fathers, which had relatively low adrenal masses compared to virgin males and relatively high thymus masses compared to virgin males and pair-bonded males under control conditions. A previous study in our lab found lower adrenal masses in California mouse fathers compared to both virgin males and males housed with tubally ligated females (Harris, 2012). To our knowledge, we are the first to report such a finding in paternal mammals, and we can only speculate about the causes. It is possible that an unknown factor associated with becoming a parent caused adrenal atrophy and thymus hypertrophy in male California mice; however, pregnancy and lactation in female rodents are not associated with significant alterations in adrenal masses (D.A. Slattery, personal communication), and are associated with a strong reduction in thymus mass (McLean et al., 1974; Kendall and Clarke, 2000). Alternatively, non-fathers (especially virgin males) may have been suffering from stress-induced adrenal hypertrophy and thymus atrophy in response to the mild and transient stressors of the control condition (repeated handling, cage cleaning, blood sampling, and ongoing activity in the colony room), whereas fathers were less affected. The finding that CVS did not (further) increase adrenal mass or reduce thymus mass in non-fathers, but strongly affected these organ masses in fathers, supports the latter hypothesis. Thus, although new fathers did respond to CVS with alterations in organ masses (as well as changes in body mass, basal plasma corticosterone levels, and expression of AVP mRNA in the PVN), they may have been somewhat protected against the effects of mild and transient stress. Since adrenal hypertrophy is associated with increased corticosterone responses to ACTH in rats (Ulrich-Lai et al., 2006) and thymus atrophy is associated with declines in oligoclonal peripheral T cell repertoire and constricted host immunity in mice (Gruver and Sempowski, 2008), prevention of these changes in organ morphology might protect the health of fathers and thus could potentially increase the survival rate of offspring. As such, this hypothetical protection against mild stressors could be an adaptation in the context of the biparental care system. Future studies could test this hypothesis using a chronic mild stress paradigm compared to a strictly stress-free control condition, in combination with a more detailed analysis of adrenal and thymus histology.
The present study is, to our knowledge, the first that investigates the effects of chronic variable stress in males of a monogamous and biparental species. The results confirm that our modified CVS paradigm successfully causes chronic stress in adult male California mice. The experiment did not, however, reveal consistent differences in responses to CVS between adult male California mice cohabiting with a female pair mate or male cage mate or between pair-bonded males with or without a newborn litter of pups. These findings are consistent with previous experiments in male California mice, in which similar housing conditions did not differentially influence the process of wound healing or responses to an acute stressor (Martin et al., 2006; Chauke et al., 2011; Harris, 2012), suggesting that the (lack of) effects of a pair bond or fatherhood do(es) not depend on the type or length of the stressor. Although it is possible that social buffering does not occur at all in California mice, it seems more likely that the effects of social support provided by male cage mates or female pair mates are indistinguishable from one another. On the other hand, the marked stress hyporesponsiveness during late pregnancy and the early post-natal period in some mammalian species (Slattery and Neumann, 2008) may be less pronounced in fathers, possibly due to the lack of strong hormonal surges (prolactin, oxytocin) that occur during parturition and lactation in females and dampen the HPA axis. However, some hormonal changes associated with the onset of fatherhood (Storey and Walsh, 2012) may protect the males and their offspring against mild stress.
Together, these results elucidate the role of pair bonds and fatherhood in stress responses in males of a monogamous and biparental rodent species. In addition, the findings provide a foundation for future studies focusing on the effects of chronic stress on paternal behavior and offspring development in California mice. Results from the present and future experiments will increase our understanding of how family life affects, and is affected by, chronic stress in biparental species, including humans.
Acknowledgments
We would like to thank the UCR vivarium staff and Dr. Akiko Sato for their help with animal care. We also thank Vanessa Yang, Julia Cho, Omar Aldaas, Aaron Stamp, Gavrielle Concepcion, Saif Hossain, Samantha Zamora, and Dr. Miyetani Chauke for their help with various aspects of experimental preparation and data collection. In addition, we thank two anonymous reviewers for their careful scrutiny of the manuscript and their excellent suggestions to improve it.
This work was supported by NIH grant 1R21MH087806
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
TdJ wrote the manuscript. TdJ, BH and WS designed the experiment. TdJ and BH coordinated the experiment and performed the majority of the experimental manipulations, measurements, and data analyses. JPPR assisted in the experimental manipulations and performed the in situ hybridizations. WS obtained the grant that supported this study, and supervised all aspects of the design and execution of the experiment as well as data analysis and manuscript writing. All authors contributed to and have approved the final manuscript.
No conflict of interest to report.
