Abstract
Dyspnea is the predominant symptom for patients with acute heart failure and initial treatment is largely directed towards the alleviation of this. Contrary to conventional belief, not all patients present with fluid overload and the approach to management is rapidly evolving from a solitary focus on diuresis to one that more accurately reflects the complex interplay of underlying cardiac dysfunction and acute precipitant. Effective treatment thus requires an understanding of divergent patient profiles and an appreciation of various therapeutic options for targeted patient stabilization. The key principle within this paradigm is directed management that aims to diminish the work of breathing through situation appropriate ventillatory support, volume reduction and hemodynamic improvement. With such an approach, clinicians can more efficiently address respiratory discomfort while reducing the likelihood of avoidable harm.
Keywords: Acute heart failure, dyspnea, congestion, clinical profile, preload, afterload, pump failure, nitrovasodilators, nitroglycerin, nesiritide, angiotensin converting enzyme (ACE) inhibitors, calcium channel blockers, relaxin, loop diuretic, furosemide, bumetanide, torsemide, vasopressin antagoists, conivaptan, tolvaptan, lixivaptan, adenosine receptor antagonists, ultrafiltration, inotrope, dobutamine, milrinone, digoxin, non-invasive positive airway pressure ventilation (NIPPV), continuous positive airway pressure (CPAP), bi-level positive airway pressure (BiPAP)
Introduction
Acute heart failure (HF) represents a summary term for the rapid onset of dyspnea in patients with underlying cardiac dysfunction. 1 While other findings including signs of systemic venous congestion and/or hypoperfusion, fatigue, weakness, and chest pain may accompany breathlessness, presence or absence and the relative severity can vary greatly between patients. For this reason, primary treatment is generally directed towards alleviation of dyspnea, with delivery of additional therapy as clinically indicated. 2
Often presumed to be a direct consequence of volume overload, acute HF is more accurately depicted by as a syndrome that results from the superimposition of potentially divergent precipitants on underlying systolic, diastolic or mixed cardiac dysfunction. 1,3 While most instances (~80%) of acute HF occur in patients with chronic cardiac disease, de novo presentation is not uncommon. Thus acute HF represents more than a simple decompensation of a chronic disorder and to be effective, treatment needs to reflect the complex nature of this condition.
Perspective
Treatment of acute HF can be broadly divided into a stabilization phase, where initial intervention directed towards immediate life-threatening conditions is followed by subsequent efforts to alleviate symptoms through targeted management of acute precipitants, and an in-hospital phase which involves continued remediation of residual signs and symptoms and on-going surveillance for interval development of renal or cardiac injury. 1 The latter also includes initiation or up-titration of chronic therapy that is in accordance with existing, evidence-based guidelines such as those put forth by the Heart Failure Society of America 2, the American College of Cardiology/American Heart Association 4, or the European Society of Cardiology 5 and pre-discharge planning with an eye on transition to the early post-discharge period.
Accordingly, it is the stabilization phase that has become synonymous with acute HF treatment and it is at this point that efforts to achieve symptom reduction through a rebalancing of hemodynamics and volume status are most critical. 6 However, there is increasing appreciation that inappropriate or overly aggressive medication administration can contribute to myocardial or renal injury and possibly worsen outcomes 7–9 underscoring the need to deliver therapy targeted to specific patient needs.
Precipitants of Acute Heart Failure
The overarching goal of acute HF treatment is to deliver the right medication to the right patient at the right time. 6 This requires a basic understanding of factors that can precipitate an episode of acute HF and how such precipitants adversely affect the cardiovascular system. As shown in the accompanying Table, these can be broadly grouped into factors that result in rapid decompensation (i.e., a profound increase in blood pressure, onset of acute myocardial injury or valve dysfunction, or dysrhythmia) and those which may be more insidious in onset (i.e., gradual fluid accumulation or progressive cardiopulmonary compromise in the setting of advanced, chronic disease). 10
Table.
Common precipitants of acute heart failure and associated mechanism leading to symptom onset
| Precipitant | Mechanism Contributing to Acute Heart Failure |
|---|---|
| Abrupt rise in blood pressure | Sudden increase in afterload causes impedance to forward flow by a structurally and/or functionally compromised left ventricle; net effect is a mismatch between ventricular/vascular coupling with a rise in end-diastolic pressure and a backflow of fluid from the systemic to pulmonary vasculature (“vascular failure”); typically occurs in a patient with chronic hypertension, left ventricular hypertrophy and impaired diastolic function |
| Acute myocardial injury | Acute onset of myocardial ischemia or infarct, inflammation (such as myocarditis from an infectious and non-infectious cause) or idiopathic myocardial damage that results in rapid development of cardiac dysfunction (either regionally or globally); net effect is to limit the heart’s pumping ability which produces a precipitous decline in cardiac output (“pump failure”) |
