Abstract
Fine-tuning the immune response and maintaining tolerance to self antigens involves a complex network of co-stimulatory and co-inhibitory molecules. The recent FDA approval of ipilimumab, a monoclonal antibody blocking CTLA-4, demonstrates the impact of checkpoint regulators in disease. This is reinforced by ongoing clinical trials targeting not only CTLA-4, but also the PD-1 and B7-H4 pathways in various disease states. Recently two new B7 family inhibitory ligands, VISTA and B7-H6 were identified. Here we review recent understanding of B7 family members and their concerted regulation of the immune response to either self or foreign pathogens. We also discuss clinical developments in targeting these pathways in different disease settings, and introduce VISTA as a putative therapeutic target.
Costimulatory and co-inhibitory molecules: fine tuning the immune response
The immune response is regulated by an array of molecules required to defend the body against danger signals and foreign bodies as well as maintain tolerance to self-antigens. This is achieved in part through the regulation of T cell function. T cell activation requires two signals. The first is activation through the T-cell receptor (TCR) by recognition of antigen presented by the major histocompatibility complex (MHC) on antigen presenting cells (APCs). The second involves the ligation of co-stimulatory and co-inhibitory molecules expressed on APCs and T cells belonging to the B7 and tumor necrosis factor (TNF) families. In the absence of co-stimulation, T cells are rendered unresponsive (anergic) [1]. In addition, a subset of co-stimulatory molecules mediate bi-directional signaling through the APCs, indicating that these molecules form part of a complex network critical in preventing the onset of autoimmunity [2]. The significance of modulating these pathways during the past decade was highlighted in 2011 by the FDA approval of ipilmumab f, an antibody targeting CTLA-4 in cancer. The ability to successfully target checkpoint regulators has since led to multiple clinical trials with antibodies targeting the pathway of the B7 family member PD-1. In parallel to the advances in cancer, ongoing work is being down to utilize CTLA-4 and B7-H4 fusion proteins, respectively, for the treatment of autoimmune diseases. The aim of this article is to address the role of B7 family co-stimulatory and co-inhibitory molecules and their recent clinic advances. We also discuss their structure and expression and how they relate to one another. Finally, we introduce the novel molecule VISTA and it's significance as a putative co-inhibitory target in future clinical studies.
Co-stimulatory B7 family ligands and receptors
B7-CD28
The best-characterized co-stimulatory molecule is CD28, a glycoprotein constitutively expressed on the surface of naïve T cells [3, 4]. CD28 forms a homodimer on the cell surface and binds to B7-1 (CD80) and B7-2 (CD86) expressed on APCs such as dendritic cells, B cells and macrophages. In the immunological synapse, signaling through CD28 leads to recruitment of Src homology 2 proteins that bind to tyrosines in the immunoreceptor tyrosine activating motif (ITAM) in the CD28 tail. Signaling through CD28 lowers the T cell threshold for optimal cell activation [5], induces T cell proliferation, up-regulates the anti-apoptotic molecule (Bcl-Xl) [6], and increases IL-2 production [7].
Despite the co-stimulatory properties of CD28, the CD28 pathway as a therapeutic target has attracted interested based on the success of monoclonal CD3 antibodies (OKT3) therapy in clinical trials and the role of CD28 on survival and homeostasis of regulatory T cells (Treg) [8]. In 2006, a London based phase I clinical trial with TGN1412, a CD28 superagonist monoclonal antibody, resulted in a catastrophe. The healthy volunteers recruited to the trial developed a cytokine-release syndrome (CRS) due to the “superactivation” of T cells by TGN1412. This led to a systemic burst of pro-inflammatory cytokines with the individuals requiring life support [9]. This baffled scientific investigators worldwide as to why CRS was not detected in the preclinical studies. To address this question, follow up studies found that the ability of CD28 superagonists to increase regulatory T cells is compromised in the presence of effector memory T cells. This was not taken into consideration during the preclinical studies and explains the onset of CRS as the source of pro-inflammatory cytokines originated from CD4+ effector memory T cells [10, 11]. In addition, subsequent studies showed that another contributory factor to the adverse reaction in the volunteers was due to the dosage of TGN1412, which led to higher receptor occupancy on human T cells compared with preclinical studies in mouse and primates [11, 12].
ICOSL-ICOS
ICOSL (also known as B7h, B7-H2, B7RP-1, LICOS, GL50, and CD275) was identified as a homolog of B7 and found to be the ligand of inducible costimulator (ICOS). It is constitutively expressed on B cells, dendritic cells, and macrophages and can be induced on non-hematopoietic cells in response to inflammatory signals [13, 14]. ICOS, as the name suggests, is up-regulated on activated T cells. In humans, but not mice, ICOSL can also bind CD28 and CTLA-4 [15]. Engagement of ICOSL supports the formation of follicular helper T cells through the induction of the transcription factor Bcl6 and is critical for germinal center formation [16]. In addition, it can promote T cell production of several cytokines including IL-10, IL-4, IL-5, IFNy, and IL-17 [17-20]. It can also influence the function of both effecter and regulatory T cells that can result in contrasting results depending on which cell type the effect dominates. For example, ICOS deficient mice have exacerbated experimental autoimmune encephalomyelitis (EAE) but are also less susceptible to collagen induced arthritis (CIA) [21, 22]. On a non-obese diabetic (NOD) background, ICOS and ICOSL deficient mice are protected from developing diabetes, but do develop autoimmunity in the neuromuscular system instead [23]. These findings make it difficult to predict what the outcome of strategies to target this pathway would be.
