Abstract
Aims:
The pathophysiology of apical ballooning syndrome (ABS) remains to be elucidated. The aim of this study was to evaluate the coronary vascular reactivity of patients who were previously diagnosed with ABS.
Methods and results:
A total of 228 cases of ABS were prospectively identified, and of these, 10 patients (median age 61 years (IQR 48–75); all females) who underwent coronary vasomotion testing were included in the study. Coronary epicardial and microvascular responses to intracoronary acetylcholine (ACH; % change in diameter and % change in blood flow at doses of 10−6–10−4 mol/l), nitroglycerin (200–300 mg), and adenosine (36–60 µg) were evaluated. The median change in diameter with ACH was –9.3% (IQR –36.4, 3.2) with six patients (60%) demonstrating epicardial coronary constriction. The median increase in peak coronary blood flow in response to ACH was 13.1% (IQR –18.6, 55.0). This was markedly lower than the blood flow response seen in a reference group of 211 women from our laboratory (mean age 60 years) with normal microvascular responses to ACH: 103% (IQR 75, 149). Seven (70%) patients had <50% increase in coronary blood flow indicating abnormal microvascular response to ACH. 70% had either abnormal epicardial or microvascular response to ACH. Median coronary flow reserve was abnormal at 2.2% (IQR 2.0, 3.4; normal >2.5), and 90% had at least one abnormal measure of microvascular vasomotion.
Conclusion:
The novel observation is that coronary microvascular dysfunction is highly prevalent in patients with ABS. Thus, chronically impaired coronary vascular reactivity, especially involving the microcirculation, may be a central feature of the pathophysiology of ABS.
Keywords: Apical ballooning syndrome, endothelium, microcirculation, stress cardiomyopathy, Tako-Tsubo cardiomyopathy
Introduction
Apical ballooning syndrome (ABS), also known as Takotsubo/ stress cardiomyopathy, was first described by Sato and colleagues in Japan in the early 1990s.1–3 The cardiomyopathy uniquely occurs in the setting of a severe mental or physical stressor, predominantly affecting postmenopausal females, and in the absence of obstructive coronary artery disease. Typically, there is transient reduction in left ventricular systolic function with regional wall motion abnormalities involving the apical and mid-ventricular segments which resolve spontaneously over time. Despite being recognized as a clinical entity in the Western population for more than a decade, the underlying pathophysiological mechanisms remain to be elucidated. Multiple explanations have been proposed, and the leading hypotheses include catecholamine-induced cardiac toxicity, possibly modulated by an oestrogen-dependent mechanism, and diffuse multivessel coronary epicardial spasm and/or microvascular dysfunction.4
Recently we have demonstrated that women with a history of ABS, when compared with age-matched postmenopausal healthy controls and patients with prior myocardial infarction, have impaired peripheral endothelium-dependent vasodilation, excessive vasoconstriction, and augmented sympathetic activation after being subjected to acute mental stress. The findings indicate that women who have suffered ABS have chronically impaired peripheral vascular reactivity.5 Thus, it may be speculated that abnormal coronary vasomotion may serve as a mechanism for ABS. In order to address this hypothesis, the aim of this study was to evaluate the coronary epicardial and microvascular vasomotor function in a cohort of women with a history of ABS.
Methods
Study population
From January 2002 to January 2012, we prospectively identified patients diagnosed with ABS based on the Mayo Clinic criteria:6 (1) transient hypokinesis, akinesis, or dyskinesis of the left ventricular mid-segments with or without apical involvement (the regional wall motion abnormalities extend beyond a single epicardial vascular distribution); (2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) clinical absence of pheochromocytoma or myocarditis. A total of 228 cases met the criteria. These cases were cross-referenced with our database of patients who had undergone clinically indicated evaluation of coronary vasomotor function according to our standardized protocol. We retrospectively identified 10 patients who met these study inclusion criteria, and all had the vascular function test performed following the episode of ABS. All subjects consented to the use of their medical records for research purposes and the Mayo Clinic Institutional Review Board approved the study.