| Acute or progressive valve dysfunction | Stenotic, regurgitant, or mechanical valve abnormality which develops acutely (often from infection or, in the case of mitral regurgitation, from ischemic complications such as left ventricular dilation with leaflet tethering or papillary muscle rupture) or subacutely (typically from worsening of underlying chronic valve disease); net effect is an increase in left atrial (mitral) or ventricular (aortic) volume with backflow into the pulmonary vasculature |
| Ventricular or supraventricular dysrhythmia | Development of tachycardia (often atrial fibrillation) or less commonly, bradycardia (often medication related), which reduces the time spent in systole and/or diastole; net effect is to limit cardiac output through a decrease in ventricular filling and stroke volume |
| Progressive fluid accumulation | Neurohormonal activation, worsening renal function, dietary sodium load, excess fluid intake (or intravenous administration if acute HF develops in- hospital) or medication non-compliance, either singularly or in combination results in fluid accumulation and increased preload; net effect an increase in venous return to a left ventricle incapable of responding by the Frank-Starling mechanism; consequence is a buildup of fluid in the lungs and onset (often gradual) of clinical pulmonary congestion (“congestive failure”) |
| Cardiopulmonary deterioration in the setting of advanced chronic disease | Can be caused by a number of factors including overexertion, medication- related effects (under, over or inappropriate use), worsening renal function, indolent (i.e., “smoldering”) myocardial necrosis, or superimposed respiratory disorders (especially pneumonia); net effect is a progression of underlying advanced disease and an intolerable increase in baseline symptoms |
Identifying the specific precipitant (and hence, the acute pathophysiology to be targeted) can be facilitated by consideration of clinical variables. To make rapid but precise treatment decisions during the stabilization phase, such variables should be easily definable on presentation or available shortly after thereafter. Consideration of such variables in combination can yield clinical profiles that are more (or less) amenable to certain therapies. 11
Clinical Profiles
Defined by the presence and severity of relatively consistent features within important variable categories, clinical profiles help align treatment decisions with the desired therapeutic response. Among the features typically included are presenting signs and symptoms (pulmonary congestion, peripheral edema and hypoperfusion), hemodynamic parameters (primarily blood pressure and heart rate), and results of readily available diagnostic test (electrocardiographic changes consistent with ischemia or infarct, biomarker indicators of acute renal and myocardial stress or injury, and findings consistent with heart failure on chest radiography). 12 While echocardiography would provide additional insight into underlying cardiac structure and function, the incremental value (if any) of further subclassification during the stabilization phase has yet to be determined. 11,13
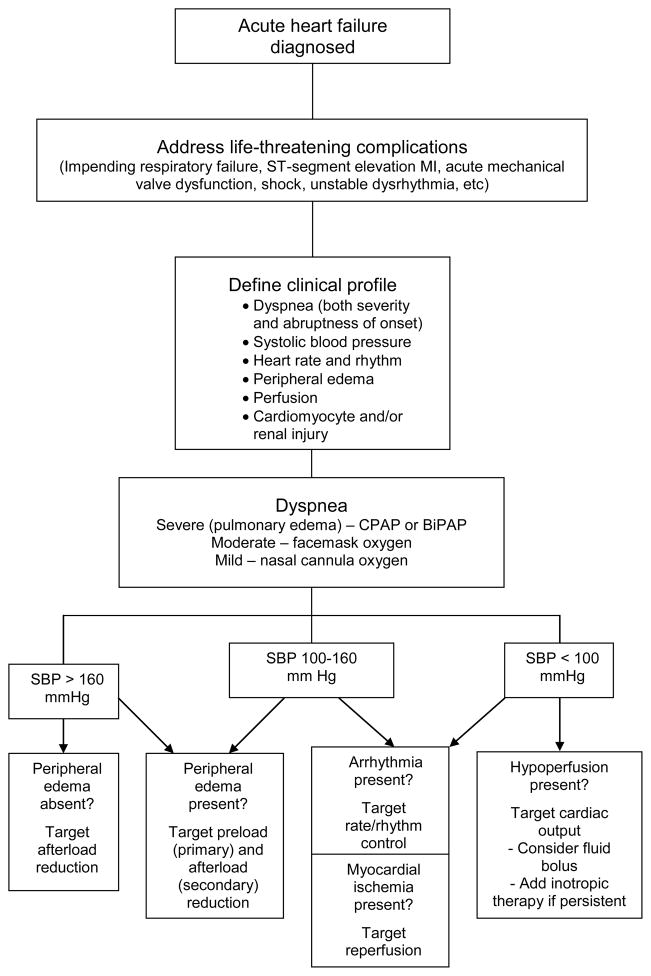
An overview of targeted management within the context of clinical profiles is provided in the accompanying Figure. As can be seen, blood pressure serves as a critical branch-point. 1,3,14 In part, this reflects the relative ease with which it can be obtained in most patients, but it also relates to the importance of blood pressure as a precipitating or contributing factor (more than 50% of all acute HF episodes are associated with a systolic blood pressure > 140 mm Hg) 15 and its relevance as a distinguishing characteristic that can predict in-hospital morbidity and mortality 16 and define divergent HF disease pathogenesis. 17
Figure.
Targeted therapy of acute heart failure based on clinical profile
Specific Targets of Therapy
Although blood pressure is a readily apparent manifestation of cardiovascular status, it is the specific hemodynamic components (i.e., afterload, preload and cardiac output) that contribute to acute HF and thus serve as actual targets of therapy.
Afterload
Patients with acute HF and excessive afterload often present with profound dyspnea but in general, respond quite well to aggressive vasodilator therapy. A number of agents can produce afterload reduction, yet only a handful have been rigorously tested in the setting of acute HF and head to head comparison trials are sorely lacking.
Nitrovasodilators
Nitrates have long been considered the first-line agents for acute hypertensive HF. As a class, these drugs work by providing an exogenous source of nitric oxide which then binds to soluble guanylate cyclase, producing cyclic GMP and vascular smooth muscle relaxation.18 At modest doses, this effect occurs predominantly in the venous circulation, resulting in increased capacitance and a marked reduction in preload. This contributes to a partial off-loading of the ventricle with a reduction in end-diastolic volume and a decrease in pulmonary capillary wedge pressure (PCWP).19,20 At higher doses (i.e, ≥ 150–250 mcg/min) arteriolar dilatation occurs which helps to improve cardiac output through a reduction in afterload and diminished impedance to forward flow.21–23 This effect may be more pronounced when systemic vascular resistance is severely elevated 24 and appears to be mediated through a dose-dependent, differential effect on the amplified pressure wave reflected back to the central circulation from the periphery when arterioles recoil (a facet of the ventricular/vascular coupling relationship termed the augmentation index). 25 Nitrate tolerance, a common but poorly understood phenomenon thought to involve O2 free-radical formation and nitric-oxide NO-synthase inhibition, can decrease the hemodynamic response to on-going administration despite up-titration 26,27 but this is of limited concern during the early part of the stabilization phase.