Currently targeting of ICOSL-ICOS pathway does not appear to be aggressively pursued for the treatment of disease. However, this pathway may be of interest to consider during the treatment with anti-CTLA-4 antibody (ipilimumab). Studies in mice suggest that the protective anti-tumor T cell responses formed during this treatment are dependent on this pathway [24].
Co-inhibitory B7 family ligands and receptors
B7-CTLA-4
Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) is a type I transmembrane protein transiently expressed on the surface of T cells within 24-48 hours after activation and constitutively expressed on Tregs [25]. It forms a homodimer and outcompetes CD28 with a higher affinity and avidity for B7-1 and B7-2. This may occur by forming a lattice structure at the immunological synapse to deliver an inhibitory signal through the T cells [26]. However, another possible mechanism suggests CTLA-4 expressing cells trans-endocytose ligands on neighboring cells preventing CD28 co-stimulation [27].
CTLA-4 is comprised of an extracellular, hydrophobic transmembrane and cytoplasmic domain/tail containing an immunoreceptor tyrosine inhibitory motif (ITIM), which recruits the phosphatases Src homology region 2 domain-containing phosphatase-1 (SHP-1) and 2 (SHP-2) to reduce T cell activation. This is achieved by increasing the threshold for activation, up-regulating indoleamine 2, 3-dioxygenase (IDO) and reducing IL-2 production [25]. CTLA-4 is critical in maintaining peripheral tolerance as demonstrated by lymphoproliferation and the onset of autoimmunity in CTLA-4-/- mice [28].
The first application of CTLA-4 as a potential therapeutic target was demonstrated using a CTLA-4-Ig fusion protein consisting of the extracellular CTLA-4 domain fused to the Fc IgG1 region, which alleviated disease in a model of collagen induced arthritis (CIA) as well as in lupus mice [29-31] This led to clinical trials with abatacept (Bristol-Myers Squibb, NJ, USA), a CTLA-4-Ig fusion protein composed of the human CTLA-4 extracellular domain linked to a modified human IgG1 domain genetically engineered to prevent signaling and effector function through the Fc portion [32]. In the clinic, abatacept was approved to treat rheumatoid arthritis (RA) patients owing to a reduction in disease activity and increase in the frequency of Tregs in RA patients [33]. CTLA-4-Ig has also been investigated in multiple sclerosis and shown to reduce the proliferation of myelin basic protein [34] and in an ongoing phase II Type 1 Diabetes Mellitus trial (NCT00505375) [35]. In addition, CTLA-4-Ig prolongs graft survival in various animal transplant models [36]. Similarly, treatment with belatacept, a human CTLA-4-Ig differing from abatacept by two amino acids and conferring a higher avidity for B7 is currently in phase II trials renal transplantation patients (NCT00035555).
In contrast to CTLA-4-Ig designed to suppress T cell activation, antibodies against CTLA-4 are attractive in cancer immunotherapy to increase T effector cell function. Clinical trials with two human anti-CTLA-4 antibodies termed ipilimumab (Yervoy) (Medarex/Bristol-Myers Squibb, Princeton, NJ, USA) and tremilimumab (Pfizer, New York, NY, USA; sublicensed by MedImmune) show the importance of targeting an inhibitory receptor. Ipilimumab, a fully human CTLA-4 IgG1 monoclonal antibody with a half life of 12-14 days, and tremelimumab, a fully human IgG2a antibody with a half life of approximately 22 days, have proved successful in clinical trials with cancer patients. In patients with advanced melanoma both antibodies reduce tumor size and metastases development although toxicity in various tissues was observed [37]. A phase III clinical trial with tremelimumab in advanced melanoma patients failed to demonstrate a significant survival rate [38]. Combinational therapy of ipilimumab with a melanoma specific gp100 peptide vaccine significantly increased survival in patients with advanced melanoma by 3.5 months [39]. This later led to the identification of ICOS as a biomarker of anti-CTLA-4 therapy in cancer patients [40, 41]. Ipilimumab (CA184-009) also effectively reduces prostate-specific antigen (PSA) levels in prostate cancer patients [42].
PD-1-PD-L1/2
In 1992, a, type I transmembrane protein termed programmed death 1 (PD-1) (CD279) was identified. PD-1 is expressed on T cells, Tregs, exhausted T cells, B cells, activated monocytes, dendritic cells (DCs), natural killer (NK) cells and natural killer T (NKT) cells. It consists of a transmembrane domain, stalk, an immunoglobulin superfamily domain and an intracellular domain containing an ITIM and immunoreceptor tyrosine-based switch motif (ITSM) [2]. PD-1 binds to two ligands: programmed death ligand -1 (PD-L1) and programmed death ligand-2 (PD-L2) [43]. PD-1-PDL-1/PD-L2 ligation results in the phosphorylation of intracellular tyrosines in ITIM and ITSM which bind SHP-1 and SHP-2 to deliver an inhibitory signal into the T cell by down regulating Bcl-XL expression and regulating T cell differentiation [2]. Thus its ability to cause cell death is indirect. PD-1 is basally expressed on the cell surface and inhibits both PI3K and Akt activity. In contrast, CTLA-4 expression is transient and targets Akt [44]. Bidirectional signaling also occurs through the PD-1 pathway. Signaling through PD-1 shuts down T cell function, and signaling through PD-L1 or PD-L2 on DCs alters cytokine production and maturation [2].