Study protocol
Coronary angiography for vasomotor function testing was performed using the standard femoral percutaneous approach with a 7-French Judkins guide using non-ionic contrast, as previously described.7–9 Heparin was given intravenously prior to initiation to maintain therapeutic activated clotting times throughout the procedure. A baseline coronary angiogram was acquired. Thereafter, once obstructive coronary disease (≥30% diameter stenosis) was excluded, a 2.2-French coronary infusion catheter was introduced into the left anterior descending artery through which a 0.014-inch Doppler guidewire was introduced to measure blood flow velocity (Volcano, Rancho Cordova, CA, USA).
The coronary vascular responses were studied in accordance to a previously reported protocol.7–9 Endothelium-independent coronary flow reserve was measured using a bolus of intracoronary adenosine (36–60 µg) via the guide catheter. Following recovery to baseline, the vasomotor response to selective intracoronary infusions of acetylcholine (ACH) at a rate of 1 ml/min at concentrations of 10–6, 10–5, and 10–4 mol/l (0.182, 1.82, and 18.2 μg/ml, respectively) for 3 min each were assessed. Finally, 200–300 mg intracoronary nitroglycerin bolus was given. At baseline and after each infusion, the following data were obtained; the heart rate and blood pressure, coronary angiogram, and Doppler coronary blood flow velocities.
Epicardial artery diameter was analysed from digitized images with a modification of the technique previously described.9 An end-diastolic still frame at each infusion (baseline, ACH, and nitroglycerin) was selected from the angiographic sequence. Epicardial diameter was measured 5 mm distal to the tip of the Doppler wire to calculate flow, and in the distal artery. The measurements were made by a trained technician who was blinded to the clinical data. Doppler flow velocity spectra were analysed online to determine time-averaged peak velocity. Volumetric coronary blood flow was determined as follows: cross-sectional area × average peak velocity × 0.5. Coronary flow reserve was calculated as the ratio of hyperaemic to basal average peak velocity following adenosine administration. The clinical, laboratory, electrocardiographic, and imaging data were obtained from the medical records.
Abnormal coronary epicardial vasomotion was defined in terms of the change in diameter in response to the maximal dose of ACH. Mild-moderate and severe abnormality was defined as change in coronary diameter between 0 and –20% and more than –20%, respectively. Abnormal microvascular response to ACH was defined by the maximum change in coronary blood flow. Mild-moderate and severe microvascular dysfunction in response to ACH was defined as 0–50% and <0% change in coronary blood flow, respectively.9
We created a comparison group of all women aged 50 or older who had a coronary vascular function test performed during the time period between October 1993 and October 2011 and found to have normal microvascular function (>50% increase in coronary blood flow in response to ACH).
Statistical analysis
All skewed continuous variables were expressed as median and interquartile range and were compared using the Wilcoxon rank-sum test. Continuous variables that demonstrated near-normal distribution are summarized as mean±standard error. Categorical variables were expressed as number of the total population (percentage) and tested with Pearson’s chi-squared test. A p-value <0.05 was considered to be statistically significant. Statistical analyses were performed using SAS 9.3 and JMP 9.0 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics and clinical course of the study population. Except for age, the clinical characteristics of the 10 patients with ABS who had invasive testing of coronary vasomotor function did not differ significantly from the remaining cohort of patients who did not have coronary vasomotor function testing. Of the study cohort, 90% was identified as having a predisposing stressor prior to ABS presentation. Six patients developed ABS after a physical stressor, while three were triggered by an emotional stressor. Left ventricular ejection fraction increased significantly from a median of 35% (IQR 25, 55) to 62% (IQR 56, 68) at median follow-up duration of 39 days (IQR 21, 54; p=0.016). Two patients developed a recurrence (follow up 8 and 57 months) and two patients died from non-cardiac causes (follow up 1 and 93 months).
Table 1.