Nitroglycerin (glyceryl trinitrate) is the most common nitrate used in the United States and is often initially given as a sublingual tablet or spray (400 mcg per dose) to enable quick absorption and rapid onset of action. For persistent symptoms, transdermal application (1–2 inches of 2% ointment) or, for more severe cases, intravenous (IV) administration may be required. Because the half-life of nitroglycerin (NTG) is short (< 5 min), a continuous infusion (rate: 20 – 400 mcg/min) may be needed to maintain effect. Higher doses of IV NTG (or isosorbide dinitrate [ISDN], an alternative form commonly used in Europe) may provide particular benefit in patients with profoundly elevated blood pressure elevation and respiratory distress (i.e., hypertensive cardiogenic pulmonary edema). When given by repeat IV bolus (every 3–5 min), both high-dose NTG (2 mg) 28 and ISDN (4 mg) 29,30 have been associated with improved outcomes (i.e., reduced need for mechanical ventilation, intensive care unit admission and hospital length of stay) without induction of harm. As indicated by a recent study of high-dose topical nitrates (2 sublingual NTG tablets followed by application of 10 NitroDerm TTS patches) which showed a substantial reduction in cardiac stress 31, the clinical benefit of such an approach may arise from effects on the aforementioned augmentation index with a consequent decrease in left ventricular impedance.
Sodium nitroprusside (NTP), another nitric-oxide donor, is an additional option for profoundly hypertensive and dyspneic patients. This agent produces both preload and afterload reduction even at lower doses and has been shown to be effective for patients with refractory hypertension.32 However, controlled trials of NTP in acute HF are lacking and, in a small comparison study (n=37), no difference in blood pressure control or transmitral Doppler flow parameters was found in hypertensive HF patients who received relatively low doses of NTG (max dose: 50 mcg/min) or NTP (max dose: 1 mcg/kg/min). 33 Because NTP can cause significant and slightly prolonged hypotension as well as reflex tachycardia, invasive arterial monitoring and close supervision are generally recommended.34 Furthermore, NTP can also increase the risk of “coronary steal syndrome” with a shunting of blood flow from the heart to the periphery as well as cyanide toxicity (though the latter can be minimized with concurrent administration of thiosulfate). Given these issues and the evidence of substantial clinical benefit with aggressively dosed NTG and ISDN, there are few (if any) circumstances where NTP would be the preferred initial agent.
Natriuretic Peptides
Nesiritide, the most studied agent in this class, is a recombinant form of brain-natriuretic peptide (BNP) that has combined neurohormonal and vasodilator effects.35 Despite a seemingly appropriate pharmacological basis, early data on the safety and efficacy of nesiritide were conflicting 36–38 prompting calls for a large-scale clinical trial of its utility in acute HF patients. This study, ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Deconpensated Heart Failure), was completed in 2010 and showed a statistical (but not clinically significant) difference in dyspnea improvement but an increased risk of hypotension with use of nesiritde. 39 While there was no additional evidence of harm, the absence of any potential benefit makes it difficult to recommend the use of nesiritide in the broad population of patients with acute HF. Other natriuretic peptide compounds including ularitide (a synthetic analogue of urodilatin, an atrial-NP derivative) and cenderitide (a chimer of c-type and d-type NP) 40 have since been developed though at present, their clinical viability has yet to be defined.
Angiotensin Converting Enzyme Inhibitors
As a class, angiotensin converting enzyme (ACE) inhibitors are effective antihypertensives and provide antagonism of the renin-angiotensin-aldosterone system making them ideal agents for HF treatment. Abundant data which show substantial benefit with use of oral ACE inhibitors in chronic HF (i.e., disease regression, symptom improvement, decreased mortality) 41–43 but few studies have been conducted in acute HF patients. However, these limited trials are encouraging with demonstration of rapid dyspnea improvement after a single dose of sublingual captopril (25 mg) and significant reduction in blood pressure after IV administration of enalaprilat (1 mg).44,45 While such signals suggest efficacy, there is potential for adverse events such as sustained hypotension, renal dysfunction, and hyperkalemia. Therefore, absent further safety data, ACE inhibitors should be used with caution during the stabilization phase of acute HF management.
Calcium Channel Blockers
While it has long been recommended to avoid rapid-onset dihydropyridine calcium channel blockers (CCB’s) such as sublingual nifedipine as they produce unpredictable effects on peripheral resistance and increase the risk of coronary and cerebral hypoperfusion, 46 a new generation of short-acting IV dihydropyridine CCBs (i.e., nicardipine and clevidipine) has been developed prompting interest in their use for hypertensive HF. Data are just beginning to emerge on this group of medications but a recent analysis from the VELOCITY (The Evaluation of the Effect of Ultra-Short-Acting Clevidipine in the Treatment of Patients With Severe Hypertension) trial showed that they may be safe in the setting of acute HF. 47 Based on this, a prospective, phase III trial of clevidine (PRONTO - An Efficacy and Safety Study of Blood Pressure Control in Acute Heart Failure - A Pilot Study) was designed. This study recently completed enrollment and results should help define the role of short-acting CCBs in the management of acute hypertensive HF.
Other Agents
Relaxin is a peptide hormone released in pregnancy that helps regulate hemodynamic function and renovascular blood flow. Specific effects of relaxin include production of nitric oxide, vascular endothelial growth factor, and matrix metalloproteinases as well as inhibition of endothelin and angiotensin II. Such effects result in a number of vascular changes (especially systemic and renal vasodilation) that may be beneficial in acute hypertensive HF. 48 Preliminary study (Pre-RELAX AHF - Phase II Multicenter, Randomized, Double-blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Relaxin in Subjects With Acute Heart Failure) suggests greater dyspnea improvement and a reduction in the composite of cardiovascular death or readmission due to heart or renal failure at day 60 (2.6% [95% CI 0.4–16.8] vs 17.2% [9.6–29.6]; p=0.053) with use of relaxin (compared to placebo). 49 Further discussion of the potential utility of relaxin in acute HF however, await the results of RELAX-AHF - a recently completed phase III follow-up to the Pre-RELAX study.