PD-L1 (B7-H1, CD274) a type I transmembrane protein consisting of an IgV-like domain, IgC-like domain, signal sequence, transmembrane domain and intracellular domains. PD-L2 (B7-DC, CD273), like PD-L1, is also a type I transmembrane comprised of a signal sequence, IgV-like domain, IgC-like domain, stalk, transmembrane domain and cytoplasmic domains [2]. PD-L1 is constitutively expressed on murine and human antigen presenting cells, non-hematopoietic cells and non-lymphoid organs such as heart, lung, placenta and liver [2]. In contrast, PD-L2 expression is restricted to macrophages and DCs. In addition to binding PD-1, PD-L1 also binds to CD80 to deliver an inhibitory signal [45].
The significance of PD-1 as a negative checkpoint regulator was shown by the development of arthritis and lupus-like glomerulonephritis in PD-1-/- mice bred on a lupus prone background lpr/lpr [46] and the development of autoimmune dilated cardiomyopathy in BALB/c PD-1-/- mice [47]. In addition, murine studies show that PD-1 blockade enhances disease severity in NOD mice [48] and in the EAE model [49]. PD-1 blockade can also enhance anti-tumor immunity [50] as its ligands expression is elevated on tumor cells [2], preventing an anti-tumor immune responses [37].
There are several clinical trials investigating the PD-1-PD-L1/2 pathway. Patients with hematopoietic malignancies were treated with a humanized IgG1 monoclonal anti-PD-1 antibody termed CT-011 (Cure Tech Ltd) [51]. In addition, trials using a human IgG4 anti-PD-1 antibody termed MDX-1106 (Medarex), lacking FcR binding, showed tumor regression by increasing T cell infiltration at the metastatic sites in patients with solid cancer [52]. There are also ongoing trials for MDX-1106 therapy in patients with relapsed malignancies (NCT00441337). In addition to anti-PD-1 antibody blockade, a phase I clinical trial in cancer with an anti-PD-L1 antibody named MDX-1105 (Bristol-Myers Squibb) is currently ongoing (NCT00729664). At present there is also an ongoing phase I trial investigating the role of a PD-L2 monoclonal antibody to treat patients with stage IV melanoma (NCT00658892).
During the past three years the number of pharmaceutical companies investing in the blockade of the PD-1-PD-L1/2 pathway has excelled with data showing that toxicity is less compared with anti-CTLA-4 antibodies. This includes AMP-224 (Amplimmune and GlaxcoSmithKline). Furthermore, the safety in targeting the PD-1 pathway was highlighted by using the PD-1 blocking antibody BMS-936558. The study showed a 28% and 27% response rate in melanoma and renal cell cancer patients, respectively. Importantly low toxicity and sustained responses were observed [53]. PD-L1 expression is also observed on tumor target cells in clinical responders suggesting it could be used a biomarker for anti-PD-1 therapy.
BTLA-HVEM
B and T lymphocyte attenuator (BTLA, CD272) is a type 1 glycosylated transmembrane glycoprotein and member of the immunoglobulin superfamily. BTLA is constitutively expressed on naïve T cells, Th1 cells, T follicular cells, NK cells, NKT cells, B cells, DCs and macrophages [54, 55]. Structural analysis show that BTLA contains an ITIM and ITSM motif within the cytoplasmic tail similar to PD-1 resulting in the recruitment of SHP-1 and SHP-2 to deliver an inhibitory signal to T cells by reducing cell proliferation and IL-2 production. BTLA competes with LIGHT (TNFSF14/CD258) for binding to herpesvirus entry mediator (HVEM). Bidirectional signaling through the BTLA-HVEM pathway has been reported [55], where the signal is dependent on how the ligation is formed [56].
At present there are no clinical trials with BTLA. However preclinical studies in mice show that blocking BTLA or BTLA-/- mice increase disease onset in autoimmunity [54, 57]. In mice, BTLA prolongs the survival of MHC-mismatched cardiac allografts [58]. A recent study has shown a beneficial effect of BTLA in suppressing tumor specific T cells in individuals receiving stem cell undergoing stem cell transplantation by increasing CD8+ T cells specific for minor histocompatibility antigens (MiHA) on malignant cells [59].
B7-H3
B7-H3 (also known as CD276) was identified based on its sequence similarity to the extracellular domain of other B7 family members and is a type I transmembrane protein containing IgV and IgC-like domains [60]. In humans, but not mice, B7-H3 has an alternate isoform containing a tandem repeat of IgV and IgC domains (VCVC) and this isoform is the more common form expressed [61]. However, the functional significance of this difference is unclear. At a transcription level, B7-H3 is widely expressed in both lymphoid and non-lymphoid organs; however, detection of protein is more limited to cell types such as recently activated monocytes, T cells, B cells, and NK cells [60]. B7-H3 can also be shed under homeostatic conditions and elevated levels are correlated with some cancers and disease states such as sepsis and meningitis [62-64].