Baseline characteristics and outcomes of ABS patients with (study cohort) and without coronary vasomotor function testing.
| Variable | Study cohort (n=10) | Controls (n=218) | p-value |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 61 (48–75) | 71 (62–80) | 0.03 |
| Female | 10 (100) | 206 (94.5) | 0.45 |
| Hypertension | 6 (60) | 156 (72.2) | 0.40 |
| Diabetes mellitus | 1 (10) | 29 (13.4) | 0.75 |
| Hyperlipidaemia | 5 (50) | 93 (43.1) | 0.66 |
| Smoking | 3 (30) | 61 (29.5) | 0.97 |
| ECG findings | |||
| ST-segment elevation | 2 (20) | 98 (45.8) | 0.11 |
| Deep T-wave inversion | 4 (40) | 100 (47) | 0.88 |
| Maximum corrected QT interval | 528 (431–597) | 494 (463–591) | 0.75 |
| Biomarkers | |||
| Peak troponin (ng/ml) | 1.13 (0.26–1.52) | 0.45 (0.15–0.84) | 0.23 |
| Peak CK-MB (ng/ml) | 17.4 (7.4–34.1) | 11.6 (7.9–20.1) | 0.43 |
| Echocardiographic findings | |||
| Ejection fraction (%) | 35 (25–55) | 35 (30–45) | 0.58 |
| Wall motion score index | 2.32 (1.83–2.43) | 1.94 (1.69–2.25) | 0.15 |
| Right ventricular systolic pressure (mmHg) | 48 (31–52) | 43 (33–51) | 0.73 |
| Mitral regurgitation present | 3 (75) | 120 (64) | 0.65 |
| Clinical course | |||
| Length of hospitalization (days) | 8 (3.5–20) | 5 (3–8) | 0.64 |
| Days in the intensive care unit | 1 (1–2) | 1 (1–3) | 0.99 |
| Acute heart failure | 3 (60) | 76 (35.4) | 0.24 |
| Intra-aortic balloon pump | 1 (20) | 17 (8) | 0.33 |
| Mechanical ventilation | 2 (40) | 43 (20) | 0.28 |
Values are median (interquartile range) or n (%).
CK, creatinine kinase.
Coronary vasomotion testing
The median duration from the initial presentation with ABS to the coronary vasomotion testing was 152 days (IQR 5, 896). Five patients had angiographically normal coronary arteries, while the rest had mild luminal plaque (<30% diameter stenosis). The median change in coronary artery diameter with ACH was –9.3% (IQR –36.4, 3.2). Six patients (60%) demonstrated epicardial coronary constriction with ACH administration (Figure 1), three of which had >20% constriction indicating the presence of severe dysfunction. Four patients had epicardial endothelium-mediated vasodilation, although this was modest (range 3.2–8.3%).
Figure 1.
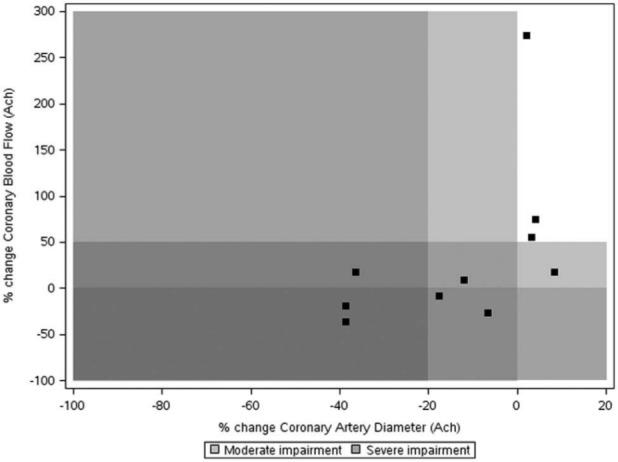
Plot of change in coronary artery diameter versus change in coronary blood flow in response to acetylcholine.
Values are percentages. The white and grey zones represent normal versus abnormal coronary vasomotion.
The median increase in peak coronary blood flow in the 10 patients was 13.1% (IQR –18.6, 55.0) (Figure 2). This was significantly lower than in the comparison group of women (n=211, mean age 60 years) with normal microvascular endothelial function from our laboratory. This comparison group’s cardiovascular risk profile was similar to the ABS cohort except that the frequency of a family history of coronary artery disease was greater (Table 2). In these subjects, the median change in coronary blood flow was 103% (IQR 75, 149) and change in coronary artery diameter was –1.7% (IQR –11.4, 4.8) in response to ACH while the median coronary flow reserve was 2.7 (IQR 2.4, 3.2).
Figure 2.