TRV120027, a beta-arrestin biased angiotensin II type 1 receptor (AT1R) ligand, is another novel agent that is being developed for patients with acute HF and elevated blood pressure. Animal data show that this agent may provide effective cardiac unloading through a reduction in systemic vascular resistance while preserving renal function. 50 A phase II trial of TRV12027, the first biased ligand to be tested in acute HF patients, is planned for early 2013.
Preload
Volume overload is a common feature in patients presenting with acute HF and relief of congestion through removal of excess fluid is an important goal of therapy. 10 Despite a lack of prospective, randomized trials, diuretics have remained the mainstay of therapy for decades and are used in the vast majority (~90%) of patients with acute HF symptoms. While alternatives have been recently investigated, none has been found to be superior in terms of safety or efficacy. Consequently, diuretics remain the de facto “standard” of care for acute HF in those with (and often without) hypervolemia. 6
Loop Diuretics
Intravenous loop diuretics (furosemide, bumetanide, torsemide and ethacrynic acid) inhibit the Na+ - K+ - 2Cl− co-transport channel in epithelial cells which line the thick ascending limb of the loop of Henle 51 and are the most common class of medication used in the treatment of acute HF. They produce an osmotic diuresis within 30 minutes of administration and achieve a peak effect 2 to 4 hours later. Non-sustained vasodilation may also be seen early (5 to 15 minutes after administration 52 but this is offset by latent vasoconstriction related, at least in part, to activation of neurohormonal factors.53 Despite this potential disadvantage, loop diuretics do effectively reduce filling pressures and induce symptomatic improvement making them widely accepted for acute HF treatment. 54
Resistance to loop diuretics as identified by diminished or absent response to high diuretic doses and refractory edema may be encountered while treating acute HF and is associated with a poor prognosis.55,56 Typically, seen in those on long-term therapy, on occasion it may occur in diuretic naïve patients with profound volume depletion and decreased renal perfusion. Adding a thiazide agent (i.e, metolazone or chlorothiazide) 57,58 can improve urinary output application of this approach to the acute setting has been insufficiently explored.
The optimal approach to diuretic dosing and administration has been a source of on-going controversy. Though some concerns with methodology exist, the best evidence to date comes from the Diuretic Optimization Strategies Evaluation (DOSE) Study which, using a 2 × 2 factorial prospectively, compared approaches to IV furosemide administration. 59 Patients with decompensated HF (n=308) who had reduced ejection fraction at baseline were randomized to receive high (2.5 times daily oral) vs. low (daily oral) dosing and intermittent bolus (every 12 hours) vs. continuous infusion for a period of at least 48 hours. While there was no statistical difference in global symptom relief, absolute change in renal function at 72 hours, or a composite of death, rehospitalization, or emergency department visits through 60 days post-discharge for either level of comparison (i.e., low vs. high dose and intermittent bolus vs. continuous infusion), a signal pointing towards better dyspnea relief, greater net weight and volume loss, an increase in the proportion free from signs of congestion and a more substantial reduction in natriuretic peptides was noted with use of the high-dose strategy. Of note, worsening renal function (defined as an increase in serum creatinine > 3 mg/dL) was more common in those who received high dose diuretics, regardless of how it was administered (23% vs. 14%; p = 0.041). Further, this renal dysfunction appeared to persist post-discharge with an incidence of nearly 20% at day 60 (with no difference between groups). Thus, according to DOSE, there may be some clinical advantage to high-dose furosemide (potentially at the expense of short-term renal dysfunction) but no clear benefit (or harm) with administration by intermittent bolus or continuous infusion.
Vasopressin Antagonists (Vaptans)
By triggering the manufacture and cell membrane insertion of aquaporin-2 molecules in renal collecting ducts, arginine vasopressin (AVP), also known as antidiuretic hormone, is a potent stimulus of free water uptake by the kidneys. Vasporessin activity is increased in many HF patients and contributes to dysregulated fluid accumulation. By blocking the action of vasopressin, conivaptan (a dual V1/V2 receptor antagonist) and tolvaptan or lixivaptan (relative V2 selective antagonists) lead to excretion of low-solute fluid and improve hyponatremia (a known risk factor in acute HF) without adversely affecting glomerular filtration rate or renal blood flow. 60 The “vaptans” thus provide a pharmacological approach to volume reduction that theoretically lacks the drawbacks of loop diuretics. Despite this, EVEREST (Efficacy of Vasopressin Antagonism in hEart failuRE: Outcome Study With Tolvaptan), a two part investigation that enrolled over 4000 patients with acute HF, only a modest improvement in dyspnea, edema, serum sodium and renal function was seen with no long-term effect on mortality or HF-related morbidity. 61,62 Consequently, vasopressin antagonism has a limited role in the treatment of acute HF at present. That said, THE BALANCE (Treatment of Hyponatremia Based on Lixivaptan in NYHA Class III/IV Cardiac Patient Evaluation) study is on-going and there may be a future indication for use in patients with acute HF complicated by hyponatremia. 63 Additional studies evaluating the benefit of tolvaptan administration in patients with increased AVP activity as determined by copeptin measurement (i.e., biomarker directed studies) are also planned.