The receptor(s) for B7-H3 has not been conclusively identified. Initial reports on B7-H3 suggested that could serve as either a co-stimulatory or co-inhibitory molecule with the results varying widely with the disease model. This gives rise to the notion that it may have more than one possible binding partner that dictates function. One group has suggested that TREM-like transcript 2 (TLT-2, TREML2) as a possible receptor that allows enhanced IL-2 and IFNy production in T cells [65]. However, another group reported that it provided negative, rather than positive, co-stimulation and could not confirm binding to TLT-2 [66].
One monoclonal antibody against B7-H3 (8H9), recognizing the isoform containing four immunoglobulin-like domains, is in development for the treatment of cancer. This clone recognizes B7-H3 expressed on several solid tumor samples, including brain cancers, but is not expressed on normal central nervous system tissue [67]. When conjugated to a radioactive isotype, it appeared to show safety and efficacy in patients with neuroblastoma that metastasized to the central nervous system [68]. There is also interest to further expand this study to patients with diffuse intrinsic pontine glioma, another aggressive brain cancer, as B7-H3 expression has been detected on some patient samples and there are very limited treatment options for this disease [69].
Therapy with an antibody against B7-H3 may also prove beneficial in cancers found outside of the central nervous system. Its expression has been reported on tumor cell lines and/or patient specimens in prostate, breast, lung, gastric, colorectal, endometrial, and skin cancers, often correlated with increased tumor size [70-77]. B7-H3 expression was also associated with decreased numbers of tumor infiltrating lymphocytes and suppression of anti-tumor T cell responses [75, 77-79]. Some work also suggests that the ability of tumors to metastasize to other sites also can also be enhanced by its expression [73, 76].
B7-H4
B7-H4 (also known as B7S1, B7×, and Vtcn1) is another B7 family member, being a type I transmembrane protein that shows some homology with other family members in its extracellular domain. By mRNA, it is detected on most non-hematopoietic tissues; however, protein expression is more limited, confining itself to induced expression on antigen presenting cells as well as expression on cancer cells [80-83]. A soluble form of B7-H4 can also be detected in the serum of rheumatoid arthritis patients and ovarian cancer patients. In mice, soluble B7-H4 correlates with increased age and disease severity in lupus-prone BWF1 mice and mice with CIA [84].
While the receptor for B7-H4 is unknown, its function consistently appears to be that of co-inhibitor molecule whose engagement decreases proliferation and IL-2 production in T cells as well as the expansion of neutrophil progenitors [85, 86]. Over-expression of B7-H4 on the pancreatic islets can protect mice from CD4 and CD8 T cell mediated autoimmunity [87, 88], and it can also prolong islet allograft survival after transplantation [89-92]. Treatment with B7-H4 Ig fusion protein can also reduce the incidence of the autoimmune diabetes in NOD mice as well as the incidence and severity of disease in a CIA model [93, 94]. In the CIA model, it was also observed that B7-H4 deficiency and, respectively, treatment with soluble B7-H4 resulted in increased disease incidence and severity [94]. A phase I study of AMP-110, a B7-H4 Ig fusion protein, for use in patients with rheumatoid arthritis is ongoing (NTC01878123).
There are indications that this molecule may also be worth targeting in some forms of cancer. In gastric cancer, tissue specimens from patients evaluated for mRNA and/or protein expression showed that higher levels of B7-H4 were associated with poor prognosis and decreased survival rates [95-97]. In one of these studies, an inverse correlation with its expression and T cells infiltrating tumors was also observed [95]. Work on lung cancer cell lines shows that B7-H4 expression by the cancer cells and on tumor associated macrophages results in inhibition of anti-tumor T cell responses [98, 99]. Patient samples show that increased B7-H4 expressing macrophages in blood correlated with tumor size and metastasis in lung carcinomas [100]. Similar observations of enhanced B7-H4 expression and impaired patient survival is also seen in esophageal squamous cell carcinoma and melanoma cases [101, 102]. In ovarian cancer, its expression was also observed, and transfection of B7-H4 into a tumor cell line showed a growth advantage in immunodeficient mice suggesting that it can promote cancer growth independently of inhibiting an adaptive immune response [103]. It was also found to be preferentially expressed in non-dividing tumor cells from brain glioma samples [104]. Ebstein-Barr virus transformed B cells can acquire B7-H4 expression and its engagement with anti-B7-H4 antibody is associated with FasL upregulation and cell arrest [105, 106].
B7-H6-NKp30
B7-H6 was originally identified as being a ligand of NKp30, a natural cytotoxicity receptor found on human NK cells. Like the other members of the B7 family, B7-H6 contains two extracellular Ig domains and sequence homology with other members [107]. The structure of B7-H6 and NKp30 interactions has been determined, showing that it comparatively has a larger region of interaction with this receptor ligand pair than with other family members and their receptors [108]. Unlike the other B7 family members, it is not detected on any normal human tissue and cell samples. However, it is present on several tumor cells lines of both hematopoietic and non-hematopoietic origin. Engagement of B7-H6 on tumor cells by NKp30 on NK cells results in increased cytotoxicity and the release of IFNγ [107].