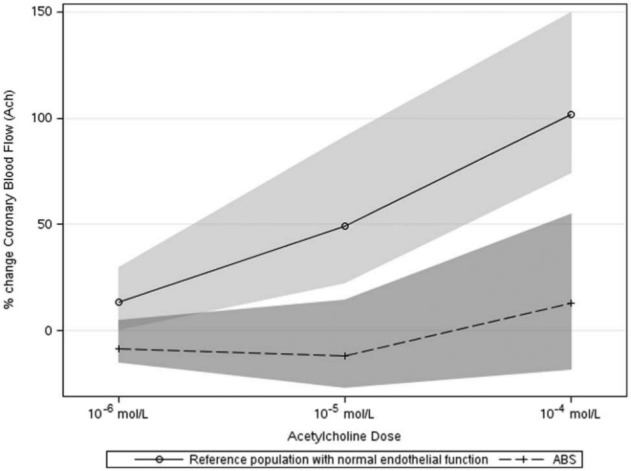
Dose–response curve for the change in coronary blood flow in response to the three doses of acetylcholine in the study cohort and a reference group of females with normal responses.
Values are median and IQR.
Table 2.
Clinical characteristics of of ABS patients with (study cohort) and without coronary vasomotor function testing.
| Variable | Study cohort (n=10) | Controls (n=211) | p-value |
|---|---|---|---|
| Age (years) | 61 (48–75) | 58 (53–66) | 0.99 |
| Female | 10 (100) | 211 (100) | |
| Body mass index (kg/m2) | 24 (22–31) | 27 (24–33) | 0.11 |
| Hypertension | 6 (60) | 113 (54) | 0.72 |
| Diabetes mellitus | 1 (10) | 13 (6) | 0.63 |
| Hyperlipidaemia | 5 (50) | 136 (65) | 0.32 |
| Smoking | 3 (30) | 98 (46) | 0.31 |
| Family history of coronary artery disease | 3 (30) | 135 (66) | 0.021 |
Values are median (interquartile range) or n (%).
Seven of the ABS patients (70%) had <50% increase in coronary blood flow in response to ACH indicating the presence of microvascular dysfunction, of whom three had severe impairment. Seven patients (70%) had at least one abnormal manifestation of vasomotor dysfunction in response to ACH, of whom five (50%) were severely impaired at either the epicardial artery or the microcirculation. Median coronary flow reserve, measured in response to adenosine, was 2.2 (IQR 2.0, 3.4). Six (60%) had a coronary flow reserve below the normal value of 2.5. Nine patients (90%) had at least one abnormal measure of microvascular vasomotion in response to either ACH or adenosine.
The microvascular response to ACH in the two patients with recurrence (peak change in blood flow –8.1% and 17.3 %) was more impaired than those without a recurrence of ABS (43.5±35.7%; (Figure 3). Similarly, microvascular endothelium-independent coronary flow reserve in the two patients with recurrence (1.9 and 2.0) was more impaired than those without a recurrence (2.7±0.3). The median increase in epicardial diameter with intracoronary nitroglycerin in all 10 ABS patients was 24.8% (IQR 5.9, 27.4).
Figure 3.
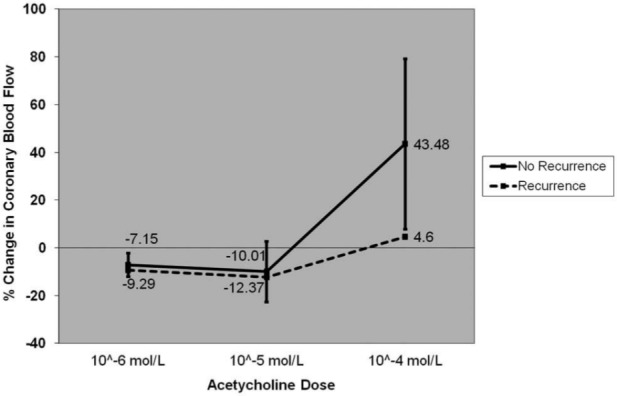
Dose–response curve for change in coronary blood flow in response to the three doses of acetylcholine in patients with and without recurrence of ABS.
Values are mean±standard error.
Discussion
The major and novel findings of this study among patients with a history of ABS are that: (i) the great majority have abnormal coronary vasomotor response to ACH; (ii) most (90%) patients have microvascular dysfunction which is frequently severe; and (iii) the magnitude of vasomotor dysfunction is greater in the microcirculation than the epicardial arteries. Thus, the current study suggests a potential primary role of coronary microvascular dysfunction in the pathophysiology of ABS.