Ultrafiltration
Mechanical fluid removal using ultrafiltration is an alternative to pharmacological diuresis and a viable option for the management of acute HF with volume overload, particularly in patients with diuretic resistance. Ultrafiltration uses veno-venous hemoconcentration to extract up to 500 mL of isotonic fluid per hour. Although the UNLOAD (Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure) study found an association with decreased readmissions 64, clear indications for the use of ultrafiltration in acute HF have not been defined. Moreover, ultrafiltration is costly (~$19,500 for device acquisition and $950 per filter with 1–2 filters required per treatment) leading to concerns about the financial implications associated with widespread implementation. 65
Cardiac Output
Myocardial ischemia, valvular dysfunction, and arrhythmia are among the most common causes of diminished cardiac output and each of these has inherent therapy that is beyond the scope of this chapter. Suffice to say, when treating HF related to such causes, the primary intervention should be directed towards the inciting factor (i.e., reperfusion for ischemia, surgery for critical valve dysfunction, and rate or rhythm control for atrial fibrillation) rather than the end manifestation. Absent these considerations, a reduction in cardiac output should be attributed to progressive pump failure. Many of these individuals will have advanced HF but low blood pressure alone should not be taken as an indication to initiate inotropic therapy, particularly when underlying coronary artery disease is present as these agents increase myocardial oxygen demand and enhance the potential for arrhythmia development.34,66–68 In fact, for those without evidence pulmonary congestion, a small bolus of isotonic normal saline (250–500cc) may be needed as these individuals frequently suffer from intravascular depletion as a result of chronic over-diuresis.
When hypoperfusion is present, inotropic therapy (i.e., dobutamine or milrinone) to improve myocardial function will be needed. Dobutamine acts through β1 and β2 adrenergic receptor stimulation to increase cardiac output by enhancing inotropy and chronotropy. 69 Milrinone, a type III phosphodiesterase inhibitor (PDEI) also improves hemodynamic function (i.e., stroke volume and cardiac output) but does so by preventing intracellular breakdown of cyclic adenosine monophosphate (cAMP).70 Though this activity is independent of adrenergic receptor stimulation, it produces similar net effects on the heart (i.e., inotropy, chronotropy and lusitropy).71 In the peripheral circulation however, vasodliatory effects predominate resulting in significant preload and afterload reduction, with the potential to worsen hypotension, particularly when intravascular volume depletion is present.72 Concurrent administration of dobutamine and milrinone (or an alternative PDEI such as amrinone or enoximone) yields an additive effect on cardiac function and may be a useful approach for those on chronic beta-blocker therapy.73,74
Positive inotropic effects can also be accomplished by targeting the mycocardial contractile apparatus itself. Cardiac glycosides (i.e., digoxin) are the time-honored approach, producing their desired effect by establishing a gradient that promotes intracellular calcium accumulation through inhibition of Na+/K+ ATPase. The relative excess of calcium enhances myocyte contractility resulting in an incremental improvement in cardiac output. Popular in decades past, digoxin use is uncommon to see in contemporary management of acute HF though there is resurgent interest. 75 Other agents that enhance myocyte contractility include levosimendan (a calcium sensitizer that functions through K+/ATP channels)76, istraroxime (a concurrent inhibitor of Na+/K+ ATPase and stimulator of sarcoendoplasmic reticulum calcium ATPase)77, and omecamtiv mecarbil (a direct-acting cardiomyosin activator).78 Of these, levosimendan has been most studied but, despite early promise in clinical trials, no benefit over dobutamine was found in SURVIVE (Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support), the definitive study of the medication. 79
Other Considerations
Oxygen Therapy and Ventilatory Support
Supplemental oxygen therapy via nasal cannula is sufficient for patients with mild dyspnea but for moderate shortness of breath a non-rebreather facemask may be needed. When profound dyspnea is present, early initiation of non-invasive positive airway pressure ventilation (NIPPV; either continuous [CPAP] of bi-level [BiPAP]) can dramatically improve symptoms and may decrease the need for endotracheal intubation. Though prior studies suggested a relative increase in the rate of myocardial infarction with use of BiPAP (vs. CPAP), several reviews 80,81 and the prospective Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial showed no difference in safety or efficacy. 82 While the latter also found no mortality benefit with CPAP or BiPAP when compared to face-mask oxygen therapy, there was clear improvement in respiratory distress with NIPPV. When using non-invasive ventilation, initial CPAP pressure is typically set at 5–7 cm H20 and BiPAP pressures at 8–10 cm H20 inspiratory and 4–5 cm H20 expiratory with up (or down) titration as needed (max = 15 cm H20 for CPAP and 20/10 cm H20 for BiPAP). In addition to reducing the work of breathing, NIPPV decreases preload helping to offset pulmonary congestion.
Overall, up to 5% of acute HF patients and nearly 40% of those with severe cardiogenic pulmonary edema will require endotracheal intubation (ETI).83–85 For most, signs of impending respiratory failure including breathlessness, tachypnea, diaphoresis, muscle fatigue and confusion will be readily apparent on arrival to the ED. Other findings that indicate a need for ETI include persistent hypoxia (SaO2 < 80) or hypoxemia (PaO2/FiO2 < 200) despite supplemental oxygen, hypercarbia (PaCO2 > 55 mmHg) and acidosis (pH < 7.25).86 While clearly necessary in some, ETI is associated with poor outcome 87 and decreases the risk of neurologically intact survival for patients with acute HF who suffer inhospital cardiac arrest. 88
Morphine Sulfate
Perhaps the most common other medication used in the treatment of acute HF is morphine sulfate. Morphine is thought to produce mild vasodilatation, induce respiratory relaxation and exert a calming effect on those with agitated dyspnea. Despite empiric use, there is limited evidence in support of morphine in acute HF and several small trials suggesting potential harm.84,89 Moreover, in ADHERE (Acute Decompensated Heart Failure Registry), morphine use was found to be an independent predictor of in-hospital mortality (adjusted odds ratio [95% CI] = 4.84 [4.52, 5.18]).90 Thus at best, morphine appears to be of marginal utility and at worst, a possible contributor to suboptimal outcomes.