Preclinical work suggests that B7-H6 may prove useful to target tumor cells expressing it. Incubation of a tumor cells with a fusion protein containing B7-H6 and 7D8, an antibody that recognizes CD20, enhanced NK cell mediated activation and cytotoxicity in vitro [109]. Furthermore, in mouse T cells transfected with a chimeric antigen receptor consisting of the extracellular portion of NKp30 and the signaling domain of CD28 can inhibit the growth of tumor cells expressing B7-H6 in vivo. This protection also appears to enhance anti-tumor responses to other antigens as mice that cleared the primary tumor were also resistant to re-challenge with tumor not expressing B7-H6 [110]. This work suggests that these techniques may prove useful to target B7-H6 expressing tumors.
VISTA
V-domain Ig suppressor of T cell activation (VISTA), also known as Differentiation of Embryonic Stem Cells 1 (Dies1), Gi24, and PD-1 homolog (PD-1H), is a 55-65kDa type 1 membrane protein belonging to the immunoglobulin family. Structural analysis shows that the extracellular domain of VISTA bears homology to PD-L1. VISTA consists of an extracellular domain containing an extracellular IgV domain linked to a stalk region, transmembrane segment and cytoplasmic domain. The IgV domain of VISTA is unique from other members of the immunoglobulin superfamily as it contains three additional cysteine amino acids. Hence, VISTA was not assigned a PD-L name due to lack of similarity[111]. The receptor for VISTA is currently unknown and is being actively investigated in our laboratory.
In contrast to PD-L1, VISTA expression is hematopoietically restricted and highly expressed on mature myeloid derived APCs and to a lesser extent on T cells and Tregs. In mice its expression is positively correlated with the marker CD11b and its found on tumor infiltrating lymphocytes. In addition, like PD-L1, VISTA induces Foxp3 expression in T cells. In vitro, VISTA expressing APCs or soluble VISTA-Ig fusion proteins inhibit T cell proliferation, cytokine production and expression of the T cell activation markers CD44 and CD69 [111] (Figure 1). It was shown to be critical for the differentiation of embryonic stem cells in particular, the bone morphogenetic protein (BMP4) signaling pathway [112]. One study also showed that its expression on tumor cells promoted tumor growth via membrane type (MT) 1- matrix metalloproteinase (MMP) by increasing cell motility [113].
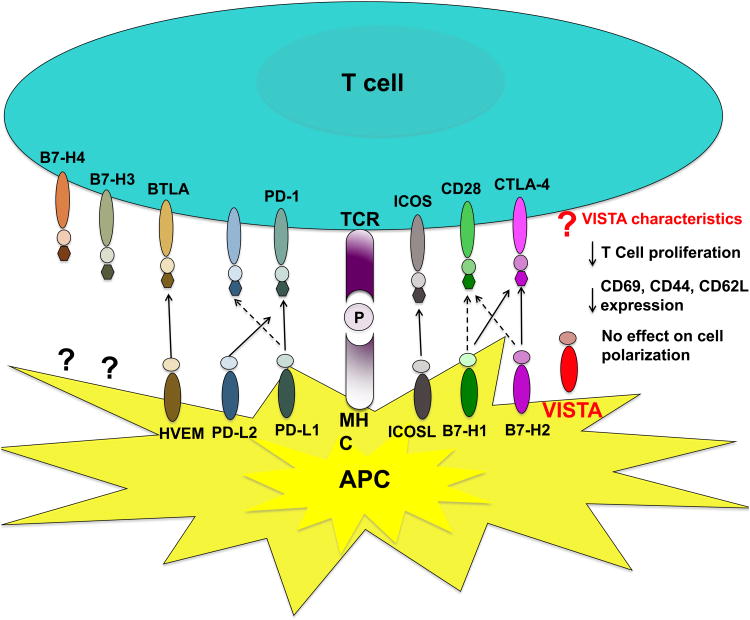
Figure 1. B7 Family of co-stimulatory and novel co-inhibitory molecules.
T cell activation requires two signals. Signal 1 is mediated by Major histocompatibility complex (MHC) molecules presenting cognate peptide (P) on antigen presenting cells (APCs) to the T cell receptor (TCR) on T cells forming an MHC-P complex. To sustain full T cell activation, a second signal is required to deliver either a co-stimulatory (B7-H1/B7-H2-CD28, inducible costimulator (ICOS) - inducible costimulator ligand (ICOSL) or co-inhibitory (B7-H1/B7-H2-cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed death ligand -1/2 (PD-L1/PD-L2) - programmed cell death 1 (PD-1), B and T lymphocyte attenuator (BTLA) - herpesvirus entry mediator (HVEM) signal. The receptors for B7-H3 and B7-H4 are unknown. Studies indicative that B7-H3 binds to putative co-stimulatory and co-inhibitory receptors. In contrast, B7-H4 binds to a putative co-inhibitory receptor only. The receptor for the inhibitory ligand V-domain Ig suppressor of T cell activation (VISTA) is unknown. Functional studies show that VISTA delivers a negative signal to the T cell.