Using non-invasive assessment of the peripheral endothelial function, we have previously demonstrated that patients with a history of ABS have abnormal microvascular vasoreactivity in response to acute mental stress leading to excessive vasoconstriction and impaired endothelium-dependent dilation during reactive hyperaemia.5 The present study extends these observations to the coronary circulation by demonstrating impaired responses to ACH with preserved epicardial responses to nitroglycerin suggesting the presence of endothelial dysfunction. These findings are underscored by a previous study which demonstrated that the coronary blood flow response to mental stress correlated with the response to acetylcholine.10 The impairment in microvascular function in our ABS cohort may be partially explained by the postmenopausal status and high prevalence of cardiovascular risk factors. However, previous studies have not demonstrated a strong relationship between these risk factors and microvascular blood responses to ACH or adenosine.8,11 Indeed, we have previously reported among a local Olmsted county cohort of ABS patients that the prevalence of cardiovascular risk factors in ABS patients is similar to that of an age- and gender-matched control group of patients with a history ST-segment elevation myocardial infarction.12 Thus, the presence of the traditional risk factors may not be the only explanation for the high frequency of abnormal ACH-mediated vasomotion in the ABS patients.
A striking characteristic of our ABS patients was the severity of impairment microvascular vasodilation with ACH (Figure 2). Virtually all patients had either no increase in blood flow or a reduction at the first two doses of ACH, possibly due to endothelial dysfunction at the microcirculation level. To further illustrate the severity of microvascular dysfunction in our cohort of ABS patients, Figure 2 shows reference data from 211 female patients who have had coronary vasomotor function testing in our laboratory and found to have normal blood flow response to ACH. The severity of epicardial vasomotor dysfunction in response to ACH in our ABS patients was relatively less, with three patients demonstrating severe vasoconstriction. These findings are consistent with that of Tsuchihashi and colleagues and Sato and colleagues who have previously reported that epicardial ‘coronary spasm’ was provoked in 10/48 (21%)13 and 8/35 (23%)14 of patients tested with ACH, although unlike our study they did not provide detailed measurement of the magnitude of vasoconstriction nor did they assess microvascular function. Overall, these data support the hypothesis that abnormal coronary vasomotion is present in patients with ABS and may play a role in the pathophysiology.
In conjunction with the abnormal microvascular responses to ACH, we also observed that microvascular endothelium-independent vasodilator response to adenosine were impaired with a mean coronary flow reserve of 2.2 (normal >2.5). Thus, 90% of our patients had evidence of microvascular dysfunction (abnormal response to ACH and/or adenosine) when tested at median duration of approximately 5 months following the acute presentation with ABS. The significance of this finding is highlighted by the fact that microvascular function was more impaired in the two patients who sustained a recurrent event compared to those who did not. These data are consistent with prior observations that coronary microvascular function is significantly abnormal during an acute episode of ABS.15 Using angiographic techniques such as myocardial blush grade, microvascular dysfunction has been detected in at least two-thirds of the patients at the time of presentation, and its severity correlates with the magnitude of troponin elevation and ECG abnormalities.15 Similarly, TIMI (Thrombolysis in Myocardial Infarction) frame count is prolonged in all three major epicardial coronary vessels in the acute setting.16,17 Single photon-emission computed tomography using thallium and sestamibi tracers and positron-emission tomography using 13N-ammonia have also consistently demonstrated impaired perfusion in the regions of the wall motion abnormality.17,18 Moreover, our study makes the important observation that coronary microvascular dysfunction is present well beyond the acute phase, even after recovery of left ventricular function has occurred. This is consistent with the finding by Barletta and colleagues19 that the cold pressor test, performed at a median duration of 688 days following the acute event, among patients who had recovered from ABS did not increase coronary blood flow. In aggregate, these data suggest that coronary microvascular dysfunction may play an important role in the pathophysiology of ABS rather than simply be an epiphenomenon.