Conclusions
Treatment of acute HF is rapidly evolving to reflect the heterogeneous nature of this complex disorder. Recognition of divergent clinical profiles despite homogeneity in presentation will help ensure delivery of the most appropriate therapy for an individual patient and improve the likelihood of optimal outcome. Within this context, effective intervention involves targeting a specific precipitant while appreciating the backdrop of underlying cardiac dysfunction. While the approach to acute HF care has progressed with improved understanding, the goals of treatment remain remarkably consistent: alleviate symptoms without causing harm.
Footnotes
Disclosure
P. Levy is a board member for the Society of Cardiovascular Patient Care and a consultant for Cornerstone Therapeutics, ekr Therapeutics, Novartis Pharma AG, Bayer Schering Pharma AG, and Travena, Inc., ane has received grants from Cardiorentis, Inc., Amgen, Inc., and Otsuka Pharmaceuticals Co Inc. He has also received honoraria from GREAT Network as well as payment for the development of educational presentations from EKR Therapeutics. A. Bellou has nothing to disclose.
Contributor Information
Phillip D. Levy, Email: plevy@med.wayne.edu, Associate Professor of Emergency Medicine, Assistant Director of Clinical Research, Cardiovascular Research Institute, Associate Director of Clinical Research, Department of Emergency Medicine, Wayne State University School of Medicine, 4201 St. Antoine; UHC – 6G, Detroit, MI 48201, Office: +1 313 993 8558, Fax: +1 313 966 8172.
Abdel Bellou, Email: abdel.bellou@voila.fr, Professor of Internal Medicine, Therapeutics, and Emergency Medicine, Head of Emergency Department, Director of Emergency Medicine Training Programme, Rennes University Hospital, 2 Rue Henri le Guilloux, 35000, Rennes, France, Office: + 3329928940.
References
- 1.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 2• •.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. Updated guidelines including an overview on acute heart failure as developed by the Heart Failure Society of America. [DOI] [PubMed] [Google Scholar]
- 3.Mebazaa A, Gheorghiade M, Pina IL, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med. 2008;36:S129–139. doi: 10.1097/01.CCM.0000296274.51933.4C. [DOI] [PubMed] [Google Scholar]
- 4••.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. Updated guidelines including an overview on acute heart failure as developed by the American College of Cardiology and the American Heart Association. [DOI] [PubMed] [Google Scholar]
- 5• •.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. Updated guidelines including an overview on acute heart failure as developed by the European Society of Cardiology. [DOI] [PubMed] [Google Scholar]
- 6• •.Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. Key review paper discussing major issues surrounding acute heart failure management from both a clinical and a research perspective. Provides insight into existing and future approaches to treatment. [DOI] [PubMed] [Google Scholar]
- 7.Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 8.Lanfear DE, Peterson EL, Campbell J, et al. Relation of worsened renal function during hospitalization for heart failure to long-term outcomes and rehospitalization. Am J Cardiol. 2011;107:74–78. doi: 10.1016/j.amjcard.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peacock F, Amin A, Granger CB, et al. Hypertensive heart failure: patient characteristics, treatment, and outcomes. Am J Emerg Med. 2011;29:855–62. doi: 10.1016/j.ajem.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Pang PS, Levy P. Pathophysiology of volume overload in acute heart failure syndromes. Congest Heart Fail. 2010;16 (Suppl 1):S1–6. [Google Scholar]
- 11.Pang PS, Hoffmann U, Shah SJ. Classification of patients with acute heart failure syndromes in the emergency department. Circ Heart Fail. 2012;5:2–5. doi: 10.1161/CIRCHEARTFAILURE.111.965830. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Braunwald E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA. 2011;305:1702–1703. doi: 10.1001/jama.2011.515. [DOI] [PubMed] [Google Scholar]
- 13.Peacock WF, Braunwald E, Abraham W, et al. National Heart, Lung, and Blood Institute working group on emergency department management of acute heart failure: research challenges and opportunities. J Am Coll Cardiol. 2010;56:343–351. doi: 10.1016/j.jacc.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Collins S, Storrow AB, Kirk JD, et al. Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department. Ann Emerg Med. 2008;51:45–57. doi: 10.1016/j.annemergmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.De Luca L, Fonarow GC, Adams KF, Jr, et al. Acute heart failure syndromes: clinical scenarios and pathophysiologic targets for therapy. Heart Fail Rev. 2007;12:97–104. doi: 10.1007/s10741-007-9011-8. [DOI] [PubMed] [Google Scholar]
- 16.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ignarro LJ. After 130 years, the molecular mechanism of action of nitroglycerin is revealed. Proc Natl Acad Sci U S A. 2002;99:7816–7817. doi: 10.1073/pnas.132271799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bussmann WD, Kaltenbach M. Sublingual nitroglycerin in the treatment of left ventricular failure and pulmonary edema. Eur J Cardiol. 1976;4:327–333. [PubMed] [Google Scholar]
- 20.Bussmann WD, Kaltenbach M. Sublingual nitroglycerin for left ventricular failure and pulmonary edema. Compr Ther. 1977;3:29–36. [PubMed] [Google Scholar]
- 21.Imhof PR, Ott B, Frankhauser P, et al. Difference in nitroglycerin dose-response in the venous and arterial beds. Eur J Clin Pharmacol. 1980;18:455–460. doi: 10.1007/BF00874655. [DOI] [PubMed] [Google Scholar]
- 22.Bayley S, Valentine H, Bennett ED. The haemodynamic responses to incremental doses of intravenous nitroglycerin in left ventricular failure. Intensive Care Med. 1984;10:139–145. doi: 10.1007/BF00265803. [DOI] [PubMed] [Google Scholar]