Use of monoclonal antibodies against VISTA has given conflicting results. In vitro studies using antibody to block its activity show enhanced T cell responses to APCs expressing VISTA. In vivo this same anti-VISTA antibody accelerates disease incidence and severity of EAE. In contrast, another group using a different monoclonal anti-VISTA antibody found that it could prevent the development of graft-versus-host disease in both semi-allogeneic and fully allogeneic murine models [114]. How in some models anti-VISTA can enhance immunity and in others suppress, is currently unresolved. It is possible that in the case of graft-versus-host disease the antibody used deletes VISTA+ cells. Further work is warranted to understand VISTA targeting in disease models to determine its effects.
Conclusions
Challenges remain for the development of products targeting B7 family checkpoint regulators. In the case of co-stimulatory molecules such as CD28, potential toxicity of agonist antibodies is a concern. Additionally, as ICOS can be both a positive and negative regulator of immune response further characterization of this molecule in various disease states is necessary if this pathway is targeted for clinical development. Receptor identification of B7-H3, B7-H4, and VISTA may also be key to fully understanding their therapeutic potential, particularly if bi-directional signaling is involved, and in its absence extensive work in preclinical models is needed to fully elucidate the effects of treatments regulating these pathways.
Over the past 10 years we have seen the birth of immunotherapeutic strategies that are substantively changing the way we treat human disease. The targeting of co-stimulatory and co-inhibitory molecules has proven to be an effective therapeutic strategy in both autoimmunity and cancer, respectively. This is exemplified by ipilimumab and ongoing clinical trials targeting the PD-1 pathway that illustrate the benefits of treatment with less toxicity. These regimens have since led to the identification of biomarkers to identify responders so treatments can to targeting to specific cancer patient populations. In addition there may be even greater benefits by targeting the use of checkpoint regulators in combination with standard of care regimens and other immunotherapeutic approaches. The use of antibodies and Ig fusion proteins targeting B7 family checkpoint regulators may prove beneficial towards the treatment of numerous human diseases.
Highlights.
In the clinic, targeting blocking the CTLA-4 and PD-1 pathway is advantageous in cancer patients.
Preclinical studies show that B7-H4 and B7-H6 are promising targets in cancer.
VISTA, a novel B7 inhibitory ligand maybe a therapeutic target in cancer and autoimmunity.
References
http://www.clinicaltrials.gov ClinicalTrials.gov Identifier (NCT Number)
- 1.Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 2.Keir ME, et al. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green JM, et al. T cell costimulation through the CD28 receptor. Proceedings of the Association of American Physicians. 1995;107:41–46. [PubMed] [Google Scholar]
- 4.Greenfield EA, et al. CD28/B7 costimulation: a review. Critical reviews in immunology. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 5.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 6.Boise LH, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 7.Rulifson IC, et al. CD28 costimulation promotes the production of Th2 cytokines. Journal of immunology. 1997;158:658–665. [PubMed] [Google Scholar]
- 8.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. Journal of immunology. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 9.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England journal of medicine. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 10.Eastwood D, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. British journal of pharmacology. 2010;161:512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romer PS, et al. Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood. 2011;118:6772–6782. doi: 10.1182/blood-2010-12-319780. [DOI] [PubMed] [Google Scholar]
- 12.Waibler Z, et al. Toward experimental assessment of receptor occupancy: TGN1412 revisited. The Journal of allergy and clinical immunology. 2008;122:890–892. doi: 10.1016/j.jaci.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Swallow MM, et al. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshinaga SK, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 15.Yao S, et al. B7-h2 is a costimulatory ligand for CD28 in human. Immunity. 2011;34:729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutloff A, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 18.McAdam AJ, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. Journal of immunology. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 19.Coyle AJ, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 20.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Nurieva RI. Regulation of immune and autoimmune responses by ICOS. Journal of autoimmunity. 2003;21:255–260. doi: 10.1016/s0896-8411(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 23.Prevot N, et al. Abrogation of ICOS/ICOS ligand costimulation in NOD mice results in autoimmune deviation toward the neuromuscular system. European journal of immunology. 2010;40:2267–2276. doi: 10.1002/eji.201040416. [DOI] [PubMed] [Google Scholar]
- 24.Fu T, et al. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer research. 2011;71:5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- 25.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 26.Salama AK, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:4622–4628. doi: 10.1158/1078-0432.CCR-10-2232. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 29.Cross AH, et al. Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA-4-Fc supports a key role for CD28 costimulation. The Journal of clinical investigation. 1995;95:2783–2789. doi: 10.1172/JCI117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knoerzer DB, et al. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. The Journal of clinical investigation. 1995;96:987–993. doi: 10.1172/JCI118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finck BK, et al. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 32.Cutolo M, Nadler SG. Advances in CTLA-4-Ig-mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmunity reviews. 2013 doi: 10.1016/j.autrev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez-Quiroga C, et al. CTLA-4-Ig therapy diminishes the frequencybut enhances the function of Treg cells in patients with rheumatoid arthritis. Journal of clinical immunology. 2011;31:588–595. doi: 10.1007/s10875-011-9527-5. [DOI] [PubMed] [Google Scholar]