References
- Aguilera G, Subburaju S, Young S, Chen J. The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog Brain Res. 2008;170:29–39. doi: 10.1016/S0079-6123(08)00403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoy M, Drickamer LC. Comparative social organization and life history of Rattus and Mus. In: Wolf JO, Sherman PW, editors. Rodent Societies: An Ecological and Evolutionary Perspective. University of Chicago Press; Chicago: 2007. pp. 380–392. [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: Behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2008;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T, Sunari D, Marshall A, Bartrop R, McKinley S, Tofler G. Physiological correlates of bereavement and the impact of bereavement interventions. Dialogues Clin Neurosci. 2012;14:129–139. doi: 10.31887/DCNS.2012.14.2/tbuckley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauke M, de Jong TR, Garland T, Jr, Saltzman W. Paternal responsiveness is associated with, but not mediated by reduced neophobia in male California mice (Peromyscus californicus) Physiol Behav. 2012;107:65–75. doi: 10.1016/j.physbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauke M, Malisch JL, Robinson C, de Jong TR, Saltzman W. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus) Horm Behav. 2011;60:128–138. doi: 10.1016/j.yhbeh.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdrey HS, Larsen PJ, Harbuz MS, Jessop DS, Aguilera G, Eckland DJ, Lightman SL. Evidence for arginine vasopressin as the primary activator of the HPA axis during adjuvant-induced arthritis. Br J Pharmacol. 1995;116:2417–2424. doi: 10.1111/j.1476-5381.1995.tb15089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- Cox BM, Alsawah F, McNeill PC, Galloway MP, Perrine SA. Neurochemical, hormonal, and behavioral effects of chronic unpredictable stress in the rat. Behav Brain Res. 2011;220:106–111. doi: 10.1016/j.bbr.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TR, Korosi A, Harris BN, Perea-Rodriguez JP, Saltzman W. Individual variation in paternal responses of virgin male California mice (Peromyscus californicus): behavioral and physiological correlates. Physiol Biochem Zool. 2012;85:740–751. doi: 10.1086/665831. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Craft TKS, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents - relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Glasper ER, DeVries AC. Social structure influences effects of pair-housing on wound healing. Brain Behav Immun. 2005;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84:915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernick DJ, Alberts JR. The biparental care system of the California mouse, Peromyscus californicus. J Comp Psychol. 1987a;101:169–177. [PubMed] [Google Scholar]
- Gubernick DJ, Alberts JR. “Resource” exchange in the biparental California mouse (Peromyscus californicus): water transfer from pups to parents. J Comp Psychol. 1987b;101:328–334. [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Perea-Rodriguez JP, Saltzman W. Acute effects of corticosterone injection on paternal behavior in California mouse (Peromyscus californicus) fathers. Horm Behav. 2011;60:666–675. doi: 10.1016/j.yhbeh.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Harris BN, Saltzman W, de Jong TR, Milnes MR. Hypothalamic-pituitary-adrenal (HPA) axis function in the California mouse (Peromyscus californicus): Changes in baseline activity, reactivity, and fecal excretion of glucocorticoids across the diurnal cycle. Gen Comp Endocrinol. 2012;179:436–450. doi: 10.1016/j.ygcen.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN. Unpublished Ph D dissertation. University of California; Riverside: 2012. Reproduction in the face of stress: mediation by the hypothalamic-pituitary-adrenal (HPA) axis; p. 235. [Google Scholar]
- Hawkley LC, Cole SW, Capitanio JP, Norman GJ, Cacioppo JT. Effects of social isolation on glucocorticoid regulation in social mammals. Horm Behav. 2012;62:314–323. doi: 10.1016/j.yhbeh.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Front Neuroendocrin. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hellemans KGC, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2010;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall MD, Clarke AG. The thymus in the mouse changes its activity during pregnancy: a study of the microenvironment. J Anat. 2000;197:393–411. doi: 10.1046/j.1469-7580.2000.19730393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, Malarkey WB. Marital stress: immunologic, neuroendocrine, and autonomic correlates. Ann N Y Acad Sci. 1998;840:656–663. doi: 10.1111/j.1749-6632.1998.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Martin LB, II, Glasper ER, Nelson RJ, DeVries AC. Prolonged separation delays wound healing in monogamous California mice, Peromyscus californicus, but not in polygynous white-footed mice, P. leucopus. Physiol Behav. 2006;87:837–841. doi: 10.1016/j.physbeh.2006.01.035. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JM, Mosley JG, Gibbs AC. Changes in the thymus, spleen and lymph nodes during pregnancy and lactation in the rat. J Anat. 1974;118:223–229. [PMC free article] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Retana-Márquez S, Bonilla-Jaime H, Vázquez-Palacios G, Martínez-García R, Velázquez-Moctezuma J. Changes in masculine sexual behavior, corticosterone and testosterone in response to acute and chronic stress in male rats. Horm Behav. 2003;44:327–337. doi: 10.1016/j.yhbeh.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav Ecol Sociobiol. 1991;29:161–166. [Google Scholar]
- Saltzman W, Abbott DH. Effects of elevated circulating cortisol concentrations on maternal behavior in common marmoset monkeys (Callithrix jacchus) Psychoneuroendocrinology. 2009;34:1222–1234. doi: 10.1016/j.psyneuen.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkiss JL, Devine DP. Responses of the HPA axis after chronic variable stress: effects of novel and familiar stressors. Neurondocrinol Lett. 2003;24:97–103. [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol. 2010;22:13–23. doi: 10.1111/j.1365-2826.2009.01933.x. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ. Biological basis of mammalian paternal behavior. In: Cabrera NJ, Tamis-LeMonda CS, editors. Handbook of Father Involvement (second edition) Routledge; New York: 2012. pp. 3–22. [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME, Raviv T, Compas BE, Connor-Smith JK. Parent and adolescent responses to poverty related stress: tests of mediated and moderated coping models. J Child Fam Stud. 2005;14:283–298. [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Zuo R, Wu Y. Pro-and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: Involvement in depression. Behav Brain Res. 2011;225:135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]