- 23.Herling IM. Intravenous nitroglycerin: clinical pharmacology and therapeutic considerations. Am Heart J. 1984;108:141–149. doi: 10.1016/0002-8703(84)90557-x. [DOI] [PubMed] [Google Scholar]
- 24.Haber HL, Simek CL, Bergin JD, et al. Bolus intravenous nitroglycerin predominantly reduces afterload in patients with excessive arterial elastance. J Am Coll Cardiol. 1993;22:251–257. doi: 10.1016/0735-1097(93)90841-n. [DOI] [PubMed] [Google Scholar]
- 25.Munir S, Guilcher A, Kamalesh T, et al. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension. 2008;51:112–118. doi: 10.1161/HYPERTENSIONAHA.107.096016. [DOI] [PubMed] [Google Scholar]
- 26.Elkayam U, Akhter MW, Singh H, et al. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high-dose nitroglycerin in patients with decompensated heart failure. Am J Cardiol. 2004;93:237–240. doi: 10.1016/j.amjcard.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 27.Gori T, Parker JD. Nitrate tolerance: a unifying hypothesis. Circulation. 2002;106:2510–2513. doi: 10.1161/01.cir.0000036743.07406.53. [DOI] [PubMed] [Google Scholar]
- 28.Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007;50:144–152. doi: 10.1016/j.annemergmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Cotter G, Faibel H, Barash P, et al. High-dose nitrates in the immediate management of unstable angina: optimal dosage, route of administration, and therapeutic goals. Am J Emerg Med. 1998;16:219–224. doi: 10.1016/s0735-6757(98)90087-0. [DOI] [PubMed] [Google Scholar]
- 30.Sharon A, Shpirer I, Kaluski E, et al. High-dose intravenous isosorbide-dinitrate is safer and better than Bi-PAP ventilation combined with conventional treatment for severe pulmonary edema. J Am Coll Cardiol. 2000;36:832–837. doi: 10.1016/s0735-1097(00)00785-3. [DOI] [PubMed] [Google Scholar]
- 31.Breidthardt T, Noveanu M, Potocki M, et al. Impact of a high-dose nitrate strategy on cardiac stress in acute heart failure: a pilot study. J Intern Med. 2010;267:322–330. doi: 10.1111/j.1365-2796.2009.02146.x. [DOI] [PubMed] [Google Scholar]
- 32.Guiha NH, Cohn JN, Mikulic E, et al. Treatment of refractory heart failure with infusion of nitroprusside. N Engl J Med. 1974;291:587–592. doi: 10.1056/NEJM197409192911201. [DOI] [PubMed] [Google Scholar]
- 33.Eryonucu B, Guler N, Guntekin U, Tuncer M. Comparison of the effects of nitroglycerin and nitroprusside on transmitral Doppler flow parameters in patients with hypertensive urgency. Ann Pharmacother. 2005;39:997–1001. doi: 10.1345/aph.1E562. [DOI] [PubMed] [Google Scholar]
- 34.Nieminen MS, Bohm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 35.Burger MR, Burger AJ. BNP in decompensated heart failure: diagnostic, prognostic and therapeutic potential. Curr Opin Investig Drugs. 2001;2:929–935. [PubMed] [Google Scholar]
- 36.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 37.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 38.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 39• •.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. Pivotal trial in acute heart failure which evaluated the safety and efficacy of nesiritide. Provides an excellent discussion of treatment paradigms and insight into the methodolgy behind the largest acute heart failure trial to date. [DOI] [PubMed] [Google Scholar]
- 40.Lisy O, Huntley BK, McCormick DJ, et al. Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–8. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 42.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 43.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton RJ, Carter WA, Gallagher EJ. Rapid improvement of acute pulmonary edema with sublingual captopril. Acad Emerg Med. 1996;3:205–212. doi: 10.1111/j.1553-2712.1996.tb03422.x. [DOI] [PubMed] [Google Scholar]
- 45.Annane D, Bellissant E, Pussard E, et al. Placebo-controlled, randomized, double-blind study of intravenous enalaprilat efficacy and safety in acute cardiogenic pulmonary edema. Circulation. 1996;94:1316–1324. doi: 10.1161/01.cir.94.6.1316. [DOI] [PubMed] [Google Scholar]
- 46.Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276:1328–1331. [PubMed] [Google Scholar]
- 47.Peacock FW, Varon J, Ebrahimi R, et al. Clevidipine for severe hypertension in acute heart failure: a VELOCITY trial analysis. Congest Heart Fail. 2010;16:55–59. doi: 10.1111/j.1751-7133.2009.00133.x. [DOI] [PubMed] [Google Scholar]
- 48.Teichman SL, Unemori E, Dschietzig T, et al. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev. 2009;14:321–329. doi: 10.1007/s10741-008-9129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teerlink JR, Metra M, Felker GM, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373:1429–39. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 50.Boerrigter G, Lark MW, Whalen EJ, et al. Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4:770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- 51.Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387–395. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 52.Dikshit K, Vyden JK, Forrester JS, et al. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. N Engl J Med. 1973;288:1087–1090. doi: 10.1056/NEJM197305242882102. [DOI] [PubMed] [Google Scholar]
- 53.Francis GS, Siegel RM, Goldsmith SR, et al. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 54.Mebazaa A, Pang PS, Tavares M, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31:832–841. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 55.Kramer BK, Schweda F, Riegger GA. Diuretic treatment and diuretic resistance in heart failure. Am J Med. 1999;106:90–96. doi: 10.1016/s0002-9343(98)00365-9. [DOI] [PubMed] [Google Scholar]
- 56.Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–38. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 57.Channer KS, McLean KA, Lawson-Matthew P, Richardson M. Combination diuretic treatment in severe heart failure: a randomised controlled trial. Br Heart J. 1994;71:146–150. doi: 10.1136/hrt.71.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dormans TP, Gerlag PG, Russel FG, Smits P. Combination diuretic therapy in severe congestive heart failure. Drugs. 1998;55:165–172. doi: 10.2165/00003495-199855020-00001. [DOI] [PubMed] [Google Scholar]