Limitations
This is a single-centre retrospective study and hence subject to potential limitations of such analyses; however, the ABS cases were identified prospectively. Only a small proportion (<5%) of patients with ABS at our institution underwent coronary vasomotor function testing, and hence our findings must be interpreted as preliminary and require validation in other cohorts. Nevertheless, the comprehensive analysis of vascular function is unique and performed by experienced operators at a centre where such studies are performed routinely as part of clinical practice. Since the coronary physiological assessment was performed after the episode of ABS, we cannot be certain as to whether the abnormality on coronary vasomotion detected was present prior to the onset of the cardiomyopathy. Our study cannot elucidate whether the impairment in the vasomotor response to ACH were exclusively due to endothelia dysfunction or the coexistence of vascular smooth muscle hyperreactivity.
Conclusions
Our data suggests that impaired vascular reactivity, especially involving the microcirculation, may contribute to the ischaemic symptoms and cardiac dysfunction that characterizes ABS. Studies with therapeutic interventions that modify vascular function would be of interest in order to establish whether recovery during an acute episode or recurrence rates may be modifiable. Preliminary evidence for this as a possibility has recently been provided by a study demonstrating that microvascular dysfunction during the acute phase of ABS can be temporarily reversed by an infusion of adenosine and is associated with improvement in myocardial function.20
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation 2007; 115: e56–e59 [DOI] [PubMed] [Google Scholar]
- 2. Sato H TH, Uchida T, Dote K, et al. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary artery spasm. In: Kodama KHK, Hori M. (eds) Clinical aspect of myocardial injury: from ischemia to heart failure. Tokyo, Japan: Kagakuhyoronsha Publishing, 1990, pp.56–64 [Google Scholar]
- 3. Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010; 55: 333–341 [DOI] [PubMed] [Google Scholar]
- 4. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004; 141: 858–865 [DOI] [PubMed] [Google Scholar]
- 5. Martin EA, Prasad A, Rihal CS, et al. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol 2010; 56: 1840–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008; 155: 408–417 [DOI] [PubMed] [Google Scholar]
- 7. Hasdai D, Gibbons RJ, Holmes DR, Jr, et al. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation 1997; 96: 3390–3395 [DOI] [PubMed] [Google Scholar]
- 8. Rubinshtein R, Yang EH, Rihal CS, et al. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur Heart J 2010; 31: 936–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000; 101: 948–954 [DOI] [PubMed] [Google Scholar]
- 10. Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med 1991; 325: 1551–1556 [DOI] [PubMed] [Google Scholar]
- 11. Han SH, Bae JH, Holmes DR, Jr, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J 2008; 29: 1359–1369 [DOI] [PubMed] [Google Scholar]
- 12. Summers MR, Lennon RJ, Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J Am Coll Cardiol 2010; 55: 700–701 [DOI] [PubMed] [Google Scholar]
- 13. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001; 38: 11–18 [DOI] [PubMed] [Google Scholar]
- 14. Sato A, Aonuma K, Nozato T, et al. Stunned myocardium in transient left ventricular apical ballooning: a serial study of dual I-123 BMIPP and Tl-201 SPECT. J Nucl Cardiol 2008; 15: 671–679 [DOI] [PubMed] [Google Scholar]
- 15. Elesber A, Lerman A, Bybee KA, et al. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J 2006; 152: 469.e9–e13 [DOI] [PubMed] [Google Scholar]
- 16. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004; 94: 343–346 [DOI] [PubMed] [Google Scholar]
- 17. Kurisu S, Inoue I, Kawagoe T, et al. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am Coll Cardiol 2003; 41: 743–748 [DOI] [PubMed] [Google Scholar]
- 18. Bybee KA, Murphy J, Prasad A, et al. Acute impairment of regional myocardial glucose uptake in the apical ballooning (takotsubo) syndrome. J Nucl Cardiol 2006; 13: 244–250 [DOI] [PubMed] [Google Scholar]
- 19. Barletta G, Del Pace S, Boddi M, et al. Abnormal coronary reserve and left ventricular wall motion during cold pressor test in patients with previous left ventricular ballooning syndrome. Eur Heart J 2009; 30: 3007–3014 [DOI] [PubMed] [Google Scholar]
- 20. Galiuto L, De Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical ballooning or Tako-Tsubo syndrome. Eur Heart J 2010; 31: 1319–1327 [DOI] [PubMed] [Google Scholar]