- 34.Viglietta V, et al. CTLA4Ig treatment in patients with multiple sclerosis: an open-label, phase 1 clinical trial. Neurology. 2008;71:917–924. doi: 10.1212/01.wnl.0000325915.00112.61. [DOI] [PubMed] [Google Scholar]
- 35.Orban T, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuzawa-Carballeda J, et al. High levels of IDO-expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplantation proceedings. 2010;42:3489–3496. doi: 10.1016/j.transproceed.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. The Journal of experimental medicine. 2012;209:201–209. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Seminars in oncology. 2010;37:450–454. doi: 10.1053/j.seminoncol.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liakou CI, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Small EJ, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 43.He YF, et al. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. Journal of immunology. 2004;173:4919–4928. doi: 10.4049/jimmunol.173.8.4919. [DOI] [PubMed] [Google Scholar]
- 44.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butte MJ, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 48.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. The Journal of experimental medicine. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salama AD, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. International immunology. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 52.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nature immunology. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 55.Steinberg MW, et al. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunological reviews. 2011;244:169–187. doi: 10.1111/j.1600-065X.2011.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Compaan DM, et al. Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. The Journal of biological chemistry. 2005;280:39553–39561. doi: 10.1074/jbc.M507629200. [DOI] [PubMed] [Google Scholar]
- 57.Oya Y, et al. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis and rheumatism. 2008;58:2498–2510. doi: 10.1002/art.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao R, et al. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. Journal of immunology. 2005;175:5774–5782. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 59.Hobo W, et al. B and T lymphocyte attenuator mediates inhibition of tumor-reactive CD8+ T cells in patients after allogeneic stem cell transplantation. Journal of immunology. 2012;189:39–49. doi: 10.4049/jimmunol.1102807. [DOI] [PubMed] [Google Scholar]
- 60.Chapoval AI, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nature immunology. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 61.Sun M, et al. Characterization of mouse and human B7-H3 genes. Journal of immunology. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 62.Zhang G, et al. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X, et al. Circulating B7-H3(CD276) elevations in cerebrospinal fluid and plasma of children with bacterial meningitis. Journal of molecular neuroscience: MN. 2009;37:86–94. doi: 10.1007/s12031-008-9133-z. [DOI] [PubMed] [Google Scholar]
- 64.Zhang G, et al. B7-H3 augments the inflammatory response and is associated with human sepsis. Journal of immunology. 2010;185:3677–3684. doi: 10.4049/jimmunol.0904020. [DOI] [PubMed] [Google Scholar]
- 65.Hashiguchi M, et al. Triggering receptor expressed on myeloid celllike transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leitner J, et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. European journal of immunology. 2009;39:1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H, et al. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer research. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kramer K, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. Journal of neurooncology. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Z, et al. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J Neurooncol. 2012;111:257–264. doi: 10.1007/s11060-012-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arigami T, et al. B7-H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer science. 2011;102:1019–1024. doi: 10.1111/j.1349-7006.2011.01877.x. [DOI] [PubMed] [Google Scholar]
- 71.Yuan H, et al. B7-H3 over expression in prostate cancer promotes tumor cell progression. The Journal of urology. 2011;186:1093–1099. doi: 10.1016/j.juro.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 72.Chavin G, et al. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:2174–2180. doi: 10.1158/1078-0432.CCR-08-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arigami T, et al. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Annals of surgery. 2010;252:1044–1051. doi: 10.1097/SLA.0b013e3181f1939d. [DOI] [PubMed] [Google Scholar]
- 74.Zhang G, et al. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung cancer. 2009;66:245–249. doi: 10.1016/j.lungcan.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Brunner A, et al. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2011;124:105–111. doi: 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Tekle C, et al. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. International journal of cancer Journal international du cancer. 2012;130:2282–2290. doi: 10.1002/ijc.26238. [DOI] [PubMed] [Google Scholar]
- 77.Sun J, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer immunology, immunotherapy: CII. 2010;59:1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen C, et al. Induced expression of B7-H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp Cell Res. 2012;319:96–102. doi: 10.1016/j.yexcr.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Katayama A, et al. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. International journal of oncology. 2011;38:1219–1226. doi: 10.3892/ijo.2011.949. [DOI] [PubMed] [Google Scholar]