- 59.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Luca L, Orlandi C, Udelson JE, et al. Overview of vasopressin receptor antagonists in heart failure resulting in hospitalization. Am J Cardiol. 2005;96:24L–33L. doi: 10.1016/j.amjcard.2005.09.067. [DOI] [PubMed] [Google Scholar]
- 61.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 62.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 63.Abraham WT, Aranda JM, Boehmer JP, et al. Rationale and Design of the Treatment of Hyponatremia Based on Lixivaptan in NYHA Class III/IV Cardiac Patient Evaluation (THE BALANCE) Study. Clin Transl Sci. 2010;3:249–253. doi: 10.1111/j.1752-8062.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 65.Kazory A, Bellamy FB, Ross EA. Ultrafiltration for acute decompensated heart failure: financial implications. Int J Cardiol. 2012;154:246–249. doi: 10.1016/j.ijcard.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 66.Burger AJ, Elkayam U, Neibaur MT, et al. Comparison of the occurrence of ventricular arrhythmias in patients with acutely decompensated congestive heart failure receiving dobutamine versus nesiritide therapy. Am J Cardiol. 2001;88:35–39. doi: 10.1016/s0002-9149(01)01581-8. [DOI] [PubMed] [Google Scholar]
- 67.Leier CV, Webel J, Bush CA. The cardiovascular effects of the continuous infusion of dobutamine in patients with severe cardiac failure. Circulation. 1977;56:468–472. doi: 10.1161/01.cir.56.3.468. [DOI] [PubMed] [Google Scholar]
- 68.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 69.Colucci WS, Wright RF, Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments - 1. N Engl J Med. 1986;314:290–299. doi: 10.1056/NEJM198601303140506. [DOI] [PubMed] [Google Scholar]
- 70.Colucci WS, Wright RF, Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments - 2. N Engl J Med. 1986;314:349–358. doi: 10.1056/NEJM198602063140605. [DOI] [PubMed] [Google Scholar]
- 71.Colucci WS, Wright RF, Jaski BE, et al. Milrinone and dobutamine in severe heart failure: differing hemodynamic effects and individual patient responsiveness. Circulation. 1986;73:175–183. [PubMed] [Google Scholar]
- 72.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert EM, Hershberger RE, Wiechmann RJ, et al. Pharmacologic and hemodynamic effects of combined beta-agonist stimulation and phosphodiesterase inhibition in the failing human heart. Chest. 1995;108:1524–1532. doi: 10.1378/chest.108.6.1524. [DOI] [PubMed] [Google Scholar]
- 74.Metra M, Nodari S, D’Aloia A, et al. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol. 2002;40:1248–1258. doi: 10.1016/s0735-1097(02)02134-4. [DOI] [PubMed] [Google Scholar]
- 75.Gheorghiade M, Braunwald E. Reconsidering the role for digoxin in the management of acute heart failure syndromes. JAMA. 2009;302:2146–2147. doi: 10.1001/jama.2009.1657. [DOI] [PubMed] [Google Scholar]
- 76.Slawsky MT, Colucci WS, Gottlieb SS, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation. 2000;102:2222–2227. doi: 10.1161/01.cir.102.18.2222. [DOI] [PubMed] [Google Scholar]
- 77.Gheorghiade M, Blair JE, Filippatos GS, et al. Hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;51:2276–2285. doi: 10.1016/j.jacc.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 78.Tavares M, Rezlan E, Vostroknoutova I, et al. New pharmacologic therapies for acute heart failure. Crit Care Med. 2008;36:S112–120. doi: 10.1097/01.CCM.0000296810.74724.8D. [DOI] [PubMed] [Google Scholar]
- 79.Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 80.Masip J, Roque M, Sanchez B, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA. 2005;294:3124–3130. doi: 10.1001/jama.294.24.3124. [DOI] [PubMed] [Google Scholar]
- 81.Collins SP, Mielniczuk LM, Whittingham HA, et al. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med. 2006;48:260–9. 9 e1–4. doi: 10.1016/j.annemergmed.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 82.Gray A, Goodacre S, Newby DE, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 83.Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114:1185–1192. doi: 10.1378/chest.114.4.1185. [DOI] [PubMed] [Google Scholar]
- 84.Sacchetti A, Ramoska E, Moakes ME, et al. Effect of ED management on ICU use in acute pulmonary edema. Am J Emerg Med. 1999;17:571–574. doi: 10.1016/s0735-6757(99)90198-5. [DOI] [PubMed] [Google Scholar]
- 85.Yan AT, Bradley TD, Liu PP. The role of continuous positive airway pressure in the treatment of congestive heart failure. Chest. 2001;120:1675–1685. doi: 10.1378/chest.120.5.1675. [DOI] [PubMed] [Google Scholar]
- 86.Masip J, Paez J, Merino M, et al. Risk factors for intubation as a guide for noninvasive ventilation in patients with severe acute cardiogenic pulmonary edema. Intensive Care Med. 2003;29:1921–1928. doi: 10.1007/s00134-003-1922-9. [DOI] [PubMed] [Google Scholar]
- 87.Adnet F, Le Toumelin P, Leberre A, et al. In-hospital and long-term prognosis of elderly patients requiring endotracheal intubation for life-threatening presentation of cardiogenic pulmonary edema. Crit Care Med. 2001;29:891–895. doi: 10.1097/00003246-200104000-00042. [DOI] [PubMed] [Google Scholar]
- 88.Levy PD, Ye H, Compton S, Chan PS, et al. Factors associated with neurologically intact survival for patients with acute heart failure and in-hospital cardiac arrest. Circ Heart Fail. 2009;2:572–581. doi: 10.1161/CIRCHEARTFAILURE.108.828095. [DOI] [PubMed] [Google Scholar]
- 89.Hoffman JR, Reynolds S. Comparison of nitroglycerin, morphine and furosemide in treatment of presumed pre-hospital pulmonary edema. Chest. 1987;92:586–593. doi: 10.1378/chest.92.4.586. [DOI] [PubMed] [Google Scholar]
- 90.Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25:205–209. doi: 10.1136/emj.2007.050419. [DOI] [PubMed] [Google Scholar]