- 80.Prasad DV, et al. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 81.Choi IH, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. Journal of immunology. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 82.Sica GL, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 83.Zang X, et al. B7×: a widely expressed B7 family member that inhibits T cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamimura Y, et al. Possible involvement of soluble B7-H4 in T cell-mediated inflammatory immune responses. Biochemical and biophysical research communications. 2009;389:349–353. doi: 10.1016/j.bbrc.2009.08.144. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, et al. B7-H4 Treatment of T Cells Inhibits ERK, JNK, p38, and AKT Activation. PloS one. 2012;7:e28232. doi: 10.1371/journal.pone.0028232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu G, et al. B7-H4-deficient mice display augmented neutrophil-mediated innate immunity. Blood. 2009;113:1759–1767. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JS, et al. B7× in the periphery abrogates pancreas-specific damage mediated by self-reactive CD8 T cells. Journal of immunology. 2012;189:4165–4174. doi: 10.4049/jimmunol.1201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei J, et al. Tissue-specific expression of B7× protects from CD4 T cell-mediated autoimmunity. The Journal of experimental medicine. 2011;208:1683–1694. doi: 10.1084/jem.20100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, et al. Endogenous expression of B7-H4 improves long-term murine islet allograft survival. Transplantation. 2013;95:94–99. doi: 10.1097/TP.0b013e318277229d. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, et al. B7-H4 induces donor-specific tolerance in mouse islet allografts. Cell Transplant. 2012;21:99–111. doi: 10.3727/096368911X582750. [DOI] [PubMed] [Google Scholar]
- 91.Wang X, et al. Local expression of B7-H4 by recombinant adenovirus transduction in mouse islets prolongs allograft survival. Transplantation. 2009;87:482–490. doi: 10.1097/TP.0b013e318195e5fa. [DOI] [PubMed] [Google Scholar]
- 92.Yuan CL, et al. B7-H4 transfection prolongs beta-cell graft survival. Transplant immunology. 2009;21:143–149. doi: 10.1016/j.trim.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Wang X, et al. Early treatment of NOD mice with B7-H4 reduces the incidence of autoimmune diabetes. Diabetes. 2011;60:3246–3255. doi: 10.2337/db11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Azuma T, et al. Potential role of decoy B7-H4 in the pathogenesis of rheumatoid arthritis: a mouse model informed by clinical data. PLoS medicine. 2009;6:e1000166. doi: 10.1371/journal.pmed.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arigami T, et al. Clinical significance of the B7-H4 coregulatory molecule as a novel prognostic marker in gastric cancer. World journal of surgery. 2011;35:2051–2057. doi: 10.1007/s00268-011-1186-4. [DOI] [PubMed] [Google Scholar]
- 96.Arigami T, et al. Expression of B7-H4 in blood of patients with gastric cancer predicts tumor progression and prognosis. Journal of surgical oncology. 2010;102:748–752. doi: 10.1002/jso.21722. [DOI] [PubMed] [Google Scholar]
- 97.Jiang J, et al. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer immunology, immunotherapy: CII. 2010;59:1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun SQ, et al. Enhanced T cell immunity by B7-H4 downregulation in nonsmall-cell lung cancer cell lines. The Journal of international medical research. 2012;40:497–506. doi: 10.1177/147323001204000211. [DOI] [PubMed] [Google Scholar]
- 99.Chen C, et al. Induced expression of B7-H4 on the surface of lung cancer cell by the tumor-associated macrophages: a potential mechanism of immune escape. Cancer letters. 2012;317:99–105. doi: 10.1016/j.canlet.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 100.Chen C, et al. Increase of circulating B7-H4-expressing CD68+ macrophage correlated with clinical stage of lung carcinomas. Journal of immunotherapy. 2012;35:354–358. doi: 10.1097/CJI.0b013e31824212c4. [DOI] [PubMed] [Google Scholar]
- 101.Chen LJ, et al. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer immunology, immunotherapy: CII. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quandt D, et al. B7-h4 expression in human melanoma: its association with patients' survival and antitumor immune response. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 103.Cheng L, et al. B7-H4 expression promotes tumorigenesis in ovarian cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2009;19:1481–1486. doi: 10.1111/IGC.0b013e3181ad0fa2. [DOI] [PubMed] [Google Scholar]
- 104.Yao Y, et al. B7-H4 is preferentially expressed in non-dividing brain tumor cells and in a subset of brain tumor stem-like cells. Journal of neuro-oncology. 2008;89:121–129. doi: 10.1007/s11060-008-9601-x. [DOI] [PubMed] [Google Scholar]
- 105.Song H, et al. B7-H4 reverse signaling induces the apoptosis of EBV-transformed B cells through Fas ligand up-regulation. Cancer letters. 2008;266:227–237. doi: 10.1016/j.canlet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 106.Park GB, et al. Cell cycle arrest induced by engagement of B7-H4 on Epstein-Barr virus-positive B-cell lymphoma cell lines. Immunology. 2009;128:360–368. doi: 10.1111/j.1365-2567.2009.03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brandt CS, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. The Journal of experimental medicine. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, et al. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. The Journal of experimental medicine. 2011;208:703–714. doi: 10.1084/jem.20102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kellner C, et al. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. Journal of immunology. 2012;189:5037–5046. doi: 10.4049/jimmunol.1201321. [DOI] [PubMed] [Google Scholar]
- 110.Zhang T, et al. An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. Journal of immunology. 2012;189:2290–2299. doi: 10.4049/jimmunol.1103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. The Journal of experimental medicine. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aloia L, et al. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. The Journal of biological chemistry. 2010;285:7776–7783. doi: 10.1074/jbc.M109.077156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sakr MA, et al. GI24 enhances tumor invasiveness by regulating cell surface membrane-type 1 matrix metalloproteinase. Cancer science. 2010;101:2368–2374. doi: 10.1111/j.1349-7006.2010.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flies DB, et al. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. Journal of immunology. 2011;187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